Abstract
In most animals, multiple genes encode protein kinase C (PKC) proteins. Pharmacological studies have revealed numerous roles for this protein family, yet the in vivo roles of specific PKC proteins and the functional targets of PKC activation are poorly understood. We find that in C. elegans, two PKC genes, pkc-1 and tpa-1, are required for mechanosensory response; the role of the nPKCε/η ortholog, pkc-1, was examined in detail. pkc-1 function is required for response to nose touch in adult C. elegans and pkc-1 likely acts in the interneurons that regulate locomotion which are direct synaptic targets of mechanosensory neurons. Previous studies have suggested numerous possible targets of pkc-1; our analysis indicates that pkc-1 may act via the ERK/MAPK pathway. We find that ERK/MAPK pathway function is required for mechanosensory response in C. elegans and that at least one component of this pathway, lin-45 Raf, acts in interneurons of the mechanosensory circuit. Genetic analysis indicates that these lin-45 and pkc-1 act together to regulate nose touch response. Thus, these results functionally link two conserved signaling pathways in adult C. elegans neurons and define distinct roles for PKC genes in vivo.
Keywords: C. elegans, PKC-1, protein kinase C, mechanosensation, MAPK, behavior, nose touch
Introduction
The mammalian protein kinase C family consists of ten serine/threonine kinases that play critical roles in cell signaling, survival and development (Newton, 1995, Nishizuka, 1995). Different PKC proteins (also known as isozymes) are placed into three groups based on sequence homology and biochemical characteristics: 1) conventional PKCs (cPKC) that require calcium and diacylglycerol (DAG) for activation, 2) novel PKCs (nPKC) that require DAG but not calcium and 3) atypical PKCs that do not respond to calcium or DAG levels for activation (Nishizuka, 1992).
Mammalian gene knockout studies have identified a few specific roles for conventional and novel PKC proteins in the nervous system. For example, loss of function of PKCγ leads to moderate spatial learning defects (Abeliovich et al., 1993) while both PKCγ and PKCε are critical for pain sensation and sensitivity (Aley et al., 2000, Cesare et al., 1999, Khasar et al., 1999, Malmberg et al., 1997). However, the majority of studies addressing neuronal PKC or the function of downstream targets utilize phorbol ester compounds; these compounds mimic the actions of DAG by binding and activating proteins containing DAG binding domains. As the majority of PKC proteins contain DAG binding domains, this technique can obscure specific roles for individual proteins in a particular pathway. To investigate individual roles of PKC proteins in vivo, we turned to the nematode C. elegans as a model system in which the functional roles for specific genes and proteins can be readily assessed.
Here, we address the role of PKC genes in the response to light touch to the nose (nose touch) - a noxious stimulus that evokes backward locomotion. In C. elegans, the bilaterally symmetric ASH sensory neurons detect nose touch, high osmolarity and other noxious stimuli (Bargmann et al., 1990, Hart et al., 1999, Hilliard et al., 2002, Kaplan & Horvitz, 1993, Sambongi et al., 2000, Troemel et al., 1997). The direct synaptic targets of the glutamatergic ASH sensory neurons include interneurons that are critical for C. elegans locomotion (Chalfie et al., 1985, White et al., 1986). Via electrical connections, these interneurons synapse directly on motorneurons that drive muscle activity in both basal and stimulus-evoked locomotion programs (Chalfie et al., 1985, White et al., 1986). In this circuit, genetic encoding of ASH-mediated aversive stimuli occurs both at the sensory neuron level (Fukuto et al., 2004, Hilliard et al., 2004, Walker et al., 2009) as well as at the interneuron level. Notably, in the interneurons, only the AMPA/kainate glutamate receptor subunits glr-1 and glr-2 and genes that regulate the expression of these receptors have been implicated in stimulus encoding (Hart et al., 1995, Maricq et al., 1995, Mellem et al., 2002, Rongo et al., 1998, Wang et al., 2008, Zheng et al., 2004). Additional genes in the interneurons likely play differential roles in stimulus response.
The C. elegans genome encodes four PKC genes: pkc-1, tpa-1, pkc-2 and pkc-3; pkc-1 and tpa-1 have been identified in C. elegans behaviors (Kindt et al., 2007, Okochi et al., 2005, Sieburth et al., 2007). For the nose touch neuronal circuit, expression pattern analysis and phenotypic rescue studies suggested that pkc-1 acts at two sites in this mechanosensory circuit (Land et al., 1994a, Okochi et al., 2005, Sieburth et al., 2007). Cell-specific rescue experiments demonstrated that restoring pkc-1 function in the ASH sensory neurons ameliorated defects in avoidance of high osmolarity, while pkc-1 function in the motor neurons is required in regulating dense core vesicular release (Okochi et al., 2005, Sieburth et al., 2007).
Herein, we reexamine the role of PKC-1 in ASH-mediated sensory responses. We demonstrate that pkc-1 function is specifically required in interneurons of adult animals for appropriate response to nose touch. We find that components of the ERK/MAPK signaling pathway are required for this behavior and that pkc-1 acts with at least one member of this pathway for touch response. Our results suggest a link between these two pathways that impacts neuronal signaling and sensory response.
Materials and Methods
Strains
Strains were maintained under standard conditions (Brenner, 1974). Strains utilized in this study include: N2 Bristol wild type, KP212 glr-1(n2461), HA1465 pkc-1(rt144), HA1898 pkc-1(ok563) 6x, IK130 pkc-1(nj3), KP3324 pkc-1(nu448), MJ563 tpa-1(k530), VC127 pkc-2(ok328), HA1796 tpa-1(k530); pkc-1(ok563), HA1795 pkc-1(ok563); pkc-2(ok328), HA1804 tpa-1(k530); pkc-1(ok563); pkc-2(ok328), HA1824 tpa-1(k530); pkc-2(ok328), VM1854 glr-2(ak10), HA1776 glr-1(n2461); pkc-1(ok563), HA1777 glr-2(ak10)/qC1; pkc-1(ok563), KP1580 nuIs25 [lin-15(+) glr-1p::glr-1::GFP], HA1719 nuIs25; pkc-1(ok563), HA1798 nuIs25; tpa-1(k530); pkc-1(ok563); pkc-2(ok328), PS5131 let-23(sy12)/mIn1, MT4866 let-60(n2021), MT8667 mek-2(n2678)/sup-11(n403) dpy-5(e61), EJ521 lin-45(dx19)/nT1, BS3347 lin-45(dx84)/nT1, HA1882 lin-45(dx19)/mIs11; pkc-1(ok563), HA1910 lin-45(dx84)/mIs11; pkc-1(ok563), BS3830 mpk-1(ga117)/qC1 KP1097 dgk-1(nu62), PS2627 dgk-1(sy428), HA1716 pkc-1(ok563); dgk-1(nu62), HA1721 pkc-1(ok563); dgk-1(sy428), TQ17 trp-1(ok323), TQ194 trp-2(sy691), HA1805 trp-2(sy691) trp-1(ok323), TM1811 mgl-1(tm1811), TM355 mgl-2(tm355), HA2117 to HA2120 pha-1(e2123); rtEx695 to rtEx698[pha-1(+), glr-1p::pkc-1(RNAi), glr-1p::tpa-1(RNAi)], HA2112 to HA2116 pha-1(e2123); rtEx690 to rtEx694[pha-1(+), glr-1p::pkc-1(RNAi), che-2p::tpa-1(RNAi)], HA2117 to HA2120 pha-1(e2123); rtEx695 to rtEx698[pha-1(+), glr-1p::pkc-1(RNAi), glr-1p::tpa-1(RNAi)], HA2121 and HA2122 pha-1(e2123); rtEx699 and rtEx700[[pha-1(+), glr-1p::pkc-1(RNAi), che-2p::tpa-1(RNAi). A small percentage of pkc-1(ok563) animals were sterile, consistent with previous RNAi results (Govindan et al., 2006).
pkc-1(rt144) genetic mapping
pkc-1(rt144) was identified as a spontaneous mutation in the genetic background of CB4856, a C. elegans mapping strain. pkc-1(rt144) animals are defective in nose touch response and resistant to serotonin-induced immobilization in swimming assays (Ranganathan et al., 2000). The latter phenotype was used only for initial mapping studies (Hyde, 2008). rt144 mapped to chromosome V using unc-60 dpy-11; 6/6 nonUnc nonDpy lines exhibited rt144 phenotypes. Three factor crosses were performed to refine the location of the rt144 allele. Dpy nonUnc progeny were picked from the unc-60 dpy-11 cross and 8/8 animals did not carry the rt144 mutation. 2/2 Unc nonDpy progeny carried the rt144 mutation. This indicated that the rt144 allele was located to the right of dpy-11.
