Abstract
Meiotic crossovers facilitate the segregation of homologous chromosomes and increase genetic diversity. The formation of meiotic crossovers was previously posited to occur via two pathways, with the relative use of each pathway varying between organisms; however, this paradigm could not explain all crossovers, and many of the key proteins involved were unidentified. Recent studies that identify some of these proteins reinforce and expand the model of two meiotic crossover pathways. The results provide novel insights into the evolutionary origins of the pathways, suggesting that one is similar to a mitotic DNA repair pathway and the other evolved to incorporate special features unique to meiosis.
Keywords: meiotic recombination, mitotic recombination
MEIOSIS is essential to maintaining the proper complement of chromosomes in sexually reproducing organisms. By following one round of DNA replication with two rounds of cellular division, meiosis effectively halves the chromosome content of participating cells. Prior to the first meiotic division, homologous chromosomes pair and, in many organisms, undergo recombination. Both crossovers, characterized by the reciprocal exchange of flanking markers, and noncrossovers, in which flanking DNA remains unchanged, result from these recombination events. Crossovers can also occur in mitotically proliferating cells during repair of certain types of DNA damage, especially double-strand breaks. Meiotic crossovers likewise are initiated from double-strand breaks, and many of the proteins used in mitotic repair are also used in meiotic recombination. This has led to the suggestion that meiotic recombination evolved from mitotic recombination (Marcon and Moens 2005). However, several modifications were necessary to give rise to meiotic recombination in its current form (reviewed in Villeneuve and Hillers 2001). First, a mechanism of generating programmed double-strand breaks to initiate recombination was needed. This was achieved through the use of Spo11, a conserved protein that generates regulated meiotic double-strand breaks (Keeney et al. 1997). Second, whereas crossovers are avoided in mitotic cells to prevent loss of heterozygosity and chromosome rearrangement, crossover formation is emphasized in meiotic recombination to facilitate the segregation of homologous chromosomes and to increase genetic diversity. Third, the preferred repair template was changed from the sister chromatid in mitotic cells to the homologous chromosome in meiotic cells, since only crossovers between homologs give the aforementioned benefits. Finally, exquisite crossover control mechanisms arose to regulate the number and distribution of crossovers across the genome and relative to one another. In particular, every chromosome pair receives at least one crossover, sometimes called an obligate crossover (Jones 1984). Also, if additional crossovers occur, they tend not to be near one another, a phenomenon called crossover interference (reviewed in Berchowitz and Copenhaver 2010).
A complication obscuring the relationship between the mitotic and meiotic recombination pathways has been the apparent existence of two meiotic crossover pathways—one pathway that produces crossovers subject to interference and another that produces noninterfering crossovers. Recent studies suggest that the interfering crossover pathway fits the scenario described above—i.e., it is a derivative of the mitotic double-strand break repair pathway that contains numerous meiosis-specific embellishments. The noninterfering pathway, however, shares striking similarities to mitotic double-strand break repair in its original form. Additional discoveries reveal functions that are essential for generating meiotic crossovers and can be carried out by different proteins in different species. These findings provide a new framework through which meiotic recombination pathways can be viewed and allow organisms previously thought to use disparate crossover pathways to be brought under the same umbrella.
Crossovers and Noncrossovers in Meiotic Recombination Models
In 1964, Holliday proposed a novel molecular model to explain how meiotic recombination could produce both crossovers and noncrossovers (Holliday 1964). The central intermediate in his model is a structure in which strands from two homologous duplexes swap pairing partners across a short region, yielding a four-stranded intermediate now known as the Holliday junction. Holliday proposed that these junctions are cleaved by DNA repair enzymes, now known as resolvases, to reestablish two separate duplexes. Depending on which strands are nicked, this process, now called resolution, could result in crossover or noncrossover products. The equally likely outcomes of resolution fit with fungal recombination studies that suggested that crossovers and noncrossovers occur in equal numbers.
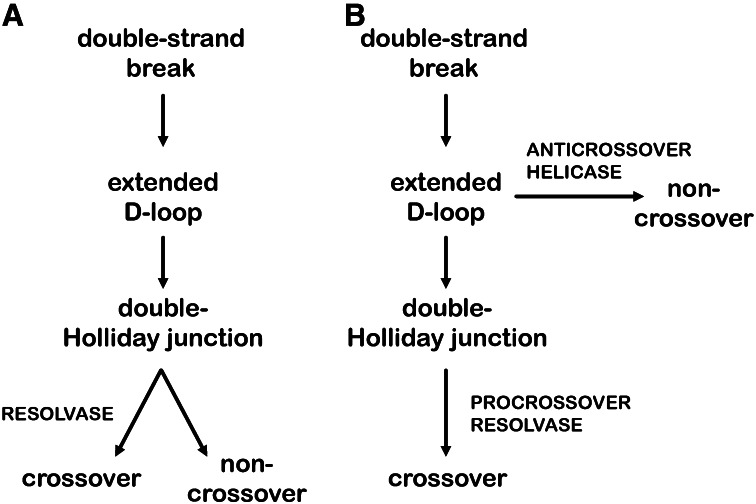
In Holliday’s model, meiotic recombination is initiated by symmetric nicks on homologous chromosomes, but this mechanism did not fit with subsequent observations (reviewed in Stahl 1994). To accommodate the new data, Szostak et al. (1983) proposed that meiotic recombination is initiated by a double-strand break on one chromatid. In the double-strand break repair model they proposed (Figure 1A), based largely on observations of double-strand gap repair in mitotic cells, the precrossover intermediate has two Holliday junctions. Each junction in this double-Holliday junction intermediate is proposed to be resolved independently, but the outcome is similar to Holliday’s model: crossovers and noncrossovers are produced in equal numbers. Strong support for the double-strand break repair model came from physical studies of meiotic recombination intermediates and products in Saccharomyces cerevisiae. These studies identified joint molecules that form between homologous chromosomes (Collins and Newlon 1994; Schwacha and Kleckner 1994). These joint molecules have many of the properties expected of double-Holliday junctions, the key intermediate in the double-strand break repair model (Schwacha and Kleckner 1995), and are widely considered to be double-Holliday junctions. In this review, we use “joint molecule” to refer to the structure detected in experiments, and “double-Holliday junction” to refer to the intermediate predicted in models.
