Abstract
Purpose
The aims of this work were to: a) develop an approach for ex-vivo MR volumetry of human brain hemispheres that does not contaminate the results of histopathological examination, b) longitudinally assess regional brain volumes postmortem, and c) investigate the relationship between MR volumetric measurements performed in-vivo and ex-vivo.
Methods
An approach for ex-vivo MR volumetry of human brain hemispheres was developed. Five hemispheres from elderly subjects were imaged ex-vivo longitudinally. All datasets were segmented. The longitudinal behavior of volumes measured ex-vivo was assessed. The relationship between in-vivo and ex-vivo volumetric measurements was investigated in seven elderly subjects imaged both ante-mortem and postmortem.
Results
The presented approach for ex-vivo MR volumetry did not contaminate the results of histopathological examination. For a period of 6 months postmortem, within-subject volume variation across time points was substantially smaller than inter-subject volume variation. A close linear correspondence was detected between in-vivo and ex-vivo volumetric measurements.
Conclusion
Regional brain volumes measured with the presented approach for ex-vivo MR volumetry remain relatively unchanged for a period of 6 months postmortem. Furthermore, the linear relationship between in-vivo and ex-vivo MR volumetric measurements suggests that the presented approach captures information linked to ante-mortem macrostructural brain characteristics.
Keywords: ex-vivo, volumetry, MRI, segmentation
Introduction
The combination of ex-vivo MRI and histopathology of human brain tissue may enhance investigation of the neuropathological correlates of brain abnormalities (1–5). Ex-vivo MRI provides images at essentially the same time-point as histological examination of the tissue, ensuring in a cost-effective manner that no additional pathology develops between imaging and histology (4). Furthermore, ex-vivo MRI is free of subject motion and allows long scan-times, thereby facilitating investigation of tissue properties without contamination from motion-induced artifacts, and with higher spatial resolution and/or signal to noise ratio compared to in-vivo imaging (4,6).
To maximize the potential of ex-vivo MRI for the purpose of volumetric studies of the human brain, whole brain or at least whole hemispheres must be imaged instead of slabs or sections of brain that may be incomplete or structurally deformed during sectioning. In addition, combination of ex-vivo MR volumetry with histopathology demands tissue preparation for ex-vivo MRI that does not interfere with histopathological examination. However, the effects of death, brain extraction from the skull and chemical fixation on the volume of different regions of a human brain hemisphere have not been investigated over time postmortem. Furthermore, the relationship between MR volumetric measurements conducted on human brain hemispheres in-vivo and ex-vivo has not been studied. Thus, the value of ex-vivo MR volumetry of human brain hemispheres remains uncertain.
In this work, an approach for ex-vivo MR volumetry of human brain hemispheres that does not contaminate the results of histopathological examination was first developed. In order to establish the longitudinal behavior of the volume of different brain regions measured with ex-vivo MR volumetry, five human brain hemispheres were imaged with MRI ex-vivo on a weekly basis over a period of three months, with an additional scan at six months postmortem. All datasets were semi-automatically segmented into a number of cortical and subcortical regions using a multi-atlas approach (7–14). Finally, the relationship between MR volumetric measurements performed in-vivo and ex-vivo was investigated using data from seven elderly subjects imaged both ante-mortem and postmortem.
Methods
Participants
Thirty-seven elderly human subjects were recruited from two longitudinal clinical-pathologic studies of aging: the Rush Memory and Aging Project (MAP) (15) and the Religious Orders Study (ROS) (16). All participants provided written informed consent and signed an anatomical gift act. The study was approved by the Institutional Review Board of Rush University Medical Center. All participants were non-demented and had no history of head trauma, brain surgery, stroke, brain tumor, Parkinson’s disease, multiple sclerosis, depression, other neurologic or psychiatric conditions.
Tissue Handling Protocol
After a subject’s death, an autopsy technician removed the brain and dura from the calvarium by severing the cranial nerves and the spinal cord at the level of the foramen magnum. Immediately after removal of the intact brain, the cerebrum was separated from the cerebellum and brainstem by cutting through the cerebral peduncles rostrally to the mammillary bodies. The cerebrum was then divided into left and right hemispheres by bisecting the corpus callosum. One of the two hemispheres was immersed in phosphate-buffered 4% formaldehyde solution (prepared from paraformaldehyde) and refrigerated at 4°C within 30 minutes after removal from the skull. While in storage, the 4% formaldehyde solution was changed weekly. Prior to ex-vivo MRI, a hemisphere was removed from refrigeration, transferred to a clear plastic container filled with 4% formaldehyde solution, and allowed to return to room temperature (approximately 8 hours). When transferring the hemisphere to the clear plastic container, air-bubbles were allowed to escape. The hemisphere was positioned with its medial aspect facing the bottom of the container, gently anchored in place using netting or plastic barriers, and imaged with MRI. Following MRI, the hemisphere was sectioned into 1cm thick coronal slabs, immersed into 4% formaldehyde solution, and refrigerated at 4°C. Finally, histopathological examination was performed within 2 weeks from ex-vivo MRI by a board-certified neuropathologist (17,18).
In-Vivo and Ex-Vivo MRI Data Acquisition
Three datasets were used in this work. Dataset 1 was used to construct 25 atlases for the purposes of multi-atlas segmentation. Dataset 2 was used to assess the behavior of the volume of different brain regions over time postmortem. Dataset 3 was used for the evaluation of multi-atlas segmentation, as well as for the investigation of the relationship between MR volumetric measurements performed in-vivo and ex-vivo. All in-vivo imaging was conducted at 1.5 Tesla (T) due to scanner proximity to the residences of the elderly participants.
Dataset 1
In-vivo T1-weighted 3D magnetization-prepared rapid gradient-echo (MP-RAGE) data was collected on 25 elderly subjects (13 men; 87.69±4.3 years of age; age-range 77.7–93.5 years) using a 1.5T General Electric MRI scanner (Waukesha, WI, USA) and the following parameters: TE=2.8 ms, TR=6.3 ms, preparation time=1000 ms, flip angle=8°, repetitions=2, field of view (FOV)=24 cm × 24 cm, 160 sagittal slices, slice thickness=1 mm, 224×192 acquisition matrix reconstructed to a 256×256 image matrix, scan time=22 min.
Dataset 2
Ex-vivo proton density and T2-weighted 2D fast spin-echo (FSE) data was collected on cerebral hemispheres from 5 elderly subjects that died of natural causes (Table 1), using a 3T General Electric MRI scanner (Waukesha, WI, USA) and the following parameters: TE1=13 ms, TE2=52 ms, TR=3.6 s, echo-train-length (ETL)=6, FOV=16 cm × 16 cm, slice thickness=1.5 mm, 256×256 acquisition matrix reconstructed to a 512×512 image matrix, repetitions=6, scan time=31 min. All hemispheres were imaged on a weekly basis for 3 months, with an additional scan at 6 months postmortem. The only deviation from the tissue handling protocol presented above was that slabbing and histopathological examination of the 5 hemispheres in Dataset 2 were performed after all ex-vivo imaging was completed.
Table 1.
