Abstract
Autophagy refers to a group of processes that involve degradation of cytoplasmic components including cytosol, macromolecular complexes, and organelles, within the vacuole or the lysosome of higher eukaryotes. The various types of autophagy have attracted increasing attention for at least two reasons. First, autophagy provides a compelling example of dynamic rearrangements of subcellular membranes involving issues of protein trafficking and organelle identity, and thus it is fascinating for researchers interested in questions pertinent to basic cell biology. Second, autophagy plays a central role in normal development and cell homeostasis, and, as a result, autophagic dysfunctions are associated with a range of illnesses including cancer, diabetes, myopathies, some types of neurodegeneration, and liver and heart diseases. That said, this review focuses on autophagy in yeast. Many aspects of autophagy are conserved from yeast to human; in particular, this applies to the gene products mediating these pathways as well as some of the signaling cascades regulating it, so that the information we relate is relevant to higher eukaryotes. Indeed, as with many cellular pathways, the initial molecular insights were made possible due to genetic studies in Saccharomyces cerevisiae and other fungi.
Keywords: autophagy, macroautophagy, membrane, organelle, protein degradation, protein trafficking, stress, vacuole
ALTHOUGH, historically, greater attention has focused on biosynthetic processes, it is clear that cellular homeostasis requires a balance between anabolism and catabolism. Thus, cells have an array of processes for breaking down proteins and other macromolecules, as well as organelles, and each of these distinct processes differ with regard to the machinery involved, the nature of the substrate, and the site of sequestration. The two primary mechanisms for subcellular degradation are the ubiquitin-proteasome system (UPS) and autophagy. A third, less-well-characterized, mechanism is the vacuole import and degradation (Vid) pathway (Hoffman and Chiang 1996), for which the most critical substrate is fructose-1,6-bisphosphatase (Fbp1), the key enzyme in gluconeogenesis, but other target proteins include Pck1, Mdh2, and Icl1 (Hung et al. 2004; Brown et al. 2010). Degradation of Fbp1 in the absence of glucose prevents futile cycling where the cell attempts to generate glucose under conditions where the carbon source is limiting. In the Vid pathway, which occurs under conditions of prolonged glucose starvation, Fbp1 is translocated into 30-nm cytosolic vesicles that subsequently fuse with the vacuole, releasing their contents into the lumen, where Fbp1 is degraded (Huang and Chiang 1997). The mechanism by which Fbp1 is translocated into the completed Vid vesicles remains unknown. The UPS can also target Fbp1 (Horak et al. 2002; Regelmann et al. 2003; Hung et al. 2004) and many other proteins, principally those with a short half-life (Ravid and Hochstrasser 2008). The targets are again individual proteins, but in this case they are tagged with ubiquitin chains and are not sequestered within a vesicle, but rather are recognized by, and degraded within, the proteasome, a multisubunit protein channel that includes deubiquitinating enzymes and proteases. In contrast with the Vid pathway and autophagy, UPS-mediated degradation occurs in the cytosol (or the nucleus), not in the vacuole.
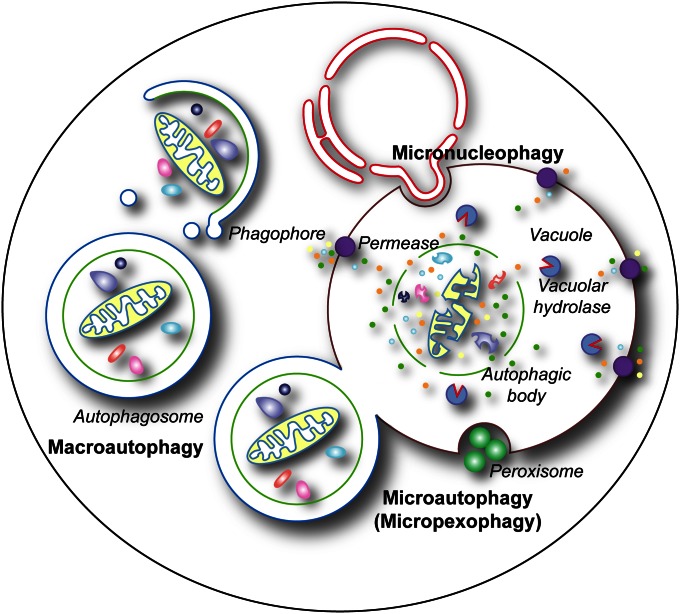
Autophagy can be divided into two main types, microautophagy and macroautophagy (Figure 1), and both of these include nonselective and selective processes (Shintani and Klionsky 2004a). Nonselective microautophagy is not well defined with regard to the machinery involved or its physiological role. In this process the vacuole membrane invaginates and scissions to produce intravacuolar vesicles that are subsequently degraded (Kunz et al. 2004; Uttenweiler and Mayer 2008). This type of microautophagy does not rely directly on the cellular components involved in selective types of microautophagy or macroautophagy. In contrast, selective microautophagy has more in common with macroautophagy since both processes share most of the same machinery (Table 1). Selective microautophagy is used in the turnover of mitochondria (Deffieu et al. 2009), parts of the nucleus (Krick et al. 2008b), and peroxisomes (Dunn et al. 2005). In this case, the cargo is recruited and sequestered directly by the vacuole membrane, and the following invagination or protrusion/septation leads to its delivery into the vacuole lumen. Whereas selective microautophagy involves uptake of the cargo directly at the limiting membrane of the vacuole, the morphological hallmark of macroautophagy is the sequestration of the targeted cargo within cytosolic double-membrane vesicles that subsequently fuse with the vacuole, allowing (in most cases) breakdown of the cargo and recycling of the resulting macromolecules (Eskelinen et al. 2011). In this review, we focus on the mechanism and regulation of selective microautophagy and both selective and nonselective macroautophagy in yeast.
