Abstract
Relatively little attention has been focused on standardization of data exchange in clinical research studies and patient care activities. Both are usually managed locally using separate and generally incompatible data systems at individual hospitals or clinics. In the past decade there have been nascent efforts to create data standards for clinical research and patient care data, and to some extent these are helpful in providing a degree of uniformity. Nevertheless these data standards generally have not been converted into accepted computer-based language structures that could permit reliable data exchange across computer networks. The National Cardiovascular Research Infrastructure (NCRI) project was initiated with a major objective of creating a model framework for standard data exchange in all clinical research, clinical registry, and patient care environments, including all electronic health records. The goal is complete syntactic and semantic interoperability. A Data Standards Workgroup was established to create or identify and then harmonize clinical definitions for a base set of standardized cardiovascular data elements that could be used in this network infrastructure. Recognizing the need for continuity with prior efforts, the Workgroup examined existing data standards sources. A basic set of 353 elements was selected. The NCRI staff then collaborated with the two major technical standards organizations in healthcare, the Clinical Data Interchange Standards Consortium and Health Level 7 International, as well as with staff from the National Cancer Institute Enterprise Vocabulary Services. Modeling and mapping were performed to represent (instantiate) the data elements in appropriate technical computer language structures for endorsement as an accepted data standard for public access and use. Fully implemented, these elements will facilitate clinical research, registry reporting, administrative reporting and regulatory compliance, and patient care.
1. Introduction
Clinical research studies are usually organized as separate and distinct efforts conducted locally at independent individual sites. Clinical information used in patient care also typically is managed locally using separate, distinct, and generally incompatible data systems at each individual institution. There has been relatively little attention focused on data exchange both in the clinical research and patient care domains. Although some limited clinical data standards exist and can be helpful in standardizing certain aspects of clinical data and providing a certain amount of uniformity, for the most part these have not been converted into accepted computer-based language structures that could be used interchangeably across computer networks. So while clinicians in different locations may think, act, and talk alike in their activities, the basic computer systems which they use to store and retrieve data locally do not, and for the most part cannot, transmit, receive, combine, analyze, and use shared data as information. As a consequence, a robust infrastructure for conducting clinical research using commonly defined and electronically exchangeable data derived directly from clinical sources does not exist in the United States.
In 2009, the National Cardiovascular Research Infrastructure (NCRI) project was initiated by the Duke Clinical Research Institute (DCRI) and the American College of Cardiology Foundation (ACCF) in order to create a model infrastructure for clinical research, clinical registries, and patient care. (1) Initial funding was provided by a grant through the American Recovery and Reinvestment Act (ARRA). The four goals of NCRI are: 1) replace the repetitive assembly and disassembly of short-lived clinical investigator networks with a stable and enduring operational infrastructure for clinical research; 2) standardize and harmonize cardiovascular data to achieve complete syntactic and semantic interoperability throughout the network; 3) coordinate and facilitate the transfer of selected, standardized cardiovascular data into existing and future national registries; 4) develop an enduring library of content for education and training of clinical investigators and site personnel. The NCRI seeks to overcome limitations of current approaches, including the absence of streamlined, one-time data collection activities at each independent site, lack of common data terms used by all, and the inability to transmit, receive, combine, analyze, and use shared data in comparable and interchangeable formats (interoperability).
One critical aspect of NCRI is establishing a universal vocabulary of cardiovascular data elements. This includes establishing all the formal technical features that are required of a controlled vocabulary that can operate on multiple computer networks in the healthcare environment, achieving both syntactic interoperability (format, packaging, transmission) as well as semantic interoperability (unambiguous shared meanings). (2, 3) This also includes disseminating widely the selected data elements and their definitions, and then eliciting feedback from, and facilitating acceptance by, all relevant parties, including investigators, sponsors, regulatory bodies, clinicians, policymakers, payors, and the general public. We describe here the methodology and principal results of the project to identify and harmonize clinical definitions of a base set of standardized cardiovascular data elements applicable to clinical research, registries, and patient care. We also seek to engage the community in efforts to absorb and integrate this distinct advance. Our work continues and expands upon recent work by the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) Task Force on Clinical Data Standards that previously established a base cardiovascular vocabulary of key data elements and definitions for electronic health records (EHR). (4) That initiative identified 99 key terms that should be available in every general purpose EHR, terms that are interoperable and applicable to every cardiovascular subspecialty EHR, and which have maximal utility across the widest spectrum of clinical settings, including clinical care and clinical research, as well as in local institutional, state, regional, and national registries and all data interchange environments. The NCRI Data Standards Workgroup followed these same principles in its efforts to build upon that foundation.
2. Methodology
2.1 Workgroup Composition
The principal investigators of NCRI collaborated with ACCF leadership to identify appropriate members for a Data Standards Workgroup charged with undertaking this project. The 8 members selected have overlapping expertise in clinical research and clinical care, information technologies, informatics, clinical registries, data standards development, and statistical analyses. The present document was composed and written by the Workgroup.
2.2 Relationships With Industry and Other Entities
The ACCF, DCRI, NCRI, and their committees, task forces, workgroups, and other bodies all make every effort to avoid actual or potential conflicts of interest. Specifically, all members of a workgroup are required to file statements disclosing current and recent relationships that may be perceived as relevant real or potential conflicts of interest, and the same is required of all peer reviewers of a document. These disclosures for members of this Workgroup are listed in Appendix 2. Comprehensive disclosure information is available online at: www.cardiosource.org/ACC/About-ACC/Leadership/Guidelines-and-Documents-Task-Forces.aspx.
2.3 Review of Literature and Existing Data Elements
This Workgroup identified several tasks involved in establishing the library of core universal cardiovascular concepts (i.e. vocabulary) to be developed for this project. The first task was identifying key clinical terms from among the many available data element concepts. To begin, the Workgroup examined the data dictionaries of the ACCF National Cardiovascular Data Registry (NCDR) and the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Registry, and then systematically examined all the current existing cardiovascular data dictionaries and standards documents published by these and other professional societies. (4–11) Criteria for inclusion of a specific term (data element) from these sources was that the key clinical concept embodied in the term had the broadest utility and therefore would be collected commonly in cardiovascular clinical research investigations, including both randomized clinical trials and registries. Selection of terms from these sources was achieved by consensus of the group after review and discussion. In general, basic (simple, singular, or atomic) terms were preferred over composite terms. Once selected, all data elements were grouped into standard categories as previously outlined. (4, 12) These categories indicate the clinical context in which the data element is expected to be obtained or collected, and reflect the usual workflow organization of information in typical clinical settings for a single episode of care. Categories are: Personal History and Family History, Physical Examination (Clinical Condition) at the time of the encounter, Laboratory Values, Diagnostic Procedures, Therapeutic Procedures, Adverse Events, Medications, Discharge Information, and Outcomes.
