Cocaine-dependent individuals anecdotally appear aged and their mortality rates are estimated up to eight times higher than in the healthy population.1 Psychological and physiological changes typically associated with old age such as cognitive decline, brain atrophy, or immunodeficiency are also seen in middle-aged cocaine-dependent individuals.2,3 These observations raise the question of whether cocaine abuse might accelerate the process of normal ageing. Although this is a little-studied area, there are several reasons for assuming that chronic cocaine exposure interferes with the processes of brain ageing.
We compared the effects of age on gray matter volume in 120 individuals aged 18–50 years (Supplementary Information). Half of the sample met the standard diagnostic criteria for cocaine dependence of the DSM-IV-TR;4 whereas the other half had no history of substance misuse disorders or major psychiatric disorders. The two groups were matched for age (t118=−0.12, P=0.905), gender (χ2=2.8, P=0.148), and verbal IQ (t115=−0.36, P=0.716), as described elsewhere.5 All participants underwent a structural MRI brain scan, which was analyzed using voxel-based morphometry5 to produce whole-brain maps of age-related change in gray matter volume.
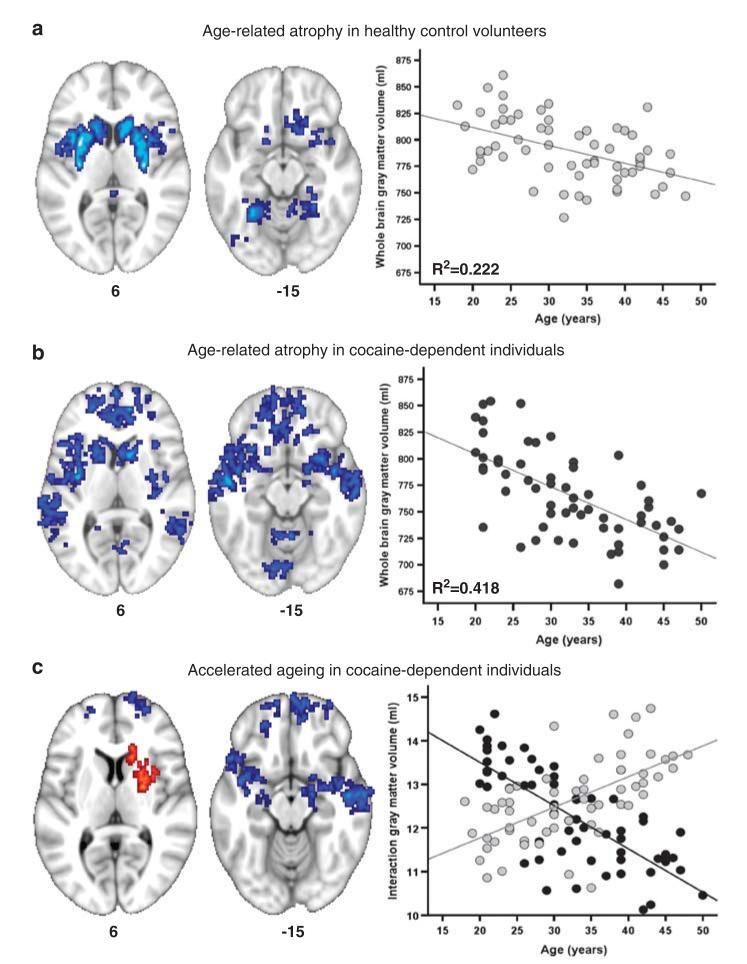
All participants showed a reduction of gray matter volume in cortical and subcortical regions as a linear function of increasing calendar age (Figures 1a and b). However, the annual rate of global gray matter volume loss in cocaine-dependent individuals was almost twice the rate of healthy volunteers (that is, 3.08 ml per year (standard error (s.e.): 0.49 ml) versus 1.69 ml per year (s.e.: 0.41 ml)). Consequently, the rate of age-related gray matter volume loss in cocaine-dependent individuals was significantly greater than in healthy volunteers (F1,116=4.7, P=0.031); this interaction remained significant after excluding 16 individuals with comorbid alcohol dependence (F1,100=6.4, P=0.013). Accelerated ageing in cocaine-dependent individuals was also demonstrated by a significant age-by-group interaction on gray matter volume of the regions affected by age (P<0.001, see Figure 1c). Cocaine-dependent individuals showed a significantly greater-than-normal age-related decline in gray matter in prefrontal and temporal regions compared with healthy controls. By contrast, parts of the striatum appeared resistant to age-related volume decline in the cocaine-using group. Enlarged striatal volume has frequently been reported in stimulant-dependent individuals,5,6 possibly reflecting a marker of reduced dopamine neurotransmission in this dopamine-rich brain region where drugs like cocaine work. Decline in striatal dopamine receptor density has been associated with normal age-related cognitive decline.7 The relative absence of age-related changes in the striatum of cocaine-dependent people may thus reflect another feature of an abnormal brain ageing process.