Figure 1.
Models of meiotic double-strand break repair. (A) In the Szostak et al. (1983) model, recombination initiates with a double-strand break that is processed into an extended displacement loop (D-loop) and then a double Holliday junction structure. The double-Holliday junction is resolved into either a crossover or noncrossover with equal probability. (B) In the revised model of Allers and Lichten (2001), some extended D-loops are unwound by an anticrossover helicase to produce noncrossovers, and double-Holliday junctions are resolved by a procrossover resolvase into crossovers.
Subsequent studies of joint molecules also led to a major challenge to the double-strand break repair model. Allers and Lichten (2001) discovered that noncrossovers arose at the same time as joint molecules and prior to crossovers, a finding incompatible with the Szostak et al. (1983) double-strand break repair model. In light of this finding, Allers and Lichten (2001) suggested that noncrossovers do not come from double-Holliday junctions, as in the double-strand break repair model, but from an earlier intermediate in the pathway, the extended displacement loop (D-loop) (Figure 1B). A D-loop is formed when a single-stranded DNA end invades a homologous duplex, annealing to one strand and displacing the other. Allers and Lichten (2001) suggested that meiotic noncrossovers arise via synthesis-dependent strand annealing, a process proposed to be a major mechanism through which crossovers are avoided in mitotic double-strand break repair (reviewed in Pâques and Haber 1999). In synthesis-dependent strand annealing, after the invading strand is extended by DNA synthesis, helicases can disrupt the D-loop, freeing the nascent strand to anneal to the other end of the double-strand break.
Allers and Lichten (2001) noted another departure from the original double-strand break repair model: Most joint molecules are processed into crossovers (Figure 1B). Although this discovery opposes the notion that resolution of a double-Holliday junction can produce a crossover or a noncrossover with equal probability, it more readily accommodates the finding that noncrossovers outnumber crossovers, sometimes by a factor of 10 or more (reviewed in Cole et al. 2012b). Thus, in the revised model of Allers and Lichten (2001), the backbone of the original double-strand break repair model is intact, but double-Holliday junctions are now preferentially repaired as crossovers, and noncrossovers arise via synthesis-dependent strand annealing instead of double-Holliday junction resolution. In this revised model, helicases that promote synthesis-dependent strand annealing act as anticrossover factors and Holliday junction resolvases become procrossover factors rather than proteins that produce both crossovers and noncrossovers.
Rise of the Two-Pathway Paradigm
Another major impact on meiotic recombination models came from studies of the S. cerevisiae ZMM (Zip1–Zip4, Msh4–Msh5, Mer3) proteins. Msh4 and Msh5 are two widely conserved ZMM proteins that form a meiosis-specific complex (Pochart et al. 1997). Notably, loss of Msh4 or Msh5, like loss of other ZMM proteins, does not eliminate crossovers, but merely reduces them by ∼50–70% (Ross-Macdonald and Roeder 1994; Hollingsworth et al. 1995). In Caenorhabditis elegans, however, Msh4 and Msh5 seem to be essential for all meiotic crossovers (Zalevsky et al. 1999; Kelly et al. 2000). To reconcile these organismal differences and explain the remaining crossovers in S. cerevisiae msh4 and msh5 mutants, Zalevsky et al. (1999) proposed that there are two different pathways for meiotic crossover formation (Table 1). The first pathway, which requires Msh4–Msh5, is responsible for a majority of crossovers in S. cerevisiae and all crossovers in C. elegans; the second, independent of Msh4–Msh5, produces the remaining crossovers in S. cerevisiae msh4 and msh5 mutants.
Table 1. Percentage of crossovers attributed to each pathway in the early two-pathway paradigm.
| Class | Type of crossover | Defining proteins | Percentage of crossovers |
||||
|---|---|---|---|---|---|---|---|
| Sc | Sp | Ce | At | Dm | |||
| I | Interfering | Msh4–Msh5 | 50–70 | 0 | 100 | 75–85 | 0a |
| II | Noninterfering | Mus81–Mms4 | 20 | 100 | 0 | 9–12 | <10 |
Sc, Saccharomyces cerevisiae; Sp, Schizosaccharomyces pombe; Ce, Caenorhabditis elegans; At, Arabidopsis thaliana; Dm, Drosophila melanogaster. See text for references.
A recent study shows that at least 90% of crossovers in Drosophila melanogaster require the mei-MCM complex, which appears to perform a function similar to that of Msh4–Msh5 (Kohl et al. 2012).
