Abstract
Context
There are no effective pharmacotherapies for stimulant dependence but there are many plausible targets for development of novel therapeutics. We hypothesized that dopamine-related targets are relevant for treatment of stimulant dependence, and there will likely be individual differences in response to dopaminergic challenges.
Objective
To measure behavioral and brain functional markers of drug-related attentional bias in stimulant-dependent individuals studied repeatedly after short-term dosing with dopamine D2/D3 receptor antagonist and agonist challenges.
Design
Randomized, double-blind, placebo-controlled, parallel-groups, crossover design using pharmacological functional magnetic resonance imaging.
Setting
Clinical research unit (GlaxoSmithKline) and local community in Cambridge, England.
Participants
Stimulant-dependent individuals (n=18) and healthy volunteers (n=18).
Interventions
Amisulpride (400 mg), pramipexole dihydrochloride (0.5 mg), or placebo were administered in counterbalanced order at each of 3 repeated testing sessions.
Main Outcome Measures
Attentional bias for stimulant-related words was measured during functional magnetic resonance imaging by a drug-word Stroop paradigm; trait impulsivity and compulsivity of dependence were assessed at baseline by questionnaire.
Results
Drug users demonstrated significant attentional bias for drug-related words, which was correlated with greater activation of the left prefrontal and right cerebellar cortex. Attentional bias was greater in people with highly compulsive patterns of stimulant abuse; the effects of dopaminergic challenges on attentional interference and related frontocerebellar activation were different between high- and low-compulsivity subgroups.
Conclusions
Greater attentional bias for and greater prefrontal activation by stimulant-related words constitute a candidate neurocognitive marker for dependence. Individual differences in compulsivity of stimulant dependence had significant effects on attentional bias, its brain functional representation, and its short-term modulation by dopaminergic challenges.
Chronic stimulant drug abuse is associated with major adverse health, social, legal, and economic consequences.1-3 Yet the pharmacological and psychological treatments available for stimulant dependence are characterized by high dropout and relapse rates.4-6 There is a clear need to improve treatment efficacy for the growing number of stimulant-dependent individuals.3
Advances in neuroimaging research have contributed to a better understanding of the neurobiological basis of stimulant dependence and have indicated some plausible targets for pharmacological intervention. On the hypothesis that stimulant dependence may be associated with a loss of “top-down” inhibitory control over lower-level reward processing,7,8 several studies have investigated brain and behavioral responses to tasks, such as the color-word Stroop paradigm, that entail attentional control to override an automatic but incorrect response tendency.9-18 In studies of stimulant dependence, a drug-related version of the Stroop test has been used to measure the degree of involuntary attention, or attentional bias, toward drug-related words compared with neutral words.19-23 The increased salience of drug-related cues, as measured by attentional bias in drug-word Stroop paradigms, is thought to contribute to the maintenance of drug-taking behavior7,24 and has demonstrated predictive value for the risk of relapse.12,21,25-27
The exact neurochemical mechanisms underpinning attentional bias to drug-related stimuli in stimulant-dependent individuals (SDIs) are unknown, but attentional bias on the Stroop paradigm is responsive to dopaminergic modulation.28-30 Dopamine is generally released in response to reward-predicting or salient events31-33 and also in response to drug-related cues in drug-dependent individuals.34,35 Accumulating evidence indicates that striatal dopamine systems are disrupted in stimulant dependence.36-38 For example, studies using raclopride positron emission tomography have shown that SDIs have a significantly lower binding potential of striatal dopamine D2 receptors,39-41 which has usually been interpreted as a marker of reduced postsynaptic receptor density.42 Reduced striatal dopamine receptor density appears to be directly related to decreased metabolic activity in the orbitofrontal cortex,39,40 a brain area implicated in compulsive behavior and craving.43
Collectively, these studies suggest that attentional bias predisposing to stimulant dependence could be related to abnormal dopamine transmission and could therefore be treated by drugs targeting dopamine receptors. There are rationales for both dopamine agonist and antagonist drugs, and both have been tried, but the findings are inconsistent,5,44-47 in most cases providing no more than weak evidence for therapeutic efficacy. One possible explanation for this discrepancy between the theoretical strength of the target and the apparent lack of clinical benefit is that stimulant dependence is a heterogeneous syndrome. There are important psychological dimensions of difference between individuals, which may modulate their response to treatment and attenuate the power of clinical trials involving small numbers of psychologically undifferentiated SDIs. Two psychological dimensions of particular interest in this respect are impulsivity and compulsivity: both are known to be abnormal in SDIs compared with nondependent people,24,48-50 and both have been linked to dopaminergic mechanisms.51-54Impulsivity can be defined as loss of inhibitory control over the response to rewarding or distracting stimuli and may play a role as both a cause and a consequence of substance abuse55,56; compulsivity is defined as persistence or perseverance of behavior in the absence of reward or despite punishment and may be particularly important in the maintenance of drug-taking habits.54 Both impulsivity and drug-related compulsivity have been linked with alterations in the dopamine system52,57,58 and are thought to account for the variability of dopaminergic drug effects in SDIs.51
Herein, we tested 2 hypothetical predictions: (1) attentional bias for drug-related cues, and related brain activation, would be abnormal in people with stimulant dependence and (2) the effects of dopaminergic challenge drugs on attentional bias and related brain systems would be influenced by individual differences in impulsivity and compulsivity among a group of SDIs. We conducted a double-blind, placebo-controlled, pharmacological functional magnetic resonance imaging (fMRI) study to evaluate the effects on drug-word Stroop task performance of single doses of a dopamine D2/D3 receptor agonist (pramipexole dihydrochloride, 0.5 mg) and a D2/D3 antagonist (amisulpride, 400 mg) administered in a within-subjects crossover design to SDIs and healthy comparison volunteers.
METHODS
PARTICIPANTS
Thirty-six right-handed volunteers participated in this study: 18 individuals with a chronic history of stimulant drug abuse, satisfying the DSM-IV-TR59 criteria for dependence on either crack cocaine (n=5), cocaine (n=5), or amphetamines (n=8) and 18 matched healthy controls. Stimulant-dependent individuals were non–treatment seeking and recruited from the local community. Prior to study enrolment, all participants had a satisfactory medical review and were screened for any other current Axis I psychiatric disorder using the Structured Clinical Interview for the DSM-IV-TR Axis I Disorders.60 Participants were excluded on the basis of any current Axis I psychiatric disorder, other than substance dependence in SDIs. Participants were also screened for normal appearance of structural MRI scans (http://www.neuroscience.cam.ac.uk/directory/profile.php?ke220). All volunteers provided written informed consent and received monetary compensation for participation. The study was approved by the Cambridge Research Ethics Committee (REC06/Q0108/130; principal investigator, T.W.R.).
SHORT-TERM DOPAMINERGIC DRUG TREATMENTS
Amisulpride is a selective antagonist at the dopamine D2/D3 receptors. We administered a single oral dose of 400 mg of amisulpride; no serious adverse events were reported. Pramipexole is a selective agonist at the dopamine D2/D3 receptors61; we administered a single oral dose of 1.5 mg of pramipexole dihydrochloride to the first 6 participants enrolled in the study (3 SDIs, 3 nondependent volunteers). However, this dose of pramipexole was poorly tolerated in the 3 nondependent volunteers, who were unable to perform the tasks at this treatment session because of nausea, vomiting, sweating, and tiredness. Subsequently, the dose of pramipexole dihydrochloride was reduced to 0.5 mg orally for all participants (ie, for 18 controls [including the 3 volunteers who did not tolerate the higher dose] and 15 SDIs). All participants were also administered 30 mg of domperidone orally at each treatment session to prevent emetic effects of dopamine receptor agonism (http://www.neuroscience.cam.ac.uk/directory/profile.php?ke220).
BASELINE ASSESSMENTS
All participants completed the Barratt Impulsiveness Scale as a measure of self-reported impulsivity. In SDIs, drug-related compulsivity was assessed using the Obsessive Compulsive Drug Use Scale,62,63 an equivalent to the Yale-Brown Obsessive Compulsive Scale64 of symptom severity in obsessive-compulsive disorder. The Obsessive Compulsive Drug Use Scale measures the amount of time, interference, and distress caused by stimulant-related thoughts or urges and the efforts to resist them. Obsessive-compulsive tendencies in controls were assessed by the Yale-Brown Obsessive Compulsive Scale (http://www.neuroscience.cam.ac.uk/directory/profile.php?ke220).
