Abstract
In a study on 3-day maize (Zea mays) seedlings, grown on nitrate, requirements were established for the maximum extraction and optimum stabilization of nitrate reductase in vitro. With the primary root, 5 mm cysteine were required in the extraction medium, but for the scutellum, which has a high level of endogenous thiol, the use of additional thiol resulted in a reduced yield of a more labile enzyme. Activity of the root and scutella nitrate reductase was obtained with either NADH or NADPH, but that of the root enzyme with NADPH was only demonstrated in the absence of phosphate.
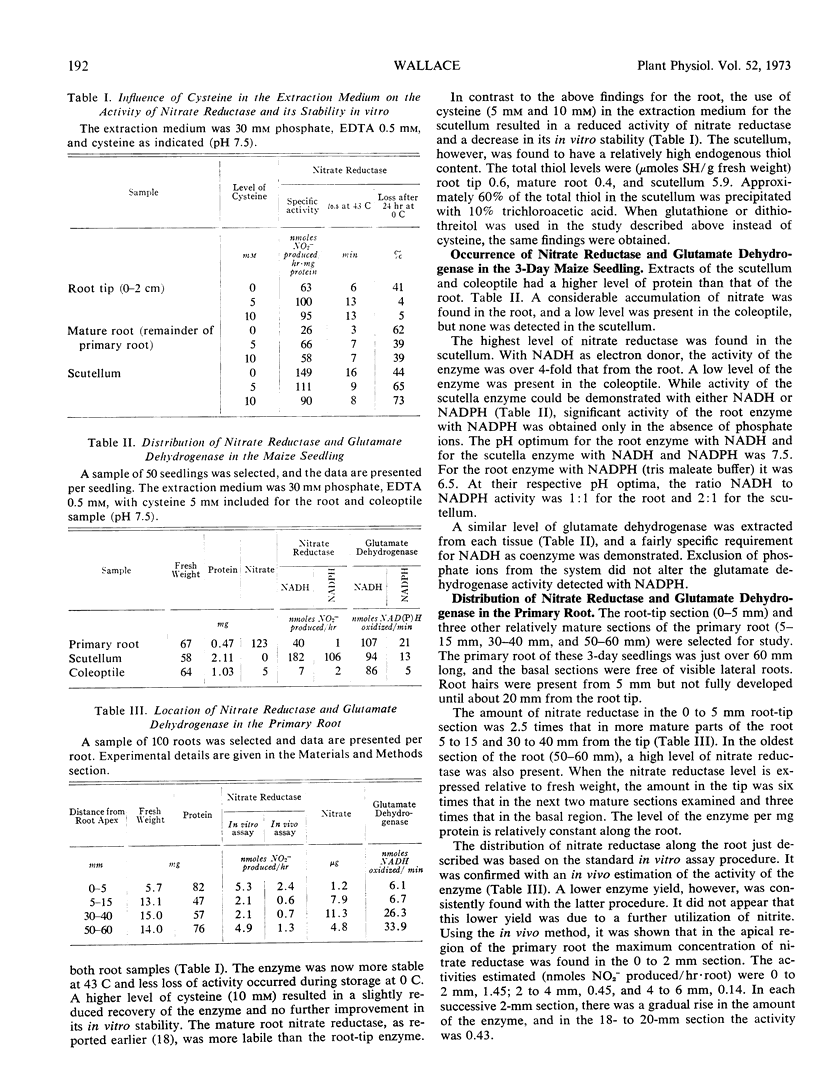
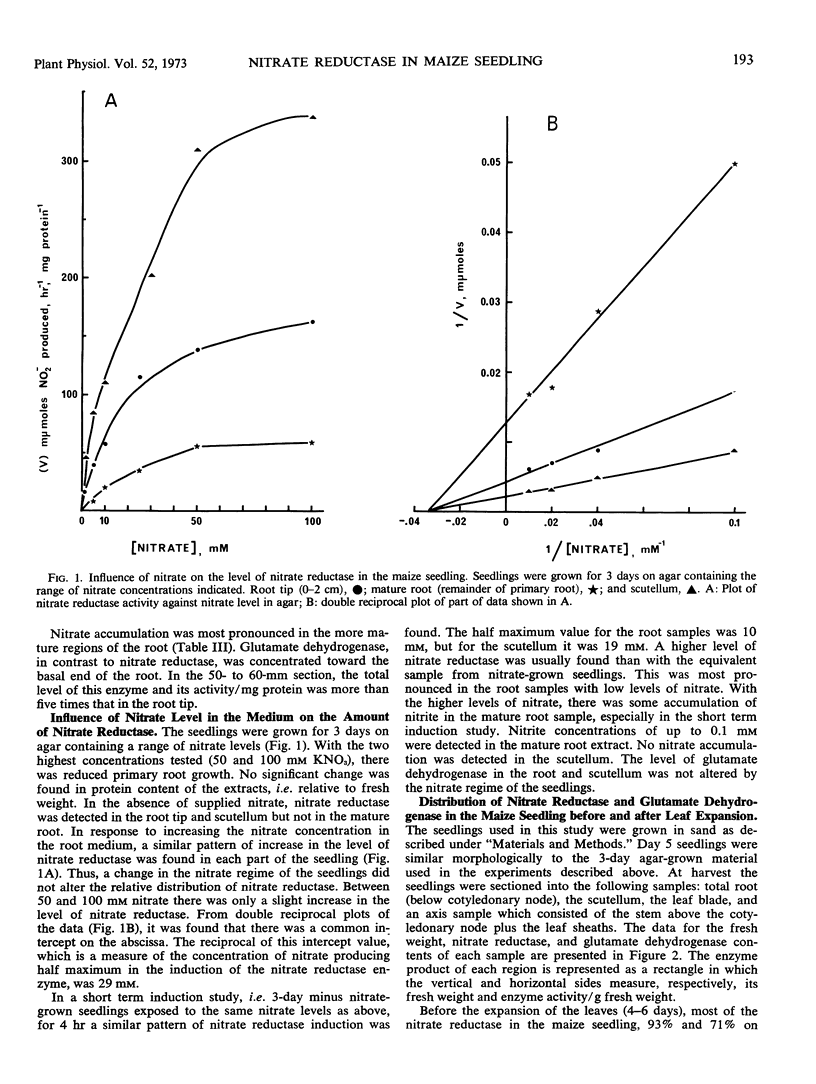
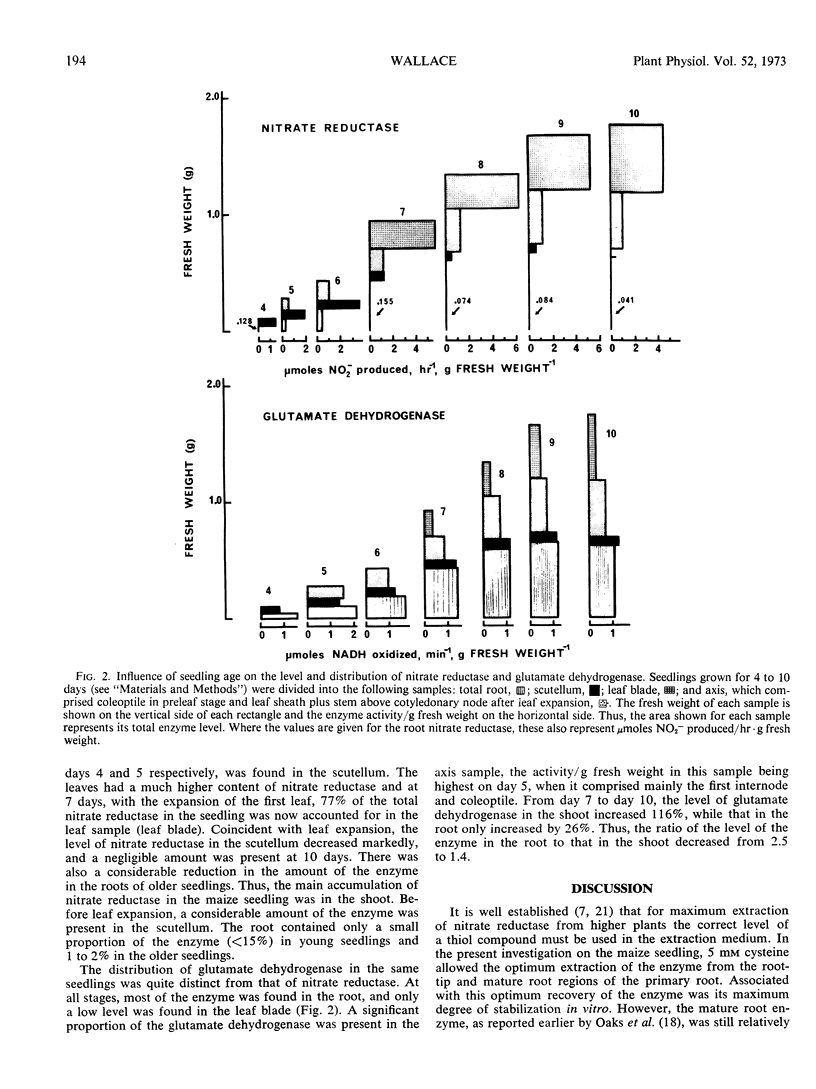
Before leaf expansion, the nitrate reductase in the maize seedling was mainly in the scutellum. The enzyme present in the primary root was predominantly in the apical region (0-2 mm). In contrast, glutamate dehydrogenase was concentrated in the mature basal region of the root (30-60 mm). A high level of nitrate (approximately 100 mm) was required to