The idea of two meiotic crossover pathways helped explain additional seemingly disparate findings in other organisms. The fission yeast Schizosaccharomyces pombe lacks orthologs of Msh4 and Msh5 (Villeneuve and Hillers 2001). Instead, most or all crossovers are dependent on the Mus81–Mms4 resolvase (the ortholog of Mms4 is called Eme1 in S. pombe and many other organisms; for simplicity, we use the S. cerevisiae protein name here) (Boddy et al. 2001; Smith et al. 2003). In budding yeast, loss of Mus81–Mms4 causes only ∼20% reduction in meiotic crossovers (Argueso et al. 2004). In light of the two-pathway paradigm, these results suggested that Mus81–Mms4 functions in one of the two meiotic crossover pathways, and that this pathway is responsible for all meiotic crossovers in S. pombe but only a subset of crossovers in S. cerevisiae. This begged the question of whether Mus81–Mms4 and Msh4–Msh5 function in the same meiotic crossover pathway or in two different pathways. In both S. cerevisiae and Arabidopsis thaliana, double mutants that lack both the Msh4–Msh5 and Mus81–Mms4 complexes have more severely reduced crossover levels than mutants lacking either one, strongly supporting the existence of two pathways—one dependent on Msh4–Msh5 (class I) and another on Mus81–Mms4 (class II) (de los Santos et al. 2003; Berchowitz et al. 2007).
The nature of the crossovers produced by the two pathways was also in question: If there are two meiotic crossover pathways, do the crossovers produced by them have different properties? A clue to the answer came from mathematical modeling of crossover interference. Copenhaver et al. (2002) were able to fit Arabidopsis data to a counting model for interference (Foss et al. 1993) if they assumed two types of crossovers: some that participate in interference and some that do not. Consistent with this prediction, experimental studies demonstrated that the residual crossovers in Arabidopsis and budding yeast msh4 and msh5 mutants do not display interference (Novak et al. 2001; Argueso et al. 2004; Higgins et al. 2004; Lu et al. 2008), as predicted by Zalevsky et al. (1999). Conversely, Mus81–Mms4-independent crossovers in S. cerevisiae and Arabidopsis do exhibit interference (de los Santos et al. 2003; Berchowitz et al. 2007). These results suggested that the Msh4–Msh5-dependent pathway produces crossovers subject to interference, whereas the Mus81–Mms4-dependent pathway produces noninterfering crossovers (Table 1). This formulation explains the finding that crossovers are noninterfering in S. pombe (Munz 1994), as these crossovers are produced from the Mus81–Mms4 pathway, and the strong interference of all crossovers in C. elegans (Meneely et al. 2002), as these are all produced by the Msh4–Msh5 pathway.
Although the paradigm of two meiotic crossover pathways helped to explain many observations, this model also had some weaknesses. First, not all meiotic crossovers fit into these two pathways. S. cerevisiae mutants lacking both Msh4–Msh5 and Mus81–Mms4 still have some residual crossovers (de los Santos et al. 2003). In addition, though mathematical models of recombination in Drosophila fit best if most or all crossovers are interfering (Copenhaver et al. 2002), the Drosophila genome lacks Msh4 and Msh5 (Sekelsky et al. 2000), raising the possibility that another pathway generates interfering crossovers in this species.
Another shortcoming of the two-pathway paradigm is that the proteins used to define these pathways have very different functions: Mus81–Mms4 is a Holliday junction resolvase whose activity presumably directly produces crossover products (Boddy et al. 2001) (i.e., it is a procrossover resolvase). Msh4–Msh5, however, does not directly produce crossovers, but instead stabilizes crossover-specific intermediates, thus blocking unwinding by anticrossover helicases (i.e., it is an anti-anticrossover complex; see discussion below). Notably, the procrossover resolvase that acts in the Msh4–Msh5-dependent pathway was unknown. Furthermore, the relationship between these pathways and the revised model of Allers and Lichten (2001) for meiotic crossovers was unclear. Does the model fit both class I and class II pathways, with different proteins used for each, or is a second model necessary? These apparent weaknesses in the two-pathway paradigm for meiotic crossovers have largely been solved in the past year, as studies in a number of laboratories using different model organisms have clarified the roles and identities of procrossover resolvases, anticrossover helicases, and anti-anticrossover complexes.
Anticrossover and Procrossover Activities of Sgs1
Studies of anticrossover helicases have been particularly illuminating. Crossovers are a beneficial product of meiotic recombination, but they are avoided during mitotic recombination because they can cause genome instability. Double-strand breaks in nonmeiotic cells are preferentially repaired into noncrossovers, largely through the action of various anticrossover helicases. One key anticrossover protein is the Bloom syndrome helicase, BLM (reviewed in Andersen and Sekelsky 2010). Although BLM likely has many anticrossover functions, two activities are relevant to double-strand break repair. First, studies in Drosophila suggested that BLM promotes synthesis-dependent strand annealing, probably by disrupting D-loops after repair DNA synthesis (Adams et al. 2003; McVey et al. 2004), an activity human BLM has been shown to have in vitro (van Brabant et al. 2000; Bachrati et al. 2006). Second, in vitro studies demonstrated that BLM, together with topoisomerase IIIα and other proteins, can catalyze double-Holliday junction dissolution, a process in which the two Holliday junctions are migrated toward one another and then decatenated (Wu and Hickson 2003). Unlike resolution of double-Holliday junctions by cleavage, dissolution generates only noncrossovers.