ATTENTIONAL CONTROL TASKS
We used 2 versions of the Stroop task as tests of attentional control, based on the paradigm used by Compton et al,65 in which participants were asked to indicate the font color of a word displayed on the screen using a 4-button box. Each button corresponded to 1 of 4 possible font colors: red, blue, yellow, or green. The drug-word Stroop task involved 2 lists of target words: 16 amphetamine-related and 16 cocaine-related words. Both lists were matched with a list of 16 neutral words with regard to length and frequency. Equivalent to the drug-word version, the color-word Stroop task also involved 2 lists of color words, each including 8 distinct color words that were presented twice per block. Each color word was always displayed in a font color incongruent with its meaning. Both lists of color words were matched with neutral words for length and frequency (http://www.neuroscience.cam.ac.uk/directory/profile.php?ke220).
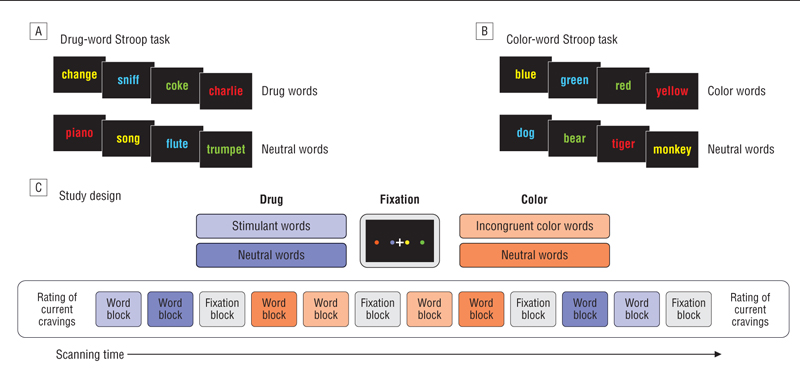
Both the drug-word and the color-word Stroop tasks were presented as a blocked periodic paradigm during fMRI (Figure 1). This design was chosen because interference effects in emotional Stroop paradigms are thought to rely on a slow disengagement process66 that generalizes to drug-related words,67 and the block design has therefore been the method of choice in many prior studies investigating attentional interference in an emotional context.65,68-70 For each word trial, the interstimulus interval was 2.2 seconds, including the presentation of a fixation cross for 0.3 second followed by the presentation of a word for 1.9 seconds, during which time the participants had to register a response by button press. Each block of words lasted 35.2 seconds, and 2 blocks of each word type were followed by a 38-second fixation block (Figure 1). The order of the words within each block was randomized and the order of the blocks was counterbalanced across participants and testing sessions. The allocation of the font colors to the words was randomized; each of the 4 colors occurred equally often in each word category. All participants were trained on the task prior to each scanning session with a practice run of at least 1 color-word block and 1 block of different neutral words to familiarize themselves with the task and to minimize practice effects. Acute Stroop task–induced stimulant cravings were measured immediately before and after the Stroop task by asking the following question: “How would you rate your current state of craving on a scale from 0 to 100?”
Figure 1.
Schematic of the Stroop paradigms. A, In the drug-word Stroop task, participants were asked to identify the font colors of the drug-related words and the neutral words. B, In the color-word Stroop task, participants were asked to identify the font colors of the color words and the neutral words. C, Blocks of drug-related words, color words (which were always incongruent with their font color), and neutral words were interspersed with blocks of fixation for task presentation during functional magnetic resonance imaging.
Task performance was measured in terms of accuracy and latency of response to each trial. Attentional bias for drug-related words was quantified by an interference score, calculated for each individual as the median latency of correct responses to the drug-related words minus the median latency of correct responses to the neutral words. Likewise, attentional interference in the color-word Stroop task was calculated as the median latency of correct responses to color words minus the median latency of correct responses to neutral words. The median is generally more robust than the mean as a measure of central location in small samples; however, the mean interference scores were also calculated for comparative purposes (Table 1 and Table 2).
Table 1.
Task Performance on the Drug-Word Stroop and Color-Word Stroop Tests in Stimulant-Dependent Individuals and Healthy Comparison Volunteersa
| Dependent Variable | Effect | df | F | P Value |
|---|---|---|---|---|
| Drug-word Stroop task | ||||
| Attentional interference score (median latency) | Drug | 2, 62 | 1.75 | .18 |
| Drug×group | 2, 62 | 0.28 | .97 | |
| Group | 1, 31 | 10.87 | .02a | |
| Attentional interference score (mean latency) | Drug | 2, 62 | 1.24 | .30 |
| Drug×group | 2, 62 | 0.27 | .77 | |
| Group | 1, 31 | 10.87 | .002a | |
| Error rate, % | Drug | 2, 62 | 1.32 | .27 |
| Drug×group | 2, 62 | 4.04 | .02a | |
| Group | 1, 31 | 3.82 | .06 | |
| Color-word Stroop task | ||||
| Attentional interference score (median latency) | Drug | 2, 62 | 6.14 | .004a |
| Drug×group | 2, 62 | 0.74 | .48 | |
| Group | 1, 31 | 0.24 | .63 | |
| Attentional interference score (mean latency) | Drug | 2, 60 | 3.08 | .053 |
| Drug×group | 2, 60 | 0.86 | .43 | |
| Group | 1, 30 | 0.01 | .94 | |
| Error rate, % | Drug | 2, 62 | 0.05 | .96 |
| Drug×group | 2, 62 | 1.19 | .31 | |
| Group | 1, 31 | 3.01 | .09 |
Abbreviation: BDI-II, Beck Depression Inventory–II.71
Analysis of covariance modeling of factorial effects on attentional interference and accuracy (covariates: BDI-II total scores, years of education, and plasma levels of pramipexole dihydrochloride).
Significant.
Table 2.
Task Performance on the Drug-Word Stroop Test in High- and Low-Compulsivity and High- and Low-Impulsivity Subgroups of Stimulant-Dependent Individualsa
| Dependent Variable on Drug-Word Stroop Task | Effect | df | F | P Value |
|---|---|---|---|---|
| Compulsivity | ||||
| Attentional interference score (median latency) | Drug | 2, 30 | 1.97 | .16 |
| Drug×group | 2, 30 | 4.35 | .02b | |
| Group | 1, 15 | 7.84 | .01b | |
| Attentional interference score (mean latency) | Drug | 2, 30 | 3.23 | .053 |
| Drug×group | 2, 30 | 5.55 | .009b | |
| Group | 1, 15 | 9.14 | .009b | |
| Error rate, % | Drug | 1.5, 22.1 | 1.42 | .24 |
| Drug×group | 1.5, 22.1 | 0.38 | .71 | |
| Group | 1, 15 | 2.07 | .17 | |
| Impulsivity | ||||
| Attentional interference score (median latency) | Drug | 2, 30 | 1.42 | .26 |
| Drug×group | 2, 30 | 1.36 | .27 | |
| Group | 1, 15 | 1.27 | .28 | |
| Attentional interference score (mean latency) | Drug | 2, 30 | 2.44 | .11 |
| Drug×group | 2, 30 | 1.94 | .16 | |
| Group | 1, 15 | 1.39 | .26 | |
| Error rate, % | Drug | 1.5, 21.8 | 1.79 | .20 |
| Drug×group | 1.5, 21.8 | 1.44 | .25 | |
| Group | 1, 15 | 0.20 | .66 |
Analysis of covariance modeling of factorial effects on attentional interference and accuracy (covariate: plasma levels of pramipexole dihydrochloride).
Significant.
ACQUISITION OF fMRI DATA
Whole-brain fMRI data were acquired at the Wolfson Brain Imaging Centre, University of Cambridge, Cambridge, England, using a Siemens Magnetom Tim Trio whole-body scanner operating at 3 T(Siemens Medical Solutions, Erlangen, Germany). During the performance of each Stroop task, 32 transaxial sections of gradient echo, echoplanar imaging data depicting blood oxygen level–dependent contrast were acquired parallel to the intercommissural line with the following parameters: repetition time=2000 milliseconds, echo time=30 milliseconds, flip angle=78°, slice thickness=3 mm plus 0.75-mm interslice gap, image matrix size=64×64, and within-plane voxel dimensions=3.0 mm×3.0 mm. Prior to data analysis, the first 5 echoplanar images were discarded to account for T1 equilibration effects.