Selected demographic and imaging characteristics, and pathological diagnosis for subjects in Dataset 2. The methods used for pathological diagnosis have been described elsewhere (17,18).
| Subject | A | B | C | D | E | Mean ± SD |
|---|---|---|---|---|---|---|
| Age at death (years) | 88.1 | 78 | 84.9 | 92.2 | 76.3 | 83.9 ± 6.7 |
| Postmortem interval to first scan (days) | 4.8 | 3.3 | 4 | 5 | 5.9 | 4.6 ± 1.0 |
| Hemisphere side | Right | Right | Right | Left | Left | - |
| Sex | F | F | M | M | M | - |
| Alzheimer’s | high likelihood | high likelihood | high likelihood | high likelihood | intermediate likelihood | |
| Lewy Bodies | no | yes | yes | yes | yes | |
| Cerebral Amyloid Angiopathy | mild to moderate | not assessed | moderate to severe | mild to moderate | not assessed | |
| Hippocampal sclerosis | none | none | none | none | none | |
| Macroinfarcts | none | yes | none | none | none | |
| Microinfarcts | none | none | none | yes | none |
Dataset 3
Both in-vivo and ex-vivo MRI data was collected on 7 elderly subjects (Table 2) (all subjects died of natural causes). All subjects were scanned in-vivo between 29–558 days ante-mortem using the same scanner, sequence and imaging parameters as those for Dataset 1. Ex-vivo proton density and T2-weighted 2D FSE acquisitions were performed on the 7 subjects between 31–103 days postmortem, using three different 3T scanners due to scanner updates that occurred between the time of death of the 7 elderly subjects (Table 2). For one of the subjects the scanner, sequence and imaging parameters used for the ex-vivo scans were the same as those for Dataset 2. Four of the subjects were scanned ex-vivo using a 3T Siemens MRI scanner (Erlangen, Germany) and the following parameters: TE1=12 ms, TE2=35 ms, TE3=58 ms, TR=3.75 s, ETL=2, FOV=16 cm × 16 cm, slice thickness=1.5 mm, 256×256 acquisition and image matrix, repetitions=4, scan time=32 min. The remaining two subjects were scanned ex-vivo using a 3T Philips MRI scanner (Best, The Netherlands) and the following parameters: TE1=16.5 ms, TE2=33 ms, TE3=49.5 ms, TE4=66 ms, TE5=82.5 ms, TR=4.055 s, ETL=5, FOV=16 cm × 16 cm, slice thickness=1.5 mm, 260×258 acquisition matrix reconstructed to a 288×288 image matrix, repetitions=2, scan time=35 min (Table 2).
Table 2.
Selected demographic and imaging characteristics, and pathological diagnosis for subjects in Dataset 3. The methods used for pathological diagnosis have been described elsewhere (17,18).
| Subject | A | B | C | D | E | F | G | Mean ± SD |
|---|---|---|---|---|---|---|---|---|
| Ante-mortem interval (days) | 29 | 361 | 33 | 292 | 303 | 131 | 558 | 244 ± 192 |
| Age at death (years) | 89.4 | 91.5 | 87.6 | 89.1 | 89.7 | 86.3 | 87.4 | 88.7 ± 1.7 |
| Postmortem interval to imaging (days) | 103 | 56 | 31 | 53 | 33 | 41 | 33 | 50 ± 25 |
| Hemisphere side | Left | Left | Right | Left | Right | Left | Right | - |
| Sex | M | M | M | M | M | F | M | - |
| MRI Scanner | GE | Siemens | Siemens | Siemens | Siemens | Philips | Philips | - |
| Alzheimer’s | low likelihood | low likelihood | intermediate likelihood | low likelihood | low likelihood | low likelihood | intermediate likelihood | |
| Lewy Bodies | none | none | none | none | none | none | none | |
| Cerebral Amyloid Angiopathy | mild to moderate | none | mild to moderate | mild to moderate | none | mild to moderate | none | |
| Hippocampal Sclerosis | yes | none | none | none | none | none | none | |
| Macroinfarcts | none | yes | none | none | none | none | none | |
| Microinfarcts | none | none | none | none | none | yes | none |
Segmentation of Ex-Vivo MRI Data
Segmenting ex-vivo MRI data on human brain hemispheres using existing software typically leads to severe errors, due to substantial differences in the anatomy and environment of a brain hemisphere imaged ex-vivo compared to those of a whole brain imaged in-vivo. Therefore, a multi-atlas approach was used instead for segmentation of ex-vivo MRI data (7–14). In multi-atlas segmentation, multiple atlases are registered to a target volume, the resulting spatial transformations are used to propagate the labels of each atlas to the target’s space, and the rate of occurrence of each label in a voxel is used for the final labeling (segmentation) of the target volume. In this work, Dataset 1 was used to construct 25 atlases for the purposes of multi-atlas segmentation. First, the T1-weighted image volumes from all 25 subjects of Dataset 1 were segmented using FreeSurfer (http://surfer.nmr.mgh.harvard.edu) (19) into the regions listed in Table 3. The FreeSurfer results were reviewed, and errors were corrected. Atlases of the left and right human brain hemispheres were obtained from the 25 image volumes and corresponding label volumes, by separating regions in left from those in right hemispheres according to the assigned FreeSurfer labels, and by masking out voxels labeled as ventricles, brain stem, cerebellum, blood vessels and optic chiasm, since those regions were not imaged ex-vivo.
Table 3.
List of gray matter regions segmented in this work. Cerebral white matter was also segmented.
| Subcortical Regions |
|---|
| Thalamus-Proper; Caudate; Putamen; Pallidum; Hippocampus; Amygdala; Accumbens Area; Ventral DC |
| Cortical Regions |
| Temporal Lobe – Medial Aspect |
| Entorhinal cortex; Parahippocampal gyrus; Temporal pole; Fusiform gyrus |
| Temporal Lobe – Lateral Aspect |
| Superior temporal gyrus; Middle temporal gyrus; Inferior temporal gyrus; Transverse temporal cortex; Banks of the superior temporal sulcus |
| Frontal Lobe |
| Superior frontal gyrus |
| Middle Frontal Gyrus |
| Rostral division; Caudal division |
| Inferior Frontal Gyrus |
| Pars opercularis; Pars orbitalis; Pars triangularis |
| Orbitofrontal Cortex |
| Lateral division; Medial division |
| Frontal Pole |
| Precentral Gyrus |
| Paracental Lobule |
| Parietal Lobe |
| Postcentral gyrus; Supramarginal gyrus; Superior parietal cortex; Inferior Parietal Cortex; Precuneus cortex |
| Occipital Lobe |
| Lingual gyrus; Pericalcarine cortex; Cuneus cortex; Lateral occipital cortex |
| Cingulate cortex |
| Rostral anterior division; Caudal anterior division; Posterior division; Isthmus division |
For each hemisphere imaged ex-vivo (target hemisphere), the image volume collected at an echo-time of approximately 50 ms (referred to as V50 in the following) was used for the purposes of segmentation. T2 maps of the target hemisphere were generated using the image volumes collected at different echo-times. Voxels of V50 of the target hemisphere containing signals from formaldehyde solution were masked out by rejecting T2 values higher than 150 ms. The result was reviewed and residual formaldehyde signals were manually removed from V50. The contrast of V50 was inverted by changing the sign of its intensities and adding a large positive number. All atlases from the same side of the brain as the target hemisphere were then registered to the pre-processed V50 of that hemisphere in two steps (7,8). In the first step, affine registration was performed using FMRIB’s Linear Image Registration Tool (FLIRT) (20). In the second step, non-linear alignment was performed using the Automatic Registration Toolbox (ART) (21,22). The resulting transformations were applied to the label volumes of the 25 atlases using nearest neighbor interpolation, propagating the 25 label volumes to the target hemisphere’s space. The final segmentation was obtained by assigning labels with the highest rate of occurrence to voxels of the target hemisphere (7–9,23). When two or more labels had the same rate of occurrence in a voxel, one of those labels was assigned to that voxel randomly (7–9). The resulting segmentation was further improved by limiting gray and white matter labels within gray and white matter tissue boundaries, respectively, using tissue maps of the pre-processed V50 of the target hemisphere, generated with FMRIB’s Automated Segmentation Tool (FAST) (24). Gray and white matter tissue boundaries were reviewed and manually corrected. Maps of the final segmentation were generated using a different color for each label. To visualize the confidence in the labeling decisions made throughout gray matter of a brain hemisphere, maps of the following ratio were also produced:
| [1] |
where NSelected is the number of times any of the 25 atlases assigned the finally selected label to the voxel under study, and NTotal is the number of times any label was assigned to that voxel. A ratio equal to one suggests that the labeling decision was unanimous, while a low ratio suggests that one or more labels other than the selected one were often assigned to that voxel, indicating low confidence in the final labeling decision.