As the rt144 allele was identified in the CB4856 genetic background, recombinant lines from three factor crosses were assessed for the presence or absence of CB4856 single nucleotide polymorphisms (SNPs). rt144 males were crossed with dpy-11 unc-76 animals; the rt144 region was narrowed down to base pairs 12,000,807 and 12,034,227 on chromosome V using SNP analysis from eight lines. Sequencing this region revealed that pkc-1(rt144) contained a G to A transition at 1502 bp in pkc-1B cDNA. pkc-1(rt144) was backcrossed eight times before undertaking the behavioral analysis described herein.
Behavioral assays
Nose touch response assays were performed as previously described (Kaplan & Horvitz, 1993). Ten to fifteen well-fed young adult animals were transferred from the bacterial lawn of an uncrowded plate to an NGM plate prepared as follows: OP50 was grown overnight at 37 degrees without shaking to an OD value between 0.2 and 0.4. 100μl of this OP50 was placed at the center of the plate. This plate was dried under a laminar flow hood for approximately 20 min to 1 hour. Plates that took less than 20 min to dry were discarded. These assay plates were allowed to sit on the bench for 10 minutes before transferring animals. All animals were grown at 23-25 °C on standard NGM plates, unless otherwise indicated. The thick end of a paintbrush hair (Loel-Cornell 9000 Kolinsky 7, Teaneck NJ) was taped to the wooden end of a cotton swab. The tapered end of the hair was placed in front of an animal as it moved forward on the assay plate, perpendicular direction of movement. If the animal failed to initiate backward locomotion before crossing the hair, response was scored as no response; each animal was assayed ten times.
Octanol response assays were performed as previously described (Chao et al., 2004). For response to high osmolarity, assays were performed as follows: A 3/8th inch diameter ring of 8M glycerol was made at the center of an unseeded NGM plate. Five well-fed young adult animals were placed in the center of the ring and the average time for animals to escape was measured. Assays were halted at the 15 min. mark due to diffusion of the 8M glycerol ring. Animals that did not escape the ring were assigned an escape time of 15 min. While pkc-1(nj3) responses to high osmolarity are significantly different than wild type responses, the magnitude of response was different than previously reported by Okochi et al., 2005. These differences may be attributed to slight variations in assays and quantification methods. In contrast to our methods, Okochi et al., utilized ten animals were used in the osmotic ring assay and numbers of animals remaining within the ring were scored after 3, 6, 9, 12, and 15 min.
All behavioral assays were repeated on at least two separate days in parallel with controls where the experimenter was blinded to genotype and/or treatment for at least one trial. For each genotype, 20-45 animals were assayed, unless otherwise noted. For pairwise comparisons, the Student’s t-test was used. For multigroup comparisons, one-way ANOVA analysis was used in combination with Fisher’s planned least significant difference (PLSD) in which α was set to p≤0.05. StatPlus for Macintosh was used for statistical analysis (AnalystSoft, Vancouver, British Columbia, Canada).
PMA treatment
Plates containing phorbol 12-myristate 13-acetate (PMA; Sigma) were made as previously described (Miwa et al., 1982). Before the nose touch assay, animals were placed on E. coli (OP50) seeded plates containing 1 μg/ml PMA or DMSO (vehicle). After a 2 hr incubation period, animals were transferred to nose touch assay plates.
Glutamate receptor localization
Animals were immobilized with 2,3-butanedione monoxamine (30 mg/mL; Sigma) prior to imaging. Digital images of young adult animals were acquired and processed using Metamorph 4.5 software (Universal Imaging) as described (Burbea et al., 2002). Intensity values were normalized to the background fluorescence of each slide. Puncta intensities, cord intensities, puncta widths, and puncta densities were measured within 60–70 μm of the ventral nerve cord posterior to the RIG neuron cell bodies using custom-written software (Burbea et al., 2002). Student’s t-test was used for statistical analysis.
Plasmid Construction
For construction of RNAi plasmids, PCR amplication of cDNA or exons of genomic DNA was performed. Targeted regions were compared to other C. elegans sequences to eliminate the possibility of multigenic knockdown. Forward and reverse primers were engineered with restriction enzyme sites such that when the PCR fragments were inserted into plasmids, the fragments were in an antisense orientation with respect to the promoter. The Fire vector pPD49.26 containing sra-6, che-2 or glr-1 promoters was chosen as the plasmid. For pkc-1, a 461bp fragment corresponding to bases 31-492 in pkc-1B cDNA was amplified by PCR from cDNA. The forward primer contained a KpnI site, while the reverse primer contained a NheI site. For lin-45, a 340bp fragment corresponding to bases 300-639 of lin-45 cDNA was amplified from cDNA using a forward primer containing NcoI and a reverse primer containing NheI. For tpa-1, a 502 bp fragment amplified from genomic DNA was generated using a forward primer containing a SacI site and a reverse primer containing a NheI site. This region, which corresponds to chromosome IV, bases 105652-106153, contains no introns. PCR amplified DNA cloned into plasmids was sequenced.
The pkc-1(gf) cDNA which creates A160E in PKC-1B was kindly provided by Y. Okochi and I. Mori. The pkc-1(gf) fragment was inserted into a pPD49.26 vector containing the glr-1 promoter (pKP#1380) using NheI and KpnI creating plasmids pHA#500. Strains carrying this transgene were lost prior to freezing.
Transgenesis
Transgenic animals were generated as described (Mello et al., 1991). Rescuing plasmids were injected at 30 ng/μl, RNAi plasmids and glr-1p::pkc-1(gf) were injected at 75ng/μl. 15–20 ng/μl of pPD48.33 myo-2p::GFP or 120 ng/μl of pBX#1 pha-1(+) were used as co-injection markers. Neither myo-2p::GFP expression nor pBX#1 interferes with nose touch response. myo-2p::GFP was used in all transgenic lines except for RNAi studies, where pBX#1 was used. Transgenic animals were recognized by GFP fluorescence in the pharynx due to myo-2p::GFP expression or viability due to pha-1 rescue. Multiple independent transgenic lines were established from each injection. Information on each plasmid is available upon request. No lethality, sterility, or vulval defects were observed in glr-1p::lin-45(RNAi) animals suggesting that dsRNA does not spread outside of the glr-1 expressing interneurons and consistent with previous results (Chao et al., 2005).
Heat shock experiments
Animals were raised at 23°C and shifted to 33°C at the L4 stage for 2 hours twice with a 30 min recovery interval at 23°C between heat shocks. Animals were grown at 23°C for 9 hrs prior to behavioral studies.
Results
pkc-1 function is required for nose touch response
When C. elegans encounter a barrier perpendicular to the nose during forward locomotion, animals halt and initiate backward locomotion; this behavior is called nose touch response (Kaplan & Horvitz, 1993). We identified a spontaneous mutation, rt144 that dramatically decreased response to nose touch (Figs. 1a,b). rt144 is recessive and a missense allele of C. elegans pkc-1 (protein kinase C-1) gene that converts arginine 501 into glutamine. This arginine is a part of the highly conserved sequence RDLKPEN found in serine/threonine kinases (Hanks et al., 1988, Taylor et al., 1993). Perturbation of the cognate arginine in PKA dramatically decreases reduces the kinase activity in mammalian models (Gibbs & Zoller, 1991); therefore, pkc-1(rt144) likely decreases or eliminates pkc-1 function in C. elegans.
Figure 1. pkc-1 function is required for response to nose touch.
(A) Predicted pkc-1 splice variants and allele changes. Exons and introns are shown as colored boxes and lines, respectively. The corresponding location of amino acid changes are indicated by arrowheads. The pkc-1 locus encodes two isoforms, pkc-1A and pkc-1B (designated A and B). PKC-1A is 56 amino acids longer at the N terminus and the corresponding genomic DNA contains a 3.2 kb intron. The ok563 deletion creates a 1.3 kb deletion as indicated. The nj3 allele results in a nonsense mutation in the C1 domain. rt144 corresponds to a missense mutation in the kinase domain, while the nu448 allele creates a nonsense mutation in the kinase domain resulting in a truncated protein (Okochi et al., 2005, Sieburth et al., 2007). (B) pkc-1 loss of function results in defective response to nose touch. Percent response to nose touch was determined for pkc-1 mutant and control animals (wild type). glr-1(n2461) animals were included for comparison in all trials (12 ± 2% response). (C) Two pkc-1 loss of function mutations lead to minor defects in response to octanol. Control animals and pkc-1 loss of function alleles were tested for response to 100% octanol after a 10 min. incubation off food. osm-11(rt142) animals were included for comparision in all trials (average time to escape =19.1 ± 0.6 sec.). (D) Some pkc-1 alleles exhibit modest osmotic avoidance defects. Control and pkc-1 mutant animals were tested for response to high osmolarity using an 8M glycerol ring. osm-10(n1602) animals were included for comparison in all trials (6.7 ± 1.3 min. escape rate). Each error bar represents S.E.M. *p<0.001 as compared to wild type using Student’s t-test.
