Summary
Human β-defensin 2 (hBD-2) and hBD-3, encoded by DEFB4 and DEFB103A, respectively, have shown anti-HIV activity, and both genes exhibit copy number variation (CNV). Although the role of hBD-1, encoded by DEFB1, in HIV-1 infection is less clear, single nucleotide polymorphisms (SNPs) in DEFB1 may influence viral loads and disease progression. We examined the distribution of DEFB1 SNPs and DEFB4/103A CNV, and the relationship between DEFB1 SNPs and DEFB4/103A CNV using samples from two HIV/AIDS cohorts from the United States (n = 150) and five diverse populations from the Coriell Cell Repositories (n = 46). We determined the frequencies of 10 SNPs in DEFB1 by using a post-PCR, oligonucleotide ligation detection reaction-fluorescent microsphere assay, and CNV in DEFB4/103A by real-time quantitative PCR. There were noticeable differences in the frequencies of DEFB1 SNP alleles and haplotypes among various racial/ethnic groups. The DEFB4/103A copy numbers varied from 2 to 8 (median, 4), and there was a significant difference between the copy numbers of self-identified whites and blacks in the US cohorts (Mann-Whitney U test p = 0.04). A significant difference was observed in the distribution of DEFB4/103A CNV among DEFB1 -52G/A and -390T/A genotypes (Kruskal-Wallis p = 0.017 and 0.026, respectively), while not in the distribution of DEFB4/103A CNV among -52G/A_-44C/G_-20G/A diplotypes. These observations provide additional insights for further investigating the complex interplay between β-defensin genetic polymorphisms and susceptibility to, or the progression or severity of, HIV infection/disease.
Introduction
Human defensins are a family of small, β-sheeted, cationic, antimicrobial and immunoregulatory peptides, which belong to either the α or β subfamily. The former are found in azurophilic granules of phagocytic cells, while the latter are released from epithelial cells (Chen et al., 2006; Ganz, 2003; Klotman and Chang, 2006). Recent studies have suggested that human β-defensins (hBDs) may play an important role in HIV-1 susceptibility and disease progression (Feng et al., 2006; Quinones-Mateu et al., 2003; Sun et al., 2005; Zapata et al., 2008). We have demonstrated that: (1) both R5 and X4 HIV-1 phenotypes induce DEFB4 and DEFB103A mRNAs, encoding hBD-2 and -3, respectively, in normal human oral epithelial cells; (2) hBD-2 and -3 inhibit HIV-1 infection by both phenotypes, with greater activity against X4 viruses; and (3) this inhibition is due to a direct interaction with virions, and through down-modulation of the CXCR4 co-receptor (Quinones-Mateu et al., 2003). A later study confirmed the anti-HIV activity of these hBDs (Sun et al., 2005). We subsequently discovered that hBD-3 acts as an antagonist of the CXCR4 co-receptor by promoting its internalization (Feng et al., 2006). Concordant with the results of our in vitro study (Quinones-Mateu et al., 2003), our group also found that HIV-exposed seronegative and HIV-seropositive Colombian individuals expressed significantly higher levels of DEFB4 and DEFB103A transcripts in oral mucosa than did healthy controls (Zapata et al., 2008).
Single nucleotide polymorphisms (SNPs) in DEFB1, encoding hBD-1, particularly -52G/A (660G/A, rs1799946), -44C/G (668C/G, rs1800972), and -20G/A (692G/A, rs11362), may also play a role in HIV-1 susceptibility and disease progression. By comparing the genotype frequencies between HIV-positive patients and comparable healthy controls, significant associations were observed between -52G/A (Braida et al., 2004; Milanese et al., 2006; Segat et al., 2006), -44C/G (Braida et al., 2004; Segat et al., 2006), and/or -20G/A (Milanese et al., 2006) genotypes and HIV-1 infection status. However, considering the variability in associations found in different (Braida et al., 2004; Milanese et al., 2006) and even the same (Milanese et al., 2006; Segat et al., 2009) populations, it appears that the DEFB1 genotype associations may be cohort-specific. Other studies have shown that -52G/A, either singly or as haplotype with -44C/G, may influence viral loads in breast milk (Baroncelli et al., 2008) and plasma (Ricci et al., 2009), respectively. Recently, in HIV-1-infected children, -44CG genotype and -52G_-44G haplotype were significantly associated with a slower disease progression (Freguja et al., 2012).
DEFB1, DEFB4, and DEFB103A are located in a cluster on chromosome 8p23.1, and are polymorphic. DEFB1 has numerous SNPs (Jurevic et al., 2002; Kim et al., 2009; Prado-Montes de Oca, 2010), with at least 25 SNPs in the coding region (http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId=1672). Among these, -52G/A, -44C/G, and -20G/A in the exon-1 region have been the focus of a variety of studies related to functional consequences of variation in DEFB1, and DEFB1 genetic associations where these SNPs were considered either singly or as haplotypes (Kalus et al., 2009; Naslavsky et al., 2010; Naslavsky et al., 2009; Prado-Montes de Oca, 2010). In addition, with respect to size, gene content and numeric variability of DEFB, the 8p23.1 region represents one of the hotspots of copy number variation (CNV) (Abu Bakar et al., 2009; Hollox et al., 2008). DEFB1 is generally considered a single-copy gene (2 copies per diploid genome [PDG]), and CNVs are very rare (Cagliani et al., 2008). However, the 200 kb DEFB region, containing DEFB4 and DEFB103A, varies en bloc in its copy number (Groth et al., 2008). Individuals carry 2-12 copies PDG (Groth et al., 2008; Hollox et al., 2003; Linzmeier and Ganz, 2005). As with SNPs, CNVs may also affect gene expression and hence determine phenotypes (Ionita-Laza et al., 2009; Zhang et al., 2009). It is therefore not surprising that the DEFB CNV is associated with clinically important phenotypes (Hollox, 2008). In addition to CNV, sequence variations between copies (termed as multisite variations) represent a further level of complexity at DEFB (Groth et al., 2010; Huse et al., 2008). Furthermore, whether there is any relationship (association, linkage disequilibrium [LD]) between SNPs in DEFB1 and CNV in DEFB4/103A is not clear, and there is some indication that there may not be (Hollox, 2008).
