Abstract
Telomere integrity is critical for telomere function and genomic stability. We previously demonstrated that non-erythroid α-spectrin (αIISp) is present in mammalian cell nuclei where it is important in repair of DNA interstrand cross-links (ICLs) and chromosome stability. We now demonstrate that αIISp is also important for telomere maintenance after ICL damage. It localizes to telomeres in S phase after ICL damage where it has enhanced association with TRF1 and TRF2 and is required for recruitment of the ICL repair protein, XPF, to damage-induced foci at telomeres. In telomerase-positive normal cells depleted of αIISp by siRNA or in Fanconi anemia, complementation group A (FA-A) cells, where αIISp levels are 35–40% of normal, ICL damage results in failure of XPF to localize to telomeres, markedly increased telomere dysfunction-induced foci, followed by catastrophic loss of telomeres. Restoration of αIISp levels to normal in FA-A cells corrects these deficiencies. Our studies demonstrate that αIISp is critical for repair of DNA ICLs at telomeres, likely by facilitating the recruitment of repair proteins similar, but not identical, to its proposed role in repair of DNA ICLs in genomic DNA and that this function in turn is critical for telomere maintenance after DNA ICL damage.
INTRODUCTION
Spectrins are structural proteins that, in the cytoplasm of non-erythroid cells, participate in a number of cellular functions, which include providing mechanical support for the plasma membrane, protein sorting, organelle and vesicle trafficking, cell proliferation and signal transduction (1–5). We have demonstrated that non-erythroid α-spectrin (αIISp) is present in mammalian cell nuclei, where it plays an important role in repair of DNA interstrand cross-links (ICLs) and is critical for chromosome stability. It preferentially binds to DNA containing an ICL; it co-localizes with the ICL repair protein, XPF, in damage-induced nuclear foci after ICL damage; it is needed for the production of incisions produced by XPF/ERCC1 at sites of DNA ICLs; and depletion of αIISp in normal human cells by siRNA leads to chromosomal instability and cellular hypersensitivity to DNA ICL agents (6–10). We have proposed that αIISp acts as a scaffold and aids in the recruitment of repair proteins to the site of damage, enhancing the repair process and chromosome stability after DNA ICL damage (6,7,9).
An excellent model for studying the effects of a deficiency in αIISp is the genetic disorder, Fanconi anemia (FA), which is characterized by diverse congenital abnormalities, progressive bone marrow failure, chromosomal instability, a marked predisposition to develop cancer and a defect in ability to repair DNA ICLs (11–14). Of particular interest, cells from patients with FA have a deficiency in αIISp, with levels ranging from 35 to 40% of those found in normal cells, due to reduced stability of this protein, which we hypothesize is dependent on FA proteins (7,10,15–17). These reduced levels of αIISp in FA cells correlate with decreased cell survival, decreased DNA ICL repair and decreased chromosome stability after ICL damage (2,8,10,13,18,19). We have hypothesized that αIISp is critical for chromosome stability and that decreased levels of it in FA cells are a factor in the chromosome instability associated with this disorder (7,9,10).
Chromosome stability is also dependent on integrity of telomeres, which are specialized nucleoprotein structures at the ends of linear chromosomes that are critical for preserving genomic integrity by preventing chromosome ends from being treated as double-strand breaks (DSBs), thus preventing end-to-end fusions (20–25). Telomere dysfunction can be an important driving factor behind genomic instability (20–26). Human telomeres consist of tracts of multiple tandem repeats of the sequence, TTAGGG, that is bound by the telomere-specific multiprotein complex, shelterin, which helps protect telomeres and prevents telomere dysfunction (20,23,24,27). Because αIISp is critical for both repair of DNA ICLs and chromosome stability, whether it is also critical for maintenance of telomere stability and function, particularly after DNA ICL damage, is an extremely important question that is addressed in the present article. Studies were undertaken to examine whether αIISp localizes to telomeres, whether damaging cells with a DNA ICL agent influences this association and whether loss of αIISp in cells affects telomere function and stability after ICL damage. Using telomerase-positive normal human lymphoblastoid cells and these lymphoblastoid cells in which αIISp had been knocked down, we present a novel finding that a portion of αIISp in the nucleus localizes to telomeres after ICL damage and is associated with the telomere-specific proteins, TRF1 and TRF2. Involvement of αIISp in ICL repair in telomeres is demonstrated by our finding that it is needed for the recruitment of the DNA ICL repair protein, XPF, to damage-induced foci at telomeres, just as it is in genomic DNA. Our studies also suggest that the mechanism of repair of ICLs at telomeres in these normal cells may be different or modified from that in genomic DNA because FANCD2, a protein involved in ICL repair in genomic DNA (12,14,28,29), does not localize to telomeres in these cells after ICL damage.
Of particular significance in the present studies, loss of αIISp leads to telomere dysfunction after ICL damage, which is characterized by the presence of telomere dysfunction-induced foci (TIF), followed by dramatic loss of telomeres and production of sister chromatid end-to-end fusions. The physiological importance of αIISp loss is demonstrated by studies on FA complementation group A (FA-A) cells in which similar telomere dysfunction and catastrophic loss of telomeres is observed after ICL damage. Restoration of levels of αIISp to normal in FA-A cells corrects telomere dysfunction. These studies demonstrate for the first time a role for αIISp in telomere maintenance after ICL damage, which in turn is critical for chromosomal stability, and we propose that αIISp’s role in repair of DNA ICLs is important in this process.
MATERIALS AND METHODS
Cell culture and protein extracts
Normal human lymphoblastoid cells (GM3299) (Coriell Institute for Medical Research) and FA-A lymphoblastoid cells (HSC 72) (a gift from Dr. Manuel Buchwald, Toronto, Canada) were grown in RPMI 1640 medium (Hyclone) as previously described (6,15). Whole cell extracts were obtained by resuspending cells in an Extraction Buffer (Biovision Inc.) and following the manufacturer’s protocol.
Treatment of cells with ICL agents
siRNA-transfected normal and FA-A cells were treated with mitomycin C (MMC) (100–400 nM) (Sigma-Aldrich, Corp) or 8-methoxypsoralen (8-MOP) (3.5 µM) (Sigma-Aldrich) plus two doses of irradiation with UVA light 24 h after transfection, as previously described (7,9,10). Cells were collected 16 or 24 h after ICL treatment for further analysis.
Co-immunoprecipitation of proteins and immunoblot analysis
For examination of co-immunoprecipitation (IP) of TRF1 and TRF2 with αIISp, whole cell extracts from normal cells, either treated with MMC (400 nM) or untreated, were prepared. Anti-TRF1, anti-TRF2 (Santa Cruz Biotechnology)or rabbit IgG (Sigma-Aldrich Corp.) were bound to protein A-coated agarose beads (Sigma-Aldrich Corp.) and the binding reactions and IPs were carried out as previously described (6,10,15). The IPs were subjected to SDS-PAGE, transferred to nitrocellulose and immunoblotted as described (6,10,15). The primary antibody used was Anti-αIISp (mAb 1622, Chemicon). Immunoblots were developed using Pierce Ultra chemiluminescent substrate (Pierce, Thermo Scientific) and then exposed to X-ray film (6,15). Images were scanned using a Hewlett-Packard ScanJet 4c/T scanner and analyzed with ImageQuant (Molecular Dynamics).
For analysis of levels of αIISp, TRF1 and TRF2 in normal and FA-A cells, undamaged or damaged with MMC, whole cell lysates were subjected to SDS-PAGE and western blot analysis as previously described (6,10,15). Topoisomerase (Calbiochem) was used as a loading control. Immunoblots were probed with the antibodies described above.
siRNA transfection
siRNA against µ-calpain nucleotides (GUGAAGGAGUUGCGGACAA) and αIISp nucleotides (AAGAUUCCUAUCGAUUCCAGUUU) were purchased from Dharmacon and a control non-target (Nt) siRNA was from Qiagen. Normal cells were transfected with Nt or αIISp siRNA (100 pM) and FA-A cells were transfected with Nt or µ-calpain siRNA (300 pM) using Lipofectamine 2000 Transfection Reagent (Invitrogen Inc.) as previously described (9,10). Cell survival was assessed using trypan blue exclusion.
Cell synchronization using centrifugal elutriation
Normal cells were resuspended in 1× phosphate buffered saline (PBS) containing 5 mM EDTA. A Beckman JE-5.0 elutriation rotor (fed by a master flex pump) (Cole Parmer model 7520-25) was used to separate cells by centrifugal elutriation into G1, S and G2/M phase of the cell cycle. Cells were then stained with propidium iodide and analyzed using FACS Calibur (Becton Dickinson) and ModFit LT V3.1 software (Verity Software House).
Metaphase spreads and chromosome analysis
Normal cells transfected with either αIISp or Nt siRNA were incubated for 24 h at 37°C, 5% CO2, and then treated with MMC (100 nM) and incubated for an additional 24 h. Colcemid (0.1 µg/ml) (Sigma-Aldrich Corp.) was added 22 h after MMC and incubation continued for 2 h. The cells were harvested, swollen in hypotonic solution, fixed and slides stained with Giemsa as previously described (9,10). At least 100 metaphases from each group were scored for chromosomal aberrations. Metaphases were viewed using a Leitz DMRB microscope (Leica) equipped with a DEI-750 analog camera (Optronics) at 40×. Images were imported into a computerized imaging system using Image Pro-Plus 6.0 (Media Cybernetics).
Telomere FISH on metaphase spreads
Normal cells transfected with Nt or αIISp siRNA and FA-A cells transfected with Nt or µ-calpain siRNA were either undamaged or treated with MMC and incubated as above. Metaphase spreads were prepared as described above and chromosomes stained with a telomere-specific Cy3-labeled peptide nucleic acid (PNA) oligonucleotide probe with the DNA sequence [CCCTAA]3 (Panegene) as described (30,31). Briefly, slides with the metaphase spreads were dehydrated by consecutive incubations in 70, 95 and 100% ethanol. After air-drying, hybridizing solution containing the PNA probe was added and spreads were denatured for 3 min at 80°C. Spreads were hybridized for 2 h in a humidified chamber in the dark at room temperature and then washed in 70% formamide, 10 nM Tris–HCl and 0.1% bovine serum albumin (BSA), followed by two washes in 0.1 M Tris–HCl, 0.15 M NaCl and 0.08% Tween-20. After air-drying, Slowfade antifade reagent (Invitrogen) containing DAPI to counterstain the chromosomal DNA was added to the spreads. At least 4600 chromosomes from each group were scored for the presence of telomeric signals on each of the chromatids in five independent experiments. Chromosomes were viewed using a Zeiss Axiovert 200 M (Carl Zeiss international) microscope at 100×. Images were imported into a computerized imaging system using AxioVision software (Carl Zeiss International).