The predicted C. elegans PKC-1 protein is most similar to vertebrate PKCε/η (Sieburth et al., 2007) and pkc-1 likely encodes two splice isoforms, PKC-1A and PKC-1B, which differ by a 56-amino acid N-terminal extension in PKC-1A (Land et al., 1994b). To confirm that the behavioral defects of pkc-1(rt144) animals are caused by diminished pkc-1 function, we tested previously characterized pkc-1 loss of function alleles. The pkc-1(nu448) and pkc-1(nj3) nonsense alleles result in premature truncation of PKC-1 translation (Okochi et al 2005; Sieburth et al 2007). A deletion allele, pkc-1(ok563), that removes the 5′ UTR of pkc-1B including the ATG translational start site was also examined. All pkc-1 alleles resulted in nose touch response defects (Fig. 1b) indicating that pkc-1 is required for normal response to nose touch.
As the circuit responsible for nose touch response is required for responses to other sensory stimuli, the ability of pkc-1 animals to respond to 1-octanol and high osmolarity was assessed. The ok563 and rt144 alleles result in modest octanol avoidance defective phenotypes, while pkc-1(nj3) and pkc-1(rt144) animals exhibit slight defects in osmotic avoidance (Figs. 1c and 1d; Okochi et al., 2005). Although all 4 pkc-1 alleles are expected to dramatically reduce gene function, they differ in their impact on nose touch response, octanol and high osmolarity avoidance. Sensitivity to residual pkc-1 gene function may differ in various cells and functional assays. Additionally, the corresponding PKC-1 protein fragments may vary dramatically in their interactions with various target or effector proteins. As the magnitude of the osmotic and chemosensory defects was modest in our hands, therefore analysis herein was restricted to pkc-1 loss of function nose touch defects.
pkc-1 function is required primarily in interneurons for response to nose touch
pkc-1 is broadly expressed in the nervous system and is found in sensory neurons, interneurons and motor neurons (Land et al., 1994a, Okochi et al., 2005, Sieburth et al., 2007). To determine where pkc-1 function is required for nose touch response, three promoters with non-overlapping expression patterns (sra-6, glr-1 and unc-17) were used to drive pkc-1 cDNA expression in neurons in the nose touch circuit (Fig. 2a). The sra-6 promoter drives gene expression in the ASH and ASI sensory neurons as well the PVQ interneurons (Troemel et al., 1997); only the ASH sensory neurons affect mechanosensory responses to nose touch (Hilliard et al., 2005, Kaplan & Horvitz, 1993). The glr-1 promoter drives expression in 17 classes of neurons, including the interneurons responsible for locomotion (Hart et al., 1995, Kaplan & Horvitz, 1993, Maricq et al., 1995); we will refer to this subset of neurons as “interneurons.” The unc-17 promoter drives gene expression in motor neurons required for response to nose touch (Alfonso et al., 1993, Driscoll & Kaplan, 1996). Expressing pkc-1B cDNA in the sensory neurons using the sra-6 promoter partially restored nose touch response to pkc-1(ok563) animals, as responses in these transgenic animals are significantly different as compared to wild type and pkc-1(ok563) strains (Fig. 2b). In contrast, expression of the pkc-1B cDNA in interneurons of the circuit in pkc-1(ok563) animals led to nose touch responses that do not significantly differ from wild type responses (p=0.66, Fig. 2b). Expression of the pkc-1 cDNA in motor neurons using the unc-17 promoter did not improve nose touch response in pkc-1(ok563) animals (Fig. 2b, p=0.09). Taken together, these results indicate that pkc-1 function in interneurons is sufficient for nose touch response, but expression in sensory neurons is sufficient to partially restore response.
Figure 2. PKC-1 is required in interneurons of the nose touch circuit.
(A) Schematic diagram of the nose touch circuit. Triangles represent sensory neurons, octagons represent interneurons, and rectangles represent motor neurons. The sra-6 promoter drives gene expression in ASH (in red) and other sensory neurons, the glr-1 promoter drives expression in 17 classes of neurons including interneurons that drive locomotion (AVB, PVC, AVA, AVD, AVE in blue) and the unc-17 promoter drives expression in a subset of motor neurons (including DB, VB, DA, VA in green). Arrowheads indicate chemical synapses and circles represent gap junctions.
(B) Expression of pkc-1 cDNA in either sensory neurons or interneurons restores nose touch response. pkc-1B cDNA results are shown; similar results were seen with pkc-1A constructs (data not shown). Introduction of promoters alone does not impact nose touch response as sra-6p::empty and glr-1p::empty did not restore responses in pkc-1(ok563) animals (36 ± 3% and 29 ± 3%, respectively). glr-1(n2461) animals were included for comparison (7 ± 2%). ANOVA analysis indicates a significant difference between groups, p<0.001; For strain comparisons, *p<0.002 vs. wild type, #p<0.001 vs. pkc-1(ok563) using Fisher’s PLSD. (C) pkc-1 RNAi knockdown in the interneurons recapitulates pkc-1 loss of function defects. ANOVA analysis reveals a significant difference between groups, p<0.001; For strain comparisons, *p<0.001 vs. wild type. glr-1(n2461) animals were included for comparison (9 ± 2%). (D) pkc-1 function in adult animals is sufficient for nose touch response. Expressing pkc-1B cDNA under the control of the heat shock promoter in pkc-1(ok563) animals restored response 9 hrs post heat shock. Restored responses were maximal at the 9 hr time point. Heat shock does not affect responses in wild type (p=0.94), pkc-1(ok563) (p=0.58) or glr-1(n2461) controls (15 ± 3% non-heat shocked, 14 ± 2% heat shocked, p=0.80). ANOVA analysis indicates a significant difference between groups, p<0.001. Red bars represent heat shocked animals, black bars indicate non-heat shocked animals. Each error bar represents S.E.M.
It is possible that the large number of pkc-1 transgene copies on the rescuing extrachromosomal arrays might lead to inappropriately high levels of pkc-1 expression and consequently, misleading phenotypic rescue due to aberrantly high expression. Additionally, ectopic expression of pkc-1 in neurons where it is not normally expressed could also cause gain-of-function effects. To avoid these problems and as an alternative approach, RNA interference was used to selectively knock down pkc-1 in the interneurons of the nose touch circuit. A pkc-1 cDNA fragment expressed in the antisense orientation was used for RNAi knockdown; similar strategies have been successful in knocking down neuronal gene expression (Esposito et al., 2007, Ferkey et al., 2007, Tavernarakis et al., 2000). Reducing pkc-1 expression in the interneurons resulted in nose touch defects comparable to pkc-1(lf) animals, as these two responses are not statistically different (p=0.21, Fig. 2c). The effects of RNAi using this promoter are specific to glr-1 expressing cells based on previous studies (Chao et al., 2005). In addition, the che-2 sensory neuron specific promoter was used to knockdown pkc-1 in the ASH sensory neurons; no nose touch response defects were induced (p=0.52 vs. wild type, Fig. 2c). The pkc-1(RNAi) and the cell-specific rescue results described above indicate that pkc-1 is required in the interneurons for mechanosensory response. This is a new site of action for pkc-1, as studies of other behaviors found a requirement for pkc-1 function in the ASH sensory neurons and the motor neurons (Okochi et al., 2005, Sieburth et al., 2007).
pkc-1 function is required in adult animals
The nose touch response defect seen in pkc-1(lf) animals could be due to developmental changes in neuronal cell fate or connectivity. To demonstrate that pkc-1 function is required in adult animals, the full-length pkc-1B cDNA was placed under the control of a heat shock inducible promoter (Stringham et al., 1992) and introduced into pkc-1(ok563) animals. The neurons of the nose touch circuit are born and become functional in the embryo or first larval stage of development (White et al., 1986). Therefore, pkc-1 expression was induced at the final fourth stage (L4) and young adult animals were assayed 9 hrs later. Heat shock induction significantly restored touch response as compared to transgenic animals without heat shock treatment; heat shock did not alter nose touch response in non-transgenic animals (Fig. 2d). We conclude that restoring pkc-1 function in late larval or adult stages is sufficient for this behavior. The normal morphology and development of the nose touch circuit in pkc-1 animals was also confirmed by examining the sensory neurons and interneurons using dye-filling and GLR-1::GFP expression (Perkins et al., 1986, Rongo et al., 1998). No changes in morphology, location, axonal projections, or the number of neurons was observed in pkc-1(ok563) animals (data not shown). Combined, these results indicate that pkc-1 acts in adult neurons to mediate nose touch response.