Evaluation of the Multi-Atlas Segmentation Approach Using a Simulation Based on In-Vivo Data
Segmentation using the multi-atlas approach presented above was compared to that using FreeSurfer. The latter was considered to be the gold standard. This comparison was conducted using in-vivo data, since FreeSurfer segmentation of human brain hemispheres imaged ex-vivo is problematic. T1-weighted in-vivo whole brain image volumes from 7 subjects (Dataset 3) were first segmented using FreeSurfer. Based on the FreeSurfer labels, each whole brain image volume was divided into two image volumes of the individual hemispheres, simulating the data collected on brain hemispheres ex-vivo. The 25 atlases were then registered to the generated image volumes, and segmentation of the hemispheres was completed following the rest of the multi-atlas segmentation approach presented above. Finally, the results of the two segmentation methods were compared in terms of the agreement in volume and location of the segmented brain regions as described below.
To evaluate the agreement in volumes measured with the two methods, the volumes of gray matter regions from all 7 simulated hemispheres measured with multi-atlas segmentation were regressed on the corresponding volumes measured with FreeSurfer, using a linear mixed model (LMM) with no intercept term and with random effect for the slope (the logarithm with base 10 of the volumes was used in the model due to the large range in size of the segmented regions). A 95% confidence interval for the slope was obtained from this LMM. The LMM was repeated for 1000 bootstrapped copies of the set of 7 simulated hemispheres, sampling hemispheres with replacement. For each iteration, the ratio of the residual variance of the volumes measured with multi-atlas segmentation regressed on volumes measured with FreeSurfer to the total variance of the volumes measured with multi-atlas segmentation, was calculated. A low value for this ratio suggests that the volumes measured with multi-atlas segmentation can be well modeled as a linear function of the volumes measured with FreeSurfer. The mean and standard deviation of this ratio over all iterations, as well as the 95% confidence interval, were calculated. Additionally, a Bland-Altman plot was constructed to evaluate how the size of a region affected the agreement in volumes measured with the two methods.
To test the agreement in location of the brain regions segmented with the two methods, a stereology-type validation study was performed. More specifically, for each of the 7 simulated hemisphere image volumes, 2000 voxels were randomly selected from gray and white matter tissue and the ventricles, and classified based on the segmentation result of the two methods. A co-occurrence table presenting the classification of the 2000 voxels according to FreeSurfer and multi-atlas segmentation was constructed for each of the 7 subjects. Cohen’s kappa (κ) coefficient, a statistical measure of inter-method agreement for categorical data, was calculated from the co-occurrence table of each subject (25–27) as follows:
| [2] |
where PActual is the observed probability of agreement between the two segmentation methods, and PRandom is the probability of agreement purely by chance. A κ=0 suggests no agreement, and a κ=1 perfect agreement. Values greater than 0.81 are generally considered to reflect excellent agreement (26).
Longitudinal Assessment of the Volume of Different Brain Regions Measured with Ex-Vivo MR Volumetry
The multi-atlas segmentation approach was applied to the longitudinal ex-vivo MRI data of Dataset 2 to assess the behavior of the volume of different brain regions over time postmortem. Considering the measurements at all time-points, the ratio of the variation between subjects over the total variation was calculated for each region, using components of variance analysis. A value for this ratio near 1 suggests that the total variation is mainly due to differences between subjects and not due to within-subject changes over time postmortem. The ratios from all segmented regions were averaged. The same procedure was repeated 100 times using bootstrapped copies of the set of 5 hemispheres sampled with replacement. The mean and standard deviation of the averaged ratio, as well as the 95% confidence interval, were estimated. The same bootstrap method was repeated for the total volume of each hemisphere. In addition, for each subject, the volume of each region, r, at each time-point, t, V(r,t), was normalized with the volume of the same region at the first time-point, V(r,1):
| [3] |
and plotted as a function of time postmortem. The total volume of each hemisphere was also plotted as a function of time postmortem.
Relationship Between MR Volumetric Measurements Performed In-Vivo and Ex-Vivo
The multi-atlas segmentation approach was applied to the ex-vivo MRI data of Dataset 3, and the results were compared to those of FreeSurfer segmentation of the in-vivo MRI data collected on the same subjects. The volumes of gray matter regions from all hemispheres measured ex-vivo were regressed on the corresponding volumes measured in-vivo, using LMM with no intercept term and with random effects for the slope (the logarithm with base 10 of the volumes was used in the model due to the large range in size of the segmented regions). The LMM was repeated for 1000 bootstrapped copies of the set of 7 hemispheres, sampled with replacement. For each iteration, the ratio of the residual variance of the volumes measured ex-vivo regressed on volumes measured in-vivo, over the total variance of the volumes measured ex-vivo, was calculated. A low value for this ratio suggests that the volumes measured ex-vivo can be well modeled as a linear function of the volumes measured in-vivo. The mean and standard deviation of this ratio over all iterations, as well as the 95% confidence interval, were calculated.