Figure 1.
The principal types of autophagy in yeast. Macroautophagy entails the sequestration of bulk cytoplasm or specific structures into autophagomes. Autophagosomes are formed by expansion of a precursor compartment known as the phagophore, which initiates the sequestration of the cargo. Upon completion, the autophagosome fuses with the vacuole, releasing the inner autophagosome vesicle into the vacuole lumen, where it is now termed an autophagic body. During microautophagy (here micropexophagy is illustrated as an example), the structures targeted to degradation are recruited in proximity to the vacuole membrane. Protrusion/septation and/or invagination of this membrane, followed by scission, allows the cargo to be transported into the vacuolar lumen. Via a similar mechanism, micronucleophagy mediates the turnover of part of the nuclear envelope and content. In most cases, the components delivered by macroautophagy, microautophagy, and micronucleophagy into the interior of the vacuole are degraded by resident hydrolases. The resulting metabolites, i.e., amino acids, sugars, and nucleotides, are subsequently transported into the cytoplasm by permeases (although these have been identified only for amino acids) and used either as a source of energy or as building blocks for the synthesis of new macromolecules.
Table 1. Types of autophagy in yeast.
| Name | Target | Characteristics/requirements |
|---|---|---|
| Microautophagy | Bulk cytosol, vacuole membrane | Uptake by direct invagination |
| Microautophagy, selective | Mitochondria | Uptake by direct invagination or protrusion/septation |
| Peroxisomes | Uptake by direct invagination or protrusion/septation | |
| Nuclear membrane | Invagination requires Nvj1 and Vac8 | |
| Macroautophagy, nonselective | Bulk cytoplasm | Sequestration by autophagosomes |
| Macroautophagy, selective | Sequestration by autophagosomes. Uses a ligand on the cargo, and an autophagy receptor/adaptor system: | |
| Cvt pathway | Resident hydrolases | Signal in the cargo. Atg19, and Atg34 are receptors, Atg11 is a scaffold |
| Mitophagy | Mitochondria | Atg32 is a receptor, Atg11 is a scaffold |
| Pexophagy | Peroxisomes | Atg30 and Atg36 are receptors, Atg11 and Atg17 are scaffolds |
| Ribophagy | Ribosomes | Ubp3–Bre5 |
| Reticulophagy | Endoplasmic reticulum | Atg19 |
| Vid pathway | Fbp1, Icl1, Mdh2, Pck1 | Cargo uptake into 30-nm vesicles |
Physiological Roles of Autophagy
Autophagy is typically considered to be a degradative process that plays a role in the turnover of bulk cytoplasm (Mizushima and Klionsky 2007). While this pathway is primarily degradative, this view is not an adequate representation of the many functional roles of autophagy. Certainly autophagy is important as a response to starvation as cells are frequently confronted by these conditions in the wild. Thus, it is not surprising that a complex system such as autophagy is in place to allow the cell to maintain viability during nutrient depletion. Organelles can be eliminated by nonselective autophagy, but they can also be specifically targeted for degradation. This type of selective organellar autophagy may occur in response to organelle damage or dysfunction, but may also be the result of cellular adaptation to changing nutrient conditions. For example, when yeast cells are shifted from conditions under which they need peroxisomes, such as growth on methanol or oleic acid, to a preferred carbon source such as glucose, they rapidly turn over these organelles that are now in surplus (Tuttle et al. 1993; Titorenko et al. 1995). This type of degradation is beneficial to the cell because organelles are costly to maintain, and they can damage the cell when dysfunctional. Moreover, autophagy can even be involved in a biosynthetic process. The cytoplasm-to-vacuole targeting (Cvt) pathway is used for the delivery of several resident hydrolases to the vacuole, their ultimate site of function (Lynch-Day and Klionsky 2010). The machinery used for the Cvt pathway and the morphology of the process overlap extensively with that of selective macroautophagy (Harding et al. 1996; Scott et al. 1996; Baba et al. 1997). Finally, although more investigation is needed, initial observations also point to autophagosomes being able to deliver specific signaling molecules into the extracellular space through fusion with the plasma membrane.