Role of diacylglycerol in nose touch response
C. elegans pkc-1 is predicted to encode a PKC that requires diacylglycerol (DAG) for activation (Konno et al., 1989, Ohno et al., 1988). Previous studies have demonstrated that DAG regulates PKC-1 activity in vitro and in vivo (Land et al., 1994a, Okochi et al., 2005, Sieburth et al., 2007). Diacylglycerol kinases (DGKs) convert DAG into phosphatidic acid (Van Blitterswijk & Houssa, 2000). C. elegans dgk-1 is expressed in most, if not all neurons (Nurrish et al., 1999), and dgk-1 loss of function phenotypes can be mimicked by phorbol ester application or mutations that result in constitutive PKC-1 activation (Okochi et al., 2005, Sieburth et al., 2007). To determine if increased DAG levels would increase nose touch response, we examined the effect of PMA, a phorbol ester, and the nose touch responses of two strains: dgk-1 loss of function animals and transgenic animals that express a gain-of-function form of PKC-1 (A160E) in the interneurons. The latter strain will be referred to herein as pkc-1(gf). None of these manipulations increased nose touch response in otherwise wild type animals (Tables 1, 2). We also examined pkc-1(gf) and dgk-1 in a related behavioral paradigm: response to nose touch after repeated exposure. Nose touch responses of wild type animals decrease with repeated stimuli (Hart et al., 1999); it is unclear if this is due to habituation or adaptation. Similar nose touch response decrements due to repetitive stimulation were observed in dgk-1(nu62) and pkc-1(gf) animals (p=0.95 and 0.09 respectively; Table 2). dgk-1(nu62) and pkc-1(gf) animals were indistinguishable from wild type animals in both paradigms, suggesting that expressing an activated pkc-1 or increasing DAG levels through loss of dgk-1 did not impact nose touch sensitivity. (Note that the level of PKC-1(GF) protein expression was not assessed here.)
Table 1.
Phorbol esters partially restore pkc-1 nose touch response
| Genotype | PMA untreated (% response) |
PMA treated (% response) |
p-value |
|---|---|---|---|
| wild type | 80 ± 3 | 80 ± 4 | 0.87 |
| pkc-1(ok563) | 43 ± 5 | 59 ± 5 | 0.005 |
|
tpa-1(k530);
pkc-1(ok563) |
19 ± 5 | 11 ± 3 | 0.29 |
Table 2.
Loss of dgk-1 or increased PKC-1 activity does not impact nose touch response
| Genotype | Naïve (% response) |
After 30 trials (% response) |
|---|---|---|
| wild type | 85 ± 5 | 43 ± 10 |
| pkc-1(gf) | 86 ± 5 | 52 ± 6 |
| dgk-1(nu62) | 82 ± 4 | 39 ± 7 |
| pkc-1(ok563) | 50 ± 9 | 14 ± 4 |
|
pkc-1(ok563);
dgk-1(nu62) |
50 ± 9 | 27 ± 8 |
The relationship between dgk-1 and pkc-1 was also examined in double mutant animals. Responses of dgk-1;pkc-1 animals were similar to pkc-1 animals, indicating that dgk-1 loss is not sufficient to suppress pkc-1 loss of function defects in nose touch response (p=0.24, Table 2). Thus, dgk-1 likely does not play a role in pkc-1 modulation of nose touch response.
pkc-1 acts redundantly with tpa-1, the other C. elegans novel PKC
Direct pharmacological activation of PKC-1 by phorbol esters might have more profound effects than genetic manipulation due to transgene expression levels and genetic redundancy. Using phorbol esters, we determined whether DAG solely acts through PKC-1 to modulate nose touch response. If this is the case, phorbol ester treatment of pkc-1 animals should not restore response. However, PMA treatment of pkc-1(lf) animals partially restored nose touch response (Table 1). This rescue might be due to the semi-redundant function of other C. elegans PKCs. The C. elegans genome contains three additional PKC genes, tpa-1, pkc-2 and pkc-3. tpa-1 and pkc-1 act semi-redundantly in other behaviors (Okochi et al., 2005). Loss of function of pkc-3 causes embryonic lethality, thus this gene was not examined further. Loss of function of tpa-1 moderately decreased nose touch response (p<0.001 as compared to wild type), while loss of pkc-2 did not affect response (p=0.97 as compared to wild type) (Fig. 3a). To assess redundancy, double and triple mutant strains for these genes were generated and nose touch response was examined. The tpa-1; pkc-1 animals were significantly worse in their responses than either tpa-1 or pkc-1 singe mutant animals (p<0.001), while the triple tpa-1; pkc-1; pkc-2 animals exhibited no additional defects compared to tpa-1; pkc-1 animals (p=0.68) (Fig. 3a). The phenotypic similarity between tpa-1; pkc-1 and the triple tpa-1; pkc-1; pkc-2 mutant suggests that pkc-2 is not required for nose touch response. This was further confirmed by analysis of double mutant strains containing pkc-2; the residual nose touch response of tpa-1 and pkc-1 animals was not altered by loss of pkc-2 function in double mutant strains (p=0.12 and 0.07 respectively) (Fig. 3a). In addition, PMA treatment did not restore responses in tpa-1; pkc-1 animals (Table 1). Thus, the two nPKC genes, tpa-1 and pkc-1, are required for nose touch response and act semi-redundantly.
Figure 3. pkc-1 acts redundantly with tpa-1 in nose touch response.
(A) Loss of the novel PKC tpa-1, but not the conventional PKC pkc-2, impacts nose touch response. Double and triple mutant combinations were made using pkc-1, tpa-1 and pkc-2 loss of function alleles. Nose touch response was determined and glr-1(n2461) animals were included for comparison (11 ± 2%) in all assays. Error bars represent S.E.M. ANOVA analysis indicates a significant difference between groups, p<0.001. *p<0.001 as compared to wild type, #p<0.001 as compared to pkc-1(ok563) by Fisher’s PLSD. (B) tpa-1 function is required in sensory and interneurons for touch response. The che-2 and glr-1 promoters were used to knockdown tpa-1 function in sensory neurons and interneurons, respectively. glr-1(n2461) control animals exhibited responses of 14 ± 4%. ANOVA analysis indicates a significant difference between groups, p<0.001. *p<0.001 as compared to wild type, **p<0.007 as compared to wild type. Error bars represent S.E.M.
To determine if these two semi-redundant PKC genes both function in the interneurons to modulate nose touch response, the site of tpa-1 function was addressed. Double stranded RNA corresponding to tpa-1 was expressed in both sensory neurons and interneurons to selectively knockdown tpa-1. Loss of tpa-1 function in either the sensory or interneurons resulted in nose touch response defects (Fig. 3b). The simplest conclusion is that tpa-1 and pkc-1 act in a semi-redundant fashion and have partially overlapping sites of action: tpa-1 function is necessary in sensory neurons and interneurons while pkc-1 function is necessary and sufficient in interneurons for nose touch response.
Loss of PKC-1 does not alter GLR-1 localization
As pkc-1 likely functions in the interneurons to modulate nose touch, it may act by altering the function or localization of proteins required for proper touch response. Previous studies have demonstrated that two C. elegans AMPA class glutamate receptors expressed in the interneurons are required for response to nose touch. The glr-1 gene encodes an AMPA-class glutamate receptor subunit whose loss specifically perturbs response to nose touch, while glr-2 encodes an AMPA-class receptor subunit whose loss modestly perturbs responses. These genes act synergistically in other sensory behaviors (Hart et al., 1995, Maricq et al., 1995, Mellem et al., 2002). We used genetic and molecular strategies to determine if pkc-1 and AMPA receptors genetically interact to modulate nose touch response. If pkc-1 and AMPA receptors act independently, then the touch response defects of double mutant animals might be additive. As shown in Fig. 4a, the response of glr-1; pkc-1 double mutant animals was not significantly worse than glr-1 animals (p=0.80). Additionally, loss of glr-2 did not alter the response of pkc-1 animals (p=0.14). The absence of additive effects with AMPA receptors and pkc-1 is consistent with, but does not prove, that pkc-1 and AMPA receptors acting in the same genetic pathway; the low nose touch response rate and the potential role of tpa-1 complicates this analysis. Therefore, we directly examined glutamate receptor localization in animals with decreased PKC function.