Immunofluorescence and immunoFISH
Normal and FA-A cells, either non-transfected or transfected with the siRNAs described above, were treated with 400 nM MMC 24 h after transfection. The cells were harvested 16 h post damage and examined for nuclear localization of aIISp, TRF1, TRF2, XPF and FANCD2 using immunofluorescence as previously described (7,9,10). For immunoFISH, cells were processed for immunofluorescence analysis as described (7,9,10), stained with PNA probe (Panagene) to detect telomeric DNA and counterstained with DAPI for localization of chromosomal DNA, as described (30,31). Briefly, cells were fixed in 4% paraformaldehyde at room temperature, permeabilized with 0.2% Triton-X and then incubated in blocking solution (10% goat serum or 4% BSA) for 1 h followed by a 1 h incubation with anti-α-spectrin (mAb 1622, Chemicon), anti-XPF (Santa Cruz Biotechnology) or anti-FANCD2 (Novus Biologicals). After washes with PBS, a secondary antibody was added, Alexafluor 488 goat anti-mouse IgG conjugate or Alexafluor 594 donkey anti-goat IgG conjugate (Molecular Probes, Invitrogen). The cells were then incubated with anti-TRF1 or anti-TRF2 (Santa Cruz Biotech), washed in PBS and a secondary antibody applied, Alexafluor 594 goat anti-rabbit IgG conjugate (Molecular Probes, Invitrogen). Samples were mounted on slides with Slowfade antifade reagent (Molecular Probes, Invitrogen) containing DAPI. TIF analysis was performed as described (30,31). Briefly, cells were treated and prepared as described above using a primary antibody against γH2AX (Upstate Biotechnology) and secondary antibody Alexafluor 488 goat anti-mouse IgG conjugate. Slides were then incubated in hybridization buffer at 85°C for 5 min, followed by 2 h in the dark at room temperature in a humidified chamber as described above.
Images were acquired using a Zeiss Axiovert 200 M epifluorescence microscope (Carl Zeiss international). Image processing was carried out with AxioVision Rel 4.6.3 software (Zeiss). To analyze and quantitate the number of nuclear foci, images were acquired as z-stacks using a × 63/1.4 oil immersion lens (32). In each z-stack, six to eight optical slices were counted for each cell, and the total number of nuclear foci in all of the optical slices in each cell was quantitated. Three hundred cells were counted in each experiment, and each experiment was independently repeated five times. Stacks were separated by 0.2–0.5 µm.
RESULTS
Knock-down of αIISp in normal human cells leads to an increase in chromosomal aberrations (sister chromatid end-to-end fusions and breaks) after damage with a DNA ICL agent
We previously showed that knock-down of αIISp by siRNA leads to chromosome instability and a deficiency in DNA ICL repair (9). We now asked what the impact of loss of αIISp is on formation of chromosomal aberrations after damage with a DNA ICL agent. Normal human lymphoblastoid cells were transfected with αIISp siRNA or Nt siRNA. Because complete loss of αIISp from cells is lethal (9,33,34), we knocked down αIISp to levels that were 40% of normal, where cell survival was ∼85% of normal (9). These are similar to levels of αIISp found in FA-A cells (7,15). After 24 h, cells were either mock treated or treated with MMC. Metaphase spreads from cells in which αIISp had been knocked down but not treated with MMC showed an increase in chromosomal aberrations to 4.6 per metaphase compared with 0.2 in Nt siRNA-transfected normal cells (Figure 1A and B and Supplementary Table S1). This is similar to the level of spontaneous chromosomal aberrations reported for FA-A and other FA cell lines (35,36). As ICLs can arise endogenously in cells from processes of cellular metabolism, such as lipid peroxidation (37–39), these chromosomal aberrations in undamaged cells may be a result of failure to repair these lesions. After the cells were treated with MMC, however, there was a further and significant increase (P < 0.0001) in chromosomal aberrations in αIISp siRNA-treated cells to 12 aberrations per metaphase (Figure 1B and Supplementary Table S1); 100% of these cells showed chromosomal aberrations. In contrast, in Nt siRNA-treated normal cells after MMC, this number increased to only 1.4 aberrations per metaphase (Figure 1B and Supplementary Table S1). In MMC-treated cells in which αIISp had been knocked down, the highest proportion (65%) of these chromosome aberrations were sister chromatid end-to-end fusions (Figure 1B), 17% were chromatid breaks and the rest were chromosome end-to-end fusions and exchanges/radials (Supplementary Table S1). These findings indicate that knock-down of αIISp in normal human cells and their subsequent damage with a DNA ICL agent leads to a significant increase in chromosome instability as evidenced by the significantly increased chromosomal aberrations, which were observed.
Figure 1.
Knock-down of αIISp in normal cells by siRNA leads to an increase in chromosomal aberrations after DNA ICL damage. (A) Normal cells were transfected with Nt siRNA or αIISp siRNA (100 pM) and subsequently treated (24 h post transfection) with MMC (100 nM) for 24 h. Metaphase spreads were prepared and examined for chromosomal aberrations. Arrows indicate sister chromatid end-to-end fusions (arrow), breaks (bold arrow) and exchanges (hollow arrow). Side panels show higher magnification images. (B) One hundred metaphase spreads were scored for chromosomal aberrations, and the average number of sister chromatid end-to-end fusions and total chromosomal aberrations per metaphase from three independent experiments are shown. Student’s t-test was used to calculate statistical significance. Error bars: SEM ***P < 0.0001.
αIISp is present at telomeres and co-localizes and physically associates with TRF1 and TRF2 after ICL damage
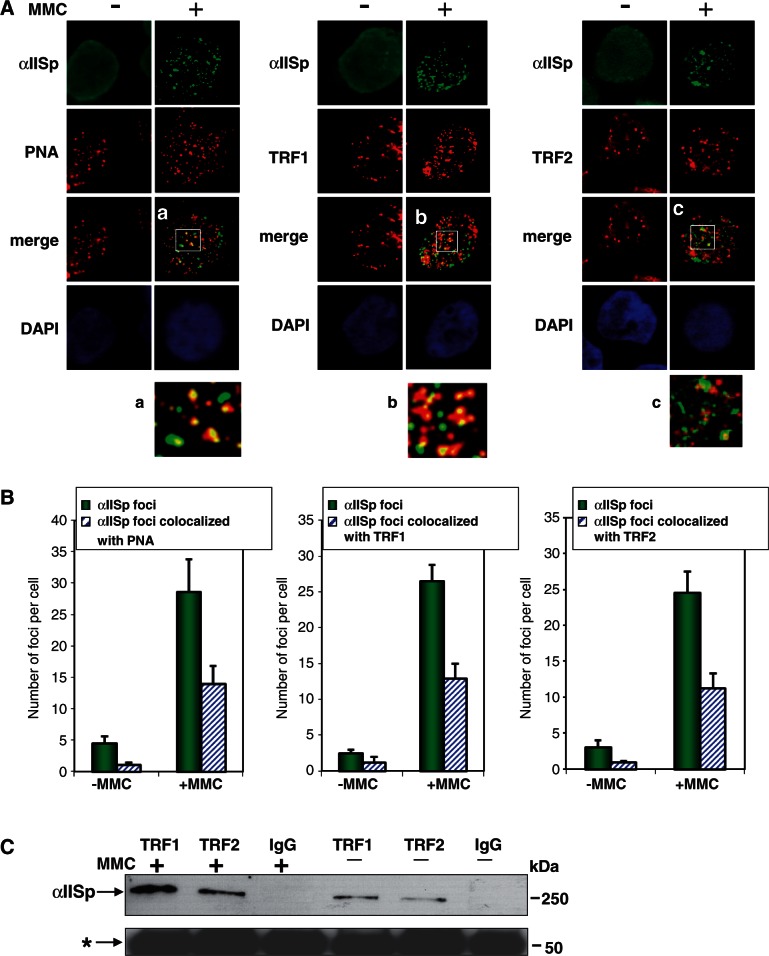
Telomeres are essential for chromosome stability, and telomere dysfunction can be an important driving factor in chromosome instability (20–24). To determine whether αIISp may have a role in telomere function, studies were undertaken to determine whether αIISp associates with telomeres and whether this association is influenced by DNA ICL damage. Immunofluorescent staining against αIISp in conjunction with fluorescence in situ hybridization (FISH) using a telomeric Cy3-conjugated peptide-nucleic acid (PNA) probe (immunoFISH) was carried out. Quantitation of co-localization of αIISp with the PNA probe was based on the total number of foci co-localizing in each cell (Figure 2B). αIISp had a diffuse pattern of localization in the nucleus in undamaged cells (Figure 2A). However, 16 h after MMC treatment, αIISp localized in discrete nuclear foci (Figure 2A), as we have previously shown and which are presumed to be at sites of DNA ICLs (7,9,10). Almost half (49%) of these foci co-localized with the PNA probe as determined by counting the number of yellow foci in the merged images of αIISp and PNA foci (Figure 2A and B). The images shown in Figure 2 represent only one optical slice from a cell. A 16 h time point was chosen because we have previously shown that at this time after ICL damage (MMC or 8-MOP plus UVA) the maximum number of αIISp foci appear (7). We then examined whether αIISp associated with two of the proteins of the shelterin complex bound to telomeres, TRF1 and TRF2, and the effects of DNA ICLs on this association. ImmunoFISH analysis using immunostaining for TRF1 (red) or TRF2 (red) showed that 16 h after MMC treatment, a portion of αIISp co-localized with TRF1 and TRF2 (Figure 2A): 49% of the αIISp foci co-localized with TFR1 foci and 46% with TRF2 foci (Figure 2B). Similar results were obtained when the co-localization of αIISp with the PNA probe and TRF1 and TRF2 foci were examined using a different DNA ICL agent, 8-MOP plus UVA light (Supplementary Figure S1). Neither MMC nor 8-MOP plus UVA light damage had an effect on the number of TRF1 or TRF2 foci observed at telomeres or on the cellular levels of TRF1 or TRF2 as determined by western blot and comparison with the loading control, topoisomerase (Supplementary Figure S2). These results indicate that αIISp co-localizes and physically associates with telomeric DNA and with TRF1 and TRF2 at telomeres after ICL damage.