Figure 1.
Age-related changes in gray matter volume in 60 healthy volunteers and 60 cocaine-dependent individuals. The brain maps show regions of age-related gray matter volume loss separately in healthy volunteers (a) and cocaine-dependent individuals (b); the scatter plots next to the brain maps show age versus the gray matter volume in these regions for each participant in each group (black circles, cocaine-dependent individuals; gray circles, healthy volunteers). (c) A direct comparison of age-related gray matter decline between the two groups revealed a significant group-by-age interaction such that cocaine-dependent individuals showed significantly greater atrophy in prefrontal and temporal-brain regions (blue regions) compared with controls and they showed a lack of normal age-related volume loss in the striatum (red regions). The scatter plot shows the mean volumes of brain regions where there was a significant group-by-age interaction. Left side of the brain is shown on the left side of each slice; the numbers denote z-coordinates for each slice in standard stereotactic space.
Abnormal ageing in chronic cocaine users is an emerging public health concern, which has received little attention. Approximately 1% (ref. 8) of the 21 million users of cocaine wordwide,9 are considered to develop cocaine dependence. These individuals may potentially be at risk of premature brain ageing. Young people taking cocaine today need to be educated about the long-term risk of ageing prematurely, specifically at a time when many developed economies are facing the demographic challenge of an ageing population. Our findings also draw attention to the increasing number of older drug users seeking treatment for drug abuse.10 Drug-treatment services, however, mainly target drug use in young people, focusing on prevention and harm reduction; the needs of older drug users are not so well catered for. As the psychological and physiological challenges of ageing may have also accelerated in individuals with long-term drug dependence,11 the effects of cocaine on the process of ageing should be recognized in order to design and administer age-appropriate treatments for older drug users.
Supplementary Material
Acknowledgments
This work was funded and sponsored by GlaxoSmithKline (RG45422) and conducted within the GlaxoSmithKline Clinical Unit Cambridge (UK) and the Behavioural and Clinical Neuroscience Institute at the University of Cambridge, which is jointly funded by the Medical Research Council and the Wellcome Trust.
Footnotes
CONFLICT OF INTEREST KDE and PSJ, who are both supported by an MRC research grant, declare no conflict of interest. GBW declares that he has no potential conflict of interest. TWR consults for Cambridge Cognition, Lilly, Lundbeck and GlaxoSmithKline, and is currently in receipt of research grants from these companies. ETB is employed part-time by GlaxoSmithKline and part-time by the University of Cambridge.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
doi: 10.1038/mp.2012.31
REFERENCES
- 1.Degenhardt L, Singleton J, Calabria B, McLaren J, Kerr T, Mehta S, et al. Drug Alcohol Depend. 2011;113:88–95. doi: 10.1016/j.drugalcdep.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 2.Verdejo-Garcia A, Perez-Garcia M. Psychopharmacology. 2007;190:517–530. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]
- 3.Reece A. Immunity Ageing. 2007;4:6. doi: 10.1186/1742-4933-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th edn., Text revision American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 5.Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Brain. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobsen LK, Giedd JN, Gottschalk C, Kosten TR, Krystal JH. Am J Psychiatry. 2001;158:486–489. doi: 10.1176/appi.ajp.158.3.486. [DOI] [PubMed] [Google Scholar]
- 7.Backman L, Nyberg L, Lindenberger U, Li SC, Farde L. Neurosci Biobehav Rev. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Bucello C, Degenhardt L, Calabria B, Nelson P, Roberts A, Medina-Mora ME, et al. NDARC Technical Report No. 30. National Drug and Alcohol Research Centre. University of NSW; Sydney: 2010. [Google Scholar]
- 9.UNDOC . Word Drug Report 2011. United Nations Publication; Sales No. E.11.XI.10. [Google Scholar]
- 10.Arndt S, Clayton R, Schultz SK. Am J Geriatr Psych. 2011;19:704–711. doi: 10.1097/JGP.0b013e31820d942b. [DOI] [PubMed] [Google Scholar]
- 11.Beynon C, Stimson G, Lawson E. Br J Gen Pract. 2010;60:481–482. doi: 10.3399/bjgp10X514710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.