Abstract
Could sympathetic hyperarousal limit treatment success in complicated grief? The present study investigated persons with complicated grief, a chronic condition with distinct symptoms including persistent intense yearning and longing for the person who died, avoidance of reminders that the person is gone, deep relentless sadness, self-blame, bitterness, or anger in connection with the death, and an inability to gain satisfaction or joy through engaging in meaningful activities or relationships with significant others. Length of bereavement did not correlate with complicated grief scores. Catecholamines (i.e., epinephrine, norepinephrine, dopamine) in plasma were assessed pre- and post-psychotherapeutic treatment. Participants with the highest levels of epinephrine at pre-treatment had the highest levels of complicated grief symptoms at post-treatment, accounting for baseline levels of symptoms. This predictive relationship was not seen for depressive symptoms. The present study supports the hypothesis that catecholamine levels are affected by bereavement, and in turn, can affect the ability of those with complicated grief to benefit from psychotherapy.
Keywords: grief, catecholamines, bereavement, psychotherapy, epinephrine, norepinephrine, dopamine
Introduction
The death of a loved one is a highly stressful event because of the permanent loss of an attachment figure. The attachment model states that when the loved one is unavailable, there is a resulting strong desire to be with the person and a sense of insecurity (Bowlby, 1969). There is evidence for the role of attachment relationships in autonomic regulatory processes (for a review, see (Hofer, 1984). Bereavement triggers acute grief, a unique biobehavioral response (Shear & Shair, 2005). Acute grief is intensely painful and disruptive, but for most people, a natural healing process slowly integrates the reality of the death. However, for a subset of bereaved people, dysfunctional thoughts, maladaptive behaviors or ineffective emotion regulation complicate and prolong the process, resulting in a disorder termed complicated grief. Little information is available regarding autonomic regulatory processes in complicated grief, but there is some evidence for increased sympatho-adrenal-medulla (SAM) system activation with acute grief, and this could be a contributor to complicated grief. The present paper investigates whether sympathetic hyperarousal limits treatment success in complicated grief.
Prior research demonstrates that acute grief is associated with increased urinary catecholamines (Jacobs, et al., 1986), increased heart rate (Buckley, et al., 2012a; O’Connor, Allen, & Kaszniak, 2002), cardiovascular disorders, including high blood pressure (Buckley, et al., 2011), stress cardiomyopathy (Wittstein, et al., 2005) and sudden cardiac death (Stroebe, Schut, & Stroebe, 2007). During emotional activation, the SAM system releases epinephrine and norepinephrine, which increases heart rate. In addition, epinephrine increases peripheral resistance, and therefore increases blood pressure. In instances of stress cardiomyopathy (usually occurring after an acute emotional trigger, most frequently, notification of the death of a loved one), plasma catecholamine levels are extremely high from SAM activation (Wittstein, 2012).
Higher grief intensity, as well as persistence and chronicity, is associated with greater physiological stress responsivity. In a small naturalistic study (n=56) of adaptation at 1and 2 months after bereavement, Jacobs and colleagues found that the subgroup whose separation anxiety symptoms worsened over the month (n=24) compared to those that did not (n=32) had higher urinary free cortisol levels (Jacobs, et al., 1987). In another study the stress of losing a family member was associated with the development of essential hypertension in the absence of other cardiovascular risk factors (Santic, Lukic, Sesar, Milicevic, & Ilakovac, 2006). These authors found that family members of deceased soldiers had a higher prevalence of arterial hypertension, accounting for a host of other cardiovascular risk factors and post-traumatic stress disorder (50.7% vs. 39.0%, p < 0.001.). The prevalence of hypertension decreased over time only in the bereaved group.
Complicated grief, which affects about 10% of bereaved people, has been recognized only a short time and is not yet in the official diagnostic nomenclature. As a result, there has been little study of the physiological profile of this chronic disabling condition (Shear, et al., 2011). Complicated grief is characterized by a prolonged acute grief accompanied by dysfunctional thoughts, behaviors or emotions related to the death that interfere with the process of adaptation. One longitudinal study found that those with complicated grief at 6 months post loss showed higher levels of hypertension at thirteen months (Prigerson, et al., 1997). We hypothesize that individuals with complicated grief continue to experience sympathetic stimulation generated by the emotional distress.