Figure 2.
αIISp associates with telomeres after DNA ICL damage. (A) Co-localization of αIISp with telomeric DNA, TRF1 and TRF2 in normal lymphoblastoid cells was examined 16 h after MMC (400 nM) treatment using immunoFISH and staining with anti-αIISp (green), anti-TRF1 (red) or anti-TRF2 (red) antibodies or a Cy3-labeled telomere-specific PNA probe (red). Nuclear DNA was counterstained with DAPI (blue). Pictures were taken by z-stack. Only one optical slice is displayed. Magnified images of αIISp co-localization (a) PNA probe, (b) TRF1 and (c) TRF2 are shown. (B) The number of αIISp nuclear foci per cell and αIISp nuclear foci co-localized with PNA, TRF1 or TRF2 per cell in normal cells before and after MMC treatment was quantitated. Three hundred cells were counted in each group. Error bars represent SEM. (C) αIISp co-immunoprecipitates with TRF1 and TRF2 after DNA ICL damage. Normal cells were either untreated or treated with MMC (400 nM). Co-immunoprecipitation of αIISp with TRF1 and TRF2 from whole cell extracts was examined by western blot analysis. αIISp was immunoprecipitated with anti-TRF1 or anti-TRF2 antibodies or rabbit IgG1. Immunoblots was probed with anti-αIISp antibody. The IgG1 heavy chain (*) was used as a loading control. Molecular weight markers are indicated to the right.
Co-immunoprecipitation studies were also undertaken to ascertain whether αIISp interacts with TRF1 or TRF2 in vivo. The results showed that αIISp had some association with TRF1 and TRF2 in extracts from undamaged normal human cells (Figure 2C), and that this association was greatly enhanced after cells were exposed to MMC. This suggests that after exposure of cells to a DNA ICL agent, a portion of αIISp in the nucleus co-localizes with TRF1 and TRF2 at telomeres.
αIISp localizes to telomeres in S phase after ICL damage
To investigate whether the localization of αIISp foci to telomeres after ICL damage occurred during any specific phase of the cell cycle, normal cells were damaged with MMC and separated into phases of the cell cycle using centrifugal elutriation. Cell cycle progression was analyzed by fluorescence-activated cell sorting. Clean separation of cells into G1, S and G2/M was observed (Figure 3). Localization of αIISp to telomeres was examined by immunoFISH. The results showed that after MMC treatment, αIISp formed foci and co-localized with TRF1 during S phase (Figure 3). As telomeres have been shown to replicate during S phase (40), these results suggest that after ICL damage, αIISp associates with telomeres when they are replicating.
Figure 3.
αIISp specifically associates with telomeres (TRF1) after ICL damage in S phase of the cell cycle. Normal cells were treated with MMC (400 nM) for 16 h and separated by centrifugal elutriation into G1, S and G2/M phase of the cell cycle. Cell cycle distribution is shown on the left panel. Formation of αIISp (green) and TRF1 (red) foci and their co-localization was examined by immunoFISH (right panel). Pictures were taken by z-stack. A magnified image of αIISp and TRF1 co-localization is shown to the right.
Knock-down of αIISp in normal cells results in loss of localization of αIISp to telomeres after ICL damage similar to that observed in FA-A cells in which there are similarly reduced levels of αIISp
We have previously shown that knock-down of αIISp by siRNA results in loss of αIISp foci in the nuclei of normal cells after MMC damage (9). The present studies show that 16 h after MMC treatment, there is also a loss of localization of αIISp to telomeres in the nuclei of normal cells transfected with αIISp siRNA (Figure 4A and C). In contrast, we saw normal co-localization of αIISp foci with TRF1 foci at telomeres in MMC-treated Nt siRNA cells (Figure 4A). As noted above, as complete loss of αIISp is lethal to cells, levels of αIISp knock-down were adjusted to 40% of normal, which were similar to the reduced levels of αIISp we have found in FA cells (9,10,15). At these levels, cell survival was 85% of normal (9). Examination of FA-A cells, in which levels of αIISp are 35–40% of normal, showed that 16 h after MMC treatment, there were few αIISp foci localized to TRF1 at the telomeres (Figure 4A).
Figure 4.
Knock-down of αIISp in normal cells leads to loss of localization of αIISp to telomeres similar to that observed in FA-A cells. (A) Normal cells, transfected with either Nt siRNA or αIISp siRNA, and FA-A cells were treated with MMC (400 nM). Co-localization of αIISp with TRF1 was examined 16 h after MMC treatment using immunoFISH and staining with anti-αIISp (green) and anti-TRF1 (red) antibodies. Nuclear DNA was counterstained with DAPI (blue). Pictures were taken by z-stack. Only one optical slice is displayed. A magnified image of co-localization of αIISp with TRF1 in MMC-treated Nt siRNA-transfected normal cells is shown to the right. (B) Knocking down µ-calpain (µ-cal) in FA-A cells restores levels of αIISp and localization of αIISp to telomeres after MMC treatment. FA-A cells were transfected with either Nt siRNA or µ-calpain siRNA and subsequently treated with MMC (400 nM). Co-localization of αIISp with TRF1 nuclear foci at telomeres was examined, as above, 16 h after MMC treatment. A magnified image of co-localization of αIISp with TRF1 in MMC-treated µ-calpain siRNA-transfected FA-A cells is shown to the right. (C) The number of αIISp nuclear foci per cell and αIISp nuclear foci that co-localized with TRF1 foci before and after MMC treatment in normal and FA-A cells was quantitated. Bar diagrams represent mean values of five independent experiments. Three hundred cells were counted in each group in each experiment. Error bars represent SEM.
Knocking down αIISp in normal cells had no effect on the total cellular levels of TRF1or TRF2, as determined by western blot analysis (Supplementary Figure S3), nor did it lead to a reduction of telomeric TRF1 or TRF2 or the PNA probe at telomeres in cells 16 h after MMC treatment as shown for TRF1 in Figure 4A and the PNA probe in Figure 5A. Similarly in FA-A cells, in which αIISp levels are reduced, levels of total cellular TRF1 and TRF2 were similar to normal in both MMC-damaged and -undamaged cells, using topoisomerase as a loading control (Supplementary Figure S2); there was also no reduction in telomeric TRF1 or TRF2 (Figure 4A) or PNA probe (Figure 6). This indicates that αIISp is not needed for association of TRF1 or TRF2 with the telomeres in either damaged or undamaged cells.
Figure 5.
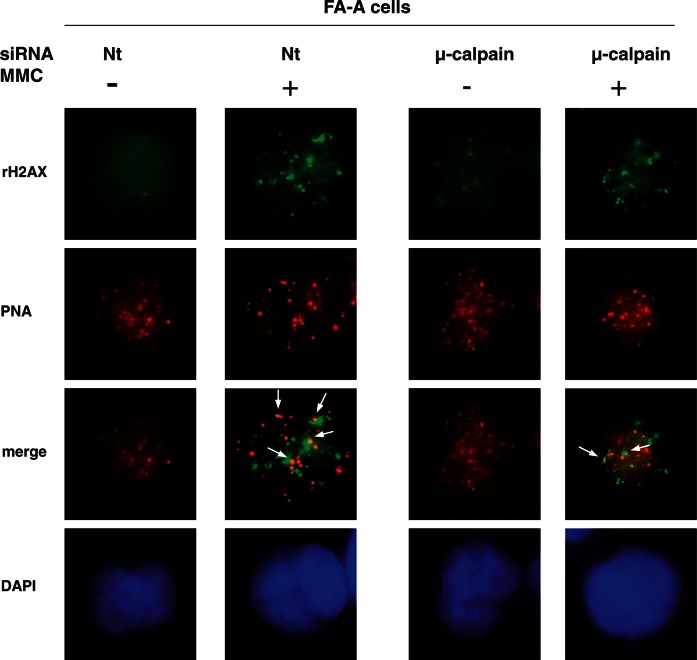
Loss of αIISp in normal cells leads to enhanced formation of telomere dysfunction-induced DNA foci (TIFs) after ICL damage. (A) Normal cells, transfected with either Nt siRNA or αIISp siRNA were treated with MMC (400 nM). Co-localization of TIF (γH2AX foci) with the PNA probe was examined 16 h after MMC treatment using immunoFISH and staining with anti-γH2AX antibody (green) and a Cy3-labeled telomere-specific PNA probe (red). Nuclear DNA was counterstained with DAPI (blue).Pictures were taken by z-stack. Only one optical slice is displayed. Arrows points to co-localizationof γH2AX with the PNA probe. (B) The percentage of TIF-positive cells containing ≥5 telomeric γH2AX foci was quantitated. Three hundred cells were scored for TIFs, and the results represent the mean of five independent experiments. Student’s t-test was used to calculate statistical significance. Error bars: SEM ***P < 0.0001.
Figure 6.
In FA-A cells, TIF formation is increased after MMC treatment, and after knock-down of µ-calpain in these cells, it is decreased. FA-A cells, transfected with either Nt siRNA or µ-calpain siRNA, were treated with MMC (400 nM). Formation of TIF (γH2AX foci) and their co-localization with a PNA probe was examined 16 h after MMC treatment using immunoFISH and staining with anti-γH2AX antibody (green) and a Cy3-labeled telomere-specific PNA probe (red). Nuclear DNA was counterstained with DAPI (blue). Pictures were taken by z-stack. Only one optical slice is displayed. Arrows point to co-localization of γH2AX with the PNA probe.
