Abstract
Objectives
Apathy is a prevalent neuropsychiatric manifestation in individuals with Alzheimer’s disease (AD) that is associated with decreased social functioning and increased caregiver burden. Olfactory deficits are also commonly observed in AD, and prior work has indicated a link between increased apathy and olfactory dysfunction in individuals with Parkinson’s disease. Here, we examined odor identification performance in patients with probable AD (n = 172), individuals with mild cognitive impairment (MCI; n = 112), and neurologically and psychiatrically healthy older adults (n = 132) and its relation to apathy, depression, and overall psychopathology.
Method
Participants were administered the Sniffin’ Sticks odor identification test and measures assessing severity of apathy, depression, and overall neuropsychiatric symptomatology.
Results
Consistent with previous research, AD and MCI patients were significantly worse at identifying odors than healthy older adults. Additionally, a sex by diagnosis interaction was observed. AD patients had significantly higher levels of apathy relative to MCI and control participants. Of note, across the entire sample odor identification deficits were correlated with level of apathy at the level of p < 0.01, but not with depression or neuropsychiatric symptom severity, when controlling for MMSE score.
Conclusion
Collectively, these data suggest that olfactory disturbance and apathy in AD may result from the progression of disease pathology in shared neural substrates.
Keywords: olfactory, smell, apathy, neuropsychiatry, sex differences
1. Introduction
Olfactory deficits are commonly observed in Alzheimer’s disease (AD) and mild cognitive impairment (MCI; Bahar-Fuchs, Moss, Rowe, & Savage, 2010; Westervelt, Bruce, Coon, & Tremont, 2008), and olfactory dysfunction can precede clinical manifestations of AD and MCI (Wilson et al., 2009). Additionally, a highly common neuropsychiatric manifestation of AD is persistent apathy (Di Iulio et al., 2010), characterized by diminished motivation, reduced goal-directed behavior and cognitive activity, blunted affect, and functional impairment (P. Robert et al., 2009). Apathy often leads to a decline in daily function and increased caregiver burden (Landes, Sperry, Strauss, & Geldmacher, 2001). Its distinction from depression is important with regards to treatment implications (Levy et al., 1998), as apathy and depression have been shown to exhibit different trajectories over the course of cognitive decline leading to AD (Wetzels, Zuidema, de Jonghe, Verhey, & Koopmans, 2010). Recently, Cramer et al. (Cramer, Friedman, & Amick, 2010) reported a relation between olfaction and apathy among individuals with Parkinson’s disease. Thus, the overall goal of this study was to evaluate olfaction as a tool in understanding apathy and other psychiatric disturbances associated with AD and its precursor, MCI.
Among olfactory measures, odor identification tasks have received the most attention, with robust odor identification deficits observed across AD and MCI samples. Patients with AD and MCI exhibit significantly worse performance on measures of odor identification when compared to healthy individuals (Devanand et al., 2010; Wesson, Wilson, & Nixon, 2010; Wilson et al., 2007). Bahar-Fuchs et al., (2010) also found that amnestic MCI patients outperformed AD patients, but only when comparing the worst nostril of participants between the two groups. Further, longitudinal studies have shown poor odor identification to predict cognitive decline leading to MCI as well as AD neuropathology (Wilson et al., 2009; Wilson et al., 2007). These findings of poor odor identification in AD and MCI as well as increased risk for cognitive decline in healthy individuals with olfactory dysfunction have raised the possibility that odor identification impairment may be a marker of vulnerability to the disorder.
In AD, apathy has been linked to reduced metabolic activity in the bilateral anterior cingulate gyrus, medial orbitofrontal cortex (OFC), and medial thalamus (Marshall et al., 2007). Similarly, olfactory dysfunction in AD has been attributed to disease-related changes in the olfactory eloquent regions, including the OFC, entorhinal cortex and hippocampus (Lehrner, Pusswald, Gleiss, Auff, & Dal-Bianco, 2009; Wilson et al., 2009). Prior research has also linked apathy with executive dysfunction in the progression of MCI and AD (Boyle et al., 2003; Ready, Ott, Grace, & Cahn-Weiner, 2003). The anatomic proximity of the olfactory network to limbic structures provides a potential explanation for the relation between olfaction and apathy, and may account for their shared dysfunction in various psychiatric disorders. In fact, many of these regions, including the amygdala, hippocampus, insula, anterior cingulate cortex, and OFC have been described as common neural substrates for emotional and olfactory processing (Soudry, Lemogne, Malinvaud, Consoli, & Bonfils, 2011). Alternatively, damage to overlapping neural circuitry may explain this association. Exploration of these observations and their underlying neural mechanisms in AD and MCI may help to illuminate the underlying neuropathophysiology of the disease.