Herein, we report the frequencies of 10 DEFB1 SNPs (Jurevic et al., 2002) and their haplotypes in two admixed, HIV/AIDS patient populations from the United States, together with five diverse populations from the Coriell Cell Repositories. We also report the distribution of DEFB4/103A CNV, and show, for the first time, that there may be a relationship between DEFB1 SNPs and DEFB4/103A CNV.
Materials and methods
Samples and genomic DNA extraction
De-identified packed blood pellets (n = 105), collected from HIV-infected adult subjects, were obtained from the Case Western Reserve University Center for AIDS Research (CFAR) specimen repository. The self-identified racial/ethnic distribution of these subjects was: whites (n = 37), blacks (n = 65), and Hispanics (n = 3). De-identified packed white blood cell pellets (n = 45), collected from HIV/AIDS patients, were obtained from the Multicenter AIDS Cohort Study (MACS) (Shepherd et al., 2008). The self-identified racial/ethnic distribution of these subjects was: white, non-Hispanic (n = 39), white, Hispanic (n = 4), and black, non-Hispanic (n = 2). DNA samples from five diverse populations (Northern European [NE, n = 10], sub-Saharan African [AfSS, n = 9], African-American [Af-Am, n = 10], Chinese [CHN, n = 10], and Mexican [MEX, n = 7]) were obtained from the Coriell Cell Repositories, Coriell Institute for Medical Research, Camden, NJ.
Genomic DNAs were extracted from 200 μl of each of the pellets using the QIAamp DNA Blood Mini Kit (Qiagen Inc., Valencia, CA). The CFAR specimen collection protocol and genetic data storage and usage were approved by the Institutional Review Board of University Hospitals Case Medical Center.
DEFB1 polymerase chain reaction (PCR) and SNP genotyping
PCR primers were designed based on the DEFB1 region of the sequence (GenBank accession #NT_023736, nucleotide coordinates 6,726,529-6,717,101) to selectively amplify the exon-1 and exon-2 regions. Sequence homology and specificity of all primer sequences were checked using the BLASTn program (http://www.ncbi.nlm.nih.gov). The primer sequences, PCR buffer, amplification conditions, and method used to perform agarose gel electrophoresis are described in supplementary Table S1.
Genotyping of six SNPs in the exon-1 region (-610G/A [102G/A, rs2741132], -390T/A [322T/A, rs2738182], -52G/A, -44C/G, -20G/A, and 79T/A [791T/A, rs2293958]), and four SNPs in the exon-2 region (1654G>A [Val38Ile, rs2738047], 1754G/A [rs1047031], 1836A/G [rs1800971], and 1873T/C [rs5743491]) (Jurevic et al., 2002) was performed by using a high-throughput, post-PCR oligonucleotide ligation detection reaction-fluorescent microsphere assay (LDR-FMA) on the Bio-Plex™ multiplex suspension array system (Bio-Rad Laboratories, Hercules, CA) (Mehlotra et al., 2007; Mehlotra et al., 2006). The LDR primers were designed based on the DEFB1 sequences available through GenBank (accession #NT_023736) and SNPper (http://snpper.chip.org/bio/export-sequence/6559); the primer sequences are provided in supplementary Table S2. The LDR conditions were 95°C for 1 min, 95°C for 15 sec and 60°C for 2 min (31×). The DEFB1 SNP genotypes were determined as previously described (Mehlotra et al., 2007; Mehlotra et al., 2006). The mean and 95% confidence interval (CI) of log-transformed fluorescent values corresponding to each genotype, using the Coriell samples (n = 46), are presented in supplementary Table S3.
DEFB4/103A CNV determination
For the determination of DEFB4 and DEFB103A copy numbers, the real-time quantitative PCR assay was used as described (Linzmeier and Ganz, 2005). Reference genes TBP (TATA-Box Binding Protein, GenBank accession #AL031259) and DEFB1, specific primer sets producing only one specific product of ~150 bp at 54°C annealing temperature, reaction mix, and conditions were used as described (Linzmeier and Ganz, 2005), and the Bio-Rad CFX96™ system (Bio-Rad Laboratories, Hercules, CA) was used for the PCR analysis. Each sample was amplified using 50 ng genomic DNA, and was run in triplicate. Data were analyzed by the comparative Ct method, and the copy numbers were calculated as described (Linzmeier and Ganz, 2005). The assay results were validated by re-running 12 of the 46 randomly-selected Coriell samples.
Statistical analysis
HaploView v4.2 (Barrett et al., 2005) was used to calculate DEFB1 SNP frequencies, the Hardy-Weinberg (H-W) equilibrium p values, and Lewontin's D′ and correlation coefficient (r2) parameters as measures of LD between SNPs. PHASE v2.1.1 (http://www.stat.washington.edu/stephens/) was used to infer DEFB1 10-SNP haplotypes and diplotypes. The QI Macros Statistical Process Control Software for Excel (QI Macros 2012) was used to calculate descriptive statistics, and to perform Chi-square (χ2) test of contingency table and Mann-Whitney U test. SAS v9.2 was used to perform the nonparametric Kruskal-Wallis test to compare DEFB4/103A CNV among DEFB1 diplotypes and SNP genotypes, and to perform Bonferroni (Dunn's) t-test for multiple comparisons. For all statistical analyses, differences were considered significant at p < 0.05.
Results
DEFB1 SNP-allele frequencies and H-W equilibrium
The DEFB1 SNP-allele frequencies in two HIV/AIDS cohorts, CFAR and MACS, and five Coriell populations are presented in Table 1. These results do not include the three Hispanic patient samples in the CFAR cohort, and the four white, Hispanic, and two black, non-Hispanic patient samples in the MACS cohort. The SNP-genotype data for all these samples (n = 9) are presented in supplementary Table S4. Taken collectively, higher frequencies of the six exon-1 SNPs were observed, compared with the frequencies of the four exon-2 SNPs (Table 1). The frequencies of the -44G and 79A alleles were noticeably higher in white HIV/AIDS patients, NE, and MEX, and those of the 1754A allele were noticeably higher in white HIV/AIDS patients, NE, and CHN. On the other hand, the frequencies of the 1654A and 1836G alleles were noticeably higher in black HIV patients, AfSS, and Af-Am. These results indicate that the DEFB1 SNPs are distributed differently among different racial/ethnic groups, although a formal test to determine the statistical significance of these frequency differences was not performed due to low sample sizes of the Coriell populations. We calculated expected genotype numbers for each of the DEFB1 SNPs in each population. For all SNPs, the expected genotype numbers did not differ significantly from the observed genotype numbers in any of the populations (data not shown), indicating no deviation from H-W equilibrium.