The parent study (NIMH MH70741) for the current report is investigating the efficacy of complicated grief treatment in older adults. We conducted a pilot study of plasma catecholamines among a subgroup of participants in this study (n=16) treated with either Complicated Grief Treatment (Shear K, 2005) or Interpersonal Psychotherapy (Shear K, 2005)1. The present paper reports results of baseline and post-treatment catecholamines in order to explore hypotheses about baseline catecholamines affecting responsiveness to treatment. To do so we measured peripheral epinephrine, norepinephrine and dopamine at pre- and post-treatment.
Methods
The data in the present report is from an ancillary study of a larger randomized clinical trial comparing Complicated Grief Treatment to Interpersonal Therapy for the treatment of complicated grief. Entry into the parent study was based upon a score of at least 30 on the 19-item Inventory of Complicated Grief (ICG; Prigerson, et al., 1999)). All participants gave informed consent, and the New York State Psychiatric Institute Institutional Review Board approved the study. The biomarker data presented here are from a subset of participants who consented to have blood drawn for neuroendocrine measures and who currently have pre and post-treatment data. The clinical trial is ongoing, and so we provide no information about which treatment the participant received. Blood for biomarkers and the ICG were measured at study intake, which occurred up to 4 weeks before the first therapy session. The Beck Depression Inventory-II (BDI-II) was completed at the first therapy session, and the ICG and BDI-II were given at week 20, following the termination of treatment.
Participants included 14 women and 2 men. This is the same percentage of males as previously seen in a randomized clinical trial of treatment for complicated grief (12%; Shear K, 2005)). They were asked not to eat or drink caffeine and not to smoke for at least 4 hours prior to blood sampling. All blood samples were drawn between 10am and 3:30pm. Participants were in the clinic for a minimum of 45 minutes prior to the blood draw, while treatment study assessment procedures were conducted. All participants were seated during the blood draw. Venipuncture collection of 4mL whole blood was drawn in k2EDTA tubes. Samples were placed on ice immediately, and plasma was separated in a 4 degrees C centrifuge within 2 hours after sampling. Plasma was initially stored at −20 degrees C, and then stored at −80 degrees C until the end of the study so that all samples could be assayed simultaneously.
Enzyme-linked immunoassay (ELISA) with a microtiter plate format was used (ALPCO Diagnostics, Salem, NH). Norepinephrine, epinephrine and dopamine were assayed. All samples were analyzed in duplicate in the same assay to minimize variability. Values below the detectable limit of the assays made up 11.8% of samples. The biomarker values were log transformed to adhere to the assumptions of parametric statistical analysis.
Results
At the pre-treatment baseline, participants had a mean age of 64 (SD = 4.3). Participants were grieving the loss of a close relative or friend, including loss of a parent (44%), spouse (31%), child (6%), sibling (13%), and other (e.g., close friend). The length of bereavement varied widely, with a mean of 87 months (SD = 123.9), but a median of 38 months. However, the length of bereavement did not correlate with depression or complicated grief scores at pre-treatment or at post-treatment, and did not correlate with any of the biomarkers.
The mean BMI was 25.7 (SD = 4.4). The paired-sample t-test for the BDI was significant (t = 8.03, p < .001). Additional demographics are in Table 1. The mean BDI score was 26.2 (SD = 7.5) at pre-treatment and 10.6 (SD = 6.5) at post-treatment. We grouped depression at post-treatment as high (BDI score of > 21) and low (BDI of < 21). Only one of the participants had a high level at post-treatment (although the BDI declined from 36 to 23). Two participants showed particularly long time since the death (425 and 344 months), and one of these had persistent levels of high depressive symptoms.
Table 1.