ANALYSIS OF DEMOGRAPHIC, PSYCHOMETRIC, AND BEHAVIORAL DATA
Using data from both groups, attentional interference scores, error, and craving data were each analyzed separately using a repeated-measures analysis of covariance (ANCOVA) model with drug treatment (3 levels: placebo, amisulpride, and pramipexole) as the within-subject factor and group (2 levels: nondependent volunteers and SDIs) as the between-subject factor. Using data from the patient group only, attentional interference was also analyzed using a repeated-measures ANCOVA model with drug treatment (3 levels: placebo, amisulpride, and pramipexole) as the within-subject factor and compulsivity subgroup (2 levels: high and low) as the between-subject factor. In all analyses of attentional interference scores, only data on correctly performed trials were included. To control for group differences in depressive mood, education, and dose of pramipexole, we included the Beck Depression Inventory scores, years of education, and plasma levels of pramipexole as covariates in all analyses involving SDIs and controls; the subgroup analyses (involving SDIs only) included plasma levels of pramipexole as a covariate. Statistical tests were conducted using SPSS (version 13; SPSS Inc, Chicago, Illinois) and were reported as significant if P<.05.
ANALYSIS OF fMRI DATA
Details of the fMRI data analysis procedures are briefly summarized herein (full details, http://www.neuroscience.cam.ac.uk/directory/profile.php?ke220). We first identified by whole-brain analysis those brain regions where functional activation was specifically related to the contrast of drug-related vs neutral words and strongly correlated with the behavioral measure of attentional interference (as defined earlier). At each voxel of these behaviorally relevant frontal and cerebellar regions, we tested the factorial effects of diagnostic group and dopaminergic drug challenge by ANCOVA using all data on both groups. Finally, we also tested each voxel of this frontocerebellar system to investigate factorial effects of compulsivity (high or low) and dopaminergic drug challenge by ANCOVA in the patient group only. These anatomically focused factorial analyses of the fMRI data are formally analogous to parallel factorial analyses of behavioral data on attentional interference (described earlier).
All data sets from both Stroop tasks for SDIs and controls under all drug conditions were initially preprocessed to correct for effects of subject motion, differential slice timing, and differences in global means.72-74 At the first single-subject level of analysis, a time series regression model specified 2 key contrasts between correctly performed trials: (1) drug-related words vs neutral words in the drug-word blocks and (2) color words vs neutral words in the color-word blocks. These contrasts are equivalent to those used to generate attentional interference scores in the behavioral data and are therefore most specific to the cognitive control processes of interest. This design matrix, convolved with a hemodynamic response function,75 was regressed at each voxel onto the corrected time series. The resulting maps of the model coefficients normalized by their standard errors for each individual were mapped into the standard space of the Montreal Neurological Institute echo-planar imaging template by linear affine transformation to obtain a group activation map. These activation maps included all participants under all drug treatments to identify brain regions that were significantly activated by contrast 1 (drug-word Stroop task) and by contrast 2 (color-word Stroop task) using a permutation test on spatial statistics (http://www.neuroscience.cam.ac.uk/directory/profile.php?ke220).
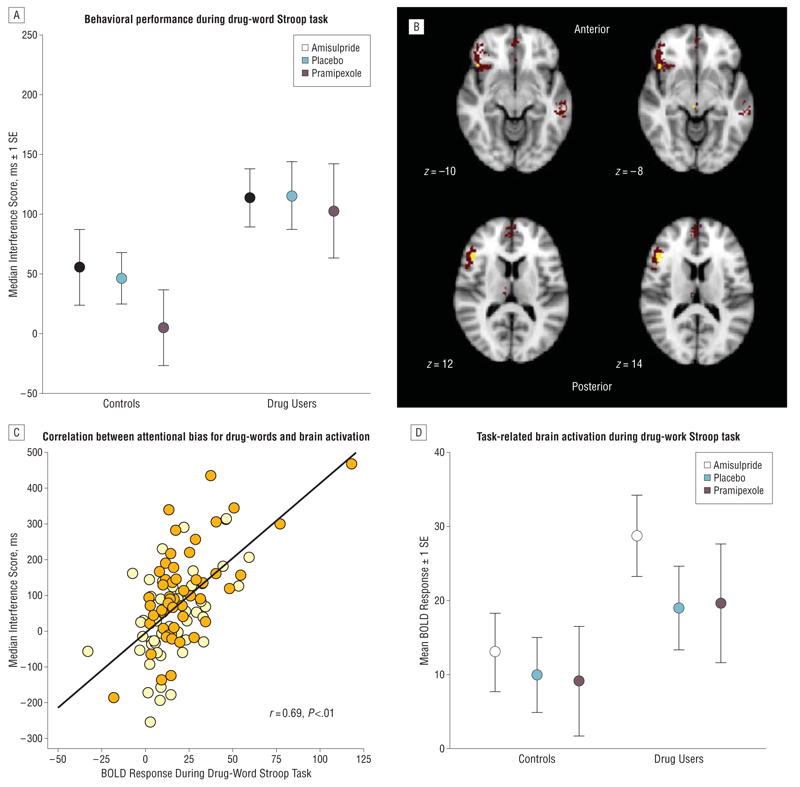
We used the activation map of the drug-word Stroop task contrast to define a mask (shown as red voxels in Figure 2) and regressed the median attentional interference score for each individual on the individual activation statistics at each voxel in this mask. This procedure identified a set of voxels that were both generically activated by the task and significantly associated with variability in attentional interference (shown as yellow voxels in Figure 2). The mean activation statistic over all voxels associated with attentional interference was calculated for each participant and used as a dependent variable in ANCOVA models that tested the effects of group, drug, and drug×group interaction on brain activation in these regions. The same regional activation statistics were also used as dependent variables in ANCOVA models that tested the effects of subgroup (eg, high or low compulsivity), drug, and drug×subgroup interaction on brain activation.
Figure 2.
Behavioral performance during the drug-word Stroop paradigm and task-related activation of a frontocerebellar system associated with attentional bias for drug words (in all participants). A, Drug users showed a significant attentional bias for drug-related words, as reflected in higher attentional interference scores compared with the healthy comparison volunteers. Attentional bias was measured by each volunteer’s median response latency of correctly identified colors of drug-related words minus the median response latency of correctly identified colors of matched neutral words. Pramipexole was given as pramipexole dihydrochloride. B, The red voxels indicate brain regions activated by the contrast between drug-related words and neutral words; yellow voxels indicate brain regions within this system where activation was positively correlated with attentional interference scores on the drug-word Stroop task. C, Scatterplot of median attentional interference score (y-axis) vs functional activation of the brain regions associated with attentional bias for drug words (x-axis), which are the left ventral prefrontal cortex (Montreal Neurological Institute coordinates [x, y, z] in millimeters: −46, 26, 12; −44, 22, −8; and −40, 6, 30) and right cerebellum (22, −80, −40). The spatial coordinates refer to the peak voxel where the effect size is greatest. BOLD indicates blood oxygen level dependent. D, Comparison of mean task-related (BOLD) activation in brain regions associated with attentional bias between stimulant-dependent individuals and healthy volunteers. Stimulant users show overactivation in the left ventral prefrontal cortex and right cerebellum compared with controls.
RESULTS
BASELINE IMPULSIVITY, COMPULSIVITY, AND MOOD
As expected from previous research,49 SDIs reported significantly higher levels of impulsivity on the Barratt Impulsiveness Scale than controls. Stimulant-dependent individuals also generally reported high levels of compulsivity of stimulant abuse on the Obsessive Compulsive Drug Use Scale, whereas healthy controls did not show strong obsessive-compulsive traits, as assessed by the Yale-Brown Obsessive Compulsive Scale (Table 3). Although participants with clinical depression were excluded from the study, SDIs reported higher scores on depressive mood, as assessed at baseline by the Montgomery-Asberg Depression Rating Scale77 and corroborated by serial scores on the Beck Depression Inventory–II71 before each treatment session (F2,34=21.50; P<.001).
Table 3.