saturate the induction of nitrate reductase in the root tip, mature root, and scutellum. The concentration of nitrate required to give half the maximum level of enzyme induced was the same for each region (29 mm).
After leaf expansion, more than 90% of the nitrate reductase was in the shoot, mainly in the leaf blade, and a marked decrease occurred in the level of the enzyme in the scutellum. A large proportion of the glutamate dehydrogenase was still found in the root.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Faull K. F., Wallace W., Nicholas D. J. Nitrite oxidase and nitrate reductase in Nitrobacter agilis. Biochem J. 1969 Jul;113(3):449–455. doi: 10.1042/bj1130449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari T. E., Varner J. E. Substrate induction of nitrate reductase in barley aleurone layers. Plant Physiol. 1969 Jan;44(1):85–88. doi: 10.1104/pp.44.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer Y. M., Filner P. Regulation of the nitrate assimilation pathway in cultured tobacco cells. 3. The nitrate uptake system. Biochim Biophys Acta. 1971 Feb 23;230(2):362–372. doi: 10.1016/0304-4165(71)90223-6. [DOI] [PubMed] [Google Scholar]
- Ingle J. The regulation of activity of the enzymes involved in the assimilation of nitrate by higher plants. Biochem J. 1966 Sep;100(3):577–588. doi: 10.1042/bj1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINSKY S. C. Induction and repression of nitrate reductase in Neurospora crassa. J Bacteriol. 1961 Dec;82:898–904. doi: 10.1128/jb.82.6.898-904.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks A., Wallace W., Stevens D. Synthesis and turnover of nitrate reductase in corn roots. Plant Physiol. 1972 Dec;50(6):649–654. doi: 10.1104/pp.50.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson G. W., Cocking E. C. Enzymic Assimilation of Nitrate in Tomato Plants. I. Reduction of Nitrate to Nitrite. Plant Physiol. 1964 May;39(3):416–422. doi: 10.1104/pp.39.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R. H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968 Oct 24;25(1):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]