Demographic Characteristics
| Percentage | |
|---|---|
| Gender (Female) | 88% |
| Ethnicity | |
| Caucasian | 81% |
| African-American | 19% |
| Marital status | |
| Never married | 19% |
| Married | 19% |
| Separated | 6% |
| Divorced | 25% |
| Widowed | 31% |
| Education | |
| Some college/AA | 13% |
| College degree | 13% |
| Some graduate school | 18% |
| Graduate/professional degree | 56% |
| Employment | |
| Full-time | 18% |
| Part-time | 38% |
| Retired | 38% |
| Unemployed | 6% |
AA=associates degree
The mean level ICG score at pre-treatment was 44.6 (SD = 11.5) and 23.5 (SD = 11.0) at post-treatment. The paired-sample t-test for the ICG was significant (t = 7.44, p < .001). We grouped ICG scores at post-treatment as high (ICG > 30) or low (ICG < 30), and 64.7% (n = 11) were low. Length of bereavement was not different between those with high or low post-treatment levels (F = 1.31, p = 0.27). Cronbach’s alpha for the ICG was 0.84 at pre-treatment, and 0.88 at post-treatment and the two scores correlated at r = 0.45, p = 0.08.
Biomarkers
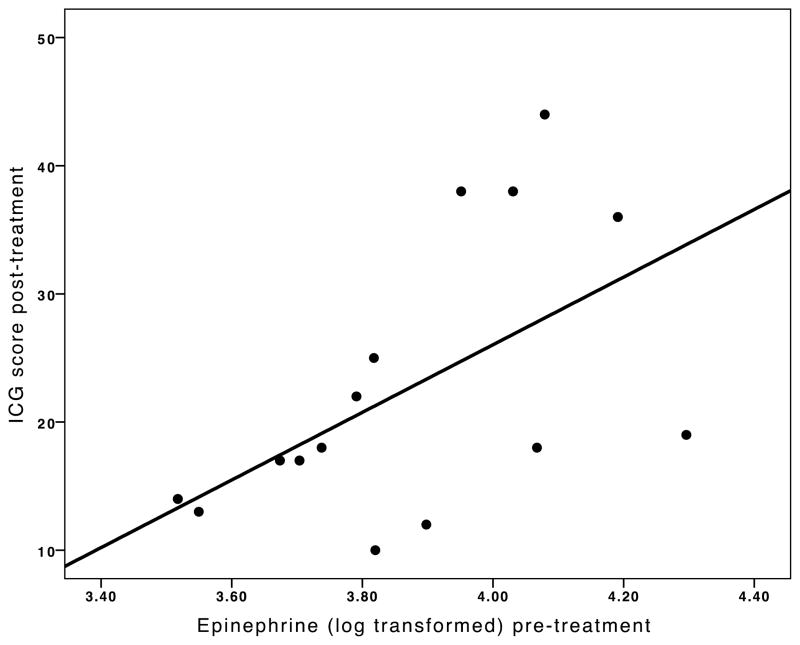
Levels of plasma catecholamines can be seen in Table 2. To determine whether the biomarkers predicted treatment outcome, regression analyses were used. Epinephrine at pre-treatment predicted the post-treatment ICG score, accounting for the pre-treatment ICG score (F (16, 2) = 6.68, p = 0.01, βlogE = 0.53, βICG = 0.48). Those participants with the highest levels of epinephrine at pre-treatment had the highest levels of complicated grief symptoms at post-treatment. Outlying values did not account for the relationship (Figure 1). Norepinephrine and dopamine at pre-treatment were not significant predictors of post-treatment ICG, once pre-treatment ICG was entered into the analysis. These results do not change if age is included in the regression analysis, and age is not a significant predictor within the model. Unlike the predictive significance of pre-treatment epinephrine, when each of the catecholamines at post-treatment was used to predict simultaneous post-treatment ICG scores, none of the post-treatment levels (i.e., epinephrine, norepinephrine and dopamine) predicted post-treatment ICG scores, once pre-treatment ICG were accounted for. None of the three biomarkers significantly predicted the post-treatment BDI score, accounting for the pre-treatment BDI score.
Table 2.
Mean levels of catecholamines at two time points.
| Mean | SD | |
|---|---|---|
| Dopamine (pre-treatment) | 81.5 | 35.0 |
| Norepinephrine (pre-treatment) | 193.9 | 243.5 |
| Epinephrine (pre-treatment) | 49.3 | 11.1 |
| Dopamine (post-treatment) | 90.0 | 44.0 |
| Norepinephrine (post-treatment) | 223.3 | 345.9 |
| Epinephrine (post-treatment) | 40.5 | 20.4 |
Figure 1.
Scatterplot of the association between complicated grief symptoms and pre-treatment epinephrine levels. ICG=Inventory of Complicated Grief.