Demographic, Psychological, and Baseline Personality Measures for the Groups of 18 Stimulant-Dependent Individuals and 18 Healthy Volunteers
| Mean (SD) [range] |
|||||
|---|---|---|---|---|---|
| Group | Healthy Comparison Volunteers | Stimulant-Dependent Individuals | F | df | P Value |
| Age, y | 32.7 (6.9) | 34.3 (7.2) | 0.47 | 1, 34 | .498 |
| Sex ratio (male:female) | 15:3 | 15:3 | a | a | >.99 |
| Ethnic ratio (white:Afro Caribbean)b | 17:1 | 16:2 | a | a | >.99 |
| Employment ratio (employed:unemployed) | 17:1 | 9:9 | a | a | .007 |
| Verbal intelligence quotient (NART) | 108.4 (6.0) | 109.0 (8.1) | 0.55 | 1, 34 | .82 |
| Education, y | 12.4 (1.8) | 11.2 (1.0) | 6.85 | 1, 34 | .01 |
| BIS-11 total score | 62.0 (7.2) | 82.0 (9.5) | 50.4 | 1, 34 | >.001 |
| MADRS total score | 0.9 (2.4) | 5.6 (8.2) | 5.49 | 1, 34 | .03 |
| BDI-II total score | 1.1 (2.4) | 9.3 (11.1) | 9.50 | 1, 34 | .004 |
| YBOCS total score | 0.1 (0.5) | ||||
| OCDUS total score | 26.5 (7.9) | ||||
| Duration of stimulant abuse, y | 11.7 (7.4) [2-26] | ||||
| Frequency of stimulant abuse, d/wk | 5.4 (2.0) | ||||
| Age at onset of stimulant abuse, y | 20.5 (5.4) [14-35] | ||||
Abbreviations: BDI-II, Beck Depression Inventory–II71; BIS-11, Barratt Impulsiveness Scale76; MADRS, Montgomery-Asberg Depression Rating Scale77; NART, National Adult Reading Test78; OCDUS, Obsessive Compulsive Drug Use Scale62; YBOCS, Yale-Brown Obsessive Compulsive Scale.64
Fisher exact test.
Ethnicity was recorded according to the standard practices of the study funder and sponsor. Ethnic background was neither an inclusion nor an exclusion criterion.
SUBJECTIVE EFFECTS OF DRUG TREATMENT
Dopaminergic challenge drugs had no significant effects on subjective alertness, contentedness, or calmness scales. There were also no significant group differences or drug×group interactions on any of these measures (http://www.neuroscience.cam.ac.uk/directory/profile.php?ke220). There was no evidence for induction of craving by performance of the drug-word Stroop task and no effect of dopaminergic challenge drugs on self-reported craving.
BEHAVIORAL DATA FOR THE STROOP TASKS
In the drug-word Stroop paradigm, SDIs showed a marked attentional bias toward drug-related cues compared with controls (Figure 2A). Thus, across all 3 short-term treatment conditions, SDIs demonstrated significantly longer latency than controls in correctly identifying the font colors of drug-related words compared with their responses to neutral words (Table 1). Drug users did not make more errors on the drug-word Stroop task than controls. The significant drug×group interaction for accuracy was driven by controls who showed a tendency to make fewer errors while taking amisulpride relative to placebo (F1,14=3.48; P=.08). On the color-word Stroop paradigm, no main effect of group and no drug×group interaction were identified (Table 1).
fMRI DATA FOR THE DRUG-WORD AND COLOR-WORD STROOP TASKS
The brain regions activated by drug words (relative to neutral words) included the left ventral and dorsal prefrontal cortex and precentral cortex; anterior cingulate, medial prefrontal, and premotor cortex; posterior cingulate and medial posterior parietal cortex; bilateral middle and inferior temporal cortex; left thalamus and caudate nucleus; and right cerebellum (Table 4 and http://www.neuroscience.cam.ac.uk/directory/profile.php?ke220). Within this pattern of brain activation, attentional bias for drug words was most strongly associated with greater activation of the left ventral prefrontal cortex (Montreal Neurological Institute coordinates [x, y, z] in millimeters: −46, 26, 12; −44, 22, −8; and −40, 6, 30) and right cerebellum (x, y, z: 22, −80, −40) (Figure 2B and C). We fitted an ANCOVA model to test for significant main and interactive effects of group and dopaminergic drug challenge on these regions of activation. Only the effect of group was significant: SDIs had greater task-related activation of the left ventral prefrontal cortex and right cerebellum compared with controls (F1,31=6.40; P<.05) (Figure 2D and http://www.neuroscience.cam.ac.uk/directory/profile.php?ke220).
Table 4.
Brain Regions Differentially Activated During the Drug-Word Stroop Task in Drug Users and Healthy Volunteers, Irrespective of Drug Treatment
| Size (Voxel) |
Max Fa | x, y, z Max Coordinates, mmb |
AAL Regions |
|---|---|---|---|
| Regions Associated With Greater Activation During Indication of Font Color of Drug-Related Words vs Neutral Words | |||
| 1030 | 44.67 | −46, 42, −12 | Bilateral inferior frontal gyrus (orbital, opercula, and triangular parts), left middle frontal gyrus (orbital part), rolandic operculum, insula, and superior temporal gyrus |
| 722 | 6.29 | −2, 50, −14 | Bilateral middle frontal gyrus (orbital part), superior frontal gyrus (medial part), rectus gyrus, and anterior cingulate gyrus |
| 221 | 2.99 | 22, −80, −46 | Right cerebellum |
| 150 | 3.81 | −4, −52, 30 | Bilateral posterior cingulate gyrus, precuneus, and left median cingulate gyrus |
| 139 | 6.59 | 0, −22, −4 | Left thalamus and caudate nucleus |
| 100 | 3.89 | −62, −46, 4 | Left middle temporal gyrus |
| 88 | 6.19 | 50, −32, −6 | Right middle temporal gyrus and inferior temporal gyrus |
| 87 | 4.29 | −52, 12, 36 | Left precentral gyrus, middle frontal gyrus, and inferior frontal gyrus (opercula part) |
| 79 | 6.55 | 0, 22, 64 | Bilateral supplementary motor area and superior frontal gyrus (medial part) |
|
| |||
| Regions Associated With Deactivation During Indication of Font Color of Drug-Related Words vs Neutral Words | |||
| 1402 | −5.36 | 2, 8, 10 | Bilateral caudate, thalamus, hippocampus, posterior cingulate, precuneus, calcarine gyrus, left parahippocampus, left lingual gyrus, left fusiform gyrus, and right cuneus |
| 639 | −3.21 | 44, −8, −8 | Right superior temporal gyrus, inferior frontal gyrus, rolandic operculum postcentral gyrus, supramarginal gyrus, Herschel gyrus, insula, and putamen |
| 427 | −2.47 | −2, −2, 48 | Bilateral cingulate gyrus, supplementary motor area, and paracentral lobule |
| 188 | −2.71 | 8, −72, 54 | Right precuneus, cuneus, superior parietal gyrus, and superior occipital gyrus |
| 130 | −3.34 | 54, −36, 52 | Right inferior parietal gyrus, postcentral gyrus, and supramarginal gyrus |
| 119 | −2.97 | −22, −12, 72 | Left precentral gyrus, superior frontal gyrus, paracentral lobule, and postcentral gyrus |
Abbreviations: AAL, Automated Anatomical Labeling79; Max, maximum.
Max F denotes the maximum F statistic (test statistics for task-related activation) over all voxels in each region.
Coordinates are given in Montreal Neurological Institute space.
In the color-word Stroop task, the only brain area activated in all participants was the left cerebellum (x, y, z: −4, −46, −40) (Table 5). We did not find a significant relationship with attentional interference scores in this locus of activation and therefore did not investigate it any further.
Table 5.
Brain Regions Differentially Activated During the Color-Word Stroop Task in Drug Users and Healthy Volunteers, Irrespective of Drug Treatment
| Size (Voxel) |
Max Fa | x, y, z Max Coordinates, mmb |
AAL Regions |
|---|---|---|---|
| Regions Associated With Greater Activation During Indication of Font Color of Incongruent Color Words vs Neutral Words | |||
| 126 | 5.69 | −4, −46, −40 | Left cerebellum and vermis |
| Regions Associated With Deactivation During Indication of Font Color of Incongruent Color Words vs Neutral Words | |||
| 431 | −4.09 | 2, 56, −10 | Bilateral middle frontal gyrus (orbital part), superior frontal gyrus (medial part), gyrus rectus, and anterior cingulate gyrus |
Abbreviations: AAL, Automated Anatomical Labeling79; Max, maximum.
Max F denotes the maximum F statistic (test statistics for task-related activation) over all voxels in each region.