Genetic studies suggested a similar anticrossover role for the S. cerevisiae BLM ortholog Sgs1 in meiosis. Crossovers are reduced in mutants lacking ZMM proteins, including Msh4–Msh5, but, remarkably, crossovers are restored in double mutants that also lack Sgs1 (Jessop et al. 2006; Oh et al. 2007). An attractive interpretation of these results is that one function of ZMMs is to antagonize the anticrossover activity of Sgs1. Thus, Msh4–Msh5 is an anti-anticrossover protein.
Although these experiments with ZMMs and Sgs1 are consistent with the known mitotic anticrossover functions of Sgs1, sgs1 mutants have only a modest increase in meiotic crossovers, much less than would be expected if all double-strand breaks were processed through a pathway in which double-Holliday junctions were produced and resolved into crossovers (Rockmill et al. 2003; Jessop et al. 2006). Novel insights into the solution to this apparent paradox came again from physical measurements of recombination intermediates and products. De Muyt et al. (2012) found that noncrossovers are still produced in sgs1 mutants, but, unlike the case in wild-type cells, these noncrossovers arise as joint molecules disappear and crossovers appear. This suggests that when Sgs1 is absent, double-Holliday junctions are resolved into crossovers and noncrossovers, as in the original double-strand break repair model.
Additional insights came from physical studies of recombination in mutants lacking the known Holliday junction resolvases. Three proteins, Mus81–Mms4, Yen1, and Slx1–Slx4, possess resolvase activity in vitro (Boddy et al. 2001; Ip et al. 2008; Fekairi et al. 2009). Mus81–Mms4 was shown to be important in generating mitotic crossovers, with Yen1 playing a compensatory or partially redundant role (Ho et al. 2010). Experiments by De Muyt et al. (2012) and Zakharyevich et al. (2012) found that single mutants lacking any one of these enzymes were still able to resolve most joint molecules and produce approximately normal numbers of crossovers. Even triple mutants lacking all three resolvases showed only a modest reduction in joint molecule resolution and crossover formation. These results suggest that the known resolvases collectively process only a small fraction of joint molecules. If these are joint molecules from the class II pathway, then most joint molecules must be generated in the class I pathway and be resolved by an unidentified resolvase.
Yet another surprise came when the same experiments were done in the absence of Sgs1. In this case, removing all three resolvases resulted in most joint molecules being left unresolved. Again, this result indicates that joint molecules produced in the absence of Sgs1 are different from those produced in the presence of Sgs1. In the absence of Sgs1, joint molecules are acted on by the known resolvases to produce both crossovers and noncrossovers, much like in the original double-strand break repair model. The known resolvases, functioning in the class II pathway, are therefore neither procrossover nor anticrossover, since they generate both outcomes. Conversely, joint molecules produced in the presence of Sgs1 (class I pathway) are cut by an unknown, procrossover resolvase to produce exclusively crossovers.
What is the identity of the procrossover resolvase that functions in the class I pathway? It had previously been suggested that the mismatch repair proteins Mlh1–Mlh3 (MutLγ complex) and Exo1 might act in double-Holliday junction resolution (Nishant et al. 2008; Zakharyevich et al. 2010). Crossovers are reduced in mlh3 mutants, but removal of Sgs1 restores crossovers, suggesting that Mlh3, like ZMMs, functions in the class I pathway (Oh et al. 2007). Consistent with this hypothesis, Zakharyevich et al. (2012) found that when all three known resolvases were removed, eliminating Mlh3 resulted in a similar reduction in crossovers as eliminating Sgs1. A parallel set of experiments suggested that Exo1 functions in a different pathway than Mus81–Mms4, putting Exo1 also in the class I pathway.
These results are consistent with Sgs1 having the expected anticrossover functions: It promotes synthesis-dependent strand annealing (in wild-type cells) and double-Holliday junction dissolution (when the three known resolvases and the putative procrossover resolvase are all missing). Unexpectedly, the results also reveal a procrossover role of Sgs1. This procrossover role may be in influencing pathway choice: In the presence of Sgs1, the ZMM-dependent class I crossover pathway can be used, but in the absence of Sgs1, the alternative class II pathway gives rise to both crossovers and noncrossovers from double-Holliday junction resolution.
Extending the Two-Pathway Paradigm
The results discussed above provided substantial support for and clarification of the two-pathway paradigm for meiotic crossovers in S. cerevisiae. Other recent results reveal the applicability of this paradigm to other model organisms. Drosophila was sometimes believed to generate interfering crossovers through a different pathway, since Msh4 and Msh5 are absent and a different resolvase may be used (see below) (e.g., Schwartz and Heyer 2011). However, Kohl et al. (2012) showed that a complex of meiotic mini-chromosome maintenance proteins (the mei-MCM complex) functionally replaces Msh4–Msh5 in Drosophila. Mutants that lack mei-MCM components have greatly reduced crossovers, but removal of the BLM/Sgs1 ortholog restores crossover formation, similar to the restoration of crossovers by Sgs1 elimination in S. cerevisiae msh4 and msh5 mutants (Jessop et al. 2006; Oh et al. 2007). Kohl et al. (2012) hypothesized that evolution of the mei-MCM complex allowed Msh4–Msh5 to be lost from Drosophila and other higher flies.