Discussion
The present study suggests that elevated epinephrine levels may be a clinically significant individual difference in complicated grief psychophysiology. Epinephrine levels may be a moderator of complicated grief treatment, such that those with higher levels of circulating epinephrine prior to therapy were the least likely to show response of complicated grief symptoms after therapy, even after accounting for the pre-treatment level of complicated grief symptoms. This moderator relationship was not seen for norepinephrine or dopamine.
A previous study of bereaved individuals showed that high mean levels of 24-hour urinary epinephrine output predicted higher hopelessness and helplessness scores at 13 and 25 months after the loss (Jacobs, et al., 1986). Similar to the current study, urinary norepinephrine had no relationship to outcome in the study by Jacobs and colleagues. Epinephrine and norepinephrine have distinct peripheral effects on the body. When an acute stress response (fight-or-flight) is triggered, the medulla releases 80 percent epinephrine and 20 percent norepinephrine. These catecholamines have unique effects depending on the particular receptors that they stimulate (in some instances inhibitory and in some instances excitatory). In tandem, they result in increased heart rate and force of heart muscle contraction and peripheral vasoconstriction. However, epinephrine and norepinephrine are not always correlated. In a study of elderly caregivers a two-week respite intervention lead to a reduction in circulating plasma epinephrine (Grant, et al., 2003). A control group of caregivers who did not receive the respite intervention showed no decline in circulating epinephrine. However, there was no significant effect of the respite intervention on norepinephrine.
It is also perhaps not surprising that dopamine showed no predictive relationship to complicated grief treatment. Dopamine is a precursor to norepinephrine and epinephrine. Dopamine in the central nervous system, involved in regulating reward behavior, is compartmentally distinct from dopamine measured in the periphery, measured in the current study.
Prior work by Bonanno and colleagues has investigated heart rate during a baseline and grief-evoking interview (Bonanno, et al., 2007). Their data show that complicated grief is associated with a decrease in heart rate from baseline to grief evocation, but this was in individuals who are bereaved just four months, and after accounting for symptoms of depression and post-traumatic stress disorder. None of these participants met our criteria for CG. Cardiovascular functioning in complicated grief may have a distinct profile in which autonomic regulation has still not been achieved and similar to the first two weeks of bereavement (Buckley, et al., 2012b).
It is notable that although BDI-II and complicated grief scores were highly correlated, catecholamines did not show a predictive relationship to depressive symptoms. This supports the well-documented difference between CG and MDD (Shear, et al., 2011). As the opening quote suggests, symptoms of grief may include prominent separation anxiety.
It is plausible that higher levels of general dysphoria, and not just epinephrine, could be predictive of complicated grief symptoms. In post hoc analyses, and despite the small sample size, we added BDI to the model. Adding the BDI at pre-treatment had no effect on the model, and it was not a significant predictor within the model. Although adding BDI at post-treatment made the model stronger (F = 13. 94, p < .001) and BDI at post-treatment was an independent predictor in the model (beta = .54, p = .003), epinephrine at baseline also remained a significant predictor (beta = .53, p = .003). This result is quite speculative, as the sample is very small, this is a large number of predictors (pre-treatment epinephrine and ICG, and post-treatment BDI), and the BDI and ICG are correlated. Nonetheless, this test did not disconfirm the relationship between lack of complicated grief treatment improvement and baseline epinephrine, suggesting that is not just explained by dysphoria.
The present study may have implications for physical health. In the MacArthur studies of aging, high epinephrine levels predicted mortality and functional decline in older adults at 3- and 7-year follow-ups (Reuben, Talvi, Rowe, & Seeman, 2000). In men, increases in epinephrine excretion over 2.5 years were associated with greater declines in cognitive function over the next 4.5 years, perhaps due to white matter ischemic damage and infarction (Karlamangla, Singer, McEwen, Rowe, & Seeman, 2002). Reuben and colleagues sent out a call for future research on the causes of epinephrine release in older adults who are not acutely ill, and the present study may provide one explanation (i.e., complicated grief) for high circulating epinephrine levels.