2.4 Data Element Definitions and Consensus Development
The second task of the Workgroup was to harmonize the definitions of elements selected, making certain that unambiguous definitions resulted. This task was intentionally focused on the needs of both the clinical care and clinical research communities, as one objective of NCRI is to promote and foster cross-domain compatibility (clinical and research) while accomplishing semantic interoperability. Nearly all clinical terms considered had multiple source definitions. However, upon closer examination many source element definitions were the same or very nearly so. This reflects prior work harmonizing the NCDR registries and the STS adult cardiac surgery registry with existing clinical data standards. Where differences remained the Workgroup used a hierarchical approach to select a final definition. Preference was given to sources as follows (sources shown in Table 1):
Table 1.
Data sources reviewed.
| Title | Reference |
|---|---|
| ACC-NCDR Registries: | |
| CathPCI Registry | www.ncdr.com/WebNCDR/elements.aspx#1 |
| ICD Registry | www.ncdr.com/WebNCDR/ICD/elements.aspx |
| ACTION-GWTG Registry | www.ncdr.com/WebNCDR/ACTION/elements.aspx |
| CARE Registry | www.ncdr.com/WebNCDR/carotidstent/elements.aspx |
| Society of Thoracic Surgeons Adult Cardiac Surgery Data Registry | www.sts.org/national-database/database-managers/adult-cardiac-surgery-database (Ref. 8) |
| ACC/AHA Data Standards documents: | |
| Adult Cardiovascular HER | Weintraub WS, et al. JACC 2011;58:202 (Ref. 4) |
| Cardiac Imaging | Hendel RC, et al. JACC 2009;53:91 (Ref. 5) |
| Electrophysiology | Buxton AE, et al. JACC 2006;48:2360 (Ref. 6) |
| Acute Coronary Syndromes | Cannon CP, et al. JACC 2001;38:2114 (Ref. 7 |
| ACS and Coronary Artery Disease | Cannon CP et al. (in press) (Ref. 9) |
| Other data standards: | |
| Clinical Data Interchange Standards Consortium (CDISC): Clinical Data Acquisition Standards Harmonization (CDASH) | www.cdisc.org/cdash (Ref. 10) |
| National Quality Forum (NQF) – Quality Data Model (QDM) | www.qualityforum.org/QualityDataModel.aspx#t=1&s=&p (Ref. 11) |
1) ACCF/AHA Adult Cardiovascular Vocabulary for EHR; (4) 2) NCDR-STS harmonized data elements; (8) 3) other ACCF/AHA Task Force on Clinical Data Standards endorsed elements, (4–9) 4) other published data standards. (10, 11) The ACCF/AHA Adult Cardiovascular Vocabulary for EHR (containing 99 elements) was given highest priority because it is the most recently completed data standardization effort and was developed specifically for EHR systems. Nevertheless, this hierarchy was not absolute and rigid; definitions were selected for best unambiguous structure and wording in the judgment of the Workgroup, regardless of source. Every element from every source was thoroughly reviewed and discussed. When inconsistencies, discrepancies, inaccuracies, ambiguity, or other substantive issues were discovered in existing data elements or definitions, the Workgroup proposed resolutions for consideration by the ACCF/AHA Task Force on Clinical Data Standards.
The Workgroup was assisted by informatics staff of ACCF and DCRI, with additional help from two other organizations (further described below): the Clinical Data Interchange Standards Consortium (CDISC) and Health Level 7 International (HL7) (13, 14). Staff members provided technical informatics support for the project, including representation of elements and terms in a standard machine readable information model developed according to the specifications in the National Cancer Institute (NCI) Data Standards Repository (caDSR). (15) Materials were assembled by staff and circulated by email. The work was conducted in a series of telephone conference calls and email exchanges beginning in June 2010 and concluding in October 2011. In addition, there was one face-to-face meeting held during the ACC Scientific Sessions in March 2011.
2.5 Relations to Other Standards
As described above, the Workgroup reviewed available published data standards and current national registry data elements. From these source materials a circumscribed set of data elements along with single best definitions was selected to serve as an initial cardiovascular data standard for computer network implementation in clinical research, clinical registries, and patient care activities.
2.6 Technical Development for Endorsement as a Recognized Data Standard
The final task for the Workgroup and supporting staff was to represent (instantiate) the selected vocabulary within accepted EHR technical language standards and publish it in a publicly available data library. (16, 17) The NCRI leadership and staff therefore contacted and collaborated with CDISC and HL7 as the two relevant international standards organizations working in this segment of the healthcare environment. Although likely not widely known among clinicians, the CDISC and HL7 technical standards are broadly accepted and have been generally adopted within the information technology platforms of both the patient care and clinical research communities. (13, 14) For example, the HL7 Reference Information Model (RIM), along with its clinical documents standards and EHR functional profile, are widely recognized as the international technical standard for clinical information systems. The CDISC Study Data Tabulation Model (SDTM) and the Clinical Data Acquisition Standards Harmonization (CDASH) are technical standards used for clinical research data collection and exchange between different organizations, for data comparisons across different clinical trials, and for electronic data submission to regulatory agencies. (10, 18) The SDTM accommodates metadata (data format and content tags), which facilitate interoperability and data exchange. The United States Food and Drug Administration (FDA) endorses submission of clinical data in this standard for regulatory review purposes. The NCRI staff therefore created a Unified Modeling Language representation of elements as a Cardiovascular Domain Analysis Model, mapping the model to the specifics required for CDISC SDTM and HL7 RIM. The NCRI data elements were then matched with concept codes assigned by NCI Enterprise Vocabulary Services (EVS). The entire set of cardiovascular concepts will be published in the NCI EVS for public access and use. (19) The data model will be imported into the NCI caDSR and linked with the metadata tags required for full and complete semantic interoperability. This means that these 353 selected cardiovascular data elements should be fully exchangeable across computer networks and within EHR structures, something that previously has not been possible.
2.7 Peer Review and Approval
Drafts of this report and the core set of cardiovascular data elements (excluding the technical representations required for CDISC and HL7 endorsement), were reviewed by the ACCF/AHA Task Force on Clinical Data Standards, and discussed at the Task Force Meeting at the ACC Scientific Sessions in March 2012, with comments transmitted back to the Workgroup. The final version was reviewed and approved by the Chairs of the Research and Publications Committees of the NCDR registries, and also by the Chair of the Science and Quality Oversight Committee. The Workgroup fully acknowledges and anticipates that these standardized data elements and definitions will require regular review and updating, as occurs with all other published guidelines, data standards, performance measures, and appropriateness criteria. NCRI staff will monitor and receive feedback, and periodically review the controlled vocabulary work product to ascertain whether modifications should be considered.