In addition to using a different anti-anticrossover complex, flies appear to use a different nuclease as their primary Holliday junction resolvase. The Drosophila Rad1/XPF endonuclease ortholog MEI-9 acts in the same pathway as the mei-MCMs and is responsible for ∼90% of meiotic crossovers (Baker and Carpenter 1972; Sekelsky et al. 1995). Thus, in Drosophila most crossovers are generated through the class I pathway, with a unique anti-anticrossover complex (mei-MCM) and a different procrossover resolvase (MEI-9).
This theme of swapping in different proteins to accomplish the same function may hold true in other organisms. Crismani et al. (2012) found that Arabidopsis mutants lacking the FANCM helicase had elevated crossovers. The additional crossovers were interference-insensitive, arose from a ZMM-independent pathway, and relied on Mus81 for formation. These findings parallel results seen in S. cerevisiae, where the absence of Sgs1 leads to crossovers being formed in an alternative, Mus81-dependent pathway. Furthermore, loss of FANCM rescues the meiotic defects of Arabidopsis zmm mutants, just like the removal of Sgs1 in S. cerevisiae zmm mutants (Crismani et al. 2012; Knoll et al. 2012). These findings strongly suggest that Arabidopsis FANCM functions as a meiotic anticrossover protein in a role that is antagonized by the ZMM proteins, similar to the role of Sgs1 in budding yeast. Thus, it appears that organisms can exchange proteins that occupy the same functional niche (in this case, swapping two anticrossover helicases) and still follow the framework of the two-pathway paradigm.
Meiotic and Mitotic Recombination in Meiosis
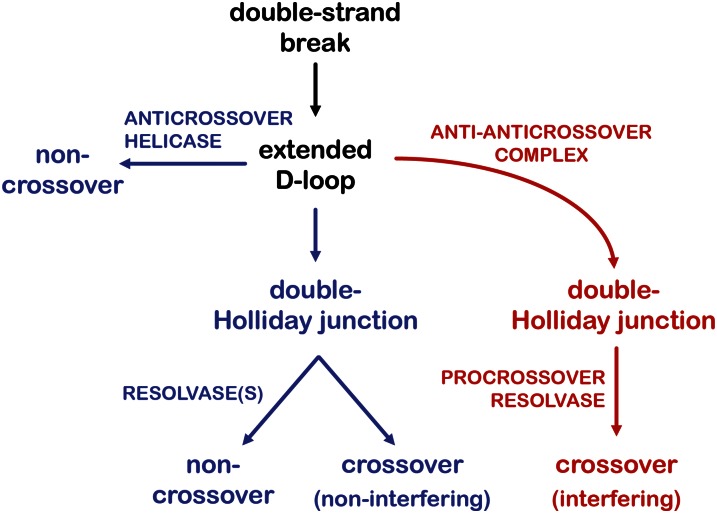
These recent findings have added much to our understanding of the two crossover pathways used in meiosis and suggest a unified model that describes the relationship between the two pathways (Figure 2). By understanding that organisms use each pathway to varying degrees and use different proteins to accomplish the same tasks, this unified model appears to be applicable to a more diverse set of model organisms than previously recognized. Furthermore, it is now apparent that the class II pathway is strikingly similar to mitotic double-strand break repair in many respects (De Muyt et al. 2012). First, noncrossovers—not crossovers—are the predominant product. This outcome is achieved through synthesis-dependent strand annealing, mediated by one or more anticrossover helicases. In instances where synthesis-dependent strand annealing does not occur and a double-Holliday junction is generated, this intermediate can be resolved in an unbiased manner by “mitotic” resolvases to give either a noncrossover or a crossover, but these crossovers are noninterfering. Despite these similarities, it should be noted that there are features of the class II pathway that are unique to meiosis. For example, double-strand breaks are generated by meiosis-specific Spo11 complexes, and engagement of DNA strands from the broken chromosome to the homologous chromosome is mediated in most species by meiosis-specific strand exchange proteins like Dmc1 (reviewed in Neale and Keeney 2006). These events, however, may occur prior to the split between the class I and class II pathways (Figure 2).
Figure 2.
Two meiotic crossover pathways. In this unified model synthesizing ideas from several sources (Börner et al. 2004; De Muyt et al. 2012; Zakharyevich et al. 2012), a double-strand break is processed into an extended displacement loop (D-loop). In the “mitotic-like” pathway (blue, class II), the extended D-loop can be unwound by an anticrossover helicase to produce noncrossovers. In some cases a double-Holliday junction is generated and then resolved by unbiased resolvases into either crossover or noncrossover products; the crossovers that are formed are noninterfering. Another possible fate of this intermediate is dissolution by a helicase and topoisomerase, to produce noncrossover products (not shown). In the meiosis-specific crossover pathway (red, class I), an anti-anticrossover complex blocks the action of anticrossover helicases to promote formation of a double-Holliday junction intermediate, which is then acted upon by a procrossover resolvase to form interfering crossovers. A double-Holliday junction is presented as a key intermediate to fit the original models (Figure 1) and the detection of joint molecules with properties of double-Holliday junctions in physical assays (Collins and Newlon 1994; Schwacha and Kleckner 1994; Schwacha and Kleckner 1995). However, there are other models that posit additional or alternative intermediates, including single Holliday junctions and multichromatid joint molecules (Osman et al. 2003; De Muyt et al. 2012; Zakharyevich et al. 2012). Variations on the two-pathway model as presented here can accommodate other intermediates and less-common fates of double-strand breaks.