Results
Tissue Handling, Ex-Vivo MRI, and Multi-Atlas Segmentation
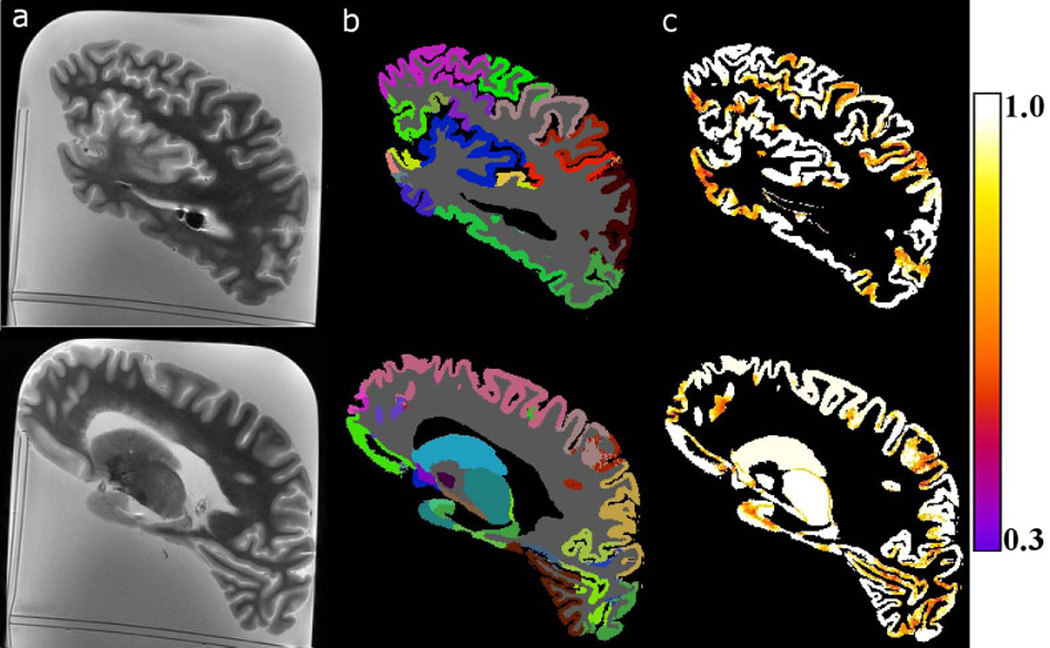
Brain hemispheres were handled following typical protocols used for the purposes of histopathological examination (17,18), with the only exception of approximately 30 minutes of MR imaging using clinical hardware and pulse sequences. No noticeable tissue changes were observed during histopathological examination. Visual inspection of the images collected ex-vivo showed sufficiently high image quality for the purposes of volumetry, comparable to, or even higher than that of images typically used for in-vivo brain MR volumetry (Fig.1A) (28). Visual inspection of ex-vivo MRI segmentation results demonstrated proper outlining of gray and white matter, and good agreement in terms of the location of regions between individual hemispheres and the corresponding FreeSurfer atlas (29) (Fig.1B). Maps of the quantity C (Eq.1) in gray matter showed high confidence in the labeling decisions made in the central portion of large gray matter regions, and lower confidence at the borders of neighboring regions, as well as throughout small gray matter regions (Fig.1C).
Figure 1.
A) Examples of sagittal T2-weighted ex-vivo MR images collected from a single brain hemisphere at TE=58 ms. B) Corresponding multi-atlas segmentation results. Each segmented brain region has been assigned a different color. C) Maps of the confidence in labeling decisions made in the two slices. The color scale on the right shows the colors corresponding to the different values of the quantity C (Eq.1).
Evaluation of the Multi-Atlas Segmentation Approach Using a Simulation Based on In-Vivo Data
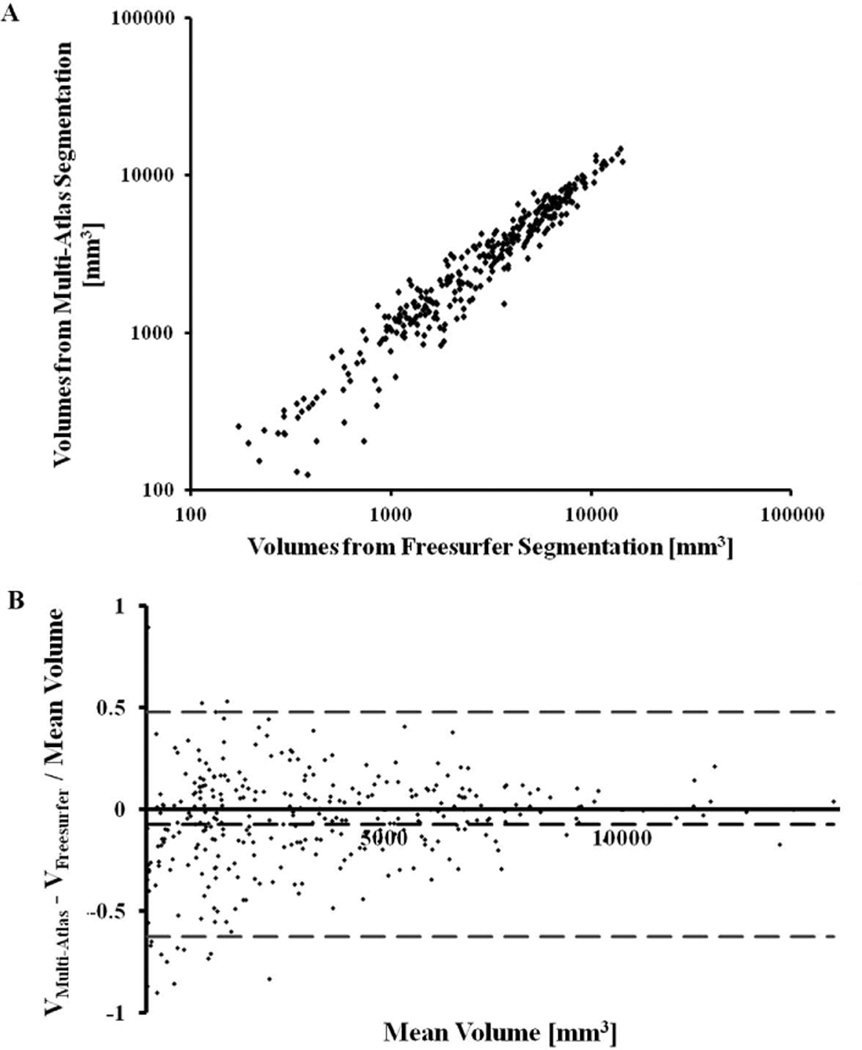
The ratio of the residual variance of the volumes measured with multi-atlas segmentation regressed on volumes measured with FreeSurfer, to the total variance of the volumes measured with multi-atlas segmentation, was relatively small at 0.067±0.011 (95% confidence interval [0.044, 0.088]). This suggests that the volumes measured with multi-atlas segmentation may be well modeled as a linear function of the volumes measured with FreeSurfer (Fig.2A). Moreover, the estimated coefficient did not differ significantly from 1 (95% confidence interval [0.9901, 1.0001]).
Figure 2.
A) Scatter-plot of the volume of all gray matter regions (Table 3) segmented with multi-atlas segmentation and FreeSurfer in hemisphere images simulated based on in-vivo data from 7 subjects (Dataset 3). Each point of the scatter-plot corresponds to a single region of a single subject. B) Bland-Altman plot of the relative difference in volume measured in a region with the two methods, as a function of the mean volume of that region (estimated as the mean of the volumes obtained from the two methods). Each point of the scatter plot corresponds to a single region of a single subject. The middle dashed line represents the mean of the relative differences in volume measured with the two methods, and the top and bottom dashed lines define the 95% confidence interval.