2.8 Intended Use
Adoption and implementation of the cardiovascular data standards presented here should improve interoperability, accuracy, and efficiency in all domains: administrative, regulatory, clinical research, and patient care. Dependable and reliable data exchange should reduce errors caused when multiple transcriptions occur, with the same data being entered into several systems. At the local site level, this will facilitate efforts to extract and review local data, and to transmit data to other entities, for example the large national registries. Combining uniform data from multiple sites for larger scale analyses will also be possible. Linkages of extracted data with administrative and long-term data records will facilitate longitudinal follow-up of specific patient groups of interest. Such linkages with outside data sources may have advantages over the direct clinical follow-up of patients, and may be more efficient and more complete, especially for larger patient groups and for very long term analyses. The Center for Medicare and Medicaid Services (CMS) Medicare Provider Analysis and Review (MEDPAR) datafiles are an example of external data linkages that might be made. Linkages with longitudinal databases may provide opportunities to assess long term mortality, hospital readmissions, subsequent procedures, and various other outcomes of interest. This is likely to enhance the study of long term safety and efficacy of drugs and devices in widespread clinical practice after initial drug or device approval. Furthermore, clinical effectiveness and patient-centered outcomes research comparing a variety of options could be conducted, and evidence-based practice recommendations developed and validated. (20, 21) Such efforts align with other national efforts to improve the clinical patient care domain, specifically the implementation of clinical decision-support tools, with the compilation and return of patient-specific, clinician-specific, and institution-specific data back to the point of care where it originates. These efforts furthermore are significant steps toward achieving the goals of the CMS ‘Meaningful Use’ program, including the use of certified EHR technologies for the purposes of exchanging health information to improve patient care. (22) All of this is consistent with the policies of the national professional societies, and conforms to the recent policy statement from the AHA on expanding the applications of existing and future clinical registries. (23)
3. National Cardiovascular Research Infrastructure Data Elements
From the various sources examined the Workgroup assembled a final list of 353 elements, including a number that are intended to exist as parent-child relationships. Elements that were judged to be the most commonly used in cardiovascular clinical research and clinical care were selected, including all 99 of the previously developed elements for the Adult Cardiovascular EHR. The Workgroup was also keenly aware of the need for parsimony. While this initial list is meant to be comprehensive, we recognize that it may not be adequate for all purposes. Furthermore, any list of data elements will always need ongoing review, with outdated ones deleted and new ones added. The underlying concepts leading to element formation also will change over time and periodic revisions are intended.
3.1 Data elements by category
The elements and their source reference locations are shown in Table 2. Only the element names along with the sources of element values and definitions are listed. Complete element specifications and definitions can be found in an online appendix (Appendix 3), as well as the NCRI website (www.ncrinetwork.org) and the HL7 website (www.hl7.org). Most of these elements were selected from existing data sources. However, nine new data elements of a minor nature were adopted by the Workgroup. These nine new elements and their definitions are shown in Table 3.
Table 2.
National Cardiovascular Research Infrastruture Data Elements
| Element Name |
Source | Element Name |
Source | Element Name |
Source |
|---|---|---|---|---|---|
| Personal history | |||||
| Hypertension | ACC/AHA CV EHR | Transient Ischemic Attack | ACC/AHA CV EHR | Hemodynamic Instability assoc. with Ventricular Tachycardia | NCDR (ICD) |
| Diabetes Mellitus | ACC/AHA CV EHR | Ischemic Stroke | ACC/AHA CV EHR | ICD | NCDR (ICD) |
| Diabetes Therapy | NCDR (ACTION-GWTG) | Hemorrhagic Stroke | ACC/AHA ACS | Initial ICD reason is Cardiac Arrest/arrhythmia Etiology Unknown | NCDR (ICD) |
| Dyslipidemia | ACC/AHA CV EHR | Undetermined Stroke | New | Initial ICD reason is Not Documented | NCDR (ICD) |
| Tobacco Use | ACC/AHA CV EHR | Syndromes at risk for sudden death | NCDR (ICD) | Initial ICD reason is Spontaneous Sustained VT | NCDR (ICD) |
| Tobacco Use-Smoked Tobacco Type | New | Sudden death syndrome type | NCDR (ICD) | Initial ICD reason is Syncope with High Risk Characteristics | NCDR (ICD) |
| Smokeless Tobacco | New | Syncope | ACC/AHA CV EHR | Initial ICD reason is Syncope with Inducible VT | NCDR (ICD) |
| Heart Failure | STS Adult Cardiac | Syncope Date | ACC/AHA CV EHR | Initial ICD reason is Ventricular Fibrillation | NCDR (ICD) |
| Heart Failure Hospital Timeframe | NCDR (ICD) | Syncope-Frequency of Episodes | ACC/AHA CV EHR | Structural Abnormality Type - Amyloidosis | NCDR (ICD) |
| CHF Hospitalization | NCDR (ICD) | Syncope-Number of Episodes | ACC/AHA CV EHR | Structural Abnormality Type - Atrial Septal Defect | NCDR (ICD) |
| Prior cardiac transplant | NCDR (ICD) | Sleep Apnea | ACC/AHA EP | Structural Abnormality Type - Chagas Disease | NCDR (ICD) |
| Heart transplant waiting list | NCDR (ICD) | Sleep Apnea-Sleep Study Diagnosis | New | Structural Abnormality Type - Common Ventricle | NCDR (ICD) |