While the class II pathway is similar to mitotic double-strand break repair, the class I pathway is a meiosis-specific double-strand break repair mechanism with embellishments to favor the formation of interfering crossovers (Börner et al. 2004; De Muyt et al. 2012; Zakharyevich et al. 2012). To ensure that double-Holliday junctions are generated, anticrossover activities of helicases are blocked by meiosis-specific anti-anticrossover proteins. These double-Holliday junctions are resolved mostly or exclusively into crossover products by a procrossover, possibly meiosis-specific, resolvase. Finally, class I crossovers exert and are sensitive to crossover interference, perhaps as a consequence of functional connections between this pathway and structural components of meiotic chromosomes, including the synaptonemal complex and meiosis-specific cohesins (Zickler and Kleckner 1999; de Boer and Heyting 2006).
The findings described above provide new insights into the evolution of meiotic recombination. Meiosis has long been thought to have evolved from mitosis (Cavalier-Smith 1981; Wilkins and Holliday 2009), and the evolution of meiotic double-strand break repair from mitotic double-strand break repair has been suggested previously (Marcon and Moens 2005). We hypothesize that early in the evolution of meiosis, meiotic recombination occurred only through the class II pathway, a method of double-strand break repair already in use in somatic cells. Over time, the class I pathway evolved to place additional constraints on meiotic recombination to promote the optimal placement of crossovers. To ensure crossover formation, additional regulation of anticrossover helicases active during mitotic double-strand break repair was developed. This functional niche was filled by meiosis-specific anti-anticrossover proteins like Msh4–Msh5 and the mei-MCMs. The class I pathway also evolved so that double-Holliday junctions are resolved in a biased way to produce crossovers but not noncrossovers. The class II pathway remained available, perhaps as a failsafe to ensure that all double-strand breaks are repaired.
Many questions about meiotic recombination remain unsolved. One key question is how the crossover/noncrossover decision (i.e., whether a given double-strand break is repaired as a crossover or a noncrossover) is made. Studies in S. cerevisiae suggest that this decision is made early in the recombination process (reviewed in Bishop and Zickler 2004), while studies in mice and C. elegans suggest that crossover/noncrossover differentiation occurs via a two-step process, wherein sites are marked early as potential crossovers but only a subset of these are subsequently designated as future crossovers (Cole et al. 2012a; Yokoo et al. 2012). In light of the two-pathway paradigm, the crossover/noncrossover decision must be enforced at or prior to divergence of the class I and class II pathways. Since Sgs1 appears to control pathway choice (De Muyt et al. 2012; Zakharyevich et al. 2012), it stands to reason that this protein may play a role in the crossover/noncrossover decision. The crossover/noncrossover decision must also be intertwined with the mechanism that mediates crossover interference. In the two-pathway model presented in Figure 2, interference could be mediated by crossovers themselves or by any precrossover intermediate specific to the class I pathway, such as D-loops or double-Holliday junctions loaded with the anti-anticrossover complex. Feedback from these precrossover intermediates or class I crossovers would have to impact the crossover/noncrossover decision of nearby double-strand break repair events to ensure they go down the noncrossover pathway. How the crossover/noncrossover decision is made, how Sgs1 mediates this decision, and how crossover interference works are important areas for future research in the meiotic recombination field. The unified view of recombination pathways depicted in Figure 2 may help to guide some of these studies.
Acknowledgments
We thank Nicole Crown, Eric Earley, and Lydia Morris for helpful comments on the manuscript. Research in the Sekelsky lab is supported by a grant from the National Institutes of Health (GM061252).
Footnotes
Communicating editor: N. Hollingsworth
Literature Cited
- Adams M. D., McVey M., Sekelsky J., 2003. Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science 299: 265–267. [DOI] [PubMed] [Google Scholar]
- Allers T., Lichten M., 2001. Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106: 47–57. [DOI] [PubMed] [Google Scholar]
- Andersen S. L., Sekelsky J., 2010. Meiotic vs. mitotic recombination: two different routes for double-strand break repair: the different functions of meiotic vs. mitotic DSB repair are reflected in different pathway usage and different outcomes. Bioessays 32: 1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso J. L., Wanat J., Gemici Z., Alani E., 2004. Competing crossover pathways act during meiosis in Saccharomyces cerevisiae. Genetics 168: 1805–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrati C. Z., Borts R. H., Hickson I. D., 2006. Mobile D-loops are a preferred substrate for the Bloom’s syndrome helicase. Nucleic Acids Res. 34: 2269–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. S., Carpenter A. T. C., 1972. Genetic analysis of sex chromosomal meiotic mutants in Drosophila melanogaster. Genetics 71: 255–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchowitz L. E., Copenhaver G. P., 2010. Genetic interference: don’t stand so close to me. Curr. Genomics 11: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchowitz L. E., Francis K. E., Bey A. L., Copenhaver G. P., 2007. The role of AtMUS81 in interference-insensitive crossovers in A. thaliana. PLoS Genet. 3: e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. K., Zickler D., 2004. Early decision; meiotic crossover interference prior to stable strand exchange and synapsis. Cell 117: 9–15. [DOI] [PubMed] [Google Scholar]