We have previously shown that levels of αIISp in FA-A cells and formation of αIISp nuclear foci in these cells after ICL damage can be restored to normal when µ-calpain, a protease that cleaves αIISp, is knocked down (10). In the present study, restoration of levels of αIISp in FA-A cells to normal by µ-calpain siRNA also led to the co-localization of αIISp with TRF1 at telomeres 16 h after MMC treatment (Figure 4B). Approximately 47% of the αIISp foci co-localized with TRF1 foci in the MMC-treated FA-A cells after µ-calpain knock-down, similar to the percentage of αIISp foci (49%) co-localizing with TRF1 in normal cells after MMC treatment (Figure 4C). These results thus show that, in FA-A cells, a deficiency in αIISp results in loss of localization of αIISp to telomeres after MMC damage and that restoration of αIISp levels to normal leads to localization of a portion of αIISp to telomeres after MMC damage, just as in normal cells. µ-Calpain knock-down had no effect on localization of TRF1 or TRF2 to telomeres in undamaged or MMC-damaged FA-A cells (Figure 4B) or on cellular levels of TRF1 or TRF2, as determined by western blot analysis and comparison with the loading control, topoisomerase (Supplementary Figure S4).
Loss of αIISp leads to telomere dysfunction and formation of TIF, especially after ICL damage
To assess whether loss of αIISp leads to telomere dysfunction, normal cells, in which αIISp had been knocked down, and FA-A cells were examined for the presence of γH2AX foci at telomeres. γH2AX foci are markers for DNA DSBs and have been used as an indicator of dysfunctional telomeres (30,41–43). Accumulation of γH2AX signals at telomeres (TIFs) was determined by examination of γH2AX foci that overlapped with telomeres and were identified by immunoFISH using γH2AX antibodies and a telomere-specific probe (Figure 5A). Cells were considered TIF positive if they contained five or more γH2AX foci that co-localized with the telomere PNA probe. These foci were imaged in consecutive z-planes and at shallow z-section to minimize coincidental overlap. Quantitation of TIFs showed that the fraction of TIF-positive Nt siRNA-transfected normal cells was 3% (Figure 5B). After knock-down of αIISp, the percentage of TIF-positive normal cells increased to 10%. Treatment of αIISp siRNA-transfected cells with MMC, however, lead to a significant increase (P < 0.0001) in TIF formation, with the percentage of TIF-positive cells increasing to 47% (Figure 5A and B). This was significantly greater (P < 0.0001) than the percentage of TIF-positive cells in MMC-treated Nt siRNA-transfected normal cells (Figure 5B). Thus, αIISp knock-down in normal cells leads to telomere dysfunction after ICL damage as evidenced by a significant increase in TIF-positive cells.
FA-A cells were also examined for telomere dysfunction after ICL damage because levels of αIISp in these cells are 35% of normal (7,15). In undamaged FA-A cells, the percentage of TIF-positive cells (11%) was similar to that observed in normal cells in which αIISp had been knocked down (Figures 5B and 6). After MMC damage, the number of TIF-positive Nt siRNA-transfected FA-A cells significantly increased (P < 0.0001) to 45%, which was similar to the percentage of TIF-positive normal cells after transfection with αIISp siRNA and treatment with MMC. To determine whether restoration of levels of αIISp in FA-A cells could result in a decrease in TIF formation after MMC damage, levels of αIISp were restored to normal by knocking down µ-calpain. In MMC-treated FA-A cells in which µ-calpain had been knocked down, there was a significant (P < 0.0001) decrease in the number of TIF-positive cells; 23% of the cells were now TIF positive, which is similar to the percentage of normal human cells (26%), which were TIF positive after MMC damage (Figures 5B and 6). These data suggest that increased TIF formation observed in FA-A cells after MMC damage is due to loss of αIISp in these cells and that restoration of levels of αIISp to normal reduces TIF formation to levels similar to normal.
αIISp deficiency leads to a catastrophic loss of telomeres after DNA ICL damage
Along with the presence of TIFs and chromatid end-to-end fusions, another indicator of telomere dysfunction is loss of telomeres or signal-free ends (SFEs). To determine whether depletion of αIISp leads to telomere loss and whether MMC damage has an effect on this, normal cells in which αIISp had been knocked down by siRNA were either mock-treated or damaged with MMC and metaphase chromosomes examined by FISH using a PNA probe. The incidence of telomere loss per chromosome, quantified as frequency of SFEs, was determined. The frequency of SFEs per chromosome in normal cells after αIISp siRNA was 0.7, which was greater than the 0.08 observed in Nt siRNA-treated normal cells (Figure 7A and B). However, after 24-h treatment with MMC, the frequency of SFEs per chromosome in normal cells after αIISp knock-down significantly increased (P < 0.0001) to three SFEs per chromosome compared with the one SFE observed in MMC damaged Nt siRNA-transfected cells (Figure 7A and B). The data are the mean values of five independent experiments in which 4600 chromosomes in 100 metaphases were counted in each experiment.
Figure 7.
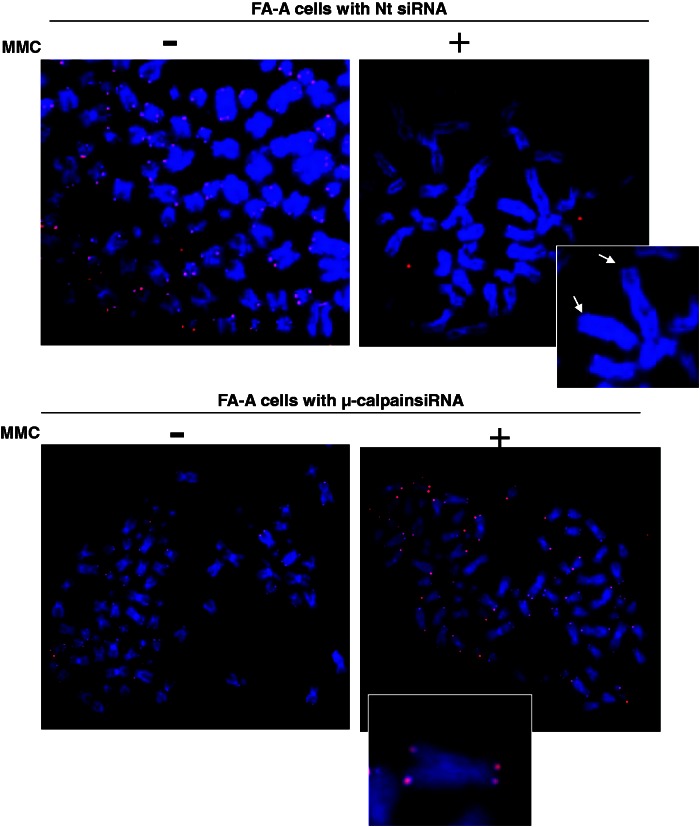
αIISp deficiency leads to enhanced loss of telomeres after ICL damage. (A) Normal cells were transfected with either Nt siRNA or αIISp siRNA and subsequently treated with MMC (400 nM) for 24 h. Metaphase spreads were prepared and chromosomes stained with DAPI (blue). Telomeric DNA was detected by FISH with a Cy3-labeled telomere-specific PNA probe (red). Inserted panels show magnified images of metaphase chromosomes. Arrowheads point to telomere SFEs. (B) Frequency of SFEs per chromosome was quantitated. Averages are shown of five independent experiments in which 4600 chromosomes were counted per experiment. Student’s t-test was used to calculate statistical significance. Error bars: SEM ***P < 0.0001.
As FA-A cells have a deficiency in αIISp, it was of particular interest to examine whether they show an increase in SFEs on chromosomes following ICL damage. In undamaged FA-A cells, the frequency of SFEs per chromosome was 0.9, which was significantly greater (P < 0.0001) than observed in undamaged Nt siRNA-transfected normal cells and was similar to that observed in undamaged αIISp siRNA-transfected cells (Figures 7B and 8). After MMC treatment for 24 h, this frequency significantly increased (P < 0.0001) to three SFEs per chromosome (Figure 7B), which was similar to the frequency of SFEs in MMC-treated normal cells in which αIISp had been knocked down. To ascertain whether reduction in levels of αIISp in FA-A cells could be a factor in telomere loss after MMC, levels of αIISp were restored to normal by knock-down of µ-calpain. This resulted in a significant decrease (P < 0.0001) in the frequency of SFEs per chromosome after MMC to ∼1, which was similar to the frequency of SFEs observed in MMC-damaged normal cells (Figures 7B and 8). These results thus indicate that αIISp depletion, due to either knock-down by siRNA or to reduction as is found in FA-A cells, leads to catastrophic loss of telomeres after MMC damage. Thus, collectively these studies indicate that loss of αIISp in cells leads to telomere dysfunction after ICL damage.
Figure 8.
In FA-A cells, loss of telomeres is enhanced after damage with MMC, and this loss is corrected after knock-down of µ-calpain. FA-A cells were transfected with Nt siRNA or µ-calpain siRNA and subsequently treated with MMC (400 nM) for 24 h. Metaphase spreads were prepared and chromosomes stained with DAPI (blue). Telomeric DNA was detected by FISH with a Cy3-labeled telomere-specific PNA probe (red). Inserted panels show magnified images of metaphase chromosomes. Arrowheads point to telomere SFEs.
αIISp is required for localization of the ICL repair protein, XPF, to telomeres after ICL damage
Telomere dysfunction after ICL damage in cells in which there is a loss of αIISp could be due to failure to repair ICLs at telomeres. We have previously shown that αIISp is involved in repair of DNA ICLs (6–10). Another protein demonstrated to play a role in ICL repair is the structure-specific endonuclease, XPF/ERCC1 (44–50). XPF/ERCC1 has also been shown to be important in regulation of telomere function and integrity; ∼1% of nuclear XPF associates with TRF2 at telomeres; the rest is dispersed throughout the nucleus (51–54). Whether XPF plays a role in the DNA damage response in telomeres after DNA ICL formation is not known. We have shown that αIISp associates with XPF/ERCC1 (55). We have also demonstrated that αIISp is needed for the localization of XPF to damage induced nuclear foci in normal cells after ICL formation and that when there is a deficiency in αIISp, as occurs in FA cells or in normal cells after αIISp knock-down, XPF foci do not form after ICL damage (7,9,10). We now show that αIISp is also needed for the localization of XPF to damage-induced foci at telomeres after ICL damage. In normal cells, XPF is present in a diffuse pattern throughout the nucleus (Figure 9A and 10A). After treatment with MMC, immunoFISH showed that a subset of XPF signals co-localized with the PNA probe as well as with TRF1 and TRF2 at telomeres (Figure 9A–D). Here, 37, 43 and 50% of the XPF foci formed after MMC damage co-localized with TRF1, TRF2 and the PNA probe, respectively (Figure 9B–D). This proportion of XPF co-localizing with telomeres compared with non-telomeric DNA 16 h after ICL damage could reflect a longer persistence of XPF foci at sites of damage due to a longer repair time. The same could hold true for αIISp. However, by 24 h after ICL damage, we have previously shown that no αIISp or XPF foci are visible, indicative of repair having taken place (7). Another possibility is that ICLs preferentially occur in telomeric DNA, hence the increased localization of αIISp and XPF at telomeres. It is also possible that there is enhanced recruitment of αIISp and XPF to ICL-damaged telomeres.