| NYHA Class | ACC/AHA CV EHR | Aorta Disease | ACC/AHA CV EHR | Structural Abnormality-Type - Ebstein's Anomaly | NCDR (ICD) |
| Chronic Kidney Disease | ACC/AHA CV EHR | Peripheral Arterial Disease | ACC/AHA CV EHR | Structural Abnormality Type - Giant Cell Myocarditis | NCDR (ICD) |
| Dialysis | ACC/AHA CV EHR | Renal Artery Disease | ACC/AHA CV EHR | Structural Abnormality Type - Hypertrophic Cardiomyopathy | NCDR (ICD) |
| Chronic Lung Disease | ACC/AHA CV EHR | Deep Venous Thrombosis | ACC/AHA CV EHR | Structural Abnormality Type - Left Ventricuar Aneurysm | NCDR (ICD) |
| Chronic Lung Disease-Home Oxygen Therapy | New | Venous thromboembolism | ACC/AHA CV EHR | Structural Abnormality Type-LV Non-compaction Syndrome | NCDR (ICD) |
| Coronary artery disease | ACC/AHA CV EHR | Pulmonary Embolism | ACC/AHA CV EHR | Structural Abnormality Type - Other | NCDR (ICD) |
| One epicardial artery > = 70% confirmed by angiography | NCDR (ICD) | Primary Valvular Disease | ACC/AHA CV EHR | Structural Abnormality Type - Right Ventricular Dysplasia (ARVD) | NCDR (ICD) |
| Myocardial Infarction | ACC/AHA CV EHR | Prior Valve Surgery/Procedure | NCDR (CathPCI) | Structural Abnormality Type - Sarcoidosis | NCDR (ICD) |
| Myocardial Infarction timeframe | NCDR (ICD) | Sinus Node Function | ACC/AHA EP | Structural Abnormality Type - Transposition of Great Vessels | NCDR (ICD) |
| PCI | NCDR (CathPCI) | Permanent Pacemaker | NCDR (ICD) | Structural Abnormality Type - Tetralogy of Fallot | NCDR (ICD) |
| CABG Surgery | NCDR (CathPCI) | Atrial arrhythmias | ACC/AHA CV EHR | Structural Abnormality Type - Ventricular Septal Defect | NCDR (ICD) |
| Cardiac Arrest | ACC/AHA CV EHR | Atrial Fibrillation | NCDR (ICD) | Depression | ACC/AHA CV EHR |
| Cardiac Arrest Date | ACC/AHA CV EHR | Atrial Fibrillation Classification | NCDR (ICD) | HIV Infection | ACC/AHA CV EHR |
| Cardiac Arrest Due to Arrhythmia | NCDR (ICD) | Atrial Flutter | NCDR (ICD) | Illicit Drug Use | ACC/AHA CV EHR |
| Previous ICD Implant site | NCDR (ICD) | Bradycardia arrest | NCDR (ICD) | Illicit Drug Use Type-Cocaine Use | NCDR (ICD) |
| Previous ICD reason | NCDR (ICD) | Ventricular arrhythmias | ACC/AHA CV EHR | Patient Life Expectancy of > = 1 year | NCDR (ICD) |
| Previous ICD type | NCDR (ICD) | Ventricular Tachycardia | NCDR (ICD) | Clinical Trial | NCDR (ICD) |
| Cardiogenic Shock | STS Adult Cardiac | Ventricular Tachycardia Type | NCDR (ICD) | ||
| Cerebral Artery Disease | ACC/AHA CV EHR | VT/VF Arrest | NCDR (ICD) | ||
| Family history | |||||
| Coronary artery disease | ACC/AHA CV EHR | Sudden Cardiac Death | ACC/AHA CV EHR | ||
| Physical exam | |||||
| Height | ACC/AHA CV EHR | Heart Rate | ACC/AHA CV EHR | Anginal classification | ACC/AHA CV EHR |
| Weight | ACC/AHA CV EHR | Heart Rate Date/Time | ACC/AHA CV EHR | Anginal Classification Date | ACC/AHA CV EHR |
| Systolic Blood Pressure | ACC/AHA CV EHR | Waist Circumferance | ACC/AHA CV EHR | Killip Class | ACC/AHA ACS |
| Diastolic Blood Pressure | ACC/AHA CV EHR | Chest Pain: Angina or Anginal Equivalent | ACC/AHA CV EHR | New York Heart Association Class | ACC/AHA CV EHR |
| Laboratory values | |||||
| Blood Urea Nitrogen | CDISC-CDASH | Total Cholesterol | CDISC-CDASH | Sodium | CDISC-CDASH |
| Creatinine | CDISC-CDASH | LDL Cholesterol | CDISC-CDASH | Potassium | CDISC-CDASH |
| Hematocrit | CDISC-CDASH | HDL Cholesterol | CDISC-CDASH | Creatine Kinase (CK) | CDISC-CDASH |
| Hemoglobin | CDISC-CDASH | Triglycerides | CDISC-CDASH | Creatine Kinase MB (CK-MB) | CDISC-CDASH |
| Glucose, any | CDISC-CDASH | Brain Naturetic Peptide (BNP) | CDISC-CDASH | Troponin | CDISC-CDASH |
| Glucose, fasting | ACC/AHA CV EHR | NT-proBNP | CDISC-CDASH | Troponin I | CDISC-CDASH |
| Hemoglobin A1c | CDISC-CDASH | Prothrombin Intl. Normalized Ratio (INR) | CDISC-CDASH | Troponin T | CDISC-CDASH |
| Diagnostic Procedures | |||||
| Cardiac diagnostic procedure | ACC/AHA CV EHR | Cardiac Rhythm-Sinus Rhythm | NCDR (ICD) | PR Interval | NCDR (ICD) |
| Date of cardiac diagnostic procedure | ACC/AHA CV EHR | Cardiac Rhythm-Atrial Tachycardia | NCDR (ICD) | PR Interval not obtainable | NCDR (ICD) |
| 12 Lead ECG | NCDR (ICD) | Cardiac Rhythm-Junctional | NCDR (ICD) | Cardiac Rhythm-Second Degree Heart Block | NCDR (ICD) |
| 12 Lead ECG Date/Time | NCDR (ICD) | Cardiac Rhythm-Idioventricular | NCDR (ICD) | Cardiac Rhythm-Third Degree Heart Block | NCDR (ICD) |
| ECG (any) | NCDR (ICD) | Cardiac Rhythm-Afib/Flutter | NCDR (ICD) | Abnormal Intraventricular Conduction | NCDR (ICD) |
| ECG (any) Date/Time | NCDR (ICD) | Cardiac Rhythm-Paced | NCDR (ICD) | Abnormal Intraventricular Conduction Type - Delay, Nonspecific | NCDR (ICD) |
| ECG Timing with STEMI or STEMI Equivalent | NCDR (ACTION GWTG) | Pacing Type | NCDR (ICD) | Abnormal Intraventricular Conduction Type - Left Anterior Fascicular Block | NCDR (ICD) |
| ECG Findings for NSTEMI | NCDR (ACTION-GWTG) | Underlying Atrial Rhythm | NCDR (ICD) | Abnormal Intraventricular Conduction Type - Left Posterior Fascicular Block | NCDR (ICD) |
| ECG Findings for STEMI | NCDR (ACTION-GWTG) | Only Ventricular Paced QRS Complexes | NCDR (ICD) | Abnormal Intraventricular Conduction Type - Left Bundle Branch Block | NCDR (ICD) |
| Electrophysiology Study | NCDR (ICD) | QRS Duration (Non-ventricular Paced Complexes) | NCDR (ICD) | Abnormal Intraventricular Conduction Type - Right Bundle Branch Block | NCDR (ICD) |
| Ventricular Arrhythmias Induced | NCDR (ICD) | Ventricular Paced QRS Duration | NCDR (ICD) | Abnormal Intraventricular Conduction Type - Ventricular Paced Rhythm | NCDR (ICD) |
| Non-Invasive Stress Testing | NCDR (ACTION-GWTG) | Stress Echo Imaging Results | NCDR (CathPCI) | Cardiac CTA | NCDR (CathPCI) |