- Boddy M. N., Gaillard P. H., McDonald W. H., Shanahan P., Yates J. R., 3rd, et al. , 2001. Mus81-Eme1 are essential components of a Holliday junction resolvase. Cell 107: 537–548. [DOI] [PubMed] [Google Scholar]
- Börner G. V., Kleckner N., Hunter N., 2004. Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell 117: 29–45. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T., 1981. The origin and early evolution of the eukaryotic cell, pp. 33–84 in Molecular and Cellular Aspects of Microbial Evolution, edited by Carlile M. J., Collins J. F., Moseley B. E. B. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Cole F., Kauppi L., Lange J., Roig I., Wang R., et al. , 2012a Homeostatic control of recombination is implemented progressively in mouse meiosis. Nat. Cell Biol. 14: 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole F., Keeney S., Jasin M., 2012b Preaching about the converted: how meiotic gene conversion influences genomic diversity. Ann. N. Y. Acad. Sci. 1267: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins I., Newlon C. S., 1994. Meiosis-specific formation of joint DNA molecules containing sequences from homologous chromosomes. Cell 76: 65–75. [DOI] [PubMed] [Google Scholar]
- Copenhaver G. P., Housworth E. A., Stahl F. W., 2002. Crossover interference in Arabidopsis. Genetics 160: 1631–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crismani W., Girard C., Froger N., Pradillo M., Santos J. L., et al. , 2012. FANCM limits meiotic crossovers. Science 336: 1588–1590. [DOI] [PubMed] [Google Scholar]
- de Boer E., Heyting C., 2006. The diverse roles of transverse filaments of synaptonemal complexes in meiosis. Chromosoma 115: 220–234. [DOI] [PubMed] [Google Scholar]
- de los Santos T., Hunter N., Lee C., Larkin B., Loidl J., et al. , 2003. The Mus81/Mms4 endonuclease acts independently of double-Holliday junction resolution to promote a distinct subset of crossovers during meiosis in budding yeast. Genetics 164: 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Muyt A., Jessop L., Kolar E., Sourirajan A., Chen J., et al. , 2012. BLM helicase ortholog Sgs1 is a central regulator of meiotic recombination intermediate metabolism. Mol. Cell 46: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekairi S., Scaglione S., Chahwan C., Taylor E. R., Tissier A., et al. , 2009. Human SLX4 is a Holliday junction resolvase subunit that binds multiple DNA repair/recombination endonucleases. Cell 138: 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss E., Lande R., Stahl F. W., Steinberg C. M., 1993. Chiasma interference as a function of genetic distance. Genetics 133: 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. D., Armstrong S. J., Franklin F. C., Jones G. H., 2004. The Arabidopsis MutS homolog AtMSH4 functions at an early step in recombination: evidence for two classes of recombination in Arabidopsis. Genes Dev. 18: 2557–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C. K., Mazon G., Lam A. F., Symington L. S., 2010. Mus81 and Yen1 promote reciprocal exchange during mitotic recombination to maintain genome integrity in budding yeast. Mol. Cell 40: 988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R., 1964. A mechanism for gene conversion in fungi. Genet. Res. 78: 282–304. [DOI] [PubMed] [Google Scholar]
- Hollingsworth N. M., Ponte L., Halsey C., 1995. MSH5, a novel MutS homolog, facilitates meiotic reciprocal recombination between homologs in Saccharomyces cerevisiae but not mismatch repair. Genes Dev. 9: 1728–1739. [DOI] [PubMed] [Google Scholar]
- Ip S. C., Rass U., Blanco M. G., Flynn H. R., Skehel J. M., et al. , 2008. Identification of Holliday junction resolvases from humans and yeast. Nature 456: 357–361. [DOI] [PubMed] [Google Scholar]
- Jessop L., Rockmill B., Roeder G. S., Lichten M., 2006. Meiotic chromosome synapsis-promoting proteins antagonize the anti-crossover activity of sgs1. PLoS Genet. 2: e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. H., 1984. The control of chiasma distribution. Symp. Soc. Exp. Biol. 38: 293–320. [PubMed] [Google Scholar]
- Keeney S., Giroux C. N., Kleckner N., 1997. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88: 375–384. [DOI] [PubMed] [Google Scholar]
- Kelly K. O., Dernburg A. F., Stanfield G. M., Villeneuve A. M., 2000. Caenorhabditis elegans msh-5 is required for both normal and radiation-induced meiotic crossing over but not for completion of meiosis. Genetics 156: 617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll A., Higgins J. D., Seeliger K., Reha S. J., Dangel N. J., et al. , 2012. The Fanconi Anemia ortholog FANCM ensures ordered homologous recombination in both somatic and meiotic cells in Arabidopsis. Plant Cell 24: 1448–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl K. P., Jones C. D., Sekelsky J., 2012. Evolution of an MCM complex in flies that promotes meiotic crossovers by blocking BLM helicase. Science 338: 1363–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Liu X., An L., Zhang W., Sun J., et al. , 2008. The Arabidopsis MutS homolog AtMSH5 is required for normal meiosis. Cell Res. 18: 589–599. [DOI] [PubMed] [Google Scholar]
- Marcon E., Moens P. B., 2005. The evolution of meiosis: recruitment and modification of somatic DNA-repair proteins. Bioessays 27: 795–808. [DOI] [PubMed] [Google Scholar]