Figure 4. Glutamate receptors may act in the same pathway as pkc-1.
(A) Loss of pkc-1 function did not exacerbate the nose touch response defects of glr-1 or glr-2 animals. Error bars represent S.E.M. ANOVA analysis indicates significant differences between groups, p<0.001. *p<0.001 as compared to wild type, #p<0.002 as compared to pkc-1(ok563) determined by Fisher’s PLSD. (B) pkc-1 loss does not alter GLR-1::GFP expression in the ventral nerve cord of adult animals. Representative images of GLR-1::GFP expression of wild type (top) and pkc-1(ok563) adult animals (bottom) in the ventral nerve cord. (C) GLR-1::GFP expression and localization is not altered in tpa-1; pkc-1; pkc-2 triple mutant animals. Representative images of GLR-1::GFP expression of wild type (top) and triple mutant animals (bottom) in the ventral nerve cord. Scale bar represents 10 μm. Quantitative analysis is found in Table 3.
Glutamate receptor localization in the post-synaptic membrane of the ventral nerve cord is also critical for response to nose touch (Juo et al., 2007, Rongo et al., 1998). To determine if pkc-1 regulates AMPA receptor levels or localization in C. elegans, we examined the synaptic localization of GLR-1::GFP, a GFP-tagged AMPA receptor in the ventral cord (as current techniques do not permit quantification of AMPA receptors at the sensory/interneuron synapses). This tagged receptor localizes to punctate structures, the majority of which correspond to postsynaptic elements at sensory-interneuron and interneuron-interneuron synapses (Burbea et al., 2002, Rongo et al., 1998). In pkc-1 loss of function animals, we observed no changes in GLR-1::GFP levels or localization (Fig. 4b, Table 3). As PKCs act redundantly in nose touch response, we also examined GLR-1 localization in animals lacking three C. elegans PKC genes: pkc-1; tpa-1; pkc-2. In these triple mutant animals, there were no significant differences in GLR-1::GFP levels or localization (Fig. 4c, Table 3). The localization of other AMPA or NMDA receptors was not examined as comparable GFP-tagged reagents are not available. These results suggest that PKC-1 loss does not alter GLR-1 levels or localization, but we do not exclude models in which pkc-1 regulates GLR-1 activity or the localization/activity of other glutamate receptors. Alternatively, PKC-1 function may be regulated by glutamate receptor activity and/or localization.
Table 3.
Quantification of GLR-1::GFP fluorescence
| Peak to cord (dF/F)% |
Density (per 10 μm) |
Width (μm) | |
|---|---|---|---|
|
|
|||
| wild type (n=17) |
190 ± 10 | 3.47 ± 0.10 | 0.69 ± 0.02 |
|
pkc-1(ok563) (n=21) |
207 ± 12 | 3.36 ± 0.09 | 0.70 ± 0.02 |
| Peak to cord (dF/F)% |
Density (per 10 μm) |
Width (μm) | |
|
|
|||
| wild type (n=14) |
209 ± 11 | 3.25 ± 0.02 | 0.69 ± 0.03 |
|
tpa-1; pkc-1; pkc-2 (n=19) |
180 ± 10 | 3.27 ± 0.11 | 0.73 ± 0.03 |
lin-45 Raf and the ERK/MAPK pathway are required for nose touch response
To identify functional targets of pkc-1 in this behavior, a subset of PKC targets identified either in C. elegans or other species was examined using the nose touch response assay. TRP channels and metabotropic glutamate receptor function are regulated by PKC in other systems (Lee & Ro, 2007, Popescu et al., 2006). Mutant alleles in corresponding C. elegans genes were tested; neither the trp-2 trp-1 double mutant nor mutations in mGluR genes mgl-1 and mgl-2 exhibited nose touch response defects (Feng et al., 2006; Supplementary Fig. 1a,b).
A previous study indicated that PKC-1 is important in neuropeptide release in C. elegans motor neurons (Sieburth et al., 2007); we investigated the possibility that the nose touch response defects in pkc-1 animals might be due to altered neuropeptide release. Animals carrying mutations in egl-3 and egl-21, two proneuropeptide processing enzymes, exhibited normal nose touch responses (Kass et al., 2001; Supplementary Fig. 2). Double mutant strains were generated for each of these neuropeptide processing enzymes with pkc-1; nose touch responses of double mutant animals were similar to pkc-1 (Supplementary Fig. 2). These results do not implicate egl-3 and egl-21 in pkc-1-mediated nose touch response.
The ERK signaling pathway is also a potential PKC target. The ERK pathway is a module of the well-characterized mitogen-activated protein kinase (MAPK) signaling pathway. In which the MAPKKK Raf that phosphorylates and activates the MAPKK MEK that in turn phosphorylates and activates ERK. These three core kinases act downstream of PKC activation in mammals in vivo and in vitro (Ferreira et al., 2005, Hu & Gereau, 2003, Hu et al., 2003, Ueda et al., 1996). In C. elegans, the classical ERK pathway was defined in development studies (Sundaram, 2006) and is required in adult chemosensory neurons for response to attractive chemicals (Hirotsu & Iino, 2005, Hirotsu et al., 2000) although it is unclear if the ERK and PKC-1 pathways are functionally connected in C. elegans neurons. Precedence for C. elegans PKC function via the MAPK pathway does exist, as tpa-1 acts upstream of MAPK function in muscles (You et al., 2006). However, a role for the MAPK module in mechanosensory response has not been previously examined.
Using previously described complete or strong loss of function alleles, we found that loss of the let-23 EGF receptor, lin-45 Raf, mek-2 MAPKK or mpk-1 ERK resulted in nose touch response defects similar to the defects observed in pkc-1 loss of function animals (Fig. 5a). A partial loss-of-function allele of let-60 Ras modestly decreased nose touch response; complete loss of function of this gene is lethal. These results suggest that the core kinases of the ERK/MAPK signaling pathway are required for nose touch response. In addition, the requirement for MAPK function in other ASH-mediated behaviors was examined; mpk-1 complete loss of function animals exhibited modest octanol avoidance defects and strong osmotic avoidance defects (Fig. 5b). Thus, the ERK MAPK pathway appears to be critical for response for multiple stimuli detected by the ASH sensory circuit.
Figure 5. The ERK signaling pathway is required for nose touch response.
(A) The ability to respond to nose touch was determined for animals carrying strong loss of function or null alleles of genes in the ERK pathway. (B) let-23, lin-45 mek-2 and mpk-1 are required for nose touch response based on the behavior of viable, complete loss of function animals. let-60(n2021), a partial loss-of-function allele, has a modest nose touch defect; complete loss of function animals are not viable. glr-1(n2461) animals were used for comparison in all assays (10 ± 1%). (C) Loss of mpk-1 impacts octanol avoidance and response to high osmolarity. osm-11(rt142) animals were used for comparison in both octanol and osmotic avoidance assays; average response times were 17 ± 1 sec for octanol and 4.7 ± 0.7 min for osmotic avoidance. Error bars represent S.E.M. ANOVA analysis indicates significant differences between groups, p<0.001. *p<0.001 as compared to wild type, **p=0.03 as compared to wild type and p=0.001 as compared to pkc-1(ok563) using using Fisher’s PLSD.
As work in other systems has suggested a direct role for PKC in the modulation of Raf function (Cai et al., 1997, Kolch et al., 1993), the role of lin-45 Raf in nose touch response was further examined.
lin-45 function is required in interneurons for nose touch response
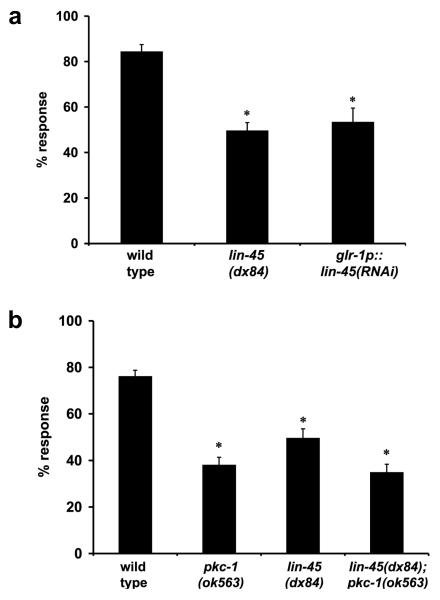
If pkc-1 and lin-45 act in the same pathway to modulate nose touch response, then they should act in the same subset of cells. To test this, dsRNA was used to knockdown lin-45 in the glr-1-expressing interneurons. Driving lin-45(RNAi) expression with the glr-1 promoter resulted in nose touch defects similar to those seen in lin-45 null animals (Fig. 6a) suggesting that lin-45 Raf function is required in the interneurons for proper touch response.