Coordinates are given in Montreal Neurological Institute space.
EFFECTS OF IMPULSIVITY AND COMPULSIVITY ON STIMULANT DEPENDENCE
To investigate possible effects of individual differences in baseline measurements of impulsivity on drug-related attentional bias, we divided the SDIs into low- and high-impulsivity subgroups by a median split on their total Barratt Impulsiveness Scale scores. The high- and low-impulsivity subgroups were not significantly different on any other demographic or clinical variables (http://www.neuroscience.cam.ac.uk/directory/profile.php?ke220). There were no significant differences between impulsivity subgroups in latency or accuracy of response to the drug-word Stroop test and there were no significant drug×impulsivity subgroup interactions (Table 2).
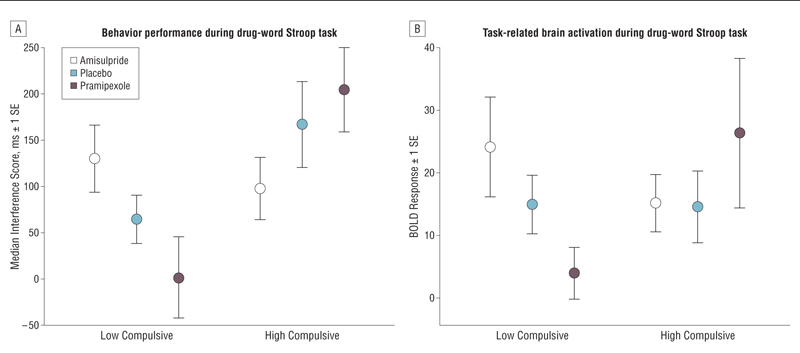
Likewise, we also divided the SDIs into high- and low-compulsivity subgroups by a median split on their total Obsessive Compulsive Drug Use Scale scores; the compulsivity subgroups were not significantly different on any other baseline variables (http://www.neuroscience.cam.ac.uk/directory/profile.php?ke220). However, there was a significant subgroup effect on attentional interference during the drug-word Stroop test; the high-compulsivity subgroup demonstrated significantly greater attentional bias to drug-related related words (Figure 3A) (Table 2). There was also a significant drug×compulsivity subgroup interaction whereby the dopaminergic agents had markedly different effects on attentional bias in high- and low-compulsivity subgroups. Post hoc analysis indicated that this interaction was due to different effects of pramipexole in the 2 subgroups: low-compulsivity SDIs showed no attentional bias, whereas in high-compulsivity SDIs, response latencies for drug-related words were increased (F1,15=9.80; P<.05).
Figure 3.
Behavioral performance and task-related activation of a frontocerebellar system during the drug-word Stroop task in high- and low-compulsivity drug users. Pramipexole was given as pramipexole dihydrochloride. A, High-compulsivity drug users showed a different profile in response to short-term dopaminergic treatment compared with low-compulsivity drug users, as reflected in greater attentional bias for drug-related words relative to neutral words. Attentional bias was measured by each volunteer’s median response latency of correctly identified colors of drug-related words minus the median response latency of correctly identified colors of matched neutral words. B, Dopaminergic drug effects on the left prefrontal cortex during the drug-word Stroop task are modulated by compulsivity of stimulant dependence. Box plots show functional activation associated with attentional bias for drug-related words in the left prefrontal cortex (Montreal Neurological Institute coordinates [x, y, z] in millimeters: −46, 26, 12; −44, 22, −8; and −40, 6, 30) and right cerebellum (22, −80, −40) in high- and low-compulsivity subgroups, indicating differential effects of pramipexole. The spatial coordinates refer to the peak voxel where the effect size is greatest.
The effects of compulsivity on attentional bias for drug-related words were followed up by an analysis of subgroup effects on brain activation in the frontocerebellar regions previously defined. We found a significant drug×compulsivity subgroup interaction on brain activation in the left ventral prefrontal cortex and right cerebellum (F2,30=3.36; P<.05) (Figure 3B). Post hoc analysis showed that this interaction also was mainly driven by pramipexole, which caused a significant reduction in frontocerebellar activation compared with placebo in the low-compulsivity subgroup (t8=2.34; P<.05) and the opposite effect of increased prefrontal activation, in the high-compulsivity subgroup (t8=−0.77; P>.05).
COMMENT
Using 2 versions of the Stroop paradigm for testing attentional control, we confirmed that SDIs had significantly greater attentional bias in favor of drug-related cues,20,21,23,63,80 reflected in greater attentional interference scores on the drug-word Stroop test (Figure 2A). Moreover, this impairment of attentional control in people with stimulant dependence was specific to drug-related cues and did not generalize to their performance on the color-word Stroop task. Thus, it seems that there is a clinically relevant impairment of attentional control in stimulant dependence21 that does not simply reflect the global impairment in attentional function that has been associated with stimulant dependence.81,82
We were also able to show, using fMRI, that greater attentional bias for drug-related words was associated with greater activation of the right cerebellum, a brain area that is implicated in Stroop task interference control,83-86 and the left ventral prefrontal cortex or inferior frontal gyrus, a brain area that is implicated in the retrieval of semantic knowledge87-89 and the processing of word meaning.90-92 This neurocognitive association was demonstrated by the contrast between SDIs and controls: SDIs had significantly greater frontocerebellar activation (Figure 2D) as well as significantly greater attentional interference scores (Figure 2A) and attentional interference was positively correlated with frontocerebellar activation over all subjects in both groups (Figure 2C). Although abnormal inferior frontal gyrus activation during the disorder-related Stroop paradigm has been identified in various groups of psychiatric patients,68,69,93 overactivation of left inferior frontal gyrus when identifying font colors of drug-related words might be specific for stimulant dependence. Left inferior frontal gyrus overactivation in stimulant users has also been reported in response to cocaine-related videos94 or imagery95 relative to neutral cues or when a stimulant drug was administered unexpectedly.96
COMPULSIVITY OF DRUG ABUSE AND FRONTOCEREBELLAR SYSTEMS
We also found that SDIs with a highly compulsive pattern of stimulant drug abuse generally had greater attentional bias for drug-related words (Figure 3A). Moreover, the high- and low-compulsivity subgroups differed markedly in their response to single-dose challenges of pramipexole and amisulpride: most saliently, pramipexole increased attentional interference and frontocerebellar activation in highly compulsive SDIs, whereas in low-compulsivity SDIs, pramipexole had the opposite effect, tending to reduce or “normalize” attentional interference and frontocerebellar activation. How can we explain the different effects of these drugs on attentional interference and related brain activation in high- and low-compulsivity subgroups of SDIs?
At a mechanistic level, the normalizing effect of pramipexole on attentional bias and related frontocerebellar systems in low-compulsive SDIs could be achieved by 1 of 2 main actions. If there is relatively deficient striatal dopamine neurotransmission in this subgroup, consistent with findings recently reviewed by Narendran and Martinez,97 that impairs the function of the associated corticostriatal “loops,” the dopamine D2/D3 agonist could restore normal function via its postsynaptic action.98 Alternatively, given that the SDIs were not in a withdrawal state, it is conceivable that they were exhibiting upregulation of striatal dopamine neurotransmission at the synapse and that the relatively low dose of the dopamine agonist restored normal functioning by its presynaptic action at dopamine D2 autoreceptors.99,100 However, the D2/D3 receptor antagonist, amisulpride, generally had opposite effects to those of pramipexole, perhaps favoring the former hypothesis.