| Stress Test Result | ACC/AHA CV EHR | Risk/Extent of Ischemia (Stress Echo) | NCDR (CathPCI) | Cardiac CTA Results | NCDR (CathPCI) |
| Exercise Stress Test Results | NCDR (CathPCI) | Stress Test with CMR Imaging Results | NCDR (CathPCI) | Pre-test probability of coronary artery disease | ACC/AHA CV EHR |
| Spect/MPI Imaging Results | NCDR (CathPCI) | Risk/Extent of Ischemia (Stress Test with CMR) | NCDR (CathPCI) | ||
| Risk/Extent of Ischemia (Spect/MPI) | NCDR (CathPCI) | ||||
| Left Ventricular Ejection Fraction (qualitative) | ACC/AHA CV EHR | Left atrium size (quantitative) | ACC/AHA CV EHR | ||
| Left Ventricular Ejection Fraction (quantitative) | ACC/AHA CV EHR | Aortic valve stenosis severity | ACC/AHA CV EHR | Mitral valve stenosis severity | ACC/AHA CV EHR |
| Left ventricle size, end-diastole (quantitative) | ACC/AHA CV EHR | Aortic valve area | ACC/AHA CV EHR | Mitral valve area | ACC/AHA CV EHR |
| Left ventricle size, end-systole (quantitative) | ACC/AHA CV EHR | Aortic valve regurgitation severity | ACC/AHA CV EHR | Mitral valve regurgitation severity | ACC/AHA CV EHR |
| Diagnostic Catheterization | NCDR (CathPCI) | Reason for Diagnostic Catheterization_Cardiac Transplantation | NCDR (CathPCI) | Intravascular Ultrasound (IVUS) | NCDR (CathPCI) |
| Diagnostic Catheterization Status | NCDR (CathPCI) | Reason for Diagnostic Catheterization_Cardiac Transplant Evaluation Type | NCDR (CathPCI) | Fractional Flow Reserve Reserve Ratio | NCDR (CathPCI) |
| Left Heart Catheterization | NCDR (CathPCI) | Reason for Diagnostic Catheterization_Cardiomyopathy or Left ventricular systolic dysfunction evaluation | NCDR (CathPCI) | Fractional Flow Reserve Ratio | NCDR (CathPCI) |
| Diagnostic Coronary Angiography | NCDR (CathPCI) | Reason for Diagnostic Catheterization_Pre-operative evaluation for non-cardiovascular surgery | NCDR (CathPCI) | ||
| Coronary Anatomy Dominance | NCDR (CathPCI) | ||||
| Coronary artery: number of diseased vessels (excludes left main disease) | ACC/AHA CV EHR | ||||
| Stenosis location | NCDR (CathPCI) | ||||
| Stenosis severity | NCDR (CathPCI) | ||||
| Therapeutic Procedures | |||||
| Cardiac Therapeutic Procedure | ACC/AHA CV EHR | Percutaneous Coronary Intervention | NCDR (CathPCI) | Primary reason reperfusion therapy not indicated-Urgent Cardiac Surgery | NCDR (ACTION-GWTG) |
| Date of Cardiac Therapeutic Procedure | ACC/AHA CV EHR | PCI Indication | NCDR (CathPCI) | Non-system reason for Delay in PCI | NCDR (CathPCI) |
| Coronary Artery Bypass Graft Surgery | NCDR (CathPCI) | PCI Status | NCDR (CathPCI) | Culprit Lesion | NCDR (CathPCI) |
| Coronary Bypass Graft Surgery Status | NCDR (CathPCI) | Coronary lesions treated | NCDR (CathPCI) | Pre-Procedure TIMI Flow | NCDR (CathPCI) |
| Coronary graft anastomoses | ACC/AHA CV EHR | Lesion Complexity Description | NCDR (CathPCI) | Post-Procedure TIMI Flow | NCDR (CathPCI) |
| Stent Placed in affected coronary artery | NCDR (CathPCI) | Bifurcation Lesion | NCDR (CathPCI) | Percent Stenosis | NCDR (CathPCI) |
| Stent placed in previous lesion | NCDR (CathPCI) | Chronic Total Occlusion | NCDR (CathPCI) | Lesion Length | NCDR (CathPCI) |
| Stent Placed in Previous PCI | NCDR (CathPCI) | Lesion in Graft | NCDR (CathPCI) | Guidewire Across Lesion | NCDR (CathPCI) |
| Stent Type | NCDR (CathPCI) | Location in Graft | NCDR (CathPCI) | Intracoronary Device Used | NCDR (CathPCI) |
| Previously Treated Lesion | NCDR (CathPCI) | Intra-aortic Balloon Pump (IABP) | NCDR (CathPCI) | Device Deployment | NCDR (ICD) |
| Previous treatment type_Stent | NCDR (CathPCI) | Intra-aortic Balloon Pump (IABP) Timing | NCDR (CathPCI) | Device Diameter | NCDR (ICD) |
| Reason for current treatment of previously treated lesion_In-stent Restenosis | NCDR (CathPCI) | Mechanical Ventricular Support-Other | NCDR (CathPCI) | Device Length | NCDR (CathPCI) |
| Reason for current treatment of previously treated lesion_in-stent Thrombus | NCDR (CathPCI) | Mechanical Ventricular Support–Other, Timing | NCDR (CathPCI) | Contrast Volume | NCDR (CathPCI) |
| Arterial Access Site | NCDR (CathPCI) | Fluoroscopy Dose | NCDR (CathPCI) | ||
| Arterial Access Closure Method | NCDR (CathPCI) | Fluoroscopy Time | NCDR (CathPCI) | ||
| Electrophysiology Procedure | NCDR (ICD) | VT Ablation Performed | NCDR (ICD) | ||
| ICD | NCDR (ICD) | ATP or Shock Therapy Appropriate | NCDR (ICD) | Lead Abnormality_Oversensing with Shock or ATP | NCDR (ICD) |
| ICD Procedure Indication | NCDR (ICD) | ATP or Shock Therapy Delivered | NCDR (ICD) | Lead Abnormality_Oversensing with out Shock or ATP | NCDR (ICD) |
| Device Implanted | NCDR (ICD) | ATP Therapy Successful | NCDR (ICD) | Lead Abnormality_Defibrillation Issues | NCDR (ICD) |
| Device Explanted | NCDR (ICD) | Shock Therapy Successful | NCDR (ICD) | Lead Abnormality_Extracardiac Stimulation | NCDR (ICD) |
| Device Manufacturer | NCDR (ICD) | CS/LV Lead Successful | NCDR (ICD) | Lead Abnormality_Failure to Capture | NCDR (ICD) |
| Device Model Name | NCDR (ICD) | Reason CS/LV Lead Not Implanted | NCDR (ICD) | Lead Abnormality_Failure to Pace | NCDR (ICD) |
| Device Model Number | NCDR (ICD) | Battery Voltage | NCDR (ICD) | Lead Abnormality_Oversensing | NCDR (ICD) |
| Device Returned to Manufacturer | NCDR (ICD) | Conductor Failure | NCDR (ICD) | Lead Abnormality_Undersensing | NCDR (ICD) |
| Device Serial Number | NCDR (ICD) | Defribillation Threshold/Lowest Energy Tested | NCDR (ICD) | Lead Dislodgement Requiring Reposition/Reoperation | NCDR (ICD) |
| Lead Returned to Manufacturer | NCDR (ICD) | Upper Limit of Vulnerability | NCDR (ICD) | Lead Erosion | NCDR (ICD) |
| Manufacturer Advisory/Recall | NCDR (ICD) | Failed to Shock with Inadequate DFT Safety Margin | NCDR (ICD) | Lead Infection | NCDR (ICD) |