Figure 6. pkc-1 and lin-45 act in the same genetic pathway for touch response.
(A) lin-45 function is required in glr-1-expressing interneurons. A 340 base pair antisense fragment of lin-45 cDNA was expressed using the glr-1 promoter. Transgenic animals were defective in nose touch response and similar to lin-45(dx84) complete loss of function animals (p=0.34); development was unperturbed suggesting that dsRNA did not spread. Transgenic animals carrying a glr-1p::empty construct had normal nose touch responses (data not shown). glr-1(n2461) animals were tested in parallel in all assays (17 ± 3%). ANOVA analysis indicates significant differences between groups, p<0.001. Significance was determined using Fisher’s PLSD. *p<0.001 as compared to wild type animals. (B) Loss of pkc-1 function does not exacerbate lin-45 loss of function nose touch response defects. Responses from pkc-1(ok563) animals do not differ significantly from the pkc-1; lin-45 double (p=0.48). Similar results were obtained using pkc-1(nj3) and lin-45(dx19) loss of function alleles (data not shown). Error bars represent S.E.M. glr-1(n2461) animals were tested for comparison (8 ± 1%). Significance was determined using Student’s t-test. *p<0.001 as compared to wild type animals.
To ascertain if C. elegans pkc-1 acts via the ERK pathway for nose touch response, we examined the consequence of losing both lin-45 and pkc-1. If pkc-1 and lin-45 function in separate pathways, then the nose touch response defects of double mutant animals would be additive. Although the slight difference between lin-45(dx84) and the lin-45; pkc-1 double mutant is significant (p=0.001), the responses of the lin-45; pkc-1 double mutant do not significantly differ from those of pkc-1 loss of function animals (p=0.48) (Fig. 6b). Thus, it is likely that pkc-1 and the ERK pathway function in the same genetic pathway in this behavioral circuit.
Discussion
Here, we demonstrate that pkc-1 is required for nose touch response in adult animals. pkc-1 function is required in sensory neurons for other C. elegans sensory responses including olfaction, thermotaxis and avoidance of high osmolarity (Okochi et al., 2005). Our results define both a new cellular focus of pkc-1 action and a novel downstream target of C. elegans pkc-1, namely the ERK pathway.
To determine the cellular site of pkc-1 function, we manipulated gene expression in subsets of C. elegans neurons. Expression of pkc-1 cDNA in either sensory neurons or interneurons significantly restored nose touch response. To discriminate between ectopic over-expression rescue versus redundant function, we also utilized cell specific RNAi knockdown studies. Only pkc-1 knockdown in glr-1-expressing neurons recapitulated pkc-1 loss of function defects. The simplest interpretation of the pkc-1 phenotypic rescue and RNAi knockdown studies is that pkc-1 function is required in the locomotory interneurons for response to nose touch. Genes expressed in this group of cells are plausible pkc-1 targets in nose touch response.
Novel PKC family members, including PKC-1, require DAG for activation (Land et al., 1994a). Phorbol ester (PMA) application, which mimics the effect of DAG, increases PKC-1 signaling in other behavioral contexts (Okochi et al., 2005, Sieburth et al., 2007). For nose touch response, increasing PKC-1 signaling through PMA application did not alter responses in wild type animals, which is consistent with behavioral analysis of strains with increased PKC-1 signaling presented herein. In contrast, PMA partially restored responses in pkc-1 loss of function animals. This difference may be attributed to a number of factors. In olfactory adaptation, the effects of PMA treatment could only be recapitulated when dgk-1 loss was coupled with loss of dgk-3 (Matsuki et al., 2006). It is possible that dgk-3 or additional diacylglycerol kinases act in a similarly redundant manner for nose touch response. Secondly, acute pharmacological manipulation might preclude compensatory changes resulting from chronic increases in DAG levels. Lastly, additional proteins with DAG binding domains may be involved in nose touch response. Both the synaptic vesicle priming protein UNC-13 as well as other PKC proteins are candidates for acting in concert with PKC-1 (Okochi et al., 2005, Sieburth et al., 2007). Based on studies presented here, the novel PKC encoded by tpa-1 acts in a semi-redundant fashion in nose touch response with pkc-1.
How might PKC-1 regulate nose touch response? PKC proteins are important for vesicle release (Morgan & Burgoyne, 1992, Xue et al., 2009). As C. elegans PKC-1 function impacts neuropeptide secretion elsewhere (Sieburth et al., 2007), the effect of neuropeptide loss on nose touch response was examined. Although disruption of neuropeptide processing due to loss of egl-3 and egl-21 did not cause nose touch response defects, PKC-1 could affect touch response by altering secretion of neuropeptides processed by other enzymes. Additionally, PKC-1 may regulate neurotransmitter release in the interneurons.
Mammalian studies suggest an important role for PKC phosphorylation of AMPA-type glutamate receptors in regulating receptor trafficking (Boehm et al., 2006, Chung et al., 2003, Lee et al., 2007, Roche et al., 1996). As two AMPA-type glutamate receptors, glr-1 and glr-2, are required in the interneurons for response to nose touch, we examined whether this relationship might be conserved in C. elegans (Hart et al., 1995, Maricq et al., 1995, Mellem et al., 2002). Loss of either perturbs nose touch response and double mutant studies suggested that glr-1 and glr-2 might act in the same genetic pathway as pkc-1 for nose touch; the order of gene action remain unclear. However, using previously defined techniques, we were unable to find differences in GLR-1 expression in the ventral nerve cord for pkc-1 loss of function animals or for animals lacking three PKC genes. Although previous work has revealed roles for genes required for GLR-1 ventral cord localization and proper nose touch responses (Juo et al., 2007, Rongo et al., 1998), it is possible that changes specific to the ASH-interneuron synapses are altered in pkc-1 loss of function animals. Localization of glr-1 receptors at synapses between the ASH sensory neurons and interneurons is technically challenging and has never been reported. Alternatively, PKC-1 might alter GLR-1 activity without altering receptor localization as there are examples of other proteins that modulate GLR-1 function in this manner (Wang et al., 2008, Zheng et al., 2004). It should be noted that the C. elegans genome encodes nine additional ionotropic glutamate receptor genes (Brockie et al., 2001), thus pkc-1 may act on another glutamate receptor to modulate nose touch response.
We favor a model in which pkc-1 acts in nose touch response through the ERK signaling pathway as loss of function and null alleles of ERK pathway genes result in nose touch response defects and genetic studies reveal interactions between these two pathways. Links between PKC and ERK signaling have been suggested previously. PKCs have been shown to regulate ERK activation both in mammalian neuronal cell culture and in other C. elegans tissues (Hu & Gereau, 2003, Hu et al., 2003, You et al., 2006). The analysis presented here is one of the first demonstrations of this interaction in vivo in neurons.
Upon which ERK pathway component might PKC-1 act in nose touch response? Two in vitro studies point to Raf, as PKC regulates Raf activation through the Raf kinase inhibitory protein (RKIP) (Corbit et al., 2003). RKIP is proposed to disrupt the protein interaction between Raf-1 and MEK, thus prohibiting Raf-1 phosphorylation of MEK. PKC phosphorylation releases RKIP from Raf-1, allowing Raf-1 to bind to MEK leading to ERK activation. Our genetic analysis is consistent with this interaction, as the loss of C. elegans Raf does not exacerbate defects seen in pkc-1 animals. Further genetic and biochemical studies will be required to determine if PKC regulates RKIP across species and to provide additional evidence linking PKC and the ERK signaling pathway in neuronal function.
Supplementary Material
Supplementary Figure 1: TRP channels and metabotropic glutamate receptors are not required for nose touch response.
(A) C. elegans genes encoding transient receptor potential channels (TRPC) orthologs were not required for nose touch response. trp-1 and trp-2 function is required in the interneurons of the nose touch circuit for response to nicotine (Feng et al. 2006). Error bars representing S.E.M. **p<0.002 compared to wild type using the Student’s t-test. (B) C. elegans genes encoding metabotropic glutamate receptors (mGluR) were not required for nose touch response. mgl-1 encodes a Group II mGluR, while mgl-2 encodes a Group I mGluR. Error bars represent S.E.M. ANOVA analysis reveals no significant differences between groups, p=0.74.