Table 1.
DEFB1 SNP-allele frequencies in various groups of samples
| Region | SNP | f (Allele) | Group of samples† |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CFAR |
MACS |
Coriell |
||||||||
| White(n = 37) | Black (n = 65) | (n = 39) | NE (n = 10) | AfSS (n = 9) | Af-Am (n = 10) | CHN (n = 10) | MEX (n = 7) | |||
| Exon-1 | -610G/A | f (G) | 0.69 | 0.77 | 0.74 | 0.75 | 0.78 | 0.75 | 0.45 | 0.57 |
| f (A) | 0.31 | 0.23 | 0.26 | 0.25 | 0.22 | 0.25 | 0.55 | 0.43 | ||
| -390T/A | f (T) | 0.61 | 0.46 | 0.72 | 0.70 | 0.50 | 0.40 | 0.40 | 0.50 | |
| f (A) | 0.39 | 0.54 | 0.28 | 0.30 | 0.50 | 0.60 | 0.60 | 0.50 | ||
| -52G/A | f (G) | 0.61 | 0.47 | 0.72 | 0.70 | 0.50 | 0.40 | 0.40 | 0.50 | |
| f (A) | 0.39 | 0.53 | 0.28 | 0.30 | 0.50 | 0.60 | 0.60 | 0.50 | ||
| -44C/G | f (C) | 0.82 | 0.92 | 0.72 | 0.70 | 0.94 | 0.90 | 0.95 | 0.79 | |
| f (G) | 0.18 | 0.08 | 0.28 | 0.30 | 0.06 | 0.10 | 0.05 | 0.21 | ||
| -20G/A | f (G) | 0.58 | 0.65 | 0.54 | 0.60 | 0.61 | 0.70 | 0.65 | 0.71 | |
| f (A) | 0.42 | 0.35 | 0.46 | 0.40 | 0.39 | 0.30 | 0.35 | 0.29 | ||
| 79T/A | f (T) | 0.81 | 0.88 | 0.74 | 0.70 | 0.89 | 0.90 | 0.95 | 0.79 | |
| f (A) | 0.19 | 0.12 | 0.26 | 0.30 | 0.11 | 0.10 | 0.05 | 0.21 | ||
| Exon-2 | 1654G>A | f (G) | 0.97 | 0.92 | 1.00 | 1.00 | 0.89 | 0.90 | 1.00 | 0.93 |
| f (A) | 0.03 | 0.08 | 0 | 0 | 0.11 | 0.10 | 0 | 0.07 | ||
| 1754G/A | f (G) | 0.89 | 0.95 | 0.85 | 0.80 | 0.94 | 0.95 | 0.70 | 0.93 | |
| f (A) | 0.11 | 0.05 | 0.15 | 0.20 | 0.06 | 0.05 | 0.30 | 0.07 | ||
| 1836A/G | f (A) | 0.95 | 0.85 | 0.97 | 0.95 | 0.72 | 0.80 | 0.95 | 0.93 | |
| f (G) | 0.05 | 0.15 | 0.03 | 0.05 | 0.28 | 0.20 | 0.05 | 0.07 | ||
| 1873T/C | f (T) | 0.97 | 0.96 | 1.00 | 1.00 | 1.00 | 0.90 | 1.00 | 1.00 | |
| f (C) | 0.03 | 0.04 | 0 | 0 | 0 | 0.10 | 0 | 0 | ||
NE = Northern European, AfS S = African sub- Sahara, Af-Am = African-American, CHN = Chinese, MEX = Mexican.
CFAR patients of white and black races were self-identified.
MACS patients were white, non-Hispanic.
DEFB1 SNP-SNP LD patterns
We quantified the extent of LD among the DEFB1 SNP pairs in all populations (Figure 1A-1H). Strong LD, defined by high values for both D′ (≥ 0.8) and r2 (≥ 0.5) parameters (Ferlin et al., 2010), was observed between SNP pairs -390T/A – -52G/A and -44C/G – 79T/A in all populations. Inter-population differences in the LD patterns occurred: strong LD was observed between SNP pairs -610G/A – -390T/A and -610G/A – -52G/A in white HIV/AIDS patients, NE, CHN, and MEX, but not in black HIV patients, AfSS, and Af-Am; strong LD was also observed between a number of additional SNP pairs in CHN, which was not evident in any other population; and white HIV/AIDS patients and black HIV patients/Af-Am showed differences in pattern and extent of LD when compared with NE and AfSS, respectively. It is well-known that in the US populations, demographic factors, such as recent migration and admixture, have contributed to the distribution and extent of disequilibrium (Ardlie et al., 2002).
Fig. 1A-1H.
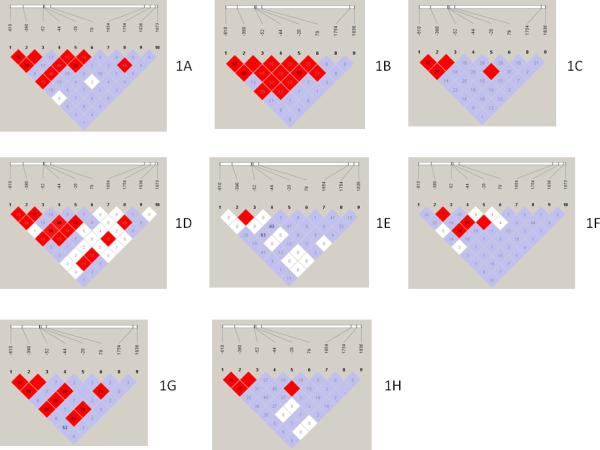
Heat maps of pairwise LD measurements for the 10 SNPs in DEFB1. 1A, CFAR white patients; 1B, MACS white, non-Hispanic patients; 1C, NE; 1D, CFAR black patients; 1E, AfSS; 1F, Af-Am; 1G, CHN; and 1H, MEX. Color scheme: bright red, D′ = 1.0 and LOD score ≥ 2.0; blue, D′ = 1.0 and LOD score < 2.0; and white, D′ < 1.0 and LOD score < 2.0. Numbers represent r2 values. r2 values of 1.0 are not shown (the box is empty).