Previous studies have also highlighted sex differences with regards to olfactory functioning in healthy individuals as women tend to perform better on olfactory tests than men (Doty, Shaman, Applebaum, et al., 1984; Murphy et al., 2002; Stockhorst & Pietrowsky, 2004). Sex differences in olfactory performance are thought to be present across age groups (Ship & Weiffenbach, 1993). Although increasing olfactory dysfunction is considered a normal part of aging (Ship & Weiffenbach, 1993), Doty et al. (1997) showed that men tend to experience age-related decline in olfactory functioning at an earlier age than women. Therefore, investigation of sex differences on tests of odor identification in AD and MCI may provide additional insight into the deterioration of OFC processes in these populations.
In this study we examined the effects of group and sex on odor identification ability and apathy severity. Prior studies have reported differences in both domains across AD, MCI, and healthy control groups (Apostolova & Cummings, 2008; Devanand et al., 2010; Starkstein, Jorge, Mizrahi, & Robinson, 2006; Westervelt et al., 2008; Wilson et al., 2007) and between men and women (Doty, Shaman, & Dann, 1984; Lavretsky et al., 2004; Murphy et al., 2002; Stockhorst & Pietrowsky, 2004), but to our knowledge no one has investigated the influence of dementia severity (group) and sex on both olfaction and apathy in the same sample. We further investigated relations between odor identification and apathy, depression, and overall psychopathology within the entire sample to determine whether odor identification is related to apathy severity and, if so, whether this relation is specific to apathy as compared to other psychiatric symptomatology. Thus, the two main objectives of the current study were to examine the contribution of dementia severity and sex to olfaction and apathy and to investigate the relation between odor identification ability and psychopathology in individuals with probable AD, MCI, and a sample of healthy older adults.
2. Methods
2.1 Recruitment and Participants
AD patients (n = 172), MCI patients (n = 112), and neurologically and psychiatrically healthy older adults (n = 132), ages 50 to 100 years, were recruited by the Alzheimer’s Disease Center at the University of Pennsylvania. Consensus best-estimate diagnoses for AD and MCI were established using data gathered during clinical interview, neuropsychological evaluation involving a short psychometric battery of measures assessing memory, attention, and language from the CERAD neuropsychological battery (Chandler et al., 2005), neurological examination, MRI or PET scan, and available information from medical record review, family, and care providers. The consensus group was comprised of neurologists, neuropsychiatrists, neuropsychologists, and social workers. Eligible participants were those with either the presence of a progressive cognitive impairment or demonstrating normal cognition based on performance on a standard psychometric assessment and confirmation by the Alzheimer’s Disease Center (ADC). All participants were screened for good bilateral nasal patency before administration of the olfactory measure to ensure adequate nasal airflow. Participants were evaluated at the Penn Memory Center or off-site at one of two collaborating continuing care retirement communities, and agreed to annual clinical evaluations of their cognitive and functional status. No dependent measures were used to determine diagnostic categories of participants. Following a full explanation of study procedures, written informed consent was obtained in compliance with guidelines established by the University of Pennsylvania Institutional Review Board and in accordance with The Code of Ethics of the World Medical Association (1964 Declaration of Helsinki).
2.2 Materials and Study Procedures
Participants were administered the Sniffin’ Sticks Odor Identification test (Hummel, Sekinger, Wolf, Pauli, & Kobal, 1997; Kobal et al., 1996), a commercially available test that has been used extensively in Europe for the assessment of chemosensory function. Task development and psychometric properties are described in detail elsewhere and show strong test-retest reliability (0.73) and significant relations to a previously established measure of odor identification (0.50), with higher sensitivity to changes in olfaction associated with age than this measure (Hummel et al., 1997). During the odor identification task, subjects were presented with 16 odor-impregnated markers, which they smelled and identified in a four-alternative multiple-choice format. Odorants were presented bi-rhinally (to both nostrils simultaneously) by a trained technician, who placed the pen under the participant’s nares and recorded his or her response. 149 AD participants, 101 MCI participants, and 121 control participants completed the Neuropsychiatric Inventory Questionnaire (NPI-Q; Cummings et al., 1994), a structured interview conducted with an informant that assesses overall psychopathology. Informants were almost always a spouse, partner, or first-degree relative. However, when this was not available, report of a close friend or neighbor familiar with the participant's functioning was utilized. The apathy scale from this questionnaire was used to determine level of apathy for all participants. 150 AD participants, 97 MCI participants, and 118 control participants completed the Geriatric Depression Scale (GDS; Lach, Chang, & Edwards, 2010), a “yes” or “no” self-report instrument used to identify depression in older adults.