- McVey M., Larocque J. R., Adams M. D., Sekelsky J. J., 2004. Formation of deletions during double-strand break repair in Drosophila DmBlm mutants occurs after strand invasion. Proc. Natl. Acad. Sci. USA 101: 15694–15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneely P. M., Farago A. F., Kauffman T. M., 2002. Crossover distribution and high interference for both the X chromosome and an autosome during oogenesis and spermatogenesis in Caenorhabditis elegans. Genetics 162: 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz P., 1994. An analysis of interference in the fission yeast Schizosaccharomyces pombe. Genetics 137: 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale M. J., Keeney S., 2006. Clarifying the mechanics of DNA strand exchange in meiotic recombination. Nature 442: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishant K. T., Plys A. J., Alani E., 2008. A mutation in the putative MLH3 endonuclease domain confers a defect in both mismatch repair and meiosis in Saccharomyces cerevisiae. Genetics 179: 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak J. E., Ross-Macdonald P. B., Roeder G. S., 2001. The budding yeast Msh4 protein functions in chromosome synapsis and the regulation of crossover distribution. Genetics 158: 1013–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. D., Lao J. P., Hwang P. Y., Taylor A. F., Smith G. R., et al. , 2007. BLM ortholog, Sgs1, prevents aberrant crossing-over by suppressing formation of multichromatid joint molecules. Cell 130: 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman F., Dixon J., Doe C. L., Whitby M. C., 2003. Generating crossovers by resolution of nicked Holliday junctions: a role for Mus81-Eme1 in meiosis. Mol. Cell 12: 761–774. [DOI] [PubMed] [Google Scholar]
- Pâques F., Haber J. E., 1999. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63: 349–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochart P., Woltering D., Hollingsworth N. M., 1997. Conserved properties between functionally distinct MutS homologs in yeast. J. Biol. Chem. 272: 30345–30349. [DOI] [PubMed] [Google Scholar]
- Rockmill B., Fung J. C., Branda S. S., Roeder G. S., 2003. The Sgs1 helicase regulates chromosome synapsis and meiotic crossing over. Curr. Biol. 13: 1954–1962. [DOI] [PubMed] [Google Scholar]
- Ross-Macdonald P., Roeder G. S., 1994. Mutation of a meiosis-specific MutS homolog decreases crossing over but not mismatch correction. Cell 79: 1069–1080. [DOI] [PubMed] [Google Scholar]
- Schwacha A., Kleckner N., 1994. Identification of joint molecules that form frequently between homologs but rarely between sister chromatids during yeast meiosis. Cell 76: 51–63. [DOI] [PubMed] [Google Scholar]
- Schwacha A., Kleckner N., 1995. Identification of double Holliday junctions as intermediates in meiotic recombination. Cell 83: 783–791. [DOI] [PubMed] [Google Scholar]
- Schwartz E. K., Heyer W. D., 2011. Processing of joint molecule intermediates by structure-selective endonucleases during homologous recombination in eukaryotes. Chromosoma 120: 109–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekelsky J., McKim K. S., Chin G. M., Hawley R. S., 1995. The Drosophila meiotic recombination gene mei-9 encodes a homologue of the yeast excision repair protein Rad1. Genetics 141: 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekelsky J., Brodsky M. H., Burtis K. C., 2000. DNA Repair in Drosophila. Insights from the Drosophila genome sequence. J. Cell Biol. 150: F31–F36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R., Boddy M. N., Shanahan P., Russell P., 2003. Fission yeast Mus81.Eme1 Holliday junction resolvase is required for meiotic crossing over but not for gene conversion. Genetics 165: 2289–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F. W., 1994. The Holliday junction on its thirtieth anniversary. Genetics 138: 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W., 1983. The double-strand-break repair model for recombination. Cell 33: 25–35. [DOI] [PubMed] [Google Scholar]
- van Brabant A. J., Ye T., Sanz M., German I. J., Ellis N. A., et al. , 2000. Binding and melting of D-loops by the Bloom syndrome helicase. Biochemistry 39: 14617–14625. [DOI] [PubMed] [Google Scholar]
- Villeneuve A. M., Hillers K. J., 2001. Whence meiosis? Cell 106: 647–650. [DOI] [PubMed] [Google Scholar]
- Wilkins A. S., Holliday R., 2009. The evolution of meiosis from mitosis. Genetics 181: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Hickson I. D., 2003. The Bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature 426: 870–874. [DOI] [PubMed] [Google Scholar]
- Yokoo R., Zawadzki K. A., Nabeshima K., Drake M., Arur S., et al. , 2012. COSA-1 reveals robust homeostasis and separable licensing and reinforcement steps governing meiotic crossovers. Cell 149: 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharyevich K., Ma Y., Tang S., Hwang P. Y., Boiteux S., et al. , 2010. Temporally and biochemically distinct activities of Exo1 during meiosis: double-strand break resection and resolution of double Holliday junctions. Mol. Cell 40: 1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharyevich K., Tang S., Ma Y., Hunter N., 2012. Delineation of joint molecule resolution pathways in meiosis identifies a crossover-specific resolvase. Cell 149: 334–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalevsky J., MacQueen A. J., Duffy J. B., Kemphues K. J., Villeneuve A. M., 1999. Crossing over during Caenorhabditis elegans meiosis requires a conserved MutS-based pathway that is partially dispensable in budding yeast. Genetics 153: 1271–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickler D., Kleckner N., 1999. Meiotic chromosomes: integrating structure and function. Annu. Rev. Genet. 33: 603–754. [DOI] [PubMed] [Google Scholar]