DEFB1 haplotype profiles
We inferred DEFB1 10-SNP haplotypes using PHASE v2.1.1 (Table 2a). Assuming random associations among the eight polymorphic SNPs (Table 1), one can predict 256 different haplotypes (28). However, only nine haplotypes (8 + 1) would be possible if mutations are the only evolutionary forces acting to create new alleles, and other forces, such as recombination, recurrent mutation, and gene conversion, do not occur (Bonnen et al., 2002). We inferred a total of 13 haplotypes in our study populations, which is closer to the theoretical minimum. Of these, four haplotypes, GTGCATGGAT (0.05-0.31), GTGCATGAAT (0.05-0.2), GTGGGAGGAT (0.05-0.3), and AAACGTGGAT (0.22-0.55), together accounted for the majority of total chromosomes examined. Considerable differences in the haplotype profiles were noticed between the populations of European ancestry (white HIV/AIDS patients and NE) and African ancestry (black HIV patients, AfSS, and Af-Am).
Table 2a.
DEFB1 10-SNP haplotype frequencies in various groups of samples
| Haplotype | Group of samples† |
|||||||
|---|---|---|---|---|---|---|---|---|
| CFAR |
MACS |
Coriell |
||||||
| White (n = 37) | Black (n = 65) | (n = 39) | NE (n = 10) | AfSS (n = 9) | Af-Am (n = 10) | CHN (n = 10) | MEX (n = 7) | |
| GTGCATGGAT | 0.31 | 0.30 | 0.28 | 0.20 | 0.28 | 0.25 | 0.05 | 0.21 |
| GTGCATGAAT | 0.11 | 0.05 | 0.15 | 0.20 | 0.06 | 0.05 | 0.30 | 0.07 |
| GTGGGAGGAT | 0.18 | 0.08 | 0.26 | 0.30 | 0.06 | 0.10 | 0.05 | 0.21 |
| AAACGTGGAT | 0.31 | 0.23 | 0.26 | 0.25 | 0.22 | 0.25 | 0.55 | 0.43 |
| GAACGTAGGT | 0.03 | 0.08 | 0 | 0 | 0.11 | 0.10 | 0 | 0.07 |
| GAACGTGGGT | 0.03 | 0.06 | 0.03 | 0.05 | 0.11 | 0.10 | 0.05 | 0 |
| GAACGTGGAT | 0 | 0.12 | 0 | 0 | 0.06 | 0.05 | 0 | 0 |
| GAACGTGGAC | 0.03 | 0.04 | 0 | 0 | 0 | 0.10 | 0 | 0 |
| GTGCGAGGAT | 0.01 | 0.03 | 0 | 0 | 0.06 | 0 | 0 | 0 |
| GTGCATGGGT | 0 | 0 | 0 | 0 | 0.06 | 0 | 0 | 0 |
| GAGCGTGGGT | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 |
| GAACATGGAT | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 |
| GTGGATGGAT | 0 | 0 | 0.03 | 0 | 0 | 0 | 0 | 0 |
As described in Table 1.
We then determined the frequencies of 3-SNP haplotypes (-52G/A_-44C/G_-20G/A) (Table 2b). As expected from the 10-SNP haplotype data, GCA (0.29-0.44), GGG (0.05-0.3), and ACG (0.28-0.6) were the predominant haplotypes among all populations.
Table 2b.
DEFB1 3-SNP haplotype frequencies in various groups of samples
| Haplotype* | Group of samples† |
|||||||
|---|---|---|---|---|---|---|---|---|
| CFAR |
MACS |
Coriell |
||||||
| White (n = 37) | Black (n = 65) | (n = 39) | NE (n = 10) | AfSS (n = 9) | Af-Am (n = 10) | CHN (n = 10) | MEX (n = 7) | |
| GCA | 0.42 | 0.35 | 0.44 | 0.40 | 0.39 | 0.30 | 0.35 | 0.29 |
| GGG | 0.18 | 0.08 | 0.26 | 0.30 | 0.06 | 0.10 | 0.05 | 0.21 |
| ACG | 0.39 | 0.52 | 0.28 | 0.30 | 0.50 | 0.60 | 0.60 | 0.50 |
| GCG | 0.01 | 0.04 | 0 | 0 | 0.06 | 0 | 0 | 0 |
| ACA | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 |
| GGA | 0 | 0 | 0.03 | 0 | 0 | 0 | 0 | 0 |
As described in Table 1.
-52G/A_-44C/G_-20G/A
DEFB4/103A CNV distribution
In all samples combined (total n = 187 [CFAR, n = 102; MACS, n = 39; Coriell, n = 46]), the copy numbers ranged from 2 to 8 (median, 4). Copy numbers 2 (n = 35), 3 (n = 54), 4 (n = 61), and 5 (n = 28) were highly prevalent, whereas 6, 7, and 8 were uncommon (n = 3 each). We compared DEFB4/103A CNV distribution between the two major racial groups in the US; i.e., self-identified whites and blacks. For this analysis, CNV results of CFAR white patients were combined with those of MACS white, non-Hispanic patients (total n = 76), and then compared with those of CFAR black patients (n = 65). We observed a significant difference in the CNV distribution between the two groups (white [median, 3], black [median, 4], Mann-Whitney U test p = 0.04). No difference was observed in the CNV distribution between CFAR white patients (n = 37) and MACS white, non-Hispanic patients (n = 39) (Mann-Whitney U test p = 0.47).