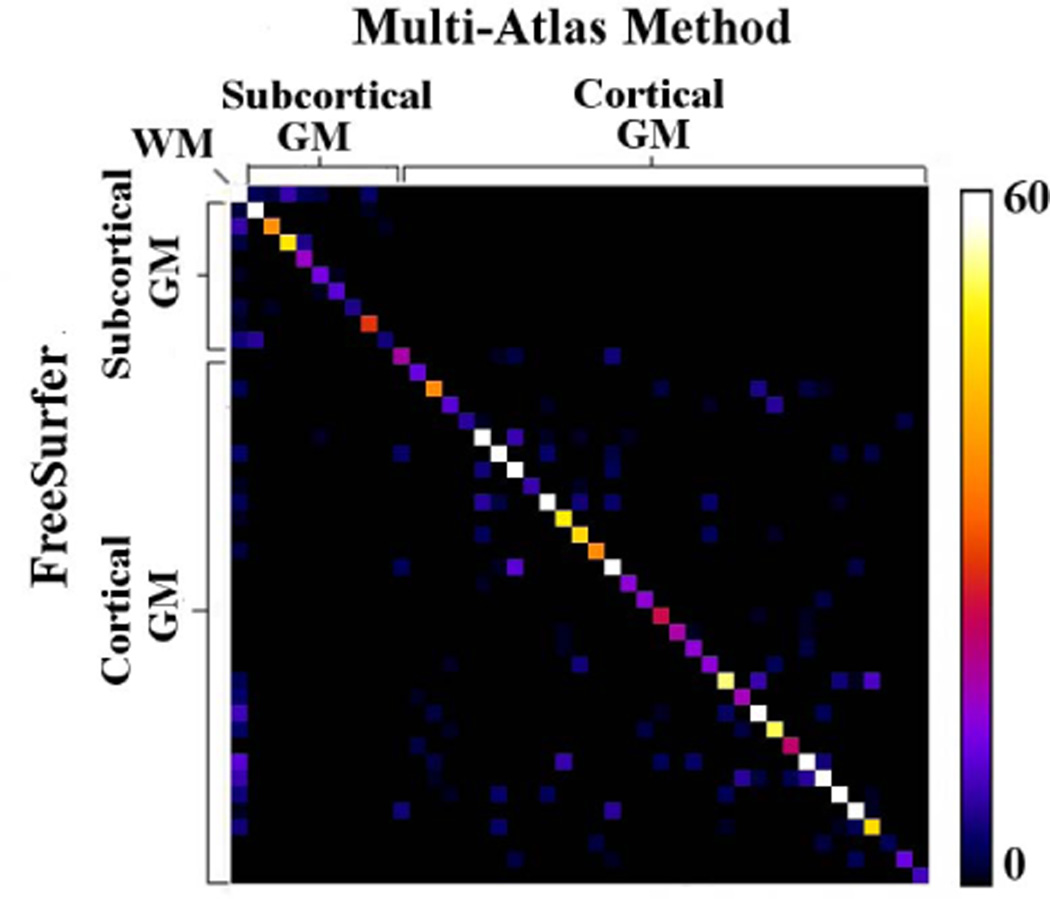
The Bland-Altman plot indicated that the size of the different brain regions did not influence the agreement in volume measurements between the two methods. The same plot demonstrated that, in smaller brain regions, the relative variation in volume measurements from one or both methods was higher than in larger regions (Fig.2B). Co-occurrence tables presenting the agreement in classification of 2000 randomly selected voxels in each of the 7 simulated hemisphere images according to multi-atlas segmentation and FreeSurfer, showed high values along the diagonal and low values in off-diagonal elements (Fig.3). Cohen’s κ coefficient ranged from 0.85 to 0.89 for the 7 simulated hemisphere image volumes.
Figure 3.
Co-occurrence table for a single simulated hemisphere image volume showing the agreement in classification of 2000 randomly selected voxels according to multi-atlas and FreeSurfer segmentation. The color bar shows the numbers of voxels assigned to labels by the two segmentation methods. Cohen’s coefficient for this particular hemisphere was κ=0.89.
Longitudinal Assessment of the Volume of Different Brain Regions Measured with Ex-Vivo MR Volumetry
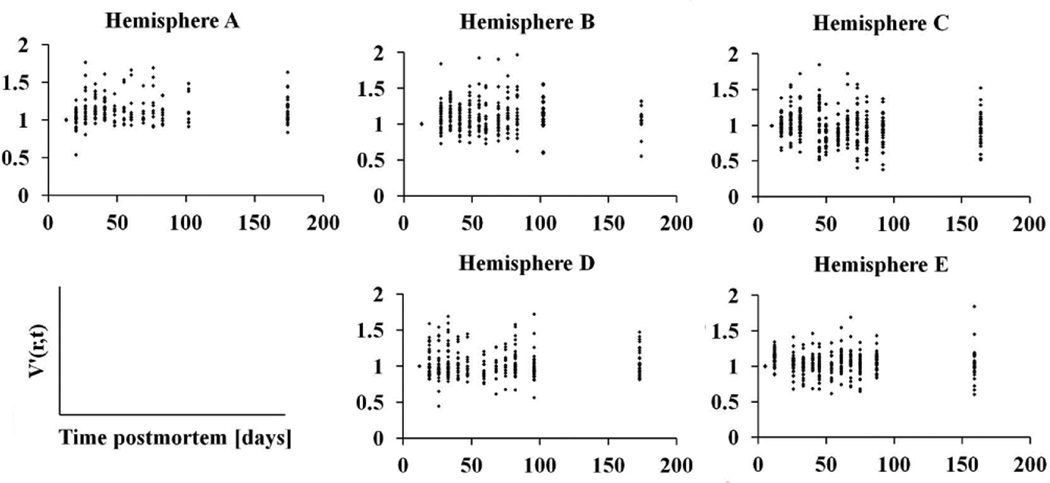
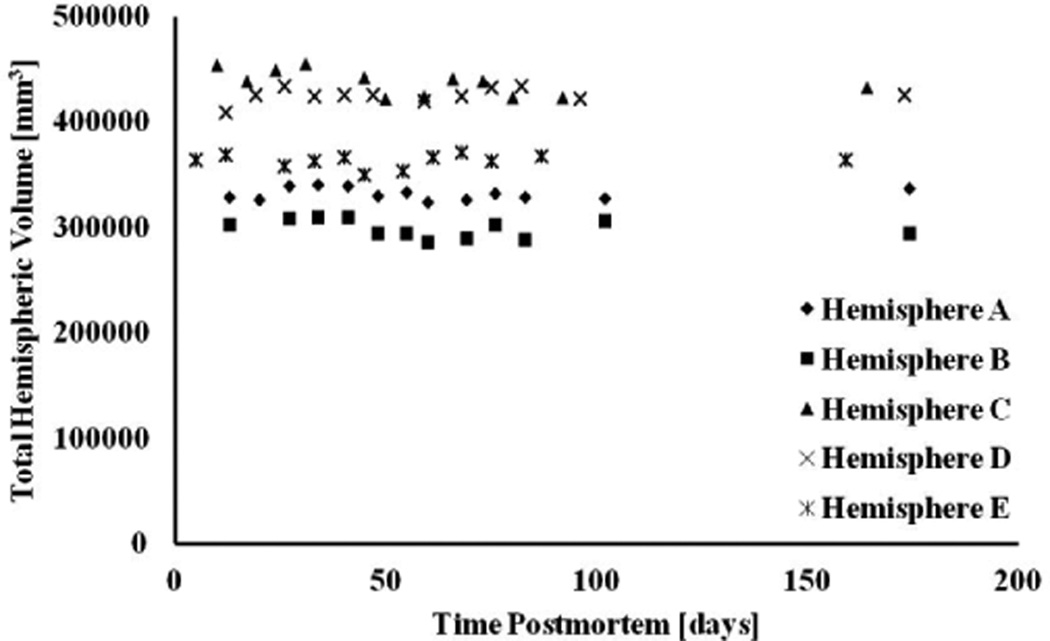
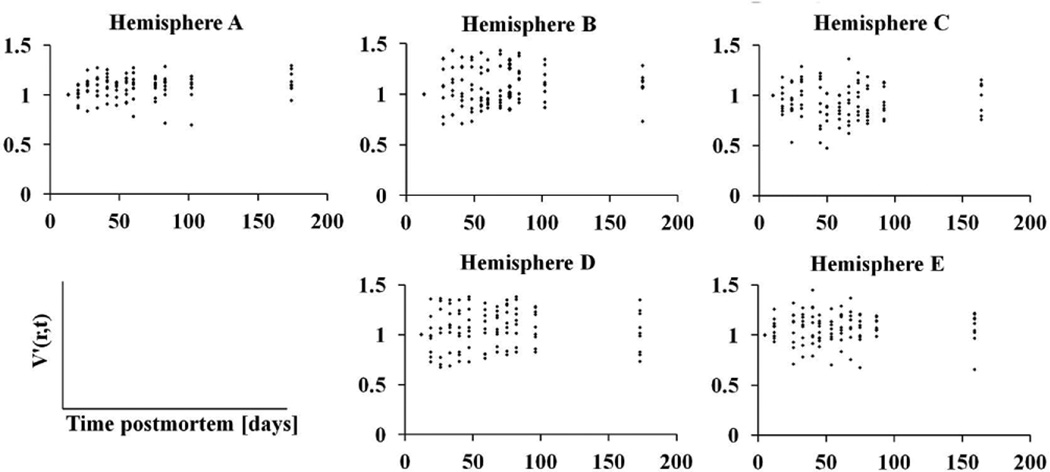
According to the bootstrap component of variance analysis, the ratio of the between-subjects variation of ex-vivo volume measurements over the total variation, averaged over all regions, was equal to 0.77±0.07 (95% confidence interval [0.51, 0.84]). The same ratio for hemispheric volumes was equal to 0.97±0.09 (95% confidence interval [0.92, 0.99]). These results suggest that the total variation in ex-vivo volume measurements was mostly due to differences between subjects and less due to within-subject changes over time postmortem (Figs.4–6).