Supplementary Figure 2: Loss of pkc-1 function impacts the nose touch response phenotypes of neuropeptide processing enzymes.
Nose touch responses were determined for animals lacking the neuropeptide processing enzymes egl-3 and egl-21. egl-3 mutant animals responded more robustly to nose touch than wild type animals (#p=0.02). Loss of pkc-1 in any of these genetic backgrounds leads to similar nose touch response defects. *p<0.001 as compared to wild type using Fisher’s PLSD. ANOVA analysis reveals significant differences between groups, p<0.001. Error bars represent S.E.M.
Acknowledgements
We are grateful to Marianne Land, Charles Rubin, Derek Sieburth, Lars Dreier, Josh Kaplan, Yoshifumi Okochi, Ikue Mori, Tim Schedl, X.Z. Shawn Xu, Mike Boxem for reagents and advice. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). We thank Komudi Singh for technical assistance and members of the Hart, van den Heuvel and Whetstine laboratories for helpful comments and discussions. This work was supported by the National Institutes of Health (NIGMS GM57918 and NINDS NS55813 to A.C.H.) and the American Psychological Association Diversity Program in Neuroscience (R.H.).
References
- Abeliovich A, Paylor R, Chen C, Kim JJ, Wehner JM, Tonegawa S. PKC gamma mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993;75:1263–1271. doi: 10.1016/0092-8674(93)90614-v. [DOI] [PubMed] [Google Scholar]
- Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso A, Grundahl K, Duerr JS, Han HP, Rand JB. The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science. 1993;261:617–619. doi: 10.1126/science.8342028. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Thomas JH, Horvitz HR. Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol. 1990;55:529–538. doi: 10.1101/sqb.1990.055.01.051. [DOI] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie PJ, Madsen DM, Zheng Y, Mellem J, Maricq AV. Differential expression of glutamate receptor subunits in the nervous system of Caenorhabditis elegans and their regulation by the homeodomain protein UNC-42. J Neurosci. 2001;21:1510–1522. doi: 10.1523/JNEUROSCI.21-05-01510.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbea M, Dreier L, Dittman JS, Grunwald ME, Kaplan JM. Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C. elegans. Neuron. 2002;35:107–120. doi: 10.1016/s0896-6273(02)00749-3. [DOI] [PubMed] [Google Scholar]
- Cai H, Smola U, Wixler V, Eisenmann-Tappe I, Diaz-Meco MT, Moscat J, Rapp U, Cooper GM. Role of diacylglycerol-regulated protein kinase C isotypes in growth factor activation of the Raf-1 protein kinase. Mol Cell Biol. 1997;17:732–741. doi: 10.1128/mcb.17.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S. The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci. 1985;5:956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MY, Komatsu H, Fukuto HS, Dionne HM, Hart AC. Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc Natl Acad Sci U S A. 2004;101:15512–15517. doi: 10.1073/pnas.0403369101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MY, Larkins-Ford J, Tucey TM, Hart AC. lin-12 Notch functions in the adult nervous system of C. elegans. BMC Neurosci. 2005;6:45. doi: 10.1186/1471-2202-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300:1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Trakul N, Eves EM, Diaz B, Marshall M, Rosner MR. Activation of Raf-1 signaling by protein kinase C through a mechanism involving Raf kinase inhibitory protein. J Biol Chem. 2003;278:13061–13068. doi: 10.1074/jbc.M210015200. [DOI] [PubMed] [Google Scholar]
- Driscoll M, Kaplan JM. Mechanotransduction. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1996. pp. 645–677. [Google Scholar]
- Esposito G, Di Schiavi E, Bergamasco C, Bazzicalupo P. Efficient and cell specific knock-down of gene function in targeted C. elegans neurons. Gene. 2007;395:170–176. doi: 10.1016/j.gene.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Feng Z, Li W, Ward A, Piggott BJ, Larkspur ER, Sternberg PW, Xu XZ. A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell. 2006;127:621–633. doi: 10.1016/j.cell.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkey DM, Hyde R, Haspel G, Dionne HM, Hess HA, Suzuki H, Schafer WR, Koelle MR, Hart AC. C. elegans G protein regulator RGS-3 controls sensitivity to sensory stimuli. Neuron. 2007;53:39–52. doi: 10.1016/j.neuron.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J, Triches KM, Medeiros R, Calixto JB. Mechanisms involved in the nociception produced by peripheral protein kinase c activation in mice. Pain. 2005;117:171–181. doi: 10.1016/j.pain.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Fukuto HS, Ferkey DM, Apicella AJ, Lans H, Sharmeen T, Chen W, Lefkowitz RJ, Jansen G, Schafer WR, Hart AC. G protein-coupled receptor kinase function is essential for chemosensation in C. elegans. Neuron. 2004;42:581–593. doi: 10.1016/s0896-6273(04)00252-1. [DOI] [PubMed] [Google Scholar]
- Gibbs CS, Zoller MJ. Rational scanning mutagenesis of a protein kinase identifies functional regions involved in catalysis and substrate interactions. J Biol Chem. 1991;266:8923–8931. [PubMed] [Google Scholar]
- Govindan JA, Cheng H, Harris JE, Greenstein D. Galphao/i and Galphas signaling function in parallel with the MSP/Eph receptor to control meiotic diapause in C. elegans. Curr Biol. 2006;16:1257–1268. doi: 10.1016/j.cub.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hart AC, Kass J, Shapiro JE, Kaplan JM. Distinct signaling pathways mediate touch and osmosensory responses in a polymodal sensory neuron. J Neurosci. 1999;19:1952–1958. doi: 10.1523/JNEUROSCI.19-06-01952.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AC, Sims S, Kaplan JM. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature. 1995;378:82–85. doi: 10.1038/378082a0. [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, Schafer WR. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. Embo J. 2005;24:63–72. doi: 10.1038/sj.emboj.7600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard MA, Bargmann CI, Bazzicalupo P. C. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Curr Biol. 2002;12:730–734. doi: 10.1016/s0960-9822(02)00813-8. [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Bergamasco C, Arbucci S, Plasterk RH, Bazzicalupo P. Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. EMBO J. 2004;23:1101–1111. doi: 10.1038/sj.emboj.7600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu T, Iino Y. Neural circuit-dependent odor adaptation in C. elegans is regulated by the Ras-MAPK pathway. Genes Cells. 2005;10:517–530. doi: 10.1111/j.1365-2443.2005.00856.x. [DOI] [PubMed] [Google Scholar]
- Hirotsu T, Saeki S, Yamamoto M, Iino Y. The Ras-MAPK pathway is important for olfaction in Caenorhabditis elegans. Nature. 2000;404:289–293. doi: 10.1038/35005101. [DOI] [PubMed] [Google Scholar]
- Hu HJ, Gereau R.W.t. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. II. Modulation of neuronal excitability. J Neurophysiol. 2003;90:1680–1688. doi: 10.1152/jn.00341.2003. [DOI] [PubMed] [Google Scholar]
- Hu HJ, Glauner KS, Gereau R.W.t. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. I. Modulation of A-type K+ currents. J Neurophysiol. 2003;90:1671–1679. doi: 10.1152/jn.00340.2003. [DOI] [PubMed] [Google Scholar]
- Hyde R. Neurobiology. Harvard University; Cambridge, Massachusetts: 2008. Genetic Analysis of Protein Kinase C and MAPK in C. elegans Mechanosensation; p. 177. [Google Scholar]
- Juo P, Harbaugh T, Garriga G, Kaplan JM. CDK-5 regulates the abundance of GLR-1 glutamate receptors in the ventral cord of Caenorhabditis elegans. Mol Biol Cell. 2007;18:3883–3893. doi: 10.1091/mbc.E06-09-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JM, Horvitz HR. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1993;90:2227–2231. doi: 10.1073/pnas.90.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass J, Jacob TC, Kim P, Kaplan JM. The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans. J Neurosci. 2001;21:9265–9272. doi: 10.1523/JNEUROSCI.21-23-09265.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt KS, Quast KB, Giles AC, De S, Hendrey D, Nicastro I, Rankin CH, Schafer WR. Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron. 2007;55:662–676. doi: 10.1016/j.neuron.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp UR. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- Konno Y, Ohno S, Akita Y, Kawasaki H, Suzuki K. Enzymatic properties of a novel phorbol ester receptor/protein kinase, nPKC. J Biochem. 1989;106:673–678. doi: 10.1093/oxfordjournals.jbchem.a122915. [DOI] [PubMed] [Google Scholar]