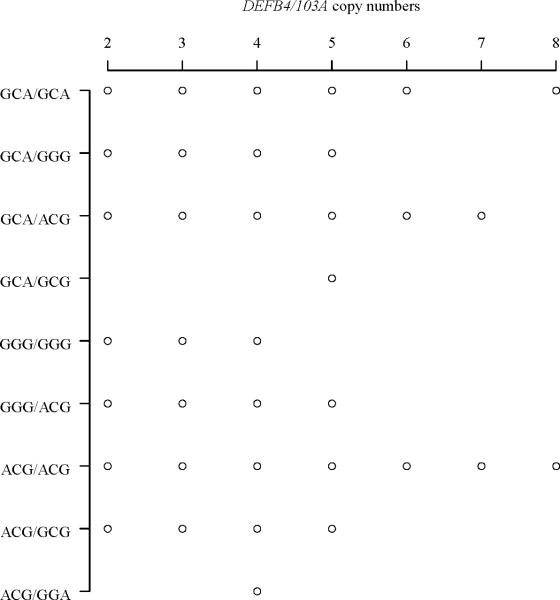
We then compared DEFB4/103A CNV distribution among DEFB1 diplotypes and SNP genotypes. For this analysis, we considered -52G/A_-44C/G_-20G/A diplotypes and the SNP genotypes of all samples combined (total n = 187). We observed a total of nine 3-SNP diplotypes among all 187 samples. The distribution of DEFB4/103A CNV among these diplotypes is presented in Figure 2 and Table 3. A χ2 test of contingency table revealed random distribution of CNV among the diplotypes (χ2 = 34.87, df = 48, p = 0.922). Comparisons of means by Kruskal-Wallis test also suggested no significant difference in the distribution of DEFB4/103A CNV among the DEFB1 diplotypes (p = 0.109).
Fig. 2.
Dot plot showing distribution of DEFB4/103A copy numbers among DEFB1 3-SNP diplotypes.
Each circle represents number of individuals, which are provided in Table 3.
Table 3.
Distribution of DEFB4/103A CNV among DEFB1 3-SNP diplotypes
| Diplotype | Copy number | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
|
Number of individuals |
||||||||
| GCA/GCA | 6 | 9 | 10 | 4 | 1 | 0 | 1 | 31 |
| GCA/GGG | 3 | 5 | 7 | 4 | 0 | 0 | 0 | 19 |
| GCA/ACG | 14 | 17 | 22 | 4 | 1 | 2 | 0 | 60 |
| GCA/GCG | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| GGG/GGG | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 6 |
| GGG/ACG | 6 | 10 | 6 | 4 | 0 | 0 | 0 | 26 |
| ACG/ACG | 3 | 9 | 11 | 9 | 1 | 1 | 2 | 36 |
| ACG/GCG | 1 | 2 | 1 | 2 | 0 | 0 | 0 | 6 |
| ACG/GGA | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
| Total | 35 | 54 | 61 | 28 | 3 | 3 | 3 | 187 |
Lastly, while no significant differences were observed in the distribution of DEFB4/103A CNV among -44C/G and -20G/A genotypes (Kruskal-Wallis p = 0.315 and 0.716, respectively), a significant difference was observed in the distribution of DEFB4/103A CNV among -52G/A genotypes (Kruskal-Wallis p = 0.017). We then performed Bonferroni (Dunn's) t-test for multiple comparisons to identify which -52G/A genotype groups differ in the distribution of DEFB4/103A CNV. This analysis revealed that -52AA (mean CNV = 4.19, n = 36) was significantly different from -52GA (mean CNV = 3.44, n = 94) (Simultaneous 95% CI, 0.19-1.33). Since -52G/A is in complete LD with -390T/A in all populations (Figure 1A-1H), a significant difference was also observed in the distribution of DEFB4/103A CNV among -390T/A genotypes (Kruskal-Wallis p = 0.026). Bonferroni (Dunn's) t-test for multiple comparisons revealed that -390AA (mean CNV = 4.16, n = 37) was significantly different from -390TA (mean CNV = 3.44, n = 93) (Simultaneous 95% CI, 0.15-1.29).
Discussion
In the present study, utilizing samples from two HIV/AIDS cohorts from the US and five diverse populations from the Coriell Cell Repositories, we made the following significant observations regarding β-defensin genetic variation: We observed that there were noticeable differences in the frequencies of DEFB1 SNP alleles (Table 1) and haplotypes (Tables 2a and 2b) among various racial/ethnic groups. To the best of our knowledge, the information regarding comparative distribution of DEFB1 polymorphisms among various populations is limited and, among admixed, HIV/AIDS patient populations from the US, is not available. The majority of studies have been conducted in European populations (Braida et al., 2004; Carter et al., 2010; Ricci et al., 2009; Schaefer et al., 2010). Limited information is available regarding the distribution of DEFB1 polymorphisms in other populations, such as Asian (Chen et al., 2007; Kim et al., 2009; Leung et al., 2006), African (Mozambican) (Baroncelli et al., 2008), Brazilian (Milanese et al., 2006; Segat et al., 2009), American, either mixed (Jurevic et al., 2003; Ozturk et al., 2010) or Caucasian-American and Af-Am (Boniotto et al., 2004), and Mexican (Prado-Montes de Oca et al., 2006). Our DEFB1 SNP-allele and haplotype frequency results are in agreement with those previously reported for comparable populations. Furthermore, our comparative DEFB1 SNP-allele and haplotype frequency results indicate noticeable differences among diverse populations, thus providing highly valuable information for future genetic studies of the etiology of diseases where hBD-1 could play an important modulatory role.