| Non-lead Related Medical/Surgical Procedure | NCDR (ICD) | Faulty Connector Header | NCDR (ICD) | Lead Perforation | NCDR (ICD) |
| Reason for Malfunction | NCDR (ICD) | Lead Location | NCDR (ICD) | ||
| Reason(s) for Reimplant | NCDR (ICD) | Existing Lead Dislodgement | NCDR (ICD) | ||
| Existing Lead Status | NCDR (ICD) | ||||
| Existing Lead Function | NCDR (ICD) | ||||
| Clinical/Adverse Events | |||||
| Significant Coronary Dissection | NCDR (CathPCI) | Myocardial infarction | NCDR (CathPCI) | Pneumothorax | NCDR (ICD) |
| Coronary Artery Perforation | NCDR (CathPCI) | Urgent cardiac surgery | NCDR (CathPCI) | Hemothorax | NCDR (ICD) |
| Coronary Thrombus | NCDR (CathPCI) | Cardiac Tamponade | NCDR (CathPCI) | Peripheral Embolus | NCDR (ICD) |
| Coronary Venous Dissection | NCDR (ICD) | Luminal/Carotid Thrombus | NCDR (CARE) | Peripheral Nerve Injury | NCDR (ICD) |
| Cardiac Valve Injury | NCDR (ICD) | Venous Obstruction | NCDR (ICD) | Infection Requiring Antibiotics | NCDR (ICD) |
| Chamber Thrombus | ACC/AHA EP | Hematoma at Access Site | NCDR (CathPCI) | Set Screw Problem | NCDR (ICD) |
| Conduction Block | NCDR (ICD) | Hematoma Size | NCDR (CathPCI) | Drug Reaction/Serious Substance-related Adverse Event | NQF-QDM |
| Cardiac Perforation | NCDR (ICD) | Hematoma Requiring Re-op | NCDR (CathPCI) | Drug/Substance Allergy | NQF-QDM |
| Pericardial Effusion | NCDR (ICD) | Red blood cell or whole blood transfusion | NCDR (CathPCI) | ||
| Medications | |||||
| At Home Medications | New | Medication Timepoint | CDISC-CDASH | Prophylactic Antibiotics Within 1 hour of procedure start time | NCDR (ICD) |
| Aspirin in First 24 hours | NCDR (ACTION-GWTG) | Medications Held or Discontinued | New | Diuretic | ACC/AHA CV EHR |
| Clopidogrel in First 24 hours | NCDR (ACTION-GWTG) | Blinded | New | Direct renin inhibitor | ACC/AHA CV EHR |
| Prasugrel in First 24 hours | NCDR (ACTION-GWTG) | Contraindication | New | Alpha blocker | ACC/AHA CV EHR |
| Ticlipodine in First 24 hours | NCDR (ACTION-GWTG) | Anticoagulant | ACC/AHA CV EHR | Steroid, systemic | ACC/AHA CV EHR |
| Beta Blocker in First 24 hours | NCDR (ACTION-GWTG) | Cyclo-oxygenase 2 inhibitor | ACC/AHA CV EHR | Nonsteroidal anti-inflammatory | ACC/AHA CV EHR |
| ACE Inhibitor in First 24 hours | NCDR (ACTION-GWTG) | P2Y12 inhibitor | ACC/AHA CV EHR | ||
| Angiotensin Receptor Blocker in First 24 hours | NCDR (ACTION-GWTG) | Beta-Blockers | CDISC-CDASH | ||
| Statin in First 24 hours | NCDR (ACTION-GWTG) | GP IIb/IIIa Inhibitor | CDISC-CDASH | ||
| Non-Statin Lipid Lowering in First 24 hours | NCDR (ACTION-GWTG) | Lipid Lowering Statin Medications | CDISC-CDASH | ||
| Aldosterone Blocking Agent in First 24 hours | NCDR (Action-GWTG) | Non Statin Lipid Lowering Medications | CDISC-CDASH | ||
| Discharge | |||||
| Vital Status | NCDR (CathPCI) | Dietary Counseling | NCDR (ACTION-GWTG) | Transfer for Procedure | NCDR (CathPCI) |
| Comfort Measures | NCDR (ACTION-GWTG) | Exercise Counseling | NCDR (ACTION-GWTG) | Transfer for Procedure Location | NCDR (CathPCI) |
| CMS Comfort Measures Timing | NCDR (ACTION-GWTG) | Smoking Counseling | NCDR (ACTION-GWTG) | ||
| CMS Discharge Disposition | NCDR (ACTION-GWTG) | Cardiac Rehabilitation Referral | NCDR (ACTION-GWTG) | ||
| Outcomes | |||||
| Death | ACC/AHA CV EHR | ||||
| Date of death | ACC/AHA CV EHR | ||||
| Cause of death | NCDR (CathPCI) | ||||
| Cardiac death | ACC/AHA CAD | ||||
| Death During Procedure | NCDR (CathPCI) | ||||
ACC/AHA ACS = ACC/AHA Acute Coronary Syndromes Data Standard (Ref. 7).
ACC/AHA CV EHR = ACC/AHA Cardiovascular Vocabulary for Electronic Health Records Data Standard. (Ref. 4).
ACC/AHA CAD = ACC/AHA Coronary Artery Disease Data Standard (Ref. 9).
CDISC-CDASH = Clinical Data Interchange Standards Consortium - Clinical Data Acquisition Standards Harmonization (Ref. 10).
STS Adult Cardiac = Society of Thoracic Surgeons Adult Cardiac Surgery Data Registry (Ref. 8).
NCDR (ACTION-GWTG) = NCDR ACTION-GWTG Registry (see text and Table 1).
NCDR (CARE) = NCDR CARE Registry (see text and Table 1).
NCDR (CathPCI) = NCDR CathPCI Registry (see text and Table 1).
NCDR (ICD) = NCDR ICD Registry (see text and Table 1).
NQF-QDM = National Quality Forum-Quality Data Model (Ref. 11).
Table 3.
Newly defined NCRI Data Elements.
| Element name | Definition | Value Domain |
|---|---|---|
| Chronic lung disease – Home oxygen therapy | Indicate if, the patient has been receiving home oxygen therapy for treatment of chronic lung disease. | Yes No |
| Sleep apnea – sleep study diagnosis | Indicate if the sleep apnea was diagnosed by a sleep study. | Yes No |
| Smoked tobacco type | Indicate the type of smoked tobacco. | Cigars Cigarettes Pipes |
| Smokeless tobacco | Indicate the use of smokeless tobacco. | Yes No |
| Undetermined stroke | Defined as a stroke with insufficient information to allow categorization as an ischemic or hemorrhagic stroke. | Yes No |
| At-home medications | Indicate if the medication was taken or started at home. | Yes No |
| Blinded | Indicate if the medication use was blinded. | Yes No |
| Contraindicated | Indicate if the medication was contraindicated. | Yes No |
| Medications held or discontinued | Indicate if the medication was held or discontinued. | Yes No |
3.2 Example representation of data elements
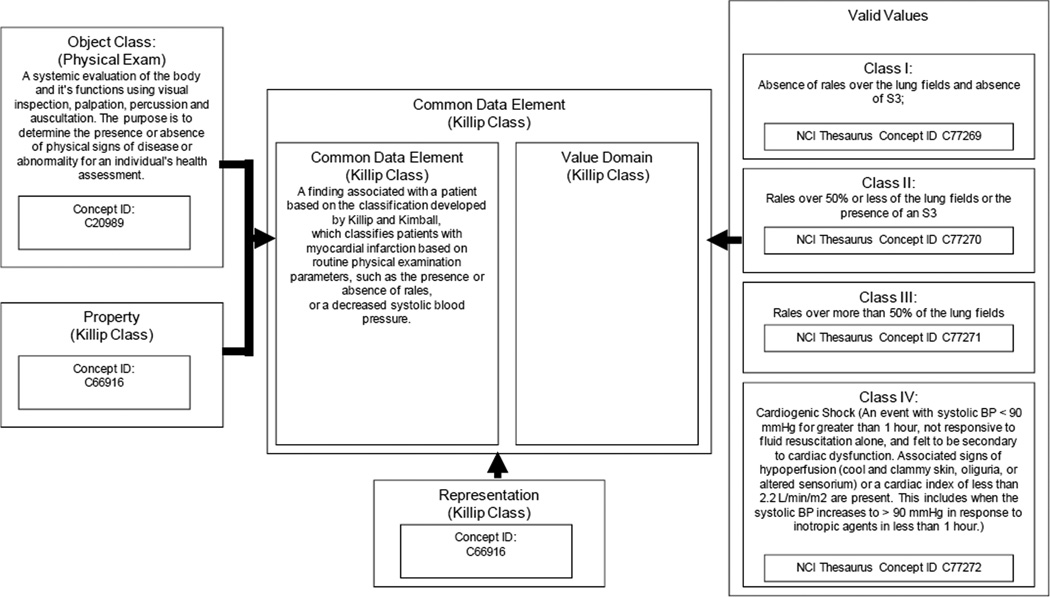
Representation of the data elements was done according to the caDSR implementation of the ISO 11179 metamodel. (15) An example of this representation for the physical examination assessment of Killip Class is shown in Figure 1. More details can be found in the online Appendix 3. A description of the cardiovascular domain analysis model (CV_DAM) is available at the HL7 website (http://www.hl7.org/implement/standards/product_brief.cfm?product_id=133
Figure 1.