Figure 4.
Plots of the cortical volumes normalized to the first time-point, V′(r,t), as a function of time postmortem, for all hemispheres of Dataset 2.
Figure 6.
Plot of total hemispheric volume as a function of time postmortem, for all hemispheres of Dataset 2.
Relationship Between MR Volumetric Measurements Performed In-Vivo and Ex-Vivo
The ratio of the residual variance of the volumes measured ex-vivo regressed on volumes measured in-vivo, over the total variance of the volumes measured ex-vivo, was relatively small at 0.086±0.006 (95% confidence interval [0.076, 0.098]). This suggests that the volumes measured ex-vivo may be well modeled as a linear function of the volumes measured in-vivo (Fig.7).
Figure 7.
Plots of the volumes of different brain regions measured ex-vivo as a function of the corresponding volumes measured in-vivo, for all hemispheres of Dataset 3. Each point in the scatter-plot represents a single brain region of a single subject.
Discussion
Ex-vivo MR volumetry has the potential to become an important tool for the assessment of the neuropathologic correlates of macrostructural brain abnormalities, since it allows volumetric investigation of brain tissue at essentially the same time-point as histopathological examination, ensuring in a cost-effective manner that no additional pathology develops between imaging and histology. This work first presented an approach for ex-vivo MR volumetry of human brain hemispheres that does not contaminate the results of histopathological examination. It was then demonstrated that, for the particular methodology used, and for a period of 6 months postmortem, within-subject volume variation across time points was substantially smaller than inter-subject volume variation. Additionally, a close linear correspondence was detected between MR volumetric measurements performed in-vivo and ex-vivo on the same subjects, suggesting that the presented approach for ex-vivo MR volumetry captures information linked to the ante-mortem macrostructural characteristics of the brain.
In order to avoid any effects on histopathology, brain hemisphere handling procedures adopted in this work generally followed typical protocols used for histopathological examination. After death, all brain tissue remained immersed in 4% formaldehyde solution, even during MR imaging. In contrast to previously published work, no exogenous contrast agents (e.g. Gd-DTPA) were used for image contrast enhancement (30,31), and brain tissue was not immersed in a perfluorocarbon liquid or in gels to minimize non-brain signals (32) and prevent movement of the tissue (30,33). The effects of these substances on brain tissue and histopathological examination have not been thoroughly investigated and therefore their use was avoided. Also, in the presented approach, all brain tissue remained refrigerated at 4°C, and was only returned to room temperature for the purposes of MRI scanning. This brief time interval at room temperature does not constitute a significant deviation from typical tissue-handling protocols used for histopathological examination. Furthermore, clinical hardware and pulse sequences were used for ex-vivo MRI, limiting the amount of energy deposited in the tissue to clinically acceptable levels. Consequently, the actual MR scanning process is not expected to have any effect on the tissue. Finally, histopathology of all brain hemispheres showed no tissue alterations or processing artifacts. Based on the above, it was concluded that the presented approach for ex-vivo MR volumetry did not contaminate the results of histopathological examination.
The image quality of the raw data obtained with the presented ex-vivo MRI approach was sufficiently high for segmentation and volume measurements (Fig.1A). Spatial resolution was higher than that typically used for in-vivo brain MR volumetry. Based on visual inspection, the contrast to noise ratio was at least comparable to that of images typically used for in-vivo brain MR volumetry (Fig.1A). Image artifacts were minor and primarily originated from trapped air-bubbles. Previous studies applied a vacuum to remove trapped air (32). In the present study, efforts were made to allow air-bubbles to escape when transferring hemispheres to containers for scanning. Due to the pulse sequences and imaging protocols used, any remaining trapped air generated only minor artifacts that did not affect volume measurements (Fig.1A). Specimen bulk motion due to scanner vibration is another potential limitation in ex-vivo MRI. Previously published microscopy studies addressed this problem by employing viscous liquids or gels to immobilize the tissue (30,33). In the current work, bulk motion due to scanner vibration was not observed between echoes, due to the relatively large weight of the hemispheres and the fact that all hemispheres were gently anchored using netting or plastic barriers. In summary, the presented ex-vivo MRI approach provided sufficient image quality in a reasonable scan time (~30 min),while using clinical hardware and software and relatively simple methodology, thereby reducing the complexity and cost of investigations conducting ex-vivo MR volumetry on large numbers of human brain hemispheres.
Ex-vivo MR images of human brain hemispheres were successfully segmented with the presented multi-atlas approach. Firstly, the gray-white matter border was well delineated in all hemispheres. The accuracy in defining this border depended on the accuracy of tissue maps generated by FAST (20), which is widely used in the neuroimaging community for segmentation of gray and white matter, and was not reevaluated here. Furthermore, tissue maps were reviewed and corrected when necessary. Secondly, in the simulation comparing segmentation results between the multi-atlas approach and FreeSurfer, the volumes of different brain regions measured with multi-atlas segmentation were linearly related to the volumes of the same regions generated with FreeSurfer, with a slope of approximately one (Fig.2A). Also, the size of the different brain regions did not influence the agreement in volume measurements between the two methods (Fig.2B). Thirdly, the locations of the different brain regions segmented with the multi-atlas approach were in good agreement between individual hemispheres and the corresponding FreeSurfer atlas, based on visual inspection. Furthermore, the co-occurrence tables and Cohen’s coefficients generated in the simulation suggested excellent agreement (25) in the location of brain regions segmented with multi-atlas segmentation and FreeSurfer. Any residual differences in segmentation using the two methods may be related to minor misregistration of the multiple atlases with the simulated images of brain hemispheres, or the fact that the simulation was based on in-vivo datasets with lower image quality than that of the actual ex-vivo MR images due to typical scan-time limitations of in-vivo imaging. It is anticipated that the agreement between the two segmentation methods will be even higher if both methods could be applied on the ex-vivo data generated with the presented approach (currently not possible for FreeSurfer). Based on the above, it was concluded that the presented multi-atlas segmentation technique appropriately segmented ex-vivo MR images of human brain hemispheres. It should be noted however, that this conclusion is based on adopting FreeSurfer and the corresponding atlas as gold standards (34–38), and does not suggest that the boundaries of the segmented regions were necessarily anatomically correct. The same segmentation approach presented here can be combined with other atlases.