2.3 Statistical Analyses
To examine the effect of dementia severity and sex on olfaction and apathy severity, two analyses of covariance (ANCOVA) were conducted with diagnosis (AD, MCI, control) and sex as between-group factors. Raw odor identification score was the dependent variable in the first ANCOVA and apathy was the dependent variable in the second ANCOVA. Age and education were included as covariates in these analyses because the diagnostic groups differed on these demographic variables (see Table 1). Next, to evaluate whether olfaction was uniquely related to apathy severity, we performed three partial correlations, controlling for Mini-Mental State Examination (MMSE) score, between olfactory performance and 1) apathy severity, 2) depression, and 3) overall level of psychopathology (NPI-Q). These partial correlations included the entire sample while partialling out MMSE score to control for level of cognitive impairment. Because three correlation analyses were performed, Bonferroni correction was applied to control for Type I error; only correlation coefficients that were significant at p < 0.02 were interpreted.
Table 1.
Demographic Characteristics
| Demographic Characteristic | AD (n = 172) |
MCI (n = 112) |
Controls (n = 132) |
ANOVA/ ANCOVA |
Group Differences |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | F, p | ||
| Age (years)* | 75.98 (7.53) | 72.63 (8.19) | 72.57 (9.52) | 8.24, <0.001 | AD > (MCI = Controls) |
| Sex (Male:Female)* | 62:110 | 44:88 | 57:55 | 9.051, 0.01 | AD ≠ (MCI = Controls) |
| Percent Caucasian | 75.00 | 75.00 | 73.21 | 8.371, 0.40 | AD = MCI = Controls |
| Education level (years)* | 14.17 (3.71) | 15.08 (4.10) | 16.06 (2.95) | 10.28, <0.001 | AD < MCI < Controls |
| Illness Duration (years) | 4.27 (3.56) | - | - | - | - |
| Age of Onset (years) | 71.73 (7.83) | - | - | - | - |
Significant group difference (p < 0.05); 1X2 value is reported here
3. Results
3.1 Participant characteristics
As shown in Table 1, the diagnostic groups differed significantly with respect to age, sex, and education, but not race or self-identified ethnicity. Follow-up analyses showed that the AD group was significantly older and less educated than the MCI and control groups. MCI patients also had significantly lower educational attainment than controls. The control group was comprised of significantly fewer female participants. With the exception of sex, which was analyzed as an independent variable in this study, other demographic variables that differed across the groups (e.g., age and education) were included as covariates in subsequent analyses.
3.2 The Effect of Group and Sex on Odor Identification Performance
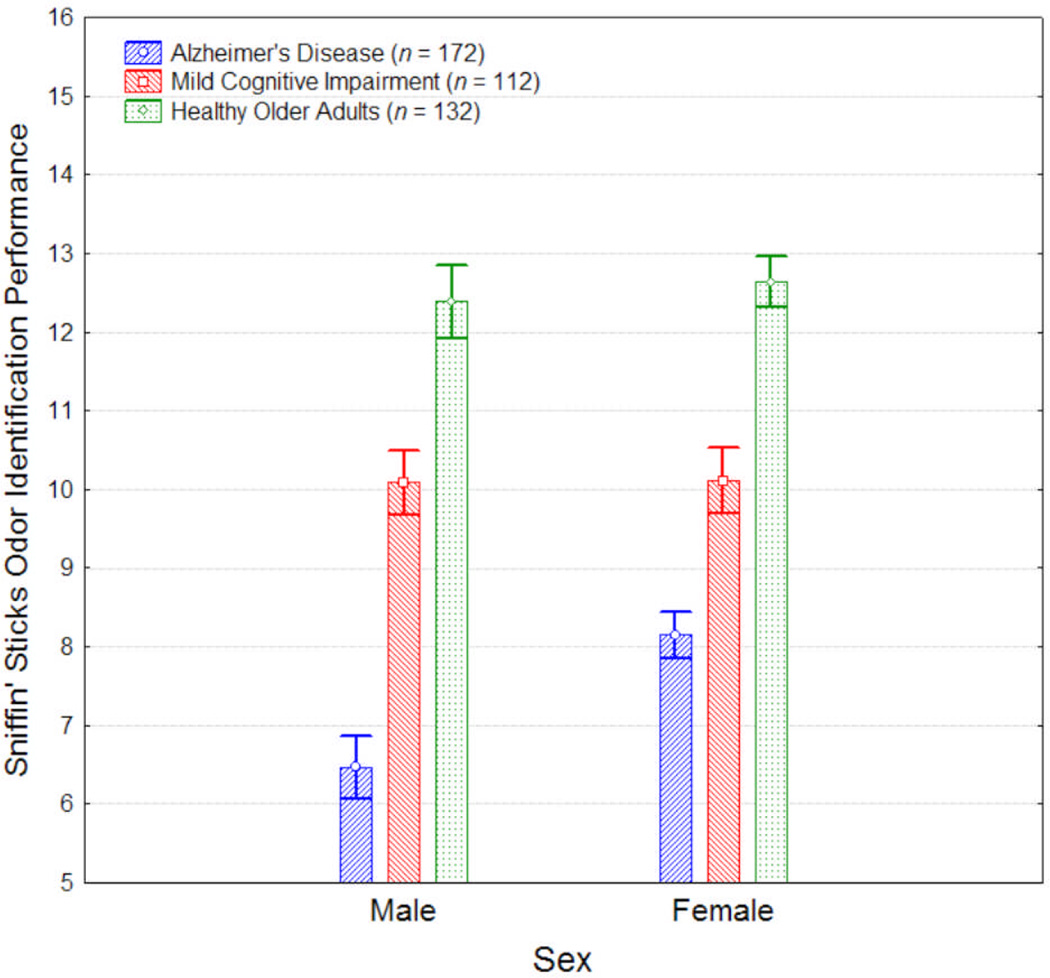