Figure 9.
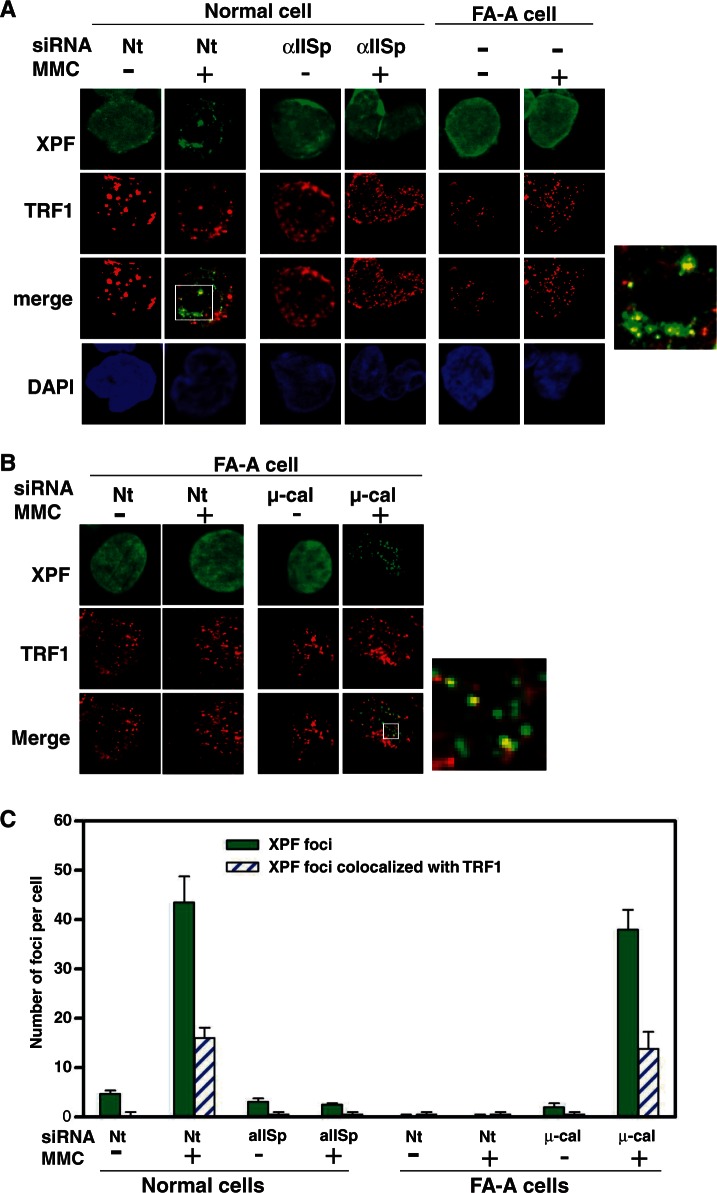
XPF localizes with telomeres after DNA ICL damage. Co-localization of XPF with telomeric DNA, TRF1 and TRF2 in normal cells was examined 16 h after MMC (400 nM) treatment using immunoFISH. (A) Cells were stained with anti-XPF (green) and anti-TRF1 (red) antibodies. Pictures were taken by z-stack. Only one optical slice is displayed. A magnified image of co-localization of XPF with TRF1 after MMC treatment is shown on the right. The number of XPF nuclear foci per cell and XPF nuclear foci per cell co-localizing with (B) TRF1, (C) the PNA probe and (D) TRF2 in normal cells before and after MMC treatment was quantitated. Three hundred cells were counted in each group. Error bars represent SEM.
Figure 10.
Knock-down of αIISp in normal cells leads to loss of localization of XPF to telomeres just as is observed in FA-A cells. (A) Normal cells, transfected with either Nt siRNA or αIISp siRNA and FA-A cells were treated with MMC (400 nM). Co-localization of XPF with TRF1 was examined 16 h after MMC treatment using immunoFISH and staining with anti-XPF (green) and anti-TRF1 (red) antibodies. Nuclear DNA was counterstained with DAPI (blue). Pictures were taken by z-stack. Only one optical slice is displayed. A magnified image of co-localization of XPF with TRF1 in MMC-treated Nt siRNA-transfected normal cells is shown to the right. (B) Knocking down µ-calpain (µ-cal) in FA-A cells restores localization of XPF to telomeres after MMC treatment. FA-A cells were transfected with either Nt siRNA or µ-calpain siRNA and subsequently treated with MMC (400 nM). Co-localization of XPF with TRF1 nuclear foci at telomeres was examined, as above, 16 h after MMC treatment. A magnified image of co-localization of XPF with TRF1 in MMC-treated µ-calpain siRNA-transfected FA-A cells is shown to the right. (C) The number of XPF nuclear foci per cell and XPF nuclear foci that co-localized with TRF1 foci before and after MMC treatment in normal and FA-A cells was quantitated. Bar diagrams represent mean values of five independent experiments. Three hundred cells were counted in each group in each experiment. Error bars represent SEM.
We then examined whether αIISp is required for the co-localization of XPF with telomeres. In cells in which αIISp had been knocked down and which were treated with MMC, XPF was present in a diffuse pattern throughout the nucleus and formed few foci; none were detectable co-localizing with TRF1 (Figure 10A). As we have previously shown, knocking down αIISp had no effect on the levels of XPF in these cells (9). The importance of αIISp in the localization of XPF to telomeres after MMC damage was further highlighted by the demonstration that in FA-A cells, in which levels of αIISp are significantly reduced, XPF was found in a diffuse pattern in the nucleus, few XPF foci were observed and there was no co-localization of XPF with TRF1 (Figure 10A). However, when levels of αIISp were restored to normal in FA-A cells by knock-down of µ-calpain, XPF formed nuclear foci and a subset of these signals co-localized with TRF2 at telomeres after MMC treatment (Figure 10B). Thirty-six percent of XPF foci co-localized with TRF1 foci, which is similar to the levels of co-localization found in normal cells after MMC damage (Figure 10C). These results indicate that αIISp is required for localization of XPF to telomeres after ICL damage and suggest that XPF may be important in the repair process and possibly in telomere stability after ICL damage.
FANCD2 does not localize to telomeres after ICL damage
A protein shown to be important in DNA ICL repair in genomic DNA is FANCD2, a component of the FA pathway, which resolves ICLs encountered during replication (14,28,29). Studies were carried out to determine whether FANCD2 localized to telomeres after ICL damage. In undamaged normal cells, some spontaneous FANCD2 foci formed (Supplementary Figure S5A and B), as has been previously shown (28,56). After treatment of these lymphoblastoid cells with MMC, immunoFISH showed that there was a significant increase (P < 0.0001) in the number of FANCD2 foci that formed in the nucleus 16 h after treatment compared with the number of foci in undamaged cells (Supplementary Figure S5A and B). However, only 10% of these foci localized with the PNA probe at telomeres (Supplementary Figure S5A and B). This suggests that FANCD2 is not involved in repair of ICLs at telomeres in these lymphoblastoid cells, in contrast to its demonstrated involvement in ICL repair in genomic (non-telomeric) DNA (12,14,28,29,56). In FA-A cells, FANCD2 did not form nuclear foci after ICL damage (Supplementary Figure S5A).
DISCUSSION
We have previously shown that αIISp is present in the nucleus of normal human cells, where it plays an important role in the repair of DNA ICLs and chromosome stability (6–10). The present studies demonstrate that αIISp is particularly important in maintaining chromosome stability after DNA ICL damage, as evidenced by our data, which show that in normal cells, after knock-down of αIISp by siRNA and subsequent damage with a DNA ICL agent, MMC, there is a significant increase in number of chromosomal aberrations, the majority of which are sister chromatid end-to-end fusions. These chromosomal aberrations could be related to a defect in repair of DNA ICLs.
Telomeres are critical structures which, with their unique complex of associated proteins, maintain chromosome stability by protecting the ends of chromosomes; loss of telomeres can lead to sister chromatid end-to-end fusions and to genomic instability (20,22–26). As our studies show that αIISp is critical for chromosome stability, particularly after ICL damage, whether it plays a role in maintenance of telomere function is an important question and one which is addressed in the present study. We have previously demonstrated that after cells are damaged with a DNA ICL agent, either MMC or 8-MOP plus UVA light, αIISp localizes to foci in the nucleus, presumably at sites of ICLs on DNA and that foci formation is maximum at 16 h after ICL damage, after which time the number of foci decrease, presumably as repair takes place, and are no longer visible by 24 h post damage (7,9). The present results present a novel finding that, at 16 h after ICL damage (either MMC or 8-MOP plus UVA light), a portion of these αIISp foci localize to telomeres, co-localizing with two telomere-specific proteins in the shelterin complex, TRF1 and TRF2. αIISp also had enhanced affinity for TRF1 and TRF2 after cells were damaged with MMC. TRF1 and TRF2 have been demonstrated to interact with or recruit specific proteins important for telomere function, including those known to act in DNA repair reactions and DNA damage signaling (20,57–60). These interactions can be transient as required for specific telomere functions (60). It is possible that after ICL damage, TRF1 and TRF2 recruit αIISp to the telomeres, where it is involved in the repair of the ICLs formed in telomeric DNA. αIISp may also be localizing directly to the site of an ICL in the telomeric DNA. We have previously demonstrated that αIISp binds directly to DNA containing a 8-MOP plus UVA light ICL (6). Human telomeres contain long tandem repeats of TTAGGG sequences. These sequences are potential targets for ICL formation by photoactivated 8-MOP, which preferentially forms ICLs at TA sequences on DNA (61,62). Studies on human fibroblasts suggest that 8-MOP plus UVA irradiation causes ICL formation at telomeres (63). Repair of ICLs at telomeres, particularly at the time of replication, could be critical for telomere maintenance and function. The kinetics and mechanism of ICL repair at telomeres, however, have not been examined and to date are unknown.