The confidence in gray matter labeling decisions was higher in the central portion of large gray matter regions than at the borders of neighboring regions, or in small regions (Fig.1C). Reduced confidence at the borders and small regions was due to uncertainty in FreeSurfer segmentation of the 25 brain volumes used as atlases, as well as misregistration of the atlases with individual hemispheres. A large number of high quality atlases and effective registration algorithms are necessary for robust multi-atlas segmentation. In this work, 25 atlases were used, which was previously suggested to be a sufficiently large number for the purposes of multi-atlas segmentation (8). All atlases were generated with FreeSurfer, which is one of the leading approaches for segmentation of in-vivo human brain MRI volumes (34–38). Also, registration of the atlases to individual brain hemispheres was achieved with ART, which was recently shown to be among the most accurate non-linear registration tools for inter-subject normalization of anatomical MRI data (39).
The presented approach for ex-vivo MR volumetry allowed postmortem longitudinal investigation of the volume of various brain regions. It was demonstrated that, from 3–6 days to approximately 6 months postmortem, within-subject volume variation across time points was substantially smaller than inter-subject volume variation. This information is crucial for future cross-sectional studies involving ex-vivo MR volumetry of human brain hemispheres imaged at different postmortem intervals (40). To our knowledge this is the first postmortem longitudinal volumetric investigation of human brain hemispheres.
The time periods from death to 3 days postmortem, and after 6 months postmortem, were not included in this study. It was recently shown that while immunohistochemical staining of several neurodegenerative disease markers is possible even after years of fixation, staining of certain markers is unsuccessful after 6 months of fixation (41–43). Thus, studies combining ex-vivo MR volumetry and histopathology may be most effective when conducted within the first 6 months postmortem. Further research is necessary to establish the behavior of ex-vivo MR volume measurements on human brain hemispheres outside the time window studied here.
Volume measurements performed ex-vivo were shown to be linearly related to volume measurements performed in-vivo on the same subjects. However, differences in image quality between the in-vivo and ex-vivo MRI data, as well as the different segmentation methods used, introduced a different bias in the volumes measured in-vivo and ex-vivo. Consequently no conclusions can be drawn from the slope of the linear relationship between MR volumetric measurements performed ex-vivo and in-vivo. Nevertheless, the linear relationship itself suggests that the presented approach for ex-vivo MR volumetry captures information that is linked to the ante-mortem macrostructural characteristics of the brain. We recently demonstrated that hippocampal volume measured postmortem in 100 brain hemispheres from elderly subjects was linked to ante-mortem measures of cognitive performance, in agreement with in-vivo studies (40). In addition, ex-vivo MRI ensures that no additional pathology develops between imaging and histology, and accomplishes that in a cost effective manner in contrast to the alternative of longitudinal in-vivo MRI. Furthermore, ex-vivo MRI allows higher image quality than in-vivo MRI, leading to more accurate volume measurements. Thus, based on the above, combination of ex-vivo MR volumetry and histopathology may become an effective tool for the assessment of the neuropathologic correlates of macrostructural brain abnormalities observed in-vivo. The main complication of this approach is access to large numbers of human brain hemispheres from well-characterized subjects. This is, however, already made feasible by projects such as MAP and ROS (15,16,40).
The current investigation also has limitations. Although FreeSurfer is one of the leading approaches for automated brain segmentation (34–38), each of the 25 atlases generated using FreeSurfer may contain minor errors in terms of the precise borders of different brain regions. However, these errors should be random across atlases, and the use of a large number of atlases should have compensated for such errors in the final multi-atlas segmentation of individual hemispheres. In future studies, custom atlases can be used in combination with the presented approach for ex-vivo MR volumetry. Another limitation of the current investigation was the use of three MRI scanners to collect ex-vivo data from the 7 subjects that were also scanned in-vivo (Dataset 3). This was inevitable due to required scanner updates between the deaths of the 7 subjects. Nevertheless, the findings from all scanners were similar (Fig.7). Finally, although the same autopsy protocol was followed for all subjects, small differences in sectioning may have occurred.
Conclusions
The present study first developed an approach for ex-vivo MR volumetry of human brain hemispheres that does not contaminate the results of histopathological examination. This approach was then used to assess the volume of different brain regions along postmortem fixation times. For a period of 6 months postmortem, within-subject volume variation across time points was substantially smaller than inter-subject volume variation. Finally, MR volumetric measurements performed ex-vivo were shown to be linearly related to those performed in-vivo on the same subjects, suggesting that the presented approach for ex-vivo MR volumetry captures information that is linked to the ante-mortem macrostructural characteristics of the brain.
Figure 5.
Plots of the subcortical volumes normalized to the first time-point, V′(r,t), as a function of time postmortem, for all hemispheres of Dataset 2.
Acknowledgements
This study was supported by National Institute on Aging grants P30AG10161, R01AG15819, R01AG17917, the Illinois Department of Public Health and the Chicago Chapter of the Achievements Reward for College Scientists Foundation. The authors thank Niranjini Rajendran for her help with segmentation of in-vivo datasets with FreeSurfer; Veronica Flores, Ryan Johnson, Catherine Ulman, Karen Skish, Dennis Brown, Kenneth Simon, Debra Magnuson and Kefiloe Tsotetsi for preparing the brain hemispheres. We also thank all participants in the Rush Memory and Aging Project and Religious Orders Study.