Although at present the randomized clinical trial comparing these two treatments is still blinded, future research should investigate which type of therapy is more effective for those who have high pre-treatment levels of epinephrine. Complicated Grief Treatment is designed to focus on issues of loss (integrating the loss into memory and reducing avoidance of emotions) and issues of restoration (restoring the life goals and positive emotions in the present day) (Shear, Frank, Houck, & Reynolds, 2005). Interpersonal psychotherapy is focused on relief of depression through discussion of the relationship with the deceased and planning for the future. Complicated Grief Treatment may be more difficult for those with high epinephrine. Alternatively, avoidance may be greater among those with high epinephrine and IPT may be ineffective when avoidance is very high. Additionally, evidence from a group CBT study of depression suggests those clients who start therapy with higher levels of catecholamines require longer (i.e., more than 12 sessions) in therapy to show improvement (Oei, Dingle, & McCarthy, 2010). High levels of catecholamines may be associated with heightened arousal that impairs cognitive functioning needed to optimally utilize cognitive therapy.
Our study has several limitations. The sample size was relatively low and we did not have pre-study hypotheses about catecholamine treatment effects. Participants in the current study underwent emotionally activating evaluation procedures prior to having their blood drawn. Nevertheless, the strong correlation between baseline epinephrine levels and post-treatment CG scores is impressive, especially since this correlation appears to be specific for CG symptoms (e.g., not found for depression) and for epinephrine (e.g., not seen with norepinephrine).
In summary, the present data contributes to a greater understanding of the physiological characterization of interpersonal loss from bereavement and suggests there may be clinically significant individual differences in psychophysiology. In addition, the data presented here supports the hypothesis that SAM activation negatively affects the ability of those with complicated grief to benefit from psychotherapy.
Highlights.
Higher epinephrine pre-therapy predicted complicated grief symptoms post-therapy.
Norepinephrine and dopamine did not predict complicated grief treatment.
The data supports the hypothesis that social stress affects autonomic functioning.
Acknowledgments
This research was supported by the National Institute of Mental Health (MH60741), the National Institute of Aging (AG028404), UCLA Claude Pepper Older Americans Independence Center funded by the National Institute of Aging (5P30AG028748), and the UCLA Cousins Center for Psychoneuroimmunology.
Footnotes
The study is ongoing so the blind cannot be broken.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bonanno GA, Neria Y, Mancini A, Coifman KG, Litz B, Insel B. Is there more to complicated grief than depression and posttraumatic stress disorder? A test of incremental validity. J Abnorm Psychol. 2007;116:342–351. doi: 10.1037/0021-843X.116.2.342. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and loss: Vol. I, Attachment. London: Hogarth Press; 1969. [Google Scholar]
- Buckley T, Mihailidou AS, Bartrop R, McKinley S, Ward C, Morel-Kopp MC, Spinaze M, Tofler GH. Haemodynamic changes during early bereavement: potential contribution to increased cardiovascular risk. Heart Lung Circ. 2011;20:91–98. doi: 10.1016/j.hlc.2010.10.073. [DOI] [PubMed] [Google Scholar]
- Buckley T, Stannard A, Bartrop R, McKinley S, Ward C, Mihailidou AS, Morel-Kopp MC, Spinaze M, Tofler G. Effect of Early Bereavement on Heart Rate and Heart Rate Variability. Am J Cardiol. 2012a doi: 10.1016/j.amjcard.2012.06.045. [DOI] [PubMed] [Google Scholar]
- Buckley T, Sunari D, Marshall A, Bartrop R, McKinley S, Tofler G. Physiological correlates of bereavement and the impact of bereavement interventions. Dialogues Clin Neurosci. 2012b;14:129–139. doi: 10.31887/DCNS.2012.14.2/tbuckley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, McKibbin CL, Taylor MJ, Mills P, Dimsdale J, Ziegler M, Patterson TL. In-home respite intervention reduces plasma epinephrine in stressed Alzheimer caregivers. Am J Geriatr Psychiatry. 2003;11:62–72. [PubMed] [Google Scholar]