Clearly, neither of these hypotheses could account for the opposite effects of pramipexole in the high-compulsivity subgroup. One hypothesis that might explain this is that highly compulsive individuals have developed a dopamine receptor sensitivity that could lead to an exaggerated and pathological response to pramipexole. Similar observations of postsynaptic supersensitivity have been described in patients with Parkinson disease who compulsively overdose their dopamine agonist medication.58 The consistent finding of reduced D2/D3 receptor binding in stimulant dependence36,38,97,101 argues against this interpretation. However, recent preclinical studies have demonstrated that a long-term regimen of amphetamine exposure does lead to an increase in activation of the high-affinity state of striatal D2 receptors.102 Regardless of these considerations, it is evident that heterogeneity in response to dopaminergic agents clearly has to be taken into account when interpreting the studies of dopamine neurotransmission in stimulant abusers and indeed may help to resolve some of the inconsistencies in the field.97
THERAPEUTIC IMPLICATIONS
One general therapeutic implication of these results is that dopaminergic drugs might have value in controlling the attentional bias toward stimulant-related cues that has previously been shown to predict relapse following a period of abstinence.21 For example, dopaminergic agents could reduce the risk of attentional capture by relapse-provoking cues and thereby promote maintenance of abstinence. The observation that we did not find a relationship between attentional bias and self-reported craving may not be unusual given that many aspects of compulsive drug taking occur through automatic or habitual processes.54,103,104 Our data further suggest that the therapeutic effects of dopaminergic drugs could be strongly influenced by the baseline compulsivity of stimulant drug use (which can vary considerably within a group homogenously defined as satisfying DSM-IV criteria for stimulant drug dependence). This finding also has potential implications for early clinical trial design in the development of new candidate therapeutics for stimulant dependence: greater statistical power for tests of therapeutic efficacy might be achieved by sampling psychologically stratified, eg, high- or low-compulsivity, groups of patients.
METHODOLOGICAL ISSUES
Strengths of the study include the balanced, gparallel-groups, crossover design; the use of psychological test paradigms theoretically focused on cognitive mechanisms of special relevance to drug dependence; the choice of pharmacologically contrasting dopaminergic challenge drugs; and the well-characterized group of stimulant users. Weaknesses of the study include modest sample size, which has probably constrained the statistical power of the experiment, although the repeated-measures design generated 108 (36×3) behavioral and neuroimaging observations to assess factorial effects of interest. This is a large number of observations by comparison with other neuroimaging studies of stimulant dependence. The dose of pramipexole dihydrochloride was reduced during the course of the experiment, from 1.5 mg to 0.5 mg, because of the occurrence of adverse effects at the starting dose. This difference in dose was associated with proportional changes in pharmacokinetic measures of exposure, which were subsequently included as covariates in ANCOVA models to control for any effects of dose on behavioral or brain functional differences between groups.
Although SDIs were carefully selected according to eligibility criteria that excluded comorbid Axis I disorders and alcohol and opiate dependence, there was still a degree of heterogeneity within the SDI group in terms of their stimulant drug of choice (cocaine or amphetamine) and its source (3 amphetamine users were prescribed amphetamine for harm reduction). This clinical heterogeneity motivates our analysis of individual differences in compulsivity and impulsivity of drug use; however, it also presents a constraint on the generalizability of our results. The observation that high- and low-compulsivity SDIs did not differ in terms of drug-taking histories or clinical symptoms may seem surprising but similar observations have been made with regard to craving in the presence of drug-related cues, which seems not to be reflected in clinical or demographic variables but in biological differences of cue-induced dopamine release in the striatum.35
In relation to the cognitive tests, one may argue that a lack of familiarity of drug words in controls may have affected the results. This seems unlikely since previous research has shown that the interference effect for the drug-word Stroop task is specific to drug-dependent individuals and independent from the familiarity of words.105,106 It is still not clear whether drug-related compulsivity is similar to compulsive behavior observed in patients with obsessive-compulsive disorder, a disorder that has also been associated with dopamine dysfunction.107-109 In our healthy controls, however, the Yale-Brown Obsessive Compulsive Scale has been shown to be insensitive for obsessive-compulsive behaviors at a subclinical level. Further studies may consider using other measures such as the Padua Inventory,110 which assesses the spectrum rather than the severity of obsessive-compulsive symptoms. Moreover, behavioral measures of compulsivity in stimulant dependence need to be developed to validate the individual differences in compulsivity of drug use measured herein by a self-report instrument (Obsessive Compulsive Drug Use Scale). For example, future studies of SDIs might usefully also include more general cognitive measures of attentional set shifting or reversal learning as more objective markers of compulsive perseveration.
Supplementary Material
Acknowledgments
Financial Disclosure: This work was funded and sponsored by GlaxoSmithKline grant RG45422 and conducted within the GlaxoSmithKline Clinical Unit Cambridge, Cambridge, England. Dr Bullmore is employed part time by the University of Cambridge and part time by GlaxoSmithKline plc and is a shareholder of GlaxoSmithKline. Dr Craig is full-time employed by P1vital Ltd and Ms Shabbir and Dr Merlo-Pich are full-time employed by GlaxoSmithKline plc. Dr Abbott has provided computing services for GlaxoSmithKline. Dr Suckling has received grant support from GlaxoSmithKline. Dr Müller has received research grant support from Janssen-Cilag and honoraria or travel expenses from Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, Pharmacia-Upjohn, and UCB Pharma. Dr Sahakian has provided consulting services to Cambridge Cognition Ltd, GlaxoSmithKline, Novartis, AstraZeneca, and Eli Lilly; she holds shares of CeNeS and has received honoraria for grand rounds in psychiatry at Massachusetts General Hospital (continuing medical education credits) and for speaking at the International Conference on Cognitive Dysfunction in Schizophrenia and Mood Disorders (2007). Dr Sahakian is also on the Medical Research Council Neurosciences and Mental Health Board and the Science Coordination Team for the Foresight Project on Mental Capital and Wellbeing and receives an honorarium as an associate editor of Psychological Medicine. Dr Robbins has provided consulting services for Cambridge Cognition Ltd and the pharmaceutical industry, including Eli Lilly and GlaxoSmithKline, and has also received honoraria from Roche, Merck Sharp and Dohme, Lundbeck, and GlaxoSmithKline. He is a past recipient of research grants from GlaxoSmithKline and Pfizer and holds shares from CeNeS and share options from Cambridge Cognition Ltd and Allon Therapeutics. He also receives an editorial honorarium from Springer-Verlag (Psychopharmacology).
Funding/Support: Software development was supported by a Human Brain Project grant from the National Institute of Mental Health and the National Institute of Biomedical Imaging and Bioengineering. Dr Ersche is a recipient of the Betty Behrens Research Fellowship at Clare Hall, University of Cambridge. Dr Sahakian is supported by a Wellcome Trust programme grant. Dr Robbins is supported by a Wellcome Trust programme grant and is a recipient of the James S. McDonnell Foundation Collaborative Award.
Footnotes
Author Contributions: Drs Ersche and Bullmore contributed equally to this work.
Previous Presentations: Data in preliminary form were presented at the International Symposium on Attention and Performance XXII; July 17, 2008; Stow, Vermont; the 21st European College for Neuropsychopharmacology Annual Conference; September 3, 2008; Barcelona, Spain; and the Collegium Internationale Neuro-Psychopharmacologicum Regional Meeting on Addiction and Psychosis; April 26, 2009; Edinburgh, Scotland.
Additional Contributions: We thank all volunteers for their participation in this study and staff at the GlaxoSmithKline Clinical Unit Cambridge and the Wolfson Brain Imaging Centre for their assistance. We are particularly grateful to Rowena Sheehan, Judy Gilbert, Rona Weston-Arnold, Sharon Crosby, and Laura Griffiths for their dedicated help with volunteer recruitment and medical support. Thanks also go to Deanna Barch, PhD, for advice on the Stroop task paradigms and to Graham Murray, MD, PhD, for providing medical cover.