We observed that in our study samples, DEFB4/103A copy numbers varied from 2 to 8 (median, 4). Using different methods, it has been consistently found that these genes vary from 2 to 12 copies PDG, and the copy numbers between 2 and 6 are common, with a modal copy number of 4 in most populations (Fode et al., 2011a; Fode et al., 2011b; Hollox, 2008; Linzmeier and Ganz, 2005; Taudien et al., 2010). Thus, our results regarding DEFB4/103A CNV, in all samples combined, are in agreement with the published findings. We also observed that there was a significant difference between the copy numbers of two major racial groups in the US; i.e., self-identified whites and blacks. Although in all populations the modal DEFB4/103A copy number is 4, there appear to be differences in the mean copy numbers, with the distribution of Chinese and Yorubans (from Ibadan, Nigeria) shifted slightly towards higher copy numbers compared with European populations (Hollox, 2008). However, in a recent study, there was no significant difference in the mean copy numbers between European and Ghanaian populations (Fode et al., 2011a). Using our Coriell samples, we compared the DEFB4/103A copy numbers between NE and the populations of African ancestry (AfSS and Af-Am, combined), and found no significant difference (Mann-Whitney U test p = 0.13). Thus, our finding that a significant difference in the copy numbers occurs between self-identified whites and blacks from the US could be unique to admixed populations or these particular sets of samples, and therefore requires further investigations.
We observed a significant difference in the distribution of DEFB4/103A CNV among DEFB1 -52G/A genotypes, but not in the distribution of DEFB4/103A CNV among -52G/A_-44C/G_-20G/A diplotypes. Furthermore, since -52G/A is in complete LD with another DEFB1 SNP -390T/A, a significant difference was observed in the distribution of DEFB4/103A CNV among -390T/A genotypes as well. To our knowledge, whether DEFB4/103A CNV has a relationship (association, LD) with DEFB1 SNPs has not been reported elsewhere. However, evidence from family studies, HapMap phase II data from four ethnically diverse populations, and genomic characteristics of the 8p23.1 region suggests that DEFB CNV is likely not associated with neighboring SNP alleles (Hollox, 2008). It is important to recognize that our results, based on comparing the means of copy numbers by a nonparametric test, Kruskal-Wallis, are not indicative of an association between DEFB4/103A CNV and DEFB1 SNPs. Nevertheless, they provide a basis for a more in-depth investigation of genetic variation in and around these loci: Determination of DEFB copy number diplotypes (Hollox, 2008) and application of LD/haplotype analysis approach (Conrad et al., 2009; de Smith et al., 2008; Menard et al., 2009) may provide a better assessment of association between DEFB4/103A CNV and DEFB1 SNPs.
In conclusion, our observations suggest that there may be an interracial difference in DEFB4/103A copy numbers between admixed populations, and a relationship between DEFB1 SNPs and DEFB4/103A CNV. Given the emerging significance of DEFB1 SNPs (Baroncelli et al., 2008; Braida et al., 2004; Freguja et al., 2012; Milanese et al., 2006; Ricci et al., 2009; Segat et al., 2009; Segat et al., 2006) and DEFB4- and DEFB103A-encoded hBD-2 and hBD-3, respectively, (Feng et al., 2006; Quinones-Mateu et al., 2003; Sun et al., 2005; Zapata et al., 2008) in influencing HIV infection and disease, these observations deserve further exploration. Future studies will help unravel the complex interplay between β-defensin genetic polymorphisms and susceptibility to, or the progression or severity of, HIV infection/disease, complementing our current efforts to further lessen the morbidity and mortality due to this global killer.
Supplementary Material
Acknowledgements
We are indebted to Michael Lederman and Benigno Rodriguez for providing the HIV-infected patient samples from the CFAR specimen repository, and to Janet Schollenberger for providing the HIV/AIDS patient samples from the MACS. We are thankful to Dave McNamara, Carolyn Myers, Tenisha Phipps, and Bangan John for thorough reading and constructive criticism of the manuscript. We sincerely thank Krufinta Bun and Kyle Logue for helping with some of the statistical analyses. This study was supported by a grant from the NIH/NIDCR (1P01DE019759, A.W.; Project 4, R.J.J.).
References
- Abu Bakar S, Hollox EJ, Armour JA. Allelic recombination between distinct genomic locations generates copy number diversity in human beta-defensins. Proceedings of the National Academy of Sciences USA. 2009;106:853. doi: 10.1073/pnas.0809073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardlie KG, Kruglyak L, Seielstad M. Patterns of linkage disequilibrium in the human genome. Nature Reviews Genetics. 2002;3:299. doi: 10.1038/nrg777. [DOI] [PubMed] [Google Scholar]
- Baroncelli S, Ricci E, Andreotti M, Guidotti G, Germano P, Marazzi MC, Vella S, Palombi L, De Rossi A, Giuliano M. Single-nucleotide polymorphisms in human beta-defensin-1 gene in Mozambican HIV-1-infected women and correlation with virologic parameters. AIDS. 2008;22:1515. doi: 10.1097/QAD.0b013e3282fd6e0c. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Boniotto M, Hazbon MH, Jordan WJ, Lennon GP, Eskdale J, Alland D, Gallagher G. Novel hairpin-shaped primer assay to study the association of the -44 single-nucleotide polymorphism of the DEFB1 gene with early-onset periodontal disease. Clinical and Diagnostic Laboratory Immunology. 2004;11:766. doi: 10.1128/CDLI.11.4.766-769.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnen PE, Wang PJ, Kimmel M, Chakraborty R, Nelson DL. Haplotype and linkage disequilibrium architecture for human cancer-associated genes. Genome Research. 2002;12:1846. doi: 10.1101/gr.483802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida L, Boniotto M, Pontillo A, Tovo PA, Amoroso A, Crovella S. A single-nucleotide polymorphism in the human beta-defensin 1 gene is associated with HIV-1 infection in Italian children. AIDS. 2004;18:1598. doi: 10.1097/01.aids.0000131363.82951.fb. [DOI] [PubMed] [Google Scholar]