αIISp may play an important role in repair of DNA ICLs at telomeres. It could act as a scaffold in recruitment of repair proteins to the sites of damage. In support of this view, the present results show that after ICL damage, αIISp is needed for the recruitment of XPF to telomeres. Loss of αIISp from cells, such as in normal cells after αIISp knock-down or in FA-A cells, results in failure of XPF to localize to telomeres after ICL damage. Restoration of levels of αIISp to normal in FA-A cells reverses this. These studies thus suggest that αIISp plays an important role in the repair process at telomeres through its recruitment of DNA repair factors such as XPF to telomeres after ICL damage similar to its role in recruitment of XPF to ICL-induced foci in genomic DNA (7).
In mammalian cells, there is no consensus as to the exact sequence of events involved in ICL repair in genomic DNA and all of the proteins involved in this process; however, the FA pathway has been shown to play an important role in the resolution of ICLs encountered during DNA replication in genomic DNA (14,29,56). A major protein involved in this pathway is FANCD2 (12,14,28,29). Although in the present study there was a significant increase in association of FANCD2 foci with genomic (non-telomeric) DNA after MMC treatment, FANCD2 foci did not localize to any extent with telomeres after ICL damage. This suggests that FANCD2 is not involved in ICL repair of telomeric DNA in these cells, as it is of genomic DNA. The lymphoblastoid cells used in these studies express telomerase. In human cells, telomeres are maintained and chromosome ends extended during DNA replication either by a pathway in which there is expression of telomerease, a ribonucleoprotein enzyme complex, or by activation of telomerase-independent pathways (64–70). Highly proliferating cells, bone marrow, peripheral blood cells, stem cells and 85–90% of cancer cells express telomerase (71,72). Results similar to the ones described here have been reported for telomerase-expressing HeLa cells (56). In these studies, after treatment of cells with MMC, FANCD2 foci did not co-localize with telomeric DNA but were found to be associated with non-telomeric DNA in the nucleus (56). These studies also showed that in three cell lines, which used a telomerase-independent pathway termed alternative lengthening of telomeres (ALT), following MMC treatment, FANCD2 foci co-localized with TRF2 at telomeres as well as with genomic DNA, suggesting an involvement of FANCD2 in the repair response at both telomeric and non-telomeric DNA. Our present results combined with these studies suggest that in telomerase-positive cells, FANCD2 is not involved in ICL repair at telomeres, whereas it is involved in telomerase-negative ALT cells. We propose that αIISp plays an important role in ICL repair at telomeres in telomerase-expressing cells, where it acts in facilitating recruitment of proteins involved in the ICL damage response similar, but not identical, to its proposed role in repair of DNA ICLs in genomic DNA. As our studies suggest that in telomerase-expressing cells FANCD2 plays a role in repair of ICLs in genomic but not telomeric DNA, we further propose that a different or modified mechanism of ICL repair could be involved in repair of ICLs at telomeric DNA compared with genomic DNA in these cells. As telomeric DNA has a different sequence structure and a unique complex of proteins associated with it, it would not be unreasonable to postulate that there could be differences in the repair pathways used at telomeres compared with genomic DNA. It will be of interest in future studies to examine the involvement of αIISp in ICL repair in telomerase-negative cells.
Our present finding that αIISp localizes to telomeres in S phase after ICL damage suggests that it is important in maintenance of telomere function during DNA replication in ICL-damaged cells. Telomeres undergo DNA replication in S phase (40). DNA ICLs can lead to blocking of DNA replication and to production of stalled replication forks in genomic DNA in S phase (29,73–75). Similarly, production of DNA ICLs during replication of telomeres may challenge replication and lead to stalled replication forks. If unrepaired, these could lead to failure to efficiently restart replication and result in production of aberrant telomeric structures and telomere dysfunction. It could be hypothesized that αIISp is recruited to ICLs at these stalled replication forks, which occur in S phase, and is needed in the recruitment of DNA damage response factors and for damage signaling and repair reactions at telomeres that would aid re-initiation of the stalled replication fork.
The importance of αIISp in telomere maintenance after ICL damage was demonstrated by studies that examined normal cells for telomere dysfunction following knock-down of αIISp by siRNA or in FA-A cells in which levels of αIISp were only 35% of normal. One of the indicators of telomere dysfunction used was examination of the TIF response in which the co-localization of γ-H2AX with a telomeric probe at telomeres was assessed. The presence of TIFs has been used as an index of telomere dysfunction (30,41–43). We found that loss of αIISp in normal cells leads to a significant increase in percentage of TIF-positive cells 16 h after damage with MMC. γ-H2AX localizes to sites of DNA DSBs and has been used as a marker for DSB induction (76,77). Thus the present studies indicate that loss of αIISp leads to accumulation of more DNA DSBs at telomeres, especially after ICL damage, as evidenced by the increased number of H2AX foci co-localizing with telomeres. This may be occurring at stalled replication forks, as discussed below, where single- and double-stranded DNA breaks arise when stalled replication forks fail to be efficiently restarted (43). In the present case, stalled replication forks may be occurring at sites of unrepaired ICLs. Aberrant DNA replication could subsequently lead to DSB production. As knock-down of αIISp and/or MMC treatment had no effect on cellular levels of TRF1 or TRF2 or their localization on telomeres, this indicates that TIF formation was not due to loss of TRF1 or TRF2 and their protective effects on the telomere.
An excellent human model for examining the physiological effects of loss of αIISp on telomeres is FA. In FA-A cells, levels of αIISp are 35% of normal owing to reduced stability of this protein, which we have proposed is dependent on FA proteins (7,10,15–17). These cells are also defective in repair of DNA ICLs (8,10,11,13–15). We found that in FA-A cells, TIF formation is significantly increased after ICL damage and levels were similar to those found in MMC damaged normal cells in which αIISp had been knocked down. That αIISp is important in preventing TIF formation and telomere dysfunction after ICL damage is demonstrated by our studies, which show that after levels of αIISp in FA-A cells were restored to normal by knocking down µ-calpain, a protease that cleaves αIISp and has elevated activity in FA-A cells (10,78,79), the percentage of TIF-positive cells after MMC treatment was reduced and was similar to that found in MMC-treated normal cells. This indicates that some repair of DNA ICLs in telomeric DNA has taken place in these cells.
Another indicator of telomere dysfunction is loss of telomeres. Loss of telomere repeat sequences can occur through a variety of mechanisms (22,26). One important mechanism proposed is that stalled replication forks within telomeres result in replication fork collapse, which leads to formation of DNA DSBs and to subsequent telomere breakage and loss (22,26,43,80). This increases the likelihood of sister chromatid fusion or other gross chromosome rearrangements (22,26). Our results demonstrate that knock-down of αIISp in normal cells leads to a catastrophic loss of telomeres, which is particularly significant 24 h after ICL damage. We have previously shown that, in normal human cells, by 24 h after ICL damage (either MMC or 8-MOP plus UVA), αIISp foci are no longer visible in the nucleus, presumably owing to repair of ICLs by that time and dispersal of the proteins in the foci at the completion of the repair process (7,9,10). However, after αIISp knock-down in normal cells, there is loss of formation of these foci at 16 h after ICL damage, which correlates with decreased repair of ICLs (9). We thus hypothesize that, by 24 h after ICL damage in αIISp siRNA-transfected cells, reduced levels of αIISp prevented efficient repair of telomeric ICLs during S phase, resulting in replication fork stalling. This in turn leads to incomplete telomere replication and formation of telomeric DSBs. The formation of DSBs in the telomeric DNA promotes the dramatic telomere loss observed by 24 h after ICL damage. αIISp would thus be critical in the processing of ICLs during telomere replication, potentially through recruitment of repair factors such as XPF, as has been demonstrated in the present study, thereby suppressing stochastic loss of telomeres and telomere dysfunction. The physiological importance of αIISp in preventing the catastrophic loss of telomeres, which occurs after ICL damage, is further shown by our studies using FA-A cells. In FA-A cells, after damage with MMC, there was also a catastrophic loss of telomeres, which was similar to that found in MMC-treated normal cells in which αIISp had been knocked down. The importance of αIISp for ICL processing events and telomere maintenance after ICL damage is additionally demonstrated by our finding that restoring levels of αIISp to normal in FA-A cells, by knocking down µ-calpain, leads to reduction in telomere loss to levels found in MMC-treated normal cells.
A similar catastrophic loss of telomeres has been observed in hematopoietic stem cells from mice in which there was deletion of a telomere-binding protein, CTC1, which was shown to facilitate telomere replication by promoting efficient restart of stalled replication forks (81). Depletion of this protein hindered efficient replication of telomeres by hindering efficient restart of stalled replication forks, which in turn led to catastrophic telomere loss (81). Telomere loss was not due to deprotection of the telomeres because localization of shelterin components on telomeres was not affected by deletion of CTC1 (81). This is similar to what we observe in our system after depletion of αIISp. We hypothesize that αIISp, like CTC1, is also needed for efficient restart of stalled replication forks at telomeres but that αIISp is specific for replication forks stalled owing to the presence of ICLs; loss of αIISp leads to failure of ICL repair, which in turn leads to failure of efficient restart of stalled replication forks and dramatic loss of telomeres.
Telomere loss can generate a variety of chromosome alterations; sister chromatid end fusions are frequent among these (22,26). We have shown that 24 h after MMC damage to cells in which αIISp has been knocked down, a number of chromosomal aberrations are observed, the most frequent of which are sister chromatid end-to-end fusions. These chromatid end fusions correlate with loss of telomeres 24 h after MMC damage. Similar types of chromosomal aberrations have been observed in FA cells after ICL damage (35,36). It is thus possible that these reported chromosomal aberrations in FA cells are in part due to loss of telomeres. The present studies thus indicate that, in FA-A cells, loss of αIISp and telomere dysfunction after ICL damage may be an important mechanism involved in development of the chromosomal instability observed in FA.