- Land M, Islas-Trejo A, Freedman JH, Rubin CS. Structure and expression of a novel, neuronal protein kinase C (PKC1B) from Caenorhabditis elegans. PKC1B is expressed selectively in neurons that receive, transmit, and process environmental signals. J Biol Chem. 1994a;269:9234–9244. [PubMed] [Google Scholar]
- Land M, Islas-Trejo A, Rubin CS. Origin, properties, and regulated expression of multiple mRNAs encoded by the protein kinase C1 gene of Caenorhabditis elegans. J Biol Chem. 1994b;269:14820–14827. [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Kameyama K, He K, Yu S, Rossetti L, Wilen D, Huganir RL. Identification and characterization of a novel phosphorylation site on the GluR1 subunit of AMPA receptors. Mol Cell Neurosci. 2007;36:86–94. doi: 10.1016/j.mcn.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Ro JY. Peripheral metabotropic glutamate receptor 5 mediates mechanical hypersensitivity in craniofacial muscle via protein kinase C dependent mechanisms. Neuroscience. 2007;146:375–383. doi: 10.1016/j.neuroscience.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peckol E, Driscoll M, Bargmann CI. Mechanosensory signalling in C. elegans mediated by the GLR-1 glutamate receptor. Nature. 1995;378:78–81. doi: 10.1038/378078a0. [DOI] [PubMed] [Google Scholar]
- Matsuki M, Kunitomo H, Iino Y. Goalpha regulates olfactory adaptation by antagonizing Gqalpha-DAG signaling in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2006;103:1112–1117. doi: 10.1073/pnas.0506954103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellem JE, Brockie PJ, Zheng Y, Madsen DM, Maricq AV. Decoding of polymodal sensory stimuli by postsynaptic glutamate receptors in C. elegans. Neuron. 2002;36:933–944. doi: 10.1016/s0896-6273(02)01088-7. [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa J, Tabuse Y, Furusawa M, Yamasaki H. Tumor promoters specifically and reversibly disturb development and behavior of Caenorhabditis elegans. J Cancer Res Clin Oncol. 1982;104:81–87. doi: 10.1007/BF00402056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A, Burgoyne RD. Interaction between protein kinase C and Exo1 (14-3-3 protein) and its relevance to exocytosis in permeabilized adrenal chromaffin cells. Biochem J. 1992;286(Pt 3):807–811. doi: 10.1042/bj2860807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- Nurrish S, Segalat L, Kaplan JM. Serotonin inhibition of synaptic transmission: Galpha(0) decreases the abundance of UNC-13 at release sites. Neuron. 1999;24:231–242. doi: 10.1016/s0896-6273(00)80835-1. [DOI] [PubMed] [Google Scholar]
- Ohno S, Akita Y, Konno Y, Imajoh S, Suzuki K. A novel phorbol ester receptor/protein kinase, nPKC, distantly related to the protein kinase C family. Cell. 1988;53:731–741. doi: 10.1016/0092-8674(88)90091-8. [DOI] [PubMed] [Google Scholar]
- Okochi Y, Kimura KD, Ohta A, Mori I. Diverse regulation of sensory signaling by C. elegans nPKC-epsilon/eta TTX-4. EMBO J. 2005 doi: 10.1038/sj.emboj.7600697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Popescu DC, Ham AJ, Shieh BH. Scaffolding protein INAD regulates deactivation of vision by promoting phosphorylation of transient receptor potential by eye protein kinase C in Drosophila. J Neurosci. 2006;26:8570–8577. doi: 10.1523/JNEUROSCI.1478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan R, Cannon SC, Horvitz HR. MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature. 2000;408:470–475. doi: 10.1038/35044083. [DOI] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Rongo C, Whitfield CW, Rodal A, Kim SK, Kaplan JM. LIN-10 is a shared component of the polarized protein localization pathways in neurons and epithelia. Cell. 1998;94:751–759. doi: 10.1016/s0092-8674(00)81734-1. [DOI] [PubMed] [Google Scholar]
- Sambongi Y, Takeda K, Wakabayashi T, Ueda I, Wada Y, Futai M. Caenorhabditis elegans senses protons through amphid chemosensory neurons: proton signals elicit avoidance behavior. Neuroreport. 2000;11:2229–2232. doi: 10.1097/00001756-200007140-00033. [DOI] [PubMed] [Google Scholar]
- Sieburth D, Madison JM, Kaplan JM. PKC-1 regulates secretion of neuropeptides. Nat Neurosci. 2007;10:49–57. doi: 10.1038/nn1810. [DOI] [PubMed] [Google Scholar]
- Stringham EG, Dixon DK, Jones D, Candido EP. Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol Biol Cell. 1992;3:221–233. doi: 10.1091/mbc.3.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram MV. RTK/Ras/MAPK signaling. WormBook. 2006:1–19. doi: 10.1895/wormbook.1.80.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernarakis N, Wang SL, Dorovkov M, Ryazanov A, Driscoll M. Heritable and inducible genetic interference by double-stranded RNA encoded by transgenes. Nat Genet. 2000;24:180–183. doi: 10.1038/72850. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Radzio-Andzelm E, Knighton DR, Ten Eyck LF, Sowadski JM, Herberg FW, Yonemoto W, Zheng J. Crystal structures of the catalytic subunit of cAMP-dependent protein kinase reveal general features of the protein kinase family. Receptor. 1993;3:165–172. [PubMed] [Google Scholar]
- Troemel ER, Kimmel BE, Bargmann CI. Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell. 1997;91:161–169. doi: 10.1016/s0092-8674(00)80399-2. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk WJ, Houssa B. Properties and functions of diacylglycerol kinases. Cell Signal. 2000;12:595–605. doi: 10.1016/s0898-6568(00)00113-3. [DOI] [PubMed] [Google Scholar]
- Walker DS, Vazquez-Manrique RP, Gower NJ, Gregory E, Schafer WR, Baylis HA. Inositol 1,4,5-trisphosphate signalling regulates the avoidance response to nose touch in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000636. doi: 10.1371/journal.pgen.1000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Walker CS, Brockie PJ, Francis MM, Mellem JE, Madsen DM, Maricq AV. Evolutionary conserved role for TARPs in the gating of glutamate receptors and tuning of synaptic function. Neuron. 2008;59:997–1008. doi: 10.1016/j.neuron.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thompson JN, Brenner S. The structure of the nervous system of the nematode C. elegans. Phil. Trans. Roy. Soc. B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Xue R, Zhao Y, Chen P. Involvement of PKC alpha in PMA-induced facilitation of exocytosis and vesicle fusion in PC12 cells. Biochem Biophys Res Commun. 2009;380:371–376. doi: 10.1016/j.bbrc.2009.01.105. [DOI] [PubMed] [Google Scholar]
- You YJ, Kim J, Cobb M, Avery L. Starvation activates MAP kinase through the muscarinic acetylcholine pathway in Caenorhabditis elegans pharynx. Cell Metab. 2006;3:237–245. doi: 10.1016/j.cmet.2006.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Mellem JE, Brockie PJ, Madsen DM, Maricq AV. SOL-1 is a CUB-domain protein required for GLR-1 glutamate receptor function in C. elegans. Nature. 2004;427:451–457. doi: 10.1038/nature02244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: TRP channels and metabotropic glutamate receptors are not required for nose touch response.
(A) C. elegans genes encoding transient receptor potential channels (TRPC) orthologs were not required for nose touch response. trp-1 and trp-2 function is required in the interneurons of the nose touch circuit for response to nicotine (Feng et al. 2006). Error bars representing S.E.M. **p<0.002 compared to wild type using the Student’s t-test. (B) C. elegans genes encoding metabotropic glutamate receptors (mGluR) were not required for nose touch response. mgl-1 encodes a Group II mGluR, while mgl-2 encodes a Group I mGluR. Error bars represent S.E.M. ANOVA analysis reveals no significant differences between groups, p=0.74.
Supplementary Figure 2: Loss of pkc-1 function impacts the nose touch response phenotypes of neuropeptide processing enzymes.
Nose touch responses were determined for animals lacking the neuropeptide processing enzymes egl-3 and egl-21. egl-3 mutant animals responded more robustly to nose touch than wild type animals (#p=0.02). Loss of pkc-1 in any of these genetic backgrounds leads to similar nose touch response defects. *p<0.001 as compared to wild type using Fisher’s PLSD. ANOVA analysis reveals significant differences between groups, p<0.001. Error bars represent S.E.M.