References
- 1.Bronge L, Bogdanovic N, Wahlund L. Postmortem MRI and histopathology of white matter changes in Alzheimer brains. Dement Geriatr Cogn Disord. 2002;13:205–212. doi: 10.1159/000057698. [DOI] [PubMed] [Google Scholar]
- 2.Seewann A, Kooi EJ, Roosendaal SD, Barkhof F, van der Valk P, Geurts JJ. Translating pathology in multiple sclerosis: the combination of postmortem imaging, histopathology and clinical findings. Acta Neurol Scand. 2009;119:349–355. doi: 10.1111/j.1600-0404.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- 3.Bobinski M, de Leon MJ, Wegiel J, Desanti S, Convit A, Saint Louis LA, Rusinek H, Wisniewski HM. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer’s disease. Neuroscience. 2000;95:721–725. doi: 10.1016/s0306-4522(99)00476-5. [DOI] [PubMed] [Google Scholar]
- 4.Pfefferbraum A, Sullivan EV, Adalsteinsson E, Garric T, Harper C. Postmortem MR imaging of formalin-fixed human brain. NeuroImage. 2004;21:1585–1595. doi: 10.1016/j.neuroimage.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Blamire AM, Rowe JG, Styles P, McDonald B. Optimising imaging parameters for postmortem MR imaging of the human brain. Acta Radiol. 1999;40:593–597. doi: 10.3109/02841859909175593. [DOI] [PubMed] [Google Scholar]
- 6.Augustinack JC, Helmer K, Huber KE, Kakunoori S, Zollei L, Fischl B. Direct Visualization of the perforant pathway in the human brain with ex-vivo diffusion tensor imaging. Frontiers in Human Neuroscience. 2010;4:42. doi: 10.3389/fnhum.2010.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gousias IS, Rueckert D, Heckemann RA, Dyet LE, Boardman JP, Edwards AD, Hammers A. Automatic segmentation of brain MRIs of 2-year-olds into 83 regions of interest. NeuroImage. 2008;40:672–684. doi: 10.1016/j.neuroimage.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 8.Heckemann RA, Hajnal JV, Aljabar P, Rueckert D, Hammers A. Automatic anatomical brain MRI segmentation combining label propagation and decision fusion. NeuroImage. 2006;33:115–126. doi: 10.1016/j.neuroimage.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 9.Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, Mitchell TN, Brooks DJ, Duncan JS. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Human Brain Mapping. 2003;19:224–347. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbøl S, Frøkjaer VG, Holm S, Paulson OB, Knudsen GM. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. NeuroImage. 2005;24:969–979. doi: 10.1016/j.neuroimage.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Rohlfing T, Brandt R, Menzel R, Maurer CR., Jr Evaluation of atlas selection strategies for atlas-based image segmentation with application to confocal microscopy images of bee brains. NeuroImage. 2004;1:1428–1442. doi: 10.1016/j.neuroimage.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Heckemann RA, Hammers A, Aljabar P, Rueckert D, Hajnal JV. The mirror method of assessing segmentation quality in atlas label propagation; International Symposium on Biomedical Imaging: From Nano to Macro; Boston, MA, USA. 2009. pp. 1194–1197. [Google Scholar]
- 13.Dawant B, Hartmann S, Thirion J, Maes F, Vandermeulen D, Demaerel P. Automatic 3-D segmentation of internal structures of the head in MR images using a combination of similarity and free-form transformations. I. Methodology and validation on normal subjects. IEEE Trans Med Imag. 1999;18:909–916. doi: 10.1109/42.811271. [DOI] [PubMed] [Google Scholar]
- 14.Artaechevarria X, Munoz-Barrutia A, Ortiz-de-Solorzano C. Combination strategies in multi-altas image segmentation: application to brain MR data. IEEE Trans Med Imag. 2009;28:1266–1277. doi: 10.1109/TMI.2009.2014372. [DOI] [PubMed] [Google Scholar]
- 15.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and Findings From the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9:646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and Findings from the Religious Orders Study. Curr Alzheimer Res. 2012;9:628–645. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed Brain Pathologies Account for Most Dementia Cases in Community-Dwelling Older Persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 18.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Education modifies the association of amyloid but not tangles with cognitive function. Neurology. 2005;65:953–955. doi: 10.1212/01.wnl.0000176286.17192.69. [DOI] [PubMed] [Google Scholar]
- 19.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 20.Jenkinson M, Smith SM. A global optimization method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 21.Ardekani BA, Bachman AH, Strother SC, Fujibayashi Y, Yonekura Y. Impact of inter-subject image registration on group analysis of fMRI data. International Congress Series. 2004;1265:49–59. [Google Scholar]
- 22.Ardekani BA. World Congress on Med Phys And Biomed Eng. Sydney, Australia: 2003. An improved method for intersubject registration in 3D volumetric brain MRI; p. 452. [Google Scholar]
- 23.Kittler J, Hatef M, Duin RPW, Matas J. On combining classifiers. IEEE Trans Pattern Anal Mach Intell. 1998;20:226–239. [Google Scholar]
- 24.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen J. A coefficient of agreement for nominal scales. Educ Psycol Meas. 1960;20:37–46. [Google Scholar]
- 26.Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A. The SRI24 multichannel atlas of normal adult human brain structure. Human Brain Mapping. 2010;31:798–819. doi: 10.1002/hbm.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 28.Kruggel F, Turner J, Muftuler LT. Alzheimer's Disease Neuroimaging Initiative. Impact of scanner hardware and imaging protocol on image quality and compartment volume precision in the ADNI cohort. NeuroImage. 2010;49:2123–2133. doi: 10.1016/j.neuroimage.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Benveniste H, Blackband S. MR microscopy and high resolution small animal MRI: applications in neuroscience research. Prog Neurobiol. 2002;67:393–420. doi: 10.1016/s0301-0082(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 31.Johnson GA, Benveniste H, Black RD, Hedlund LW, Maronpot RR, Smith BR. Histology by magnetic resonance microscopy. Magn Reson Q. 1993;9:1–30. [PubMed] [Google Scholar]
- 32.D'Arceuil HE, Westmoreland S, de Crespigny AJ. An approach to high resolution diffusion tensor imaging in fixed primate brain. NeuroImage. 2007;35:553–565. doi: 10.1016/j.neuroimage.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs RE, Fraser SE. Imaging neuronal development with magnetic resonance imaging (NMR) microscopy. J Neurosci Methods. 1994;54:189–196. doi: 10.1016/0165-0270(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 34.Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, Pacheco J, Albert M, Killiany R, Blacker D, Maguire P, Rosas D, Makris N, Gollub R, Dale A, Dickerson B, Fischl B. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. NeuroImage. 2009;46:177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han X, Jovicich J, Salat D, an der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 36.Cherbuin N, Anstey KJ, Réglade-Meslin C, Sachdev PS. In vivo hippocampal measurement and memory: a comparison of manual tracing and automated segmentation in a large community-based sample. PLoS One. 2009;4:e5265. doi: 10.1371/journal.pone.0005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, 2nd, Lewis DV, LaBar KS, Styner M, McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. NeuroImage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dewey J, Hana G, Russell T, Price J, McCaffrey D, Harezlak J, Sem E, Anyanwu JC, Guttmann CR, Navia B, Cohen R, Tate DF HIV Neuroimaging Consortium. Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. NeuroImage. 2010;51:1334–1344. doi: 10.1016/j.neuroimage.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dawe RJ, Bennett DA, Schneider JA, Arfanakis K. Neuropathologic correlates of hippocampal atrophy in the elderly: A clinical, pathologic, postmortem MRI study. PLoS One. 2011;6:e26286. doi: 10.1371/journal.pone.0026286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pikkarainen M, Martikainen P, Alafuzoff I. The effect of prolonged fixation time on immunohistochemical staining of common neurodegenerative disease markers. J Neuropathol Exp Neurol. 2010;69:40–52. doi: 10.1097/NEN.0b013e3181c6c13d. [DOI] [PubMed] [Google Scholar]
- 42.Dwork AJ, Liu D, Kaufman MA, Prohovnik I. Archival, formalin-fixed tissue: its use in the study of Alzheimer's type changes. Clin Neuropathol. 1998;17:45–49. [PubMed] [Google Scholar]
- 43.Evers P, Uylings HB. An optimal antigen retrieval method suitable for different antibodies on human brain tissue stored for several years in formaldehyde fixative. J Neurosci Methods. 1997;72:197–207. doi: 10.1016/s0165-0270(96)02204-2. [DOI] [PubMed] [Google Scholar]