A simplified view of a Common Data Element (CDE) in the National Cancer Institute Data Standards Repository (caDSR) Implementation of the ISO 11179 metamodel. This example is for a CDE that describes the physical examination assessment of Killip Class constrained to an enumerated list of values as presented by the HL7 Acute Coronary Syndrome Domain Analysis Model, Release 1. Modified from Komatsoulis et al. (15).
4. Discussion
Clinical research in the United States is an enormous enterprise of great value to the nation’s health. Yet the remarkable advances achieved over the past 80 years have been accomplished largely as a series of separate, organizationally distinct and disconnected efforts undertaken by individual public and private sponsors. For the most part these were done using data management procedures unique to each specific endeavor. Even when ultimate sponsorship has been through the federal enterprise (the National Institutes of Health and other agencies) the individual projects themselves have been dispersed and uncoordinated, and with little effort or attention focused on data interchange. There does not yet exist in the United States, Europe, or elsewhere a robust and sustainable unifying infrastructure that spans the entire translational research, clinical research, regulatory, and clinical practice continuum. Arguably, this absence leads to inefficiencies, delays, and increased costs, all of which have called into question the foundations upon which our clinical research enterprise is built. (24–26) In some instances the increasing globalization of clinical research has allowed new techniques and therapies, including some that are federally funded, to become available first to other regions of the world.
It is noteworthy that the multiple available methods of data collection, storage, and transmission, mostly remain generally incompatible with one another, even though they are parts of the same system involving administrative functions, patient care, clinical research, and regulatory reporting and compliance. Lack of full integration with clinical EHR systems has especially constrained efforts to coordinate information transfer, despite the fact that all the functional areas mentioned have become increasingly interdependent. Development of standard data elements with clear and unambiguous definitions and that are compatible with EHR systems holds great promise for addressing the current absence of interoperability. The EHR thus becomes the definitive repository of valid and fully verifiable clinical data, as well as the substrate for facilitating extraction and exchange of data across multiple systems in both the clinical research and patient care domains. Properly constructed, this substrate will enable a broadly distributed yet interconnected network to facilitate information exchange with semantic interoperability among geographically dispersed sites. In order to begin, a single authoritative set of interoperable data elements are needed as the basis for a unified nationwide infrastructure useful simultaneously in both clinical research and patient care. This portion of the NCRI project addresses that need.
Ideally, all clinical data captured via integrated clinical workflows into EHRs eventually will be subject to data standards, including those endorsed by ACCF, AHA, SCAI, STS, and other organizations. However, the task is twofold. First, the relevant clinical data standards have to be created by the appropriate clinical workgroups. Then, these clinical terms and concepts must be converted into syntactically and semantically compatible computer language structures to make them interoperable across networked computer information systems. Implementing such structures for all existing clinical data standards is a daunting task and cannot be accomplished all at once. The NCDR and STS registries together contain approximately 2,400 data elements in current use. When other officially approved data elements are added, the total could grow by hundreds and possibly thousands more. The costs of fully developing the technical specifications and obtaining endorsement for all potential data elements will be quite large. Therefore, some selectivity is required initially in order to establish the core elements for a baseline data standard that can be put into place and then periodically modified. That was the task of this Workgroup. Ultimately, the NCRI project is intended to evolve into permanent stewardship by ACCF of a fully accepted cardiovascular vocabulary. This stewardship will include mechanisms for constant oversight and periodic formal review and updating in response to research, development, and new discoveries. There will be continuing opportunities for engagement and involvement of all stakeholders. For one thing, much more work is needed to harmonize even these initial standardized cardiovascular data elements with other recognized administrative data formats, such as the Systematized Nomenclature for Medicine (SNOMED/CT), the International Classification for Diseases (ICD 9/10), the Logical Observation Identifiers Names and Codes for laboratory values (LOINC), and RxNorm for drugs and pharmacy systems. (27 – 30)
In conclusion, the NCRI Data Standards Workgroup has assembled a set of 353 cardiovascular data elements with definitions that is designed to serve as a foundation of a national cardiovascular clinical and research infrastructure. The vast majority of elements were identified from already existing sources. This work builds upon earlier efforts to establish a base cardiovascular vocabulary for electronic health records, and it includes all the technical developments required for adoption as an international standard. Once fully adopted and implemented these elements will be useful in facilitating clinical research, registry reporting, administrative reporting and regulatory compliance, and all aspects of patient care.