REFERENCES
- 1.United Nations Office on Drugs and Crime . World Drug Report 2007. United Nations Office on Drugs and Crime; New York, NY: 2007. [Google Scholar]
- 2.Nutt D, King LA, Saulsbury W, Blakemore C. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. 2007;369(9566):1047–1053. doi: 10.1016/S0140-6736(07)60464-4. [DOI] [PubMed] [Google Scholar]
- 3.European Monitoring Centre for Drugs and Drug Addiction . Cocaine and Crack Cocaine: A Growing Public Health Problem. European Monitoring Centre for Drugs and Drug Addiction; Lisbon, Portugal: 2007. [Google Scholar]
- 4.Sayre SL, Schmitz JM, Stotts AL, Averill PM, Rhoades HM, Grabowski JJ. Determining predictors of attrition in an outpatient substance abuse program. Am J Drug Alcohol Abuse. 2002;28(1):55–72. doi: 10.1081/ada-120001281. [DOI] [PubMed] [Google Scholar]
- 5.de Lima MS, de Oliveira Soares BG, Reisser AA, Farrell M. Pharmacological treatment of cocaine dependence: a systematic review. Addiction. 2002;97(8):931–949. doi: 10.1046/j.1360-0443.2002.00209.x. [DOI] [PubMed] [Google Scholar]
- 6.Kang SY, Kleinman PH, Woody GE, Millman RB, Todd TC, Kemp J, Lipton DS. Outcomes for cocaine abusers after once-a-week psychosocial therapy. Am J Psychiatry. 1991;148(5):630–635. doi: 10.1176/ajp.148.5.630. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47(suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16(4):456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verdejo-García A, Perez-Garcia M. Profile of executive deficits in cocaine and heroin polysubstance users: common and differential effects on separate executive components. Psychopharmacology (Berl) 2007;190(4):517–530. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]
- 11.Verdejo-García AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007;32(5):950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 12.Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, Tzilos G, Afshar M, Rouse ED, Tian H, Renshaw PF, Ciraulo DA, Yurgelun-Todd DA. Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology. 2008;33(4):827–836. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- 13.Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during Stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64(11):998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S. Addiction changes orbitofrontal gyrus function: involvement in response inhibition. Neuroreport. 2001;12(11):2595–2599. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horner MD. Attentional functioning in abstinent cocaine abusers. Drug Alcohol Depend. 1999;54(1):19–33. doi: 10.1016/s0376-8716(98)00141-0. [DOI] [PubMed] [Google Scholar]
- 16.Toomey R, Lyons MJ, Eisen SA, Xian H, Chantarujikapong S, Seidman LJ, Faraone SV, Tsuang MT. A twin study of the neuropsychological consequences of stimulant abuse. Arch Gen Psychiatry. 2003;60(3):303–310. doi: 10.1001/archpsyc.60.3.303. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl) 2006;188(2):162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- 18.Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, Flynn NM, Henik A, Pfefferbaum A, Sullivan EV. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Res. 2002;111(1):65–74. doi: 10.1016/s0165-1781(02)00111-7. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, Telang F, Alia-Klein N, Volkow ND. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144(4):1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hester R, Dixon V, Garavan H. A consistent attentional bias for drug-related material in active cocaine users across word and picture versions of the emotional Stroop task. Drug Alcohol Depend. 2006;81(3):251–257. doi: 10.1016/j.drugalcdep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter KM, Schreiber E, Church S, McDowell D. Drug Stroop performance: relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addict Behav. 2006;31(1):174–181. doi: 10.1016/j.addbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Vadhan NP, Carpenter KM, Copersino ML, Hart CL, Foltin RW, Nunes EV. Attentional bias towards cocaine-related stimuli: relationship to treatment-seeking for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33(5):727–736. doi: 10.1080/00952990701523722. [DOI] [PubMed] [Google Scholar]
- 23.Copersino ML, Serper MR, Vadhan N, Goldberg BR, Richarme D, Chou JC, Stitzer M, Cancro R. Cocaine craving and attentional bias in cocaine-dependent schizophrenic patients. Psychiatry Res. 2004;128(3):209–218. doi: 10.1016/j.psychres.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Lubman DI, Yucel M, Pantelis C. Addiction, a condition of compulsive behaviour? neuroimaging and neuropsychological evidence of inhibitory dysregulation. Addiction. 2004;99(12):1491–1502. doi: 10.1111/j.1360-0443.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- 25.Cox WM, Hogan LM, Kristian MR, Race JH. Alcohol attentional bias as a predictor of alcohol abusers’ treatment outcome. Drug Alcohol Depend. 2002;68(3):237–243. doi: 10.1016/s0376-8716(02)00219-3. [DOI] [PubMed] [Google Scholar]
- 26.Marissen MAE, Franken IHA, Waters AJ, Blanken P, van den Brink W, Hendriks VM. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101(9):1306–1312. doi: 10.1111/j.1360-0443.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- 27.Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychol. 2003;22(4):378–387. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franken IHA, Hendriks VM, Stam CJ, Van den Brink W. A role for dopamine in the processing of drug cues in heroin dependent patients. Eur Neuropsychopharmacol. 2004;14(6):503–508. doi: 10.1016/j.euroneuro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Hitsman B, MacKillop J, Lingford-Hughes A, Williams TM, Ahmad F, Adams S, Nutt DJ, Munafò MR. Effects of acute tyrosine/phenylalanine depletion on the selective processing of smoking-related cues and the relative value of cigarettes in smokers. Psychopharmacology (Berl) 2008;196(4):611–621. doi: 10.1007/s00213-007-0995-5. [DOI] [PubMed] [Google Scholar]
- 30.Munafò MR, Mannie ZN, Cowen PJ, Harmer CJ, McTavish SB. Effects of acute tyrosine depletion on subjective craving and selective processing of smoking-related cues in abstinent cigarette smokers. J Psychopharmacol. 2007;21(8):805–814. doi: 10.1177/0269881107077216. [DOI] [PubMed] [Google Scholar]
- 31.Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96(4):651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 32.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36(2):241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 33.Ungless MA. Dopamine: the salient issue. Trends Neurosci. 2004;27(12):702–706. doi: 10.1016/j.tins.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brasić JR, Kimes AS, Maris MA, Kumar A, Contoreggi C, Links J, Ernst M, Rousset O, Zukin S, Grace AA, Lee JS, Rohde C, Jasinski DR, Gjedde A, London ED. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31(12):2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- 36.Martinez D, Kim JH, Krystal J, Abi-Dargham A. Imaging the neurochemistry of alcohol and substance abuse. Neuroimaging Clin N Am. 2007;17(4):539–555. doi: 10.1016/j.nic.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9(6):557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- 38.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64(11):1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 39.Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine-D(2) receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14(2):169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 40.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D-2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158(12):2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 41.Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, Laruelle M, Frankel WG. Cocaine dependence and D-2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29(6):1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- 42.Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, et al. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry. 1990;147(6):719–724. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- 43.Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Ann N Y Acad Sci. 2007;1121:576–597. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- 44.Amato L, Minozzi S, Pani PP, Davoli M. Antipsychotic medications for cocaine dependence. Cochrane Database Syst Rev. 2007;(3):CD006306. doi: 10.1002/14651858.CD006306.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Soares BG, Lima MS, Reisser AA, Farrell M. Dopamine agonists for cocaine dependence. Cochrane Database Syst Rev. 2003;(2):CD003352. doi: 10.1002/14651858.CD003352. [DOI] [PubMed] [Google Scholar]
- 46.Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29(7):1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 47.Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J. Pharmacotherapy of methamphetamine addiction: an update. Subst Abus. 2008;29(3):31–49. doi: 10.1080/08897070802218554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158(11):1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- 49.Moeller FG, Dougherty DM, Barratt ES, Oderinde V, Mathias CW, Harper RA, Swann AC. Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug Alcohol Depend. 2002;68(1):105–111. doi: 10.1016/s0376-8716(02)00106-0. [DOI] [PubMed] [Google Scholar]
- 50.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 51.Cools R, Sheridan M, Jacobs E, D’Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007;27(20):5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315(5816):1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robbins TW, Everitt BJ. A role for mesencephalic dopamine in activation: commentary on Berridge (2006) Psychopharmacology (Berl) 2007;191(3):433–437. doi: 10.1007/s00213-006-0528-7. [DOI] [PubMed] [Google Scholar]