An ANCOVA, controlling for age and education, was performed to evaluate effects of group and sex on odor identification. The covariates, age and education, were both significantly related to odor identification (F1, 408 = 15.38, p < 0.001; F1, 408 = 4.54, p = 0.03, respectively). After controlling for age and education, there were significant main effects of group (see Table 2) and sex (F1,408 = 5.13, p = 0.02), as well as a statistically significant group-by-sex interaction (F2,408 = 2.96, p = 0.05; see Figure 1). Post-hoc analyses showed that, compared to controls, AD and MCI patients were both significantly more impaired at identifying odors, and AD patients were significantly more impaired than MCI patients (see Table 2). This pattern of group differences (AD < MCI < control) was observed among both men and women when analyzed separately (all p’s < 0.001). However, a significant sex difference was observed only in the AD group, in which AD male patients were significantly more impaired relative to their female counterparts (F1,408 = 12.75, p < 0.001); no sex differences were observed within the MCI or control group (all p’s > 0.60).
Table 2.
Clinical Characteristics
| Clinical Characteristic | AD (n = 172) |
MCI (n = 112) |
Controls (n = 132) |
ANCOVA (Main Effect of Group) |
AD vs. MCI | AD vs. Controls |
MCI vs. Controls |
Group Differences |
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | F, p | F, p | F, p | F, p | ||
| Odor Identification Score | 7.54 (3.53) | 10.10 (3.39) | 12.55 (2.52) | 75.55, <0.001 | 40.63, <0.001 | 149.87, <0.001 | 31.17, <0.001 | AD < MCI < Controls |
| Apathy Severity Total Score | 0.47 (0.85) | 0.03 (0.27) | 0.17 (.45) | 14.60, <0.001 | 14.21, <0.001 | 26.52, <0.001 | 1.70, 0.19 | AD > (MCI = Controls) |
Figure 1. Olfaction and Apathy in Alzheimer’s Disease, Mild Cognitive Impairment, and Healthy Older Adults Figure 1.
Olfactory task performance (± SE) in patients with Alzheimer’s disease (AD), mild cognitive impairment (MCI) and healthy older adults.
3.3 The Effect of Group and Sex on Apathy Severity
An ANCOVA, controlling for age and education, was performed to evaluate the effects of group and sex on apathy severity. Education, but not age, was significantly related to apathy severity (F1, 363 = 5.91, p < 0.02; F1, 363 = 0.95, p = 0.33, respectively). After controlling for the effects of the covariates, there was a statistically significant main effect of group (see Table 2), but no significant main effect of sex and no significant interaction (all p’s > 0.22). As shown in Table 2, post-hoc analyses revealed that AD patients had a significantly higher level of apathy severity compared to controls and MCI patients. MCI patients and controls did not differ with respect to apathy severity.