- Hofer MA. Relationships as regulators: a psychobiologic perspective on bereavement. Psychosom Med. 1984;46:183–197. doi: 10.1097/00006842-198405000-00001. [DOI] [PubMed] [Google Scholar]
- Jacobs SC, Mason J, Kosten TR, Kasl SV, Ostfeld AM, Wahby V. Urinary free cortisol and separation anxiety early in the course of bereavement and threatened loss. Biol Psychiatry. 1987;22:148–152. doi: 10.1016/0006-3223(87)90225-3. [DOI] [PubMed] [Google Scholar]
- Jacobs SC, Mason JW, Kosten TR, Wahby V, Kasl SV, Ostfeld AM. Bereavement and catecholamines. Journal of Psychosomatic Research. 1986;30:489–496. doi: 10.1016/0022-3999(86)90088-7. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline. MacArthur studies of successful aging. J Clin Epidemiol. 2002;55:696–710. doi: 10.1016/s0895-4356(02)00399-2. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Allen JJB, Kaszniak AW. Autonomic and emotion regulation in bereavement and depression. Journal of Psychosomatic Research. 2002;52:183–185. doi: 10.1016/s0022-3999(02)00292-1. [DOI] [PubMed] [Google Scholar]
- Oei TP, Dingle GA, McCarthy M. Urinary catecholamine levels and response to group cognitive behaviour therapy in depression. Behav Cogn Psychother. 2010;38:479–483. doi: 10.1017/S1352465810000093. [DOI] [PubMed] [Google Scholar]
- Prigerson HG, Bierhals AJ, Kasl SV, Reynolds CF, 3rd, Shear MK, Day N, Beery LC, Newsom JT, Jacobs S. Traumatic grief as a risk factor for mental and physical morbidity. Am J Psychiatry. 1997;154:616–623. doi: 10.1176/ajp.154.5.616. [DOI] [PubMed] [Google Scholar]
- Prigerson HG, Shear MK, Jacobs SC, Reynolds CF, 3rd, Maciejewski PK, Davidson JR, Rosenheck R, Pilkonis PA, Wortman CB, Williams JB, Widiger TA, Frank E, Kupfer DJ, Zisook S. Consensus criteria for traumatic grief. A preliminary empirical test. Br J Psychiatry. 1999;174:67–73. doi: 10.1192/bjp.174.1.67. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Talvi SL, Rowe JW, Seeman TE. High urinary catecholamine excretion predicts mortality and functional decline in high-functioning, community-dwelling older persons: MacArthur Studies of Successful Aging. J Gerontol A Biol Sci Med Sci. 2000;55:M618–624. doi: 10.1093/gerona/55.10.m618. [DOI] [PubMed] [Google Scholar]
- Santic Z, Lukic A, Sesar D, Milicevic S, Ilakovac V. Long-term follow-up of blood pressure in family members of soldiers killed during the war in Bosnia and Herzegovina. Croat Med J. 2006;47:416–423. [PMC free article] [PubMed] [Google Scholar]
- Shear K, FEHPRRCF Treatment of complicated grief: A randomized controlled trial. JAMA: The Journal of the American Medical Association. 2005;293:2601–2608. doi: 10.1001/jama.293.21.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear K, Shair H. Attachment, loss, and complicated grief. Dev Psychobiol. 2005;47:253–267. doi: 10.1002/dev.20091. [DOI] [PubMed] [Google Scholar]
- Shear MK, Frank E, Houck PR, Reynolds CF. Treatment of Complicated Grief: A Randomized Controlled Trial. Journal of the American Medical Association. 2005;293:2601–2608. doi: 10.1001/jama.293.21.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear MK, Simon N, Wall M, Zisook S, Neimeyer R, Duan N, Reynolds C, Lebowitz B, Sung S, Ghesquiere A, Gorscak B, Clayton P, Ito M, Nakajima S, Konishi T, Melhem N, Meert K, Schiff M, O’Connor MF, First M, Sareen J, Bolton J, Skritskaya N, Mancini AD, Keshaviah A. Complicated grief and related bereavement issues for DSM-5. Depression and Anxiety. 2011;28:103–117. doi: 10.1002/da.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroebe M, Schut H, Stroebe W. Health outcomes of bereavement. Lancet. 2007;370:1960–1973. doi: 10.1016/S0140-6736(07)61816-9. [DOI] [PubMed] [Google Scholar]
- Wittstein IS. Stress cardiomyopathy: a syndrome of catecholamine-mediated myocardial stunning? Cell Mol Neurobiol. 2012;32:847–857. doi: 10.1007/s10571-012-9804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittstein IS, Thiemann DR, Lima JAC, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral Features of Myocardial Stunning Due to Sudden Emotional Stress. New England Journal of Medicine. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]