- Cagliani R, Fumagalli M, Riva S, Pozzoli U, Comi GP, Menozzi G, Bresolin N, Sironi M. The signature of long-standing balancing selection at the human defensin beta-1 promoter. Genome Biology. 2008;9:R143. doi: 10.1186/gb-2008-9-9-r143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JG, West SK, Painter S, Haynes RJ, Churchill AJ. beta-Defensin 1 haplotype associated with postoperative endophthalmitis. Acta Ophthalmologica. 2010;88:786. doi: 10.1111/j.1755-3768.2009.01534.x. [DOI] [PubMed] [Google Scholar]
- Chen H, Xu Z, Peng L, Fang X, Yin X, Xu N, Cen P. Recent advances in the research and development of human defensins. Peptides. 2006;27:931. doi: 10.1016/j.peptides.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Chen QX, Lv C, Huang LX, Cheng BL, Xie GH, Wu SJ, Fang XM. Genomic variations within DEFB1 are associated with the susceptibility to and the fatal outcome of severe sepsis in Chinese Han population. Genes & Immunity. 2007;8:439. doi: 10.1038/sj.gene.6364401. [DOI] [PubMed] [Google Scholar]
- Conrad DF, Pinto D, Redon R, Feuk L, Gokcumen O, Zhang Y, et al. Origins and functional impact of copy number variation in the human genome. Nature. 2009;464:704. doi: 10.1038/nature08516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smith AJ, Walters RG, Coin LJ, Steinfeld I, Yakhini Z, Sladek R, Froguel P, Blakemore AI. Small deletion variants have stable breakpoints commonly associated with alu elements. PLoS One. 2008;3:e3104. doi: 10.1371/journal.pone.0003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Dubyak GR, Lederman MM, Weinberg A. Cutting edge: human beta defensin 3 - a novel antagonist of the HIV-1 coreceptor CXCR4. Journal of Immunology. 2006;177:782. doi: 10.4049/jimmunol.177.2.782. [DOI] [PubMed] [Google Scholar]
- Ferlin A, Ganz F, Pengo M, Selice R, Frigo AC, Foresta C. Association of testicular germ cell tumor with polymorphisms in estrogen receptor and steroid metabolism genes. Endocrine-Related Cancer. 2010;17:17. doi: 10.1677/ERC-09-0176. [DOI] [PubMed] [Google Scholar]
- Fode P, Jespersgaard C, Hardwick RJ, Bogle H, Theisen M, Dodoo D, et al. Determination of beta-defensin genomic copy number in different populations: a comparison of three methods. PLoS One. 2011a;6:e16768. doi: 10.1371/journal.pone.0016768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fode P, Stegger M, Andersen PS. Human beta-defensin 3 (DEFB103) and its influence on Staphylococcus aureus nasal carriage. International Journal of Infectious Diseases. 2011b;15:e388. doi: 10.1016/j.ijid.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Freguja R, Gianesin K, Del Bianco P, Malacrida S, Rampon O, Zanchetta M, Giaquinto C, De Rossi A. Polymorphisms of innate immunity genes influence disease progression in HIV-1 infected children. AIDS. 2012:765. doi: 10.1097/QAD.0b013e3283514350. [DOI] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nature Reviews Immunology. 2003;3:710. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Groth M, Szafranski K, Taudien S, Huse K, Mueller O, Rosenstiel P, Nygren AO, Schreiber S, Birkenmeier G, Platzer M. High-resolution mapping of the 8p23.1 beta-defensin cluster reveals strictly concordant copy number variation of all genes. Human Mutation. 2008;29:1247. doi: 10.1002/humu.20751. [DOI] [PubMed] [Google Scholar]
- Groth M, Wiegand C, Szafranski K, Huse K, Kramer M, Rosenstiel P, Schreiber S, Norgauer J, Platzer M. Both copy number and sequence variations affect expression of human DEFB4. Genes & Immunity. 2010;11:458. doi: 10.1038/gene.2010.19. [DOI] [PubMed] [Google Scholar]
- Hollox EJ. Copy number variation of beta-defensins and relevance to disease. Cytogenetics and Genome Research. 2008;123:148. doi: 10.1159/000184702. [DOI] [PubMed] [Google Scholar]
- Hollox EJ, Armour JA, Barber JC. Extensive normal copy number variation of a beta-defensin antimicrobial-gene cluster. American Journal of Human Genetics. 2003;73:591. doi: 10.1086/378157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollox EJ, Barber JC, Brookes AJ, Armour JA. Defensins and the dynamic genome: what we can learn from structural variation at human chromosome band 8p23.1. Genome Research. 2008;18:1686. doi: 10.1101/gr.080945.108. [DOI] [PubMed] [Google Scholar]
- Huse K, Taudien S, Groth M, Rosenstiel P, Szafranski K, Hiller M, et al. Genetic variants of the copy number polymorphic beta-defensin locus are associated with sporadic prostate cancer. Tumor Biology. 2008;29:83. doi: 10.1159/000135688. [DOI] [PubMed] [Google Scholar]
- Ionita-Laza I, Rogers AJ, Lange C, Raby BA, Lee C. Genetic association analysis of copy-number variation (CNV) in human disease pathogenesis. Genomics. 2009;93:22. doi: 10.1016/j.ygeno.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurevic RJ, Bai M, Chadwick RB, White TC, Dale BA. Single-nucleotide polymorphisms (SNPs) in human beta-defensin 1: high-throughput SNP assays and association with Candida carriage in type I diabetics and nondiabetic controls. Journal of Clinical Microbiology. 2003;41:90. doi: 10.1128/JCM.41.1.90-96.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurevic RJ, Chrisman P, Mancl L, Livingston R, Dale BA. Single-nucleotide polymorphisms and haplotype analysis in beta-defensin genes in different ethnic populations. Genetic Testing. 2002;6:261. doi: 10.1089/10906570260471787. [DOI] [PubMed] [Google Scholar]
- Kalus AA, Fredericks LP, Hacker BM, Dommisch H, Presland RB, Kimball JR, Dale BA. Association of a genetic polymorphism (-44 C/G SNP) in the human DEFB1 gene with expression and inducibility of multiple beta-defensins in gingival keratinocytes. BMC Oral Health. 2009;9:21. doi: 10.1186/1472-6831-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Lee JE, Namkung JH, Kim PS, Kim S, Shin ES, Cho EY, Yang JM. Single nucleotide polymorphisms and the haplotype in the DEFB1 gene are associated with atopic dermatitis in a Korean population. Journal of Dermatological Science. 2009;54:25. doi: 10.1016/j.jdermsci.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nature Reviews Immunology. 2006;6:447. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- Leung TF, Li CY, Liu EK, Tang NL, Chan IH, Yung E, Wong GW, Lam CW. Asthma and atopy are associated with DEFB1 polymorphisms in Chinese children. Genes & Immunity. 2006;7:59. doi: 10.1038/sj.gene.6364279. [DOI] [PubMed] [Google Scholar]