3.4 Relations Among Olfactory Performance, Apathy, Depression, and Overall Psychopathology
Partial correlations, controlling for MMSE and including the entire study sample, showed that reduced odor identification performance was associated with increased apathy severity but was unrelated to self-reported depression or overall neuropsychiatric symptom severity (see Table 3). The significant relation between odor identification and apathy severity met criteria for statistical significance even after Bonferroni correction.
Table 3.
Partial correlations between odor identification score and measures of apathy, depression, and overall neuropsychiatric symptomatology, controlling for overall level of cognitive impairment (MMSE)
| Apathy Severity Total Score |
GDS1 Total Score | NPI-Q2 Total Score | ||
|---|---|---|---|---|
| Odor Identification Score | rc | −0.141* | −0.017 | −0.040 |
| p | 0.007 | 0.770 | 0.448 | |
Significant group difference (p < 0.01); 1GDS = Geriatric Depression Scale; 2NPI-Q = Neuropsychiatric Inventory
4. Discussion
The current study examined the effects of dementia severity and sex on odor identification and apathy severity. Overall, results showed that dementia severity and sex influenced odor identification and apathy severity quite differently. We observed progressively lower scores on the odor identification measure with greater dementia severity among the three groups, as AD patients performed significantly worse than MCI patients, who in turn performed significantly worse than healthy individuals on the Sniffin’ Sticks Odor Identification test. Interestingly, males had significantly lower odor identification scores than females in the AD group, while olfactory performance in the MCI and healthy older adult cohorts did not differ by sex. Apathy severity was significantly higher in the AD group than the MCI and control groups, but showed no effect of or interaction with sex. When examining the relation between olfaction and psychopathology, we found a specific association between odor identification performance and apathy severity within the entire sample, even after controlling for MMSE to account for differential levels of cognitive impairment. Odor identification was not significantly associated with depression or overall psychopathology.
The finding that women outperformed men on the odor identification measure in the AD group is interesting in light of previous literature showing that women tend to perform better than men on tests of olfaction across the lifespan (Doty, Shaman, Applebaum, et al., 1984; Murphy et al., 2002; Stockhorst & Pietrowsky, 2004). The physiological basis of these sex differences in olfaction may have implications for the progression of AD, particularly considering that this difference was only found in the AD group in our study. Given that sexually dimorphic olfactory scores have been shown across the lifespan, it is unlikely that this disparity results from differences in circulating gonadal hormones (Doty, Shaman, Applebaum, et al., 1984). However, the source of these sex differences remains unknown, posing difficulties for the interpretation of our results. Existing literature has shown that the left orbitofrontal cortex (OFC) is more active in women than men when making odor valence decisions (Royet, Plailly, Delon-Martin, Kareken, & Segebarth, 2003). The OFC has also been reported as a vulnerable region in cognitive aging and one of the earliest areas to accumulate amyloid plaques in aging and AD (Resnick, Lamar, & Driscoll, 2007). Future research should investigate the mechanisms associated with these sex differences in olfaction with regards to potential implications for the progression of AD pathology.
Our results showing greater apathy severity in AD patients than healthy older adults and individuals with MCI are supported by previous research suggesting that apathy severity increases with conversion from MCI to AD (Starkstein et al., 2006). Several longitudinal studies have indicated that apathy may be an effective early predictor of risk for conversion from MCI to AD (Palmer et al., 2010; P. H. Robert et al., 2006). Moreover, it has been suggested that apathy may be one of the first markers of cognitive decline in the progression of MCI to AD (Drijgers, Verhey, Leentjens, Kohler, & Aalten, 2011). Our results showing significantly higher olfactory dysfunction in the AD group than the MCI group and in the MCI group than the control group, as well as significantly greater apathy severity in the AD and MCI groups than the control group, suggest a progressive decline in these domains as disease symptoms worsen. This parallel progression suggests the possibility of shared neural mechanisms that underlie olfaction, apathy, and AD disease pathology and may aid in our ultimate understanding of the disorder. The association of apathy with limbic brain regions also implicated in olfaction and indications of common cerebral substrates in the processing of odors and emotions further highlight the possibility that the presence of both apathy and increased olfactory dysfunction may indicate early signs of AD pathology in these areas. Further, growing preclinical evidence suggests that olfactory deficits may exaggerate affective disturbances (Glinka et al., 2012). This raises the possibility that olfactory and emotional symptoms associated with dementia may not only be driven by related neural substrates, but that they may also interact to increase clinical severity, underscoring the need to better characterize these features of illness. Future research should consider potential causal relations between apathy and olfactory dysfunction in AD and MCI.