Our studies have demonstrated that in FA-A cells knocking down µ-calpain, so as to restore αIISp levels back to normal, leads to reversal of a number of the defects observed after ICL damage in both telomeres and genomic DNA (10) and that this occurs in the absence of a functional Fanconi anemia FANCA protein. These include recruitment of repair proteins such as XPF to sites of damage, decreased TIFs and loss of telomeres, restoration of DNA repair and an increase in cell survival and chromosome stability. We have proposed that one critical function of the FANCA protein, and potentially other FA proteins, is maintenance of αIISp stability in the cell (10,16,17). This could be an end point for FANCA function. When this end point is achieved by alternate means (i.e. restoration of αIISp levels), then those functions depending on this end point, such as recruitment of repair proteins to the site of an ICL and telomere stability after ICL damage, would be enabled and the presence of FANCA would not be as critical. This view is further supported by our studies, which show that in normal cells in which αIISp has been knocked down, XPF foci do not localize to sites of damage after ICL damage and DNA repair is not carried out, even though the levels of FANCA are normal in these cells (9). This indicates that the presence of FANCA, itself, is not enough to aid in recruitment of repair proteins such as XPF to sites of damage. Hence, in these particular aspects of the repair process, the role of FANCA may be indirect.
The present studies thus demonstrate an important role for αIISp in maintenance of telomere function after ICL damage, which we propose is related to its role in repair of DNA ICLs. Three different telomeric phenotypes associated with telomere dysfunction are observed after ICL damage when there is a loss of αIISp, due either to its knock-down in normal cells or its deficiency in FA-A cells. These are as follows: (i) increased TIF formation, (ii) dramatic loss of telomeres and (iii) formation of sister chromatid end-to-end fusions. Furthermore, in FA-A cells, restoration of αIISp levels to normal leads to correction of these deficiencies. These studies also suggest that, in telomerase-expressing cells, there is a different or modified mechanism of ICL repair of telomeric DNA compared with that used by genomic DNA. Thus we propose that αIISp plays a critical role in the nucleus in repair of ICLs at telomeres in telomerase-expressing cells, where it aids in recruitment of proteins important in the DNA damage response similar, but not identical, to its proposed role in repair of DNA ICLs in genomic DNA.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Table 1 and Supplementary Figures 1–5.
FUNDING
The National Institutes of Health [RO1 HL054860 to M.W.L., R01 CA136533 to U.H.]; the Foundation of UMDNJ (to M.W.L.). Funding for open access charge: A UMDNJ university account of M.W.L.
Conflict of interest statement. None declared.
Supplementary Material
REFERENCES
- 1.Goodman SR, Zimmer WE, Clark MB, Zagon IS, Barker JE, Bloom ML. Brain spectrin: of mice and men. Brain Res. Bull. 1995;36:593–606. doi: 10.1016/0361-9230(94)00264-2. [DOI] [PubMed] [Google Scholar]
- 2.de Matteis MA, Morrow JS. Spectrin tethers and mesh in the biosynthetic pathway. Cell Sci. 2000;113:2331–2343. doi: 10.1242/jcs.113.13.2331. [DOI] [PubMed] [Google Scholar]
- 3.Gascard P, Mohandas N. New insights into functions of erythroid proteins in nonerythroid cells. Curr. Opin. Hematol. 2000;7:123–129. doi: 10.1097/00062752-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Bennet V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 2001;81:353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 5.Machnicka B, Grochowalska R, Boguslawska DM, Sikorski AF, Lecomte MC. Spectrin-based skeleton as an actor in cell signaling. Cell Mol. Life Sci. 2012;69:191–201. doi: 10.1007/s00018-011-0804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMahon LW, Sangerman J, Goodman SR, Kumaresan K, Lambert MW. Human alpha spectrin II and the FANCA, FANCC, and FANCG proteins bind to DNA containing psoralen interstrand cross-links. Biochemistry. 2001;40:7025–7034. doi: 10.1021/bi002917g. [DOI] [PubMed] [Google Scholar]
- 7.Sridharan D, Brown M, Lambert WC, McMahon LW, Lambert MW. Nonerythroid alpha II spectrin is required for recruitment of FANCA and XPF to nuclear foci induced by DNA interstrand cross-links. J. Cell Sci. 2003;116:823–835. doi: 10.1242/jcs.00294. [DOI] [PubMed] [Google Scholar]
- 8.Kumaresan K, Sridharan D, McMahon L, Lambert MW. Deficiency in incisions produced by XPF at the site of a DNA interstrand cross-link in Fanconi anemia cells. Biochemistry. 2007;46:14359–14368. doi: 10.1021/bi7015958. [DOI] [PubMed] [Google Scholar]
- 9.McMahon LW, Zhang P, Sridharan DM, Lefferts JA, Lambert MW. Knockdown of αII spectrin in normal human cells by siRNA leads to chromosomal instability and decreased DNA interstrand cross-link repair. Biochem. Biophys. Res. Commun. 2009;381:288–293. doi: 10.1016/j.bbrc.2009.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang P, Sridharan D, Lambert MW. Knockdown of µ-calpain in Fanconi anemia, FA-A, cells by siRNA restores αII spectrin levels and corrects chromosomal instability and defective DNA interstrand cross-link repair. Biochemistry. 2010;49:5570–5581. doi: 10.1021/bi100656j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joenje H, Patel KJ. The emerging genetic and molecular basis of Fanconi anaemia. Nat. Rev. Genet. 2001;2:446–457. doi: 10.1038/35076590. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi T, D’Andrea AD. Molecular pathogenesis of Fanconi anemia: recent progress. Blood. 2006;107:223–4233. doi: 10.1182/blood-2005-10-4240. [DOI] [PubMed] [Google Scholar]
- 13.de Winter JP, Joenje H. The genetic and molecular basis of Fanconi anemia. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2009;668:11–19. doi: 10.1016/j.mrfmmm.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Kim H, D’Andrea AD. Regulation of DNA cross-link repair by the Fanconia anemia/BRCA pathway. Genes Dev. 2012;26:1393–1408. doi: 10.1101/gad.195248.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMahon LW, Walsh CE, Lambert MW. Human αspectrin II and the Fanconi anemia proteins FANCA and FANCC interact to form a nuclear complex. J. Biol. Chem. 1999;274:32904–32980. doi: 10.1074/jbc.274.46.32904. [DOI] [PubMed] [Google Scholar]
- 16.Lefferts JA, Lambert MW. Fanconi anemia cell lines deficient in αII spectrin express normal levels of αII spectrin mRNA. Biochem. Biophys. Res. Commun. 2003;307:510–515. doi: 10.1016/s0006-291x(03)01213-0. [DOI] [PubMed] [Google Scholar]
- 17.Lefferts JA, Wang C, Sridharan D, Baralt M, Lambert MW. The SH3 domain of αII spectrin is a target for the Fanconi anemia protein FANCG. Biochemistry. 2009;48:254–263. doi: 10.1021/bi801483u. [DOI] [PubMed] [Google Scholar]
- 18.Kumaresan KR, Lambert MW. Fanconi anemia, complementation group A, cells are defective in ability to produce incisions at sites of psoralen interstrand cross-links. Carcinogenesis. 2000;21:741–751. doi: 10.1093/carcin/21.4.741. [DOI] [PubMed] [Google Scholar]
- 19.Niedernhofer LJ, Lalai AS, Hoeijmakers JH. Fanconi anemia (cross)linked to DNA repair. Cell. 2005;123:1191–1198. doi: 10.1016/j.cell.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 20.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 21.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat. Rev. Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 22.Murnane P. Telomere loss as a mechanism for chromosome instability in human cancer. Cancer Res. 2010;70:4255–4259. doi: 10.1158/0008-5472.CAN-09-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 24.O’Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat. Rev. Mol. Cell. Biol. 2010;11:171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 26.Murnane JP. Telomere dysfunction and chromosome instability. Mutat. Res. 2012;730:28–36. doi: 10.1016/j.mrfmmm.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sfeir A, de Lange T. Removal of shelterin reveals the telomere end-protection problem. Science. 2012;336:593–597. doi: 10.1126/science.1218498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Higaera I, Taniguchi T, Ganesan S, Meyn MS, Timmers C, Hejna J, Grompe M, D’Andrea AD. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol. Cell. 2001;7:249–262. doi: 10.1016/s1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 29.Knipscheer P, Raschle M, Smogorzeuska A, Enoiu M, Ho TV, Scharer OD, Elledge SJ, Walter J. The Fanconi anemia pathway promotes replication-dependent DNA interstrand cross-link repair. Science. 2009;236:1698–1701. doi: 10.1126/science.1182372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol. Cell. 2004;14:501–513. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 31.Konishi A, de Lange T. Cell cycle control of telomere protection and NHEJ revealed by a ts mutation in the DNA-binding domain of TRF2. Genes Dev. 2008;22:1221–1230. doi: 10.1101/gad.1634008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suram A, Kaplunov J, Patel PL, Ruan H, Cerutti A, Boccardi V, Fumagalli M, Di Micco R, Mirani N, Gurung RL, et al. Oncogene-induced telomere dysfunction enforces cellular senescence in human cancer precursor lesions. EMBO J. 2012;31:2839–2851. doi: 10.1038/emboj.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Coyne RS, Dubreuil RR, Goldstein LS, Branton D. Cell shape and interaction defects in alpha-spectrin mutants of Drosophila melanogaster. J. Cell Biol. 1993;123:1797–1809. doi: 10.1083/jcb.123.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norman KR, Moerman DG. αSpectrin is essential for morphogenesis and body wall muscle formation in Caenorhabditis elegans. J. Cell Biol. 2002;157:665–677. doi: 10.1083/jcb.200111051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howlett NG, Taniguchi T, Durkin SG, D’Andrea AD, Glover TW. The Fanconi anemia pathway is required for the DNA replication stress response and for the regulation of common fragile site stability. Hum. Mol. Genet. 2005;14:693–701. doi: 10.1093/hmg/ddi065. [DOI] [PubMed] [Google Scholar]