- Linzmeier RM, Ganz T. Human defensin gene copy number polymorphisms: comprehensive analysis of independent variation in alpha- and beta-defensin regions at 8p22-p23. Genomics. 2005;86:423. doi: 10.1016/j.ygeno.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Mehlotra RK, Bockarie MJ, Zimmerman PA. Prevalence of UGT1A9 and UGT2B7 nonsynonymous single nucleotide polymorphisms in West African, Papua New Guinean, and North American populations. European Journal of Clinical Pharmacology. 2007;63:1. doi: 10.1007/s00228-006-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlotra RK, Ziats MN, Bockarie MJ, Zimmerman PA. Prevalence of CYP2B6 alleles in malaria-endemic populations of West Africa and Papua New Guinea. European Journal of Clinical Pharmacology. 2006;62:267. doi: 10.1007/s00228-005-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard V, Eap O, Harvey M, Guillemette C, Levesque E. Copy-number variations (CNVs) of the human sex steroid metabolizing genes UGT2B17 and UGT2B28 and their associations with a UGT2B15 functional polymorphism. Human Mutation. 2009;30:1310. doi: 10.1002/humu.21054. [DOI] [PubMed] [Google Scholar]
- Milanese M, Segat L, Pontillo A, Arraes LC, de Lima Filho JL, Crovella S. DEFB1 gene polymorphisms and increased risk of HIV-1 infection in Brazilian children. AIDS. 2006;20:1673. doi: 10.1097/01.aids.0000238417.05819.40. [DOI] [PubMed] [Google Scholar]
- Naslavsky MS, Crovella S, Lima Filho JL, Rocha CR. The sound of silence: human beta-defensin-1 gene untranslated SNPs change the predicted mRNA secondary structure in a length-dependent manner. Immunology Letters. 2010;129:53. doi: 10.1016/j.imlet.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Naslavsky MS, Rocha CR, Lima Filho JL, Crovella S. Predicting alternative candidates as binding sites to DEFB1 668 (-44) SNP: a long way from statistical association with multifactorial diseases. Infection, Genetics and Evolution. 2009;9:1129. doi: 10.1016/j.meegid.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Ozturk A, Famili P, Vieira AR. The antimicrobial peptide DEFB1 is associated with caries. Journal of Dental Research. 2010;89:631. doi: 10.1177/0022034510364491. [DOI] [PubMed] [Google Scholar]
- Prado-Montes de Oca E. Human beta-defensin 1: a restless warrior against allergies, infections and cancer. International Journal of Biochemistry and Cell Biology. 2010;42:800. doi: 10.1016/j.biocel.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Prado-Montes de Oca E, Rangel-Villalobos H, Gallegos-Arreola MP, Sandoval L, Figuera LE. SNPs in human beta-defensin 1 gene (DEFB1): frequencies in a Mexican population and new PCR-RFLPs assays. International Journal of Immunogenetics. 2006;33:339. doi: 10.1111/j.1744-313X.2006.00628.x. [DOI] [PubMed] [Google Scholar]
- Quinones-Mateu ME, Lederman MM, Feng Z, Chakraborty B, Weber J, Rangel HR, et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17:F39. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- Ricci E, Malacrida S, Zanchetta M, Montagna M, Giaquinto C, De Rossi A. Role of beta-defensin-1 polymorphisms in mother-to-child transmission of HIV-1. Journal of Acquired Immune Deficiency Syndrome. 2009;51:13. doi: 10.1097/QAI.0b013e31819df249. [DOI] [PubMed] [Google Scholar]
- Schaefer AS, Richter GM, Nothnagel M, Laine ML, Ruhling A, Schafer C, et al. A 3’ UTR transition within DEFB1 is associated with chronic and aggressive periodontitis. Genes & Immunity. 2010;11:45. doi: 10.1038/gene.2009.75. [DOI] [PubMed] [Google Scholar]
- Segat L, Brandao LA, Guimaraes RL, Crovella S. Are defensin beta 1 gene polymorphisms associated with HIV infection and virus replication? AIDS. 2009;23:647. doi: 10.1097/QAD.0b013e3283277247. [DOI] [PubMed] [Google Scholar]
- Segat L, Milanese M, Boniotto M, Crovella S, Bernardon M, Costantini M, Alberico S. DEFB-1 genetic polymorphism screening in HIV-1 positive pregnant women and their children. Journal of Maternal-Fetal and Neonatal Medicine. 2006;19:13. doi: 10.1080/14767050500381123. [DOI] [PubMed] [Google Scholar]
- Shepherd JC, Jacobson LP, Qiao W, Jamieson BD, Phair JP, Piazza P, Quinn TC, Margolick JB. Emergence and persistence of CXCR4-tropic HIV-1 in a population of men from the multicenter AIDS cohort study. Journal of Infectious Diseases. 2008;198:1104. doi: 10.1086/591623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Finnegan CM, Kish-Catalone T, Blumenthal R, Garzino-Demo P, La Terra Maggiore GM, et al. Human beta-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. Journal of Virology. 2005;79:14318. doi: 10.1128/JVI.79.22.14318-14329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taudien S, Groth M, Huse K, Petzold A, Szafranski K, Hampe J, Rosenstiel P, Schreiber S, Platzer M. Haplotyping and copy number estimation of the highly polymorphic human beta-defensin locus on 8p23 by 454 amplicon sequencing. BMC Genomics. 2010;11:252. doi: 10.1186/1471-2164-11-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata W, Rodriguez B, Weber J, Estrada H, Quinones-Mateu ME, Zimermman PA, Lederman MM, Rugeles MT. Increased levels of human beta-defensins mRNA in sexually HIV-1 exposed but uninfected individuals. Current HIV Research. 2008;6:531. doi: 10.2174/157016208786501463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Gu W, Hurles ME, Lupski JR. Copy number variation in human health, disease, and evolution. Annual Review of Genomics and Human Genetics. 2009;10:451. doi: 10.1146/annurev.genom.9.081307.164217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.