In contrast to our findings with odor identification, we found no main effect of sex and no sex-by-dementia severity interaction for apathy severity. This was somewhat surprising, as sex differences in AD have been documented independent of dementia severity, showing women with AD to be more emotionally labile and demonstrate inappropriate affect, while men exhibit greater levels of apathy and changes in eating and sleep behaviors (Ott, Tate, Gordon, & Heindel, 1996). Although olfaction and apathy appear to be related with regards to disease progression and neuroanatomical correlates, the discrepant sex effects suggest important differences between these domains that should be explored in future research. One possible explanation for these findings is that sex differences in apathy may tend to be less extreme than those in olfaction and thus were not detected in our sample. The investigation of sex differences with regards to brain structures implicated in both olfaction and apathy may be useful in further characterizing AD progression.
When odor identification was examined in relation to measures of psychopathology, the apathy domain of the NPI-Q was the only measure to be significantly correlated with odor identification scores in our study. Importantly, this relation was observed even after controlling for dementia severity. Taken together with research highlighting the distinction between apathy and other seemingly related emotions such as depression (Levy et al., 1998; Wetzels et al., 2010), this finding emphasizes the importance of specifically investigating apathy as a neuropsychiatric manifestation of the progression to AD. Of note, a correlation analysis did not reveal a significant association between our measures of apathy (NPI-Q, apathy domain) and depression (GDS), ensuring that these constructs were distinctly assessed in our sample. The correlation between olfaction and apathy also further supports the possibility of shared neural mechanisms underlying these domains, and suggests that olfactory measures may be a useful probe of apathy in older adults.
Our study did pose several limitations, including our use of the NPI-Q instead of a more detailed apathy measure. However, our results are comparable to findings reported by Cramer and colleagues who used a specialized measure of apathy (Apathy Evaluation Scale; AES) and between-group analyses (Cramer et al., 2010). Moreover, it was recently shown that both the apathy subscale of the NPI-Q and the AES are successful in distinguishing apathetic from non-apathetic individuals in a sample of people with and without dementia (Leontjevas et al., 2012). Nevertheless, future studies that incorporate more comprehensive and/or interview-based measures of apathy severity, such as the AES, would be useful in capturing a greater range of apathy severity.
Additionally, we did not account for smoking differences because this information was only available for a subset of participants. However, smoking did not differ across this sub-sample of individuals, and previous research has shown olfactory performance to be unaffected by smoking differences in MCI (Wilson et al., 2007). Also of note, the lack of olfactory sex differences in our control and MCI groups is inconsistent with reported findings of sex differences across the lifespan, and may reflect a ceiling effect of testing within these groups. Further investigation of sex differences across healthy and pathological aging processes is necessary to help explain these findings. Finally, it has been observed that in addition to odor identification, odor discrimination correlates strongly with neuropsychological test performance in AD, MCI, and healthy older adults (Djordjevic, Jones-Gotman, De Sousa, & Chertkow, 2008). The use of additional olfactory probes such as odor discrimination and detection threshold tasks may be useful to further clarify the exact nature of the relation between olfaction and apathy in these populations.
These limitations notwithstanding, the current study utilized well-known measures with large samples of individuals who were assessed according to well-established diagnostic guidelines by a consensus panel. In summary, the results of this study suggest that sex differences in olfaction that arise over the course of cognitive decline may also have implications for the progression of illness. Further, they support a specific relation between olfaction and apathy, particularly with regards to AD symptoms and progression. Although future research will be necessary to more precisely determine the nature and extent of this relation, our results suggest that potentially shared pathways between olfaction and apathy may indeed underlie the symptoms of AD. Future studies should aim to clarify the associations of processes whose neural bases are better established, such as olfaction, with symptoms of AD and MCI in the hopes of elucidating the mechanism of disease.
Acknowledgements
We wish to thank Young Baek for assistance with data management and our research subject volunteers for their participation. This work was supported by the National Institutes of Health (P30AG10124) and the Marian S. Ware Alzheimer Program.