- 54.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 55.de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14(1):22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neurosci Biobehav Rev. 2004;28(3):343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 57.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 58.Evans AH, Pavese N, Lawrence AD, Tai YF, Appel S, Doder M, Brooks DJ, Lees AJ, Piccini P. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann Neurol. 2006;59(5):852–858. doi: 10.1002/ana.20822. [DOI] [PubMed] [Google Scholar]
- 59.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 60.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- 61.Mierau J, Schneider FJ, Ensinger HA, Chio CL, Lajiness ME, Huff RM. Pramipexole binding and activation of cloned and expressed dopamine D-2, D-3 and D-4 receptors. Eur J Pharmacol. 1995;290(1):29–36. doi: 10.1016/0922-4106(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 62.Franken IH, Hendriksa VM, van den Brink W. Initial validation of two opiate craving questionnaires: the Obsessive Compulsive Drug Use Scale and the Desires for Drug Questionnaire. Addict Behav. 2002;27(5):675–685. doi: 10.1016/s0306-4603(01)00201-5. [DOI] [PubMed] [Google Scholar]
- 63.Franken IHA, Kroon LY, Hendriks VM. Influence of individual differences in craving and obsessive cocaine thoughts on attentional processes in cocaine abuse patients. Addict Behav. 2000;25(1):99–102. doi: 10.1016/s0306-4603(98)00112-9. [DOI] [PubMed] [Google Scholar]
- 64.Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale, 1: development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 65.Compton RJ, Banich MT, Mohanty A, Milham MP, Herrington J, Miller GA, Scalf PE, Webb A, Heller W. Paying attention to emotion: an fMRI investigation of cognitive and emotional Stroop tasks. Cogn Affect Behav Neurosci. 2003;3(2):81–96. doi: 10.3758/cabn.3.2.81. [DOI] [PubMed] [Google Scholar]
- 66.Phaf RH, Kan KJ. The automaticity of emotional Stroop: a meta-analysis. J Behav Ther Exp Psychiatry. 2007;38(2):184–199. doi: 10.1016/j.jbtep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 67.Waters AJ, Sayette MA, Franken IHA, Schwartz JE. Generalizability of carryover effects in the emotional Stroop task. Behav Res Ther. 2005;43(6):715–732. doi: 10.1016/j.brat.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 68.van den Heuvel OA, Veltman DJ, Groenewegen HJ, Witter MP, Merkelbach J, Cath DC, van Balkom AJ, van Oppen P, van Dyck R. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Arch Gen Psychiatry. 2005;62(8):922–933. doi: 10.1001/archpsyc.62.8.922. [DOI] [PubMed] [Google Scholar]
- 69.Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R. An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disord. 2005;7(suppl 5):58–69. doi: 10.1111/j.1399-5618.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- 70.Mitterschiffthaler MT, Williams SCR, Walsh ND, Cleare AJ, Donaldson C, Scott J, Fu CH. Neural basis of the emotional Stroop interference effect in major depression. Psychol Med. 2008;38(2):247–256. doi: 10.1017/S0033291707001523. [DOI] [PubMed] [Google Scholar]
- 71.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory–II. Psychological Corp; San Antonio, TX: 1996. [Google Scholar]
- 72.Bullmore E, Long C, Suckling J, Fadili J, Calvert G, Zelaya F, Carpenter TA, Brammer M. Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum Brain Mapp. 2001;12(2):61–78. doi: 10.1002/1097-0193(200102)12:2<61::AID-HBM1004>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suckling J, Davis MH, Ooi O, Wink AM, Fadili J, Salvador R, Welchew D, Sendur L, Maxim V, Bullmore ET. Permutation testing of orthogonal factorial effects in a language-processing experiment using fMRI. Hum Brain Mapp. 2006;27(5):425–433. doi: 10.1002/hbm.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bullmore ET, Brammer MJ, Rabe-Hesketh S, Curtis VA, Morris RG, Williams SC, Sharma T, McGuire PK. Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum Brain Mapp. 1999;7(1):38–48. doi: 10.1002/(SICI)1097-0193(1999)7:1<38::AID-HBM4>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Glover GH. Deconvolution of impulse response in event-related BOLD fMRI1. Neuroimage. 1999;9(4):416–429. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- 76.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 77.Montgomery SA, Asberg M. New depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 78.Nelson HE. National Adult Reading Test Manual. NFER-Nelson; Windsor, England: 1982. [Google Scholar]
- 79.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 80.Hester R, Garavan H. Neural mechanisms underlying drug-related cue distraction in active cocaine users. Pharmacol Biochem Behav. 2009;93(3):270–277. doi: 10.1016/j.pbb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 81.Fadardi JS, Cox WM. Alcohol attentional bias: drinking salience or cognitive impairment? Psychopharmacology (Berl) 2006;185(2):169–178. doi: 10.1007/s00213-005-0268-0. [DOI] [PubMed] [Google Scholar]
- 82.Stetter F, Ackermann K, Bizer A, Straube ER, Mann K. Effects of disease-related cues in alcoholic inpatients—results of a controlled alcohol Stroop study. Alcohol Clin Exp Res. 1995;19(3):593–599. doi: 10.1111/j.1530-0277.1995.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 83.Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, Charney DS. Neural correlates of the classic color and emotional Stroop in women with abuse-related posttraumatic stress disorder. Biol Psychiatry. 2004;55(6):612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 84.Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32(10):2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- 85.Egner T, Hirsch J. The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage. 2005;24(2):539–547. doi: 10.1016/j.neuroimage.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 86.Rocca MA, Valsasina P, Ceccarelli A, Absinta M, Ghezzi A, Riccitelli G, Pagani E, Falini A, Comi G, Scotti G, Filippi M. Structural and functional MRI correlates of Stroop control in benign MS. Hum Brain Mapp. 2009;30(1):276–290. doi: 10.1002/hbm.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. J Neurosci. 1995;15(9):5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a re-evaluation. Proc Natl Acad Sci U S A. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 90.Devlin JT, Matthews PM, Rushworth MFS. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci. 2003;15(1):71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- 91.Gabrieli JDE, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci U S A. 1998;95(3):906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- 93.Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 94.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 95.Kilts CD, Gross RE, Ely TD, Drexler KPG. The neural correlates of cueinduced craving in cocaine-dependent women. Am J Psychiatry. 2004;161(2):233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- 96.Volkow ND, Wang GJ, Ma YM, Fowler JS, Zhu W, Maynard L, Telang F, Vaska P, Ding YS, Wong C, Swanson JM. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci. 2003;23(36):11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62(11):851–869. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- 98.Maj J, Rogoz Z, Skuza G, Kolodziejczyk K. The behavioural effects of pramipexole, a novel dopamine receptor agonist. Eur J Pharmacol. 1997;324(1):31–37. doi: 10.1016/s0014-2999(97)00066-6. [DOI] [PubMed] [Google Scholar]
- 99.Cannon WB. The emergency function of the adrenal medulla in pain and major emotions. Am J Physiol. 1914;33:356–372. [Google Scholar]
- 100.Mierau J, Schingnitz G. Biochemical and pharmacological studies on pramipexole, a potent and selective dopamine-D2 receptor agonist. Eur J Pharmacol. 1992;215(2-3):161–170. doi: 10.1016/0014-2999(92)90024-x. [DOI] [PubMed] [Google Scholar]
- 101.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seeman P. Dopamine D2High receptors measured ex vivo are elevated in amphetamine-sensitized animals. Synapse. 2009;63(3):186–192. doi: 10.1002/syn.20595. [DOI] [PubMed] [Google Scholar]
- 103.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2(10):695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 104.Tiffany ST. A cognitive model of drug urges and drug-use behavior—role of automatic and nonautomatic processes. Psychol Rev. 1990;97(2):147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- 105.Munafò M, Mogg K, Roberts S, Bradley BP, Murphy M. Selective processing of smoking-related cues in current smokers, ex-smokers and never-smokers on the modified Stroop task. J Psychopharmacol. 2003;17(3):310–316. doi: 10.1177/02698811030173013. [DOI] [PubMed] [Google Scholar]
- 106.Field M. Cannabis ‘dependence’ and attentional bias for cannabis-related words. Behav Pharmacol. 2005;16(5-6):473–476. doi: 10.1097/00008877-200509000-00021. [DOI] [PubMed] [Google Scholar]
- 107.Perani D, Garibotto V, Gorini A, Moresco RM, Henin M, Panzacchi A, Matarrese M, Carpinelli A, Bellodi L, Fazio F. In vivo PET study of 5HT2A serotonin and D2 dopamine dysfunction in drug-naive obsessive-compulsive disorder. Neuroimage. 2008;42(1):306–314. doi: 10.1016/j.neuroimage.2008.04.233. [DOI] [PubMed] [Google Scholar]
- 108.Olver JS, O’Keefe G, Jones GR, Burrows GD, Tochon-Danguy HJ, Ackermann U, Scott A, Norman TR. Dopamine D1 receptor binding in the striatum of patients with obsessive-compulsive disorder. J Affect Disord. 2009;114(1-3):321–326. doi: 10.1016/j.jad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 109.Denys D, van der Wee N, Janssen J, De Geus F, Westenberg HGM. Low level of dopaminergic D2 receptor binding in obsessive-compulsive disorder. Biol Psychiatry. 2004;55(10):1041–1045. doi: 10.1016/j.biopsych.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 110.Burns GL, Keortge SG, Formea GM, Sternberger LG. Revision of the Padua Inventory of obsessive compulsive disorder symptoms: distinctions between worry, obsessions, and compulsions. Behav Res Ther. 1996;34(2):163–173. doi: 10.1016/0005-7967(95)00035-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.