- 36.Godthelp B, van Buul PPW, Jaspers NGJ, Elghalbzouri-Maghrini E, van Duijn-Goedhart A, Arwert F, Joenje H, Zdzienicka MZ. Cellular characterization of cells from Fanconi anemia complementation group, FA-D1/BRCA2. Mutat. Res. 2006;601:191–201. doi: 10.1016/j.mrfmmm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Liu V, Lao Y, Yang IY, Hecht SS, Moriya M. Replication-coupled repair of crotonaldehyde/acetaldehyde-induced guanine-guanine interstrand cross-links and their mutagenicity. Biochemistry. 2006;45:12898–12905. doi: 10.1021/bi060792v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pang Q, Andreassen PR. Fanconi anemia proteins and endogenous stresses. Mutat. Res. 2009;668:42–53. doi: 10.1016/j.mrfmmm.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scharer OD. DNA interstrand crosslinks: natural and drug-induced DNA adducts that induce unique cellular responses. ChemBioChem. 2005;6:27–32. doi: 10.1002/cbic.200400287. [DOI] [PubMed] [Google Scholar]
- 40.Wright WE, Tesmer VM, Liao ML, Shay JW. Normal human telomeres are not late replicating. Exp. Cell Res. 1999;251:492–499. doi: 10.1006/excr.1999.4602. [DOI] [PubMed] [Google Scholar]
- 41.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 42.Martinez P, Thanasoula M, Munoz P, Liao C, Tejera A, McNees C, Flores JM, Fernandez-Capetillo O, Tarsounas M, Blasco MA. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Gene Dev. 2009;23:2060–2075. doi: 10.1101/gad.543509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badie S, Escandell JM, Bouwman P, Carlos AR, Thanasoula M, Gallardo MM, Suram A, Jaco I, Benitez J, Herbig U, et al. BRCA2 acts as a RAD51 loader to facilitate telomere replication and capping. Nat. Struct. Mol. Biol. 2010;17:1461–1469. doi: 10.1038/nsmb.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuraoka I, Kobertz WR, Ariza RR, Biggerstaff M, Essigmann JM, Wood RS. Repair of an interstrand DNA cross-link initiated by ERCC1-XPF repair/recombination nuclease. J. Biol. Chem. 2000;275:26632–26636. doi: 10.1074/jbc.C000337200. [DOI] [PubMed] [Google Scholar]
- 45.De Silva IV, McHugh PJ, Clingen PH, Hartley JH. Defining the roles of nucleotide excision repair and recombination in the repair of DNA interstrand crosslinks in mammalian cells. Mol. Cell. Biol. 2000;20:7980–7990. doi: 10.1128/mcb.20.21.7980-7990.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prasher JM, Lalai AS, Heijmans-Antonissen C, Ploemacher RE, Hoeijmakers JH, Touw IP, Niedernhofer LJ. Reduced hematopoietic reserves in DNA interstrand crosslink repair-deficient Ercc1-/- mice. EMBO J. 2005;24:861–871. doi: 10.1038/sj.emboj.7600542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 48.Al-Minawi AZ, Lee YF, Hakansson D, Johansson F, Lundin C, Saleh-Gohari N, Schultz N, Jenssen D, Bryant HE, Meuth M, et al. The ERCC1/XPF endonuclease is required for completion of homologous recombination at DNA replication forks stalled by inter-strand cross-links. Nucleic Acids Res. 2009;37:6400–6413. doi: 10.1093/nar/gkp705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hlavin EM, Smeaton MB, Miller PS. Initiation of DNA interstrand cross-link repair in mammalian cells. Environ. Mol. Mutagen. 2010;51:604–624. doi: 10.1002/em.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gregg SQ, Robinson AR, Niedernhofer LJ. Physiological consequences of defects in ERCC1-XPF DNA repair endonuclease. DNA Repair. 2011;10:781–791. doi: 10.1016/j.dnarep.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu XD, Niedernhofer L, Kuster B, Mann M, Hoeijmakers JH, de Lange T. ERCC1/XPF removes the 3’ overhang from uncapped telomeres and represses formation of telomeric DNA-containing double minute chromosomes. Mol. Cell. 2003;12:1489–1498. doi: 10.1016/s1097-2765(03)00478-7. [DOI] [PubMed] [Google Scholar]
- 52.Munoz P, Blanco R, Flores JM, Blasco MA. XPF nuclease-dependent telomere loss and increased DNA damage in mice overexpressing TRF2 result in premature aging and cancer. Nat. Genet. 2005;37:1063–1071. doi: 10.1038/ng1633. [DOI] [PubMed] [Google Scholar]
- 53.Wu Y, Zacal NJ, Rainbow AJ, Zhu XD. XPF with mutations in its conserved nuclease domain is defective in DNA repair but functions in TRF2-mediated telomere shortening. DNA Repair. 2007;6:157–166. doi: 10.1016/j.dnarep.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Wu Y, Mitchell TRH, Zhu XD. Human XPF controls TRF2 and telomere length maintenance through distinctive mechanisms. Mech. Ageing Dev. 2008;129:602–610. doi: 10.1016/j.mad.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Sridharan DM, McMahon LW, Lambert MW. αII-Spectrin interacts with five groups of functionally important proteins in the nucleus. Cell Biol. Intl. 2006;30:866–878. doi: 10.1016/j.cellbi.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Fan Q, Zhang F, Barrett B, Ren K, Andreassen PR. A role for monoubiquitinated FANCD2 at telomeres in ALT cells. Nucleic Acids Res. 2009;37:1740–1754. doi: 10.1093/nar/gkn995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu XD, Kuster B, Mann M, Petrini JHJ, de Lange T. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat. Genet. 2000;25:347–352. doi: 10.1038/77139. [DOI] [PubMed] [Google Scholar]
- 58.Kim H, Lee O-H, Xin H, Chen L-Y, Qin J, Chae HK, Lin S-Y, Safari A, Liu D, Songyang Z. TRF2 functions as a protein hub and regulates telomere maintenance by recognizing specific peptide motifs. Nat. Struct. Mol. Biol. 2009;16:372–379. doi: 10.1038/nsmb.1575. [DOI] [PubMed] [Google Scholar]
- 59.Ballal RD, Saha T, Fan S, Haddad BR, Rosen EM. BRCA1 localization to the telomere and its loss from the telomere in response to DNA damage. J. Biol. Chem. 2009;284:36083–36098. doi: 10.1074/jbc.M109.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diotti R, Loayza D. Shelterin complex and associated factors at human telomeres. Nucleus. 2011;2:119–135. doi: 10.4161/nucl.2.2.15135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeung AT, Jones BK, Capraro M, Chu CT. The repair of psoralen monoadducts by the Escherichia coli UvrABC endonuclease. Nucleic Acids Res. 1987;15:4957–4971. doi: 10.1093/nar/15.12.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumaresan KR, Hang B, Lambert MW. Human endonucleolytic incision of DNA 3’ and 5’ to a site-directed psoralen monoadduct and interstrand cross-link. J. Biol. Chem. 1995;270:30709–30716. doi: 10.1074/jbc.270.51.30709. [DOI] [PubMed] [Google Scholar]
- 63.Hovest MG, Bruggenolte N, Hosseini KS, Krieg T, Herrmann G. Senescence of human fibroblasts after psoralen photoactivation is mediated by ATR kinase and persistent DNA damage foci at telomeres. Mol. Biol. Cell. 2006;17:1758–1767. doi: 10.1091/mbc.E05-08-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greider CW, Blackburn EH. The telomere terminal transferase of tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;5:837–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 65.Greider C. Telomere length regulation. Annu. Rev. Biochem. 1996;65:336–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- 66.Smogorzewska A, de Lange T. Regulation of telomerase by telomeric proteins. Ann. Rev. Biochem. 2004;73:177–208. doi: 10.1146/annurev.biochem.73.071403.160049. [DOI] [PubMed] [Google Scholar]
- 67.Cech TR. Beginning to understand the end of the chromosome. Cell. 2004;116:272–279. doi: 10.1016/s0092-8674(04)00038-8. [DOI] [PubMed] [Google Scholar]
- 68.Blackburn EH. Telomeres and telomerase. FEBS Lett. 2005;579:859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 69.Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, Reddel RR. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat. Med. 1997;3:1271–1274. doi: 10.1038/nm1197-1271. [DOI] [PubMed] [Google Scholar]
- 70.Dunham MA, Neumann AA, Fasching CL, Reddel RR. Telomere maintenance by recombination in human cells. Nat. Genet. 2002;26:447–450. doi: 10.1038/82586. [DOI] [PubMed] [Google Scholar]
- 71.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Cociello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 72.Broccoli D, Young JW, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc. Natl Acad. Sci. USA. 1995;92:9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akkari YMN, Bateman RL, Reifsteck CA, Olson SB, Grompe M. DNA replication is required to elicit cellular responses to psoralen-induced DNA interstrand cross-links. Mol. Cell. Biol. 2000;20:8283–8289. doi: 10.1128/mcb.20.21.8283-8289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hlavin EM, Smeaton MB, Miller PS. Initiation of DNA interstrand cross-link repair in mammalian cells. Environ. Mol. Mutagen. 2010;51:604–624. doi: 10.1002/em.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vare D, Groth P, Carlsson R, Johansson F, Eriron K, Jenssen D. DNA interstrand crosslinks induce a potent replication block followed by formation and repair of double strand breaks in intact mammalian cells. DNA Repair. 2012;11:976–985. doi: 10.1016/j.dnarep.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 76.Kinner A, Wu W, Staudt C, Lliakis G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36:5678–5694. doi: 10.1093/nar/gkn550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuo LJ, Yang LX. Gamma-H2AX – a novel biomarker for DNA double-strand breaks. In Vivo. 2008;22:305–309. [PubMed] [Google Scholar]
- 78.Nicolas G, Fournier CM, Galand C, Malbert-Colas L, Bournier O, Kroviarski Y, Bourgeois M, Camonis JH, Dhermy D, Grandchamp B, et al. Tyrosine phosphorylation regulates alpha II spectrin cleavage by calpain. Mol. Cell. Biol. 2002;22:3527–3536. doi: 10.1128/MCB.22.10.3527-3536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Czogalla A, Sikorski AF. Spectrin and calpain: a’target’ and a ‘sniper’ in the pathology of neuronal cells. Cell. Mol. Life Sci. 2005;62:1913–1924. doi: 10.1007/s00018-005-5097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saharia A, Teasley DC, Duxin JP, Dao B, Chiappinelli KB, Stewart SA. FEN1 ensures telomere stability by facilitating replication fork re-initiation. J. Biol. Chem. 2010;285:27057–27066. doi: 10.1074/jbc.M110.112276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gu P, Min J-N, Wang Y, Huang C, Peng T, Chai W, Chang S. CTC1 deletion results in defective telomere replication, leading to catastrophic telomere loss and stem cell exhaustion. EMBO J. 2012;31:2309–2321. doi: 10.1038/emboj.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.