References
- Apostolova LG, Cummings JL. Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord. 2008;25(2):115–126. doi: 10.1159/000112509. [DOI] [PubMed] [Google Scholar]
- Bahar-Fuchs A, Moss S, Rowe C, Savage G. Olfactory performance in AD, aMCI, and healthy ageing: a unirhinal approach. Chem Senses. 2010;35(9):855–862. doi: 10.1093/chemse/bjq094. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Malloy PF, Salloway S, Cahn-Weiner DA, Cohen R, Cummings JL. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(2):214–221. [PubMed] [Google Scholar]
- Chandler MJ, Lacritz LH, Hynan LS, Barnard HD, Allen G, Deschner M, Cullum CM. A total score for the CERAD neuropsychological battery. Neurology. 2005;65(1):102–106. doi: 10.1212/01.wnl.0000167607.63000.38. [DOI] [PubMed] [Google Scholar]
- Cramer CK, Friedman JH, Amick MM. Olfaction and apathy in Parkinson's disease. Parkinsonism Relat Disord. 2010;16(2):124–126. doi: 10.1016/j.parkreldis.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Tabert MH, Cuasay K, Manly JJ, Schupf N, Brickman AM, Mayeux R. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol Aging. 2010;31(9):1593–1600. doi: 10.1016/j.neurobiolaging.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Iulio F, Palmer K, Blundo C, Casini AR, Gianni W, Caltagirone C, Spalletta G. Occurrence of neuropsychiatric symptoms and psychiatric disorders in mild Alzheimer's disease and mild cognitive impairment subtypes. Int Psychogeriatr. 2010;22(4):629–640. doi: 10.1017/S1041610210000281. [DOI] [PubMed] [Google Scholar]
- Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2008;29(5):693–706. doi: 10.1016/j.neurobiolaging.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Doty RL. Studies of human olfaction from the University of Pennsylvania Smell and Taste Center. Chem Senses. 1997;22(5):565–586. doi: 10.1093/chemse/22.5.565. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226(4681):1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32(3):489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Drijgers RL, Verhey FR, Leentjens AF, Kohler S, Aalten P. Neuropsychological correlates of apathy in mild cognitive impairment and Alzheimer's disease: the role of executive functioning. Int Psychogeriatr. 2011;23(8):1327–1333. doi: 10.1017/S1041610211001037. [DOI] [PubMed] [Google Scholar]
- Glinka ME, Samuels BA, Diodato A, Teillon J, Feng Mei D, Shykind BM, Fleischmann A. Olfactory deficits cause anxiety-like behaviors in mice. J Neurosci. 2012;32(19):6718–6725. doi: 10.1523/JNEUROSCI.4287-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. 'Sniffin' sticks': olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22(1):39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S. "Sniffin' sticks": screening of olfactory performance. Rhinology. 1996;34(4):222–226. [PubMed] [Google Scholar]
- Lach HW, Chang YP, Edwards D. Can older adults with dementia accurately report depression using brief forms? Reliability and validity of the Geriatric Depression Scale. J Gerontol Nurs. 2010;36(5):30–37. doi: 10.3928/00989134-20100303-01. [DOI] [PubMed] [Google Scholar]
- Landes AM, Sperry SD, Strauss ME, Geldmacher DS. Apathy in Alzheimer's disease. J Am Geriatr Soc. 2001;49(12):1700–1707. doi: 10.1046/j.1532-5415.2001.49282.x. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Kurbanyan K, Ballmaier M, Mintz J, Toga A, Kumar A. Sex differences in brain structure in geriatric depression. Am J Geriatr Psychiatry. 2004;12(6):653–657. doi: 10.1176/appi.ajgp.12.6.653. [DOI] [PubMed] [Google Scholar]
- Lehrner J, Pusswald G, Gleiss A, Auff E, Dal-Bianco P. Odor identification and self-reported olfactory functioning in patients with subtypes of mild cognitive impairment. Clin Neuropsychol. 2009;23(5):818–830. doi: 10.1080/13854040802585030. [DOI] [PubMed] [Google Scholar]
- Leontjevas R, Evers-Stephan A, Smalbrugge M, Pot AM, Thewissen V, Gerritsen DL, Koopmans RT. A Comparative Validation of the Abbreviated Apathy Evaluation Scale (AES-10) With the Neuropsychiatric Inventory Apathy Subscale Against Diagnostic Criteria of Apathy. J Am Med Dir Assoc. 2012;13(3):308, e301–e306. doi: 10.1016/j.jamda.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Levy ML, Cummings JL, Fairbanks LA, Masterman D, Miller BL, Craig AH, Litvan I. Apathy is not depression. J Neuropsychiatry Clin Neurosci. 1998;10(3):314–319. doi: 10.1176/jnp.10.3.314. [DOI] [PubMed] [Google Scholar]