Supplementary Material
Acknowledgments
NCRI grant: 1RC2HL101512-01
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Staff:
ACCF: Dana M. Pinchotti, BS
Arsalan Khalid, MBA
DCRI: Rebecca Wilgus, RN, MSN
Brian McCourt, BS
David F. Kong, MD, FACC
CDISC: Chris Tolk, BS
HL7: Mead Walker
NCI EVS: Erin Muhlbrandt, PhD
Theresa Quinn, RN, BS
REFERENCES
- 1.Kong DF, Peterson ED, McCourt B, Krucoff MW, Rumsfeld JS, Harrington RA. The national cardiovascular research infrastructure: a new platform for evidence generation. (in process). [Google Scholar]
- 2.Hammond WE. eHealth interoperability. Stud Health Technol Inform. 2008;134:245–253. [PubMed] [Google Scholar]
- 3.Mead CN. Data interchange standards in healthcare IT – computable semantic interoperability: now possible but still difficult, do we need a better mousetrap? J Healthc Inf Manag. 2006;20:71–78. [PubMed] [Google Scholar]
- 4.Weintraub WS, Karlsberg RP, Tcheng JE, Buxton AE, Boris JR, Dove JT, Fonarow GC, Goldberg LR, Heidenreich P, Hendel RC, Jacobs AK, Lewis W, Mirro MJ, Shahian DM. ACCF/AHA 2011 key data elements and definitions of a base cardiovascular vocabulary for electronic health records. J Am Coll Cardiol. 2011;58:202–222. doi: 10.1016/j.jacc.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Hendel RC, Budoff MJ, Cardella JF, Chambers CE, Dent JM, Fitzgerald DM, Hodgson JM, Klodas E, Kramer CM, Stillman AE, Tilkemeier PL, Ward RP, Weigold WG, White RD, Woodard PK. ACC/AHA/ACR/ASE/ASNC/HRS/NASCI/RSNA/SAIP/SCAI/SCCT/SCMR/SIR 2008 key data elements and definitions for cardiac imaging. J. Am. Coll. Cardiol. 2009;53:91–124. doi: 10.1016/j.jacc.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Buxton AE, Calkins H, Callans DJ, DiMarco JP, Fisher JD, Greene HL, Haines DE, Hayes DL, Heidenreich PA, Miller JM, Poppas A, Prystowsky EN, Schoenfeld MH, Zimetbaum PJ, Heidenreich Paul A, Goff DC, Grover FL, Malenka DJ, Peterson ED, Radford MJ, Redberg RF. ACC/AHA/HRS 2006 key data elements and definitions for electrophysiological studies and procedures. J. Am. Coll. Cardiol. 2006;48:2360–2396. doi: 10.1016/j.jacc.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, Flaherty JT, Harrington RA, Krumholz HM, Simoons ML, Van De Werf FJ, Weintraub WS, Mitchell KR, Morrisson SL, Brindis RG, Anderson HV, Cannom DS, Chitwood WR, Cigarroa JE, Collins-Nakai RL, Ellis SG, Gibbons RJ, Grover FL, Heidenreich PA, Khandheria BK, Knoebel SB, Krumholz HL, Malenka DJ, Mark DB, Mckay CR, Passamani ER, Radford MJ, Riner RN, Schwartz JB, Shaw RE, Shemin RJ, Van Fossen DB, Verrier ED, Watkins MW, Phoubandith DR, Furnelli T. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. J Am Coll Cardiol. 2001;38:2114–2130. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 8. [Accessed September 15, 2011]; Available at: www.sts.org/national-database/database-managers/adult-cardiac-surgery-database. [Google Scholar]
- 9.Cannon CP, Brindis RG, Chaitman BR, Cohen DJ, Cross JT, Jr, Drozda JP, Fesmire FM, Fintel DJ, Fonarow GC, Fox KA, Gray DT, Harrington RA, Hicks KA, Hollander J, Krumholz H, Labarthe DR, Long JB, Mascette A, Meyer C, Peterson ED, Radford MJ, Roe MT, Richmann JB, Selker HP, Shahian DM, Shaw RE, Sprenger S, Swor R, Underberg JA, Van de Werf F, Weiner BH, Weintraub WS. ACCF/AHA key elements and data definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease. (in press) [Google Scholar]
- 10. [Accessed October 14, 2011]; Information available at: www.cdisc.org/cdash. [Google Scholar]
- 11. [Accessed November 10, 2011]; Information available at: www.qualityforum.org/QualityDataModel.aspx#t=1&s=&p. [Google Scholar]
- 12.Radford MJ, Heidenreich PA, Bailey SR, Goff DC, Grover FL, Havranek EP, Kuntz RE, Malenka DJ, Peterson ED, Redberg RF, Roger VL. ACC/AHA 2007 methodology for the development of clinical data standards: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards. J Am Coll Cardiol. 2007;49:830–837. doi: 10.1016/j.jacc.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 13. [Accessed October 14, 2011]; Information available at www.cdisc.org. [Google Scholar]
- 14. [Accessed October 24, 2011]; Information available at: www.hl7.org. [Google Scholar]
- 15.Komatsoulis GA, Warzel DB, Hartel FW, Shanbhag K, Chilukuri R, Fragoso G, Coronado S, Reeves DM, Hadfield JB, Ludet C, Covitz PA. caCORE version 3: Implementation of a model driven, service-oriented architecture for semantic interoperability. J Biomed Inform. 2008;41:106–123. doi: 10.1016/j.jbi.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCourt B, Harrington RA, Fox K, Hamilton CD, Booher K, Hammond WE, Walden A, Nahm M. Data standards: at the intersection of sites, clinical research networks, and standards development initiatives. Drug Inf J. 2007;41:393–404. [Google Scholar]
- 17.Nahm M, Walden A, McCourt B, Pieper K, Honeycutt E, Hamilton CD, Harrington RA, Diefenbach J, Kisler B, Walker M, Hammond WE. Standardizing clinical data elements. Int J Functional Informatics and Personalized Medicine. 2010;3:314–341. [Google Scholar]
- 18. [Accessed October 14, 2011]; Available at: www.cdisc.org/sdtm. [Google Scholar]
- 19. [Accessed November 28, 2011]; Available at: http://evs.nci.nih.gov.
- 20.Navathe AS, Clancy C, Glied S. Advancing research data infrastructure for patient-centered outcomes research. JAMA. 2011;306:1254–1255. doi: 10.1001/jama.2011.1341. [DOI] [PubMed] [Google Scholar]
- 21.Adler-Milstein J, Jha AK. Sharing clinical data electronically: a critical challenge for fixing the health care system. JAMA. 2012;307:1695–1696. doi: 10.1001/jama.2012.525. [DOI] [PubMed] [Google Scholar]
- 22. [Accessed January 30, 2012]; Available at: www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Meaningful_Use.html.
- 23.Bufalino VJ, Masoudi FA, Stranne SK, Horton K, Albert NM, Beam C, Bonow RO, Davenport RL, Girgus M, Fonarow GC, Krumholz HM, Legnini MW, Lewis WR, Nichol G, Peterson ED, Rosamond W, Rumsfeld JS, Schwamm LH, Shahian DM, Spertus JA, Woodard PK, Yancy CW. The American Heart Association’s recommendations for expanding the applications of existing and future clinical registries: a policy statement from the American Heart Association. Circulation. 2011;123:2167–2179. doi: 10.1161/CIR.0b013e3182181529. [DOI] [PubMed] [Google Scholar]
- 24.Kim ESH, Carrigan TP, Menon V. International participation in cardiovascular randomized controlled trials sponsored by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2011;58:671–676. doi: 10.1016/j.jacc.2011.01.066. [DOI] [PubMed] [Google Scholar]
- 25.Califf RM, Harrington RA. American industry and the U.S. cardiovascular clinical research enterprise. J Am Coll Cardiol. 2011;58:677–680. doi: 10.1016/j.jacc.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 26.Probstfield JL, Frye RL. Strategies for recruitment and retention of participants in clinical trials. JAMA. 2011;306:1798–1799. doi: 10.1001/jama.2011.1544. [DOI] [PubMed] [Google Scholar]
- 27. [Accessed January 15, 2012]; Available at: www.nlm.nih.gov/research/umls/Snomed/snomed_main.html.
- 28. [Accessed January 15, 2012]; Available at: www.who.int/classifications/icd/en/.
- 29. [Accessed March 28, 2012]; Available at: http://loinc.org. [Google Scholar]
- 30. [Accessed March 28, 2012]; Available at: www.nlm.nih.gov/research/umls/rxnorm/overview.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.