Morphology
As discussed above, one of the ways to think about the different types of autophagy is in relation to the substrate and the mechanism through which the substrate is separated from the remainder of the cytoplasm and targeted for degradation. Both micro- and macroautophagy involve the movement of macromolecules and organelles from the cytosol into the vacuole lumen. Thus, these components must be translocated from the intracellular space (i.e., the cytosol) to the topological equivalent of the extracellular space. An even simpler way to look at this problem is that during autophagy folded proteins, macromolecular complexes and intact organelles must be moved across a membrane, a process that represents a substantial thermodynamic barrier. Therefore, particularly with regard to an organelle, the question is, What process can the cell use to accomplish this requirement?
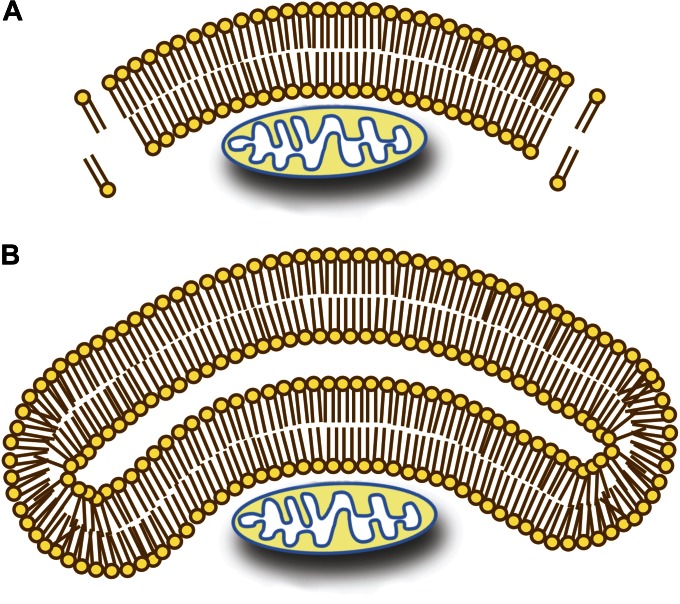
During transport throughout the secretory pathway, cargo transits within vesicles from one compartment to another. The critical issue then is getting the cargo within a vesicle. The cargo must first translocate across the ER membrane, a process that requires the protein to be unfolded and to move through a specialized channel inserted into the ER membrane. Although there are cytosolic chaperones that can unfold proteins, it is not feasible to do this on the scale needed for macroautophagy. In addition, such a mechanism cannot be used with organelles. For these reasons, during macroautophagy the vesicle must be formed around the existing cargo. A final point is that it is not possible to sequester a cytoplasmic cargo within a single-membrane vesicle; exposure of the hydrophobic core of the bilayer during the sequestration event would be thermodynamically unfavorable (Figure 2). As a consequence, this type of sequestration necessitates the use of a double membrane.
Figure 2.
Sequestration of cytoplasmic cargo requires a double-membrane compartment. (A) Exposure of the hydrophobic core of a lipid bilayer to the aqueous cytosol would make it energetically unfavorable to use a single-bilayer membrane to sequester a cytoplasmic cargo. In this scenario, it would also be unclear how the phagophore membrane would expand by lipid addition. (B) The use of a double-lipid bilayer maintains thermodynamic energy requirements, while allowing the cargo to be sequestered by expansion of the double membrane. The expansion of the phagophore could occur by lateral movement or translocation of lipids from an attached organelle, or by vesicular fusion.
Nonselective macroautophagy
Formation of the autophagosome is described as de novo to distinguish it from what happens in the secretory pathway because these double-membrane vesicles do not form by budding from a preexisting organelle (Noda et al. 2002; Kovacs et al. 2007). The process of autophagosome biogenesis is perhaps the least understood part of macroautophagy, and many aspects remain to be fully elucidated. The first issue concerns the nature of the nucleation process. The initial sequestering compartment is termed the phagophore (Figure 1) (Klionsky et al. 2011). Accordingly, the phagophore assembly site (PAS) is the name given to the presumed nucleating site. The PAS is located next to the vacuole, although it is not known if there is any significance to this particular localization. The majority of the autophagy-related (Atg) proteins (Klionsky et al. 2003) that constitute the machinery of autophagy localize at least transiently to this site based on fluorescence microscopy of fluorophore-tagged chimeras (Suzuki et al. 2001, 2007; Kim et al. 2002). This observation has led to the circular definition of the PAS as the site of Atg protein localization, with the site where Atg proteins localize being defined as the PAS. At least part of the reason for this confusion is that the PAS is otherwise uncharacterized; it is not known whether it is a membrane structure, whether it is permanent, or whether it is literally converted into a phagophore as opposed to playing a role in the formation of a separate phagophore. Nonetheless, for the purposes of this review we consider the PAS as the dynamic precursor structure that nucleates into a phagophore. In a wild-type strain only ∼30% of the cells have a detectable PAS (based on the localization of a fluorescent-tagged protein such as GFP–Atg8), whereas essentially the entire population displays a PAS when macroautophagy is blocked in an atg mutant (Shintani and Klionsky 2004b). Thus, either the PAS is a transient structure or localization of the Atg proteins to the PAS is dynamic, with proteins such as Atg8 cycling on and off.
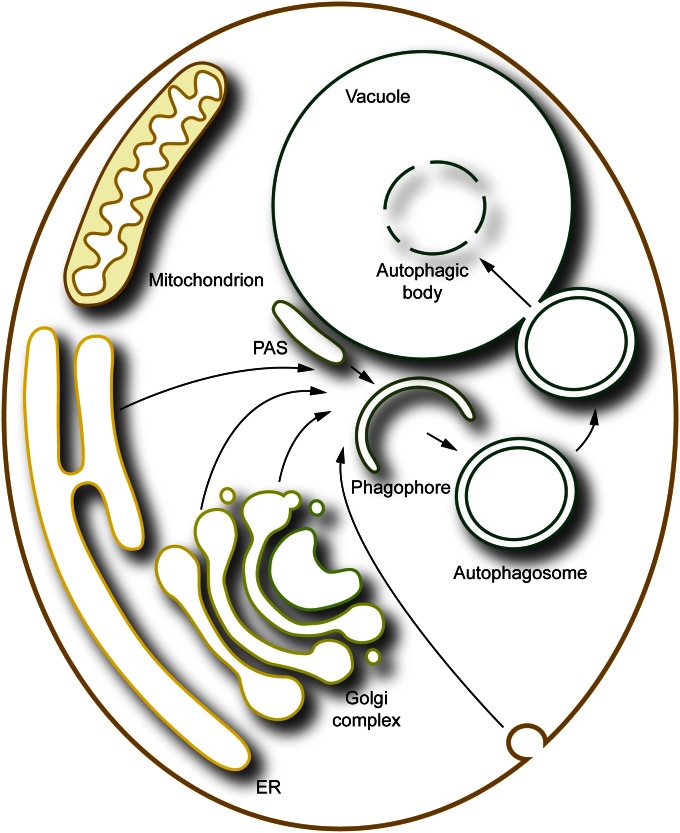
A general model for the generation of an autophagosome involves the expansion of the phagophore by the addition of lipid bilayers from one or more donor sources. Presumably the membrane is delivered to the phagophore through vesicular trafficking events in yeast, but direct translocation from an adjacent organelle cannot be excluded. SNARE proteins, which play a role in membrane fusion, are implicated in macroautophagy (Nair et al. 2011), perhaps functioning at multiple steps of the pathway, including PAS/phagophore assembly and/or phagophore expansion, in addition to the fusion of the completed autophagosome with the vacuole. The origin of the membrane(s) that allow the phagophore to be formed and expand, that is, the membranes that ultimately form the autophagosome, is another highly controversial topic (Reggiori 2006). Molecular genetic studies have implicated protein components that normally function throughout the cell. For example, the involvement of Ypt1 and the autophagy-specific TRAPPIII complex (Lynch-Day et al. 2010) suggest that the membrane is delivered to the phagophore from the endoplasmic reticulum (ER). This would not be surprising considering the role of this organelle in synthesis of phospholipids, the major component of autophagosomal membranes. However, the requirement for the conserved oligomeric Golgi (COG) complex that acts as a tether, and several components that function in protein secretion from the trans-Golgi including Sec2, Sec4, Sec7, Arf1/2 and Pik1, suggests that the Golgi apparatus is also an important membrane donor (Geng et al. 2010; Mari et al. 2010; van der Vaart et al. 2010; Yen et al. 2010; Wang et al. 2012). It is also possible that other compartments, including the plasma membrane (Taylor et al. 2012), provide material for phagophore expansion. The cell may in fact mobilize membrane from multiple sources to meet the substantial demands of macroautophagy (Figure 3).
Figure 3.
Multiple membrane sources may contribute to formation and expansion of the phagophore. Various compartments including the ER, the Golgi apparatus, and the plasma membrane may contribute to the nucleation and/or expansion of the phagophore. See the text for details.
Another issue concerns the curvature of the sequestering membrane. In yeast, no proteins containing BAR domains have been clearly associated with autophagosome formation. Similarly, this process does not appear to involve the use of a canonical protein coat such as clathrin or COPII, which may not be surprising considering that the huge size of the autophagosome would require an unusually large amount of coat components. In the case of selective autophagy the cargo may determine the curvature. One observation in favor of this possibility is the interaction between the autophagy receptors (see below) and Atg8, which is also present on the phagophore membrane. This type of protein–protein interaction may allow the forming membrane to essentially wrap around the cargo. With nonselective macroautophagy, however, this mechanism cannot be invoked. Instead, biophysical parameters may be responsible for the curvature, including the potential unequal distribution of lipids, such as phosphatidylethanolamine (PE) or phosphatidylinositol-3-phosphate (PtdIns3P), or proteins—in particular on what will become the convex surface—which would cause the lipid bilayers to form a curved structure. Autophagosomes typically fall within a particular size range of ∼400–900 nm and only the presence of an extremely large cargo could promote bending of the membrane.
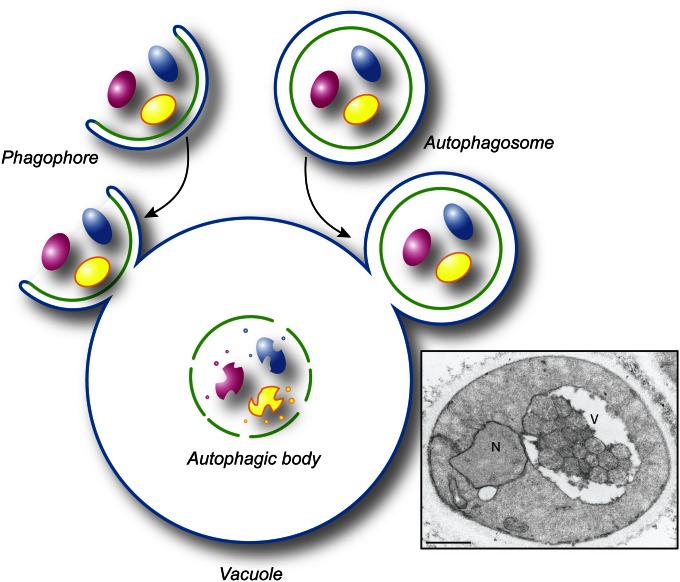
The ultimate goal of phagophore expansion is the complete sequestration of the cargo, which requires the phagophore to seal, thus forming the autophagosome. The necessity of this step can be visualized by considering the outcome of fusion between a phagophore vs. an autophagosome and the vacuole (Figure 4). In the former event, the cargo is not delivered into the vacuole lumen