- Marshall GA, Monserratt L, Harwood D, Mandelkern M, Cummings JL, Sultzer DL. Positron emission tomography metabolic correlates of apathy in Alzheimer disease. Arch Neurol. 2007;64(7):1015–1020. doi: 10.1001/archneur.64.7.1015. [DOI] [PubMed] [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288(18):2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- Ott BR, Tate CA, Gordon NM, Heindel WC. Gender differences in the behavioral manifestations of Alzheimer's disease. J Am Geriatr Soc. 1996;44(5):583–587. doi: 10.1111/j.1532-5415.1996.tb01447.x. [DOI] [PubMed] [Google Scholar]
- Palmer K, Di Iulio F, Varsi AE, Gianni W, Sancesario G, Caltagirone C, Spalletta G. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer's disease: the role of depression and apathy. J Alzheimers Dis. 2010;20(1):175–183. doi: 10.3233/JAD-2010-1352. [DOI] [PubMed] [Google Scholar]
- Ready RE, Ott BR, Grace J, Cahn-Weiner DA. Apathy and executive dysfunction in mild cognitive impairment and Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(2):222–228. [PubMed] [Google Scholar]
- Resnick SM, Lamar M, Driscoll I. Vulnerability of the orbitofrontal cortex to age-associated structural and functional brain changes. Ann N Y Acad Sci. 2007;1121:562–575. doi: 10.1196/annals.1401.027. [DOI] [PubMed] [Google Scholar]
- Robert P, Onyike CU, Leentjens AF, Dujardin K, Aalten P, Starkstein S, Byrne J. Proposed diagnostic criteria for apathy in Alzheimer's disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98–104. doi: 10.1016/j.eurpsy.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Robert PH, Berr C, Volteau M, Bertogliati C, Benoit M, Sarazin M, Dubois B. Apathy in patients with mild cognitive impairment and the risk of developing dementia of Alzheimer's disease: a one-year follow-up study. Clin Neurol Neurosurg. 2006;108(8):733–736. doi: 10.1016/j.clineuro.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Royet JP, Plailly J, Delon-Martin C, Kareken DA, Segebarth C. fMRI of emotional responses to odors: influence of hedonic valence and judgment, handedness, and gender. Neuroimage. 2003;20(2):713–728. doi: 10.1016/S1053-8119(03)00388-4. [DOI] [PubMed] [Google Scholar]
- Ship JA, Weiffenbach JM. Age, gender, medical treatment, and medication effects on smell identification. J Gerontol. 1993;48(1):M26–M32. doi: 10.1093/geronj/48.1.m26. [DOI] [PubMed] [Google Scholar]
- Soudry Y, Lemogne C, Malinvaud D, Consoli SM, Bonfils P. Olfactory system and emotion: common substrates. [Review] Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128(1):18–23. doi: 10.1016/j.anorl.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Jorge R, Mizrahi R, Robinson RG. A prospective longitudinal study of apathy in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2006;77(1):8–11. doi: 10.1136/jnnp.2005.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockhorst U, Pietrowsky R. Olfactory perception, communication, and the nose-to-brain pathway. Physiol Behav. 2004;83(1):3–11. doi: 10.1016/j.physbeh.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Wesson DW, Wilson DA, Nixon RA. Should olfactory dysfunction be used as a biomarker of Alzheimer's disease? Expert Rev Neurother. 2010;10(5):633–635. doi: 10.1586/ern.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westervelt HJ, Bruce JM, Coon WG, Tremont G. Odor identification in mild cognitive impairment subtypes. J Clin Exp Neuropsychol. 2008;30(2):151–156. doi: 10.1080/13803390701287408. [DOI] [PubMed] [Google Scholar]
- Wetzels RB, Zuidema SU, de Jonghe JF, Verhey FR, Koopmans RT. Course of neuropsychiatric symptoms in residents with dementia in nursing homes over 2-year period. [Research Support, Non-U.S. Gov't] Am J Geriatr Psychiatry. 2010;18(12):1054–1065. doi: 10.1097/jgp.0b013e3181f60fa1. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer's disease. Ann N Y Acad Sci. 2009;1170:730–735. doi: 10.1111/j.1749-6632.2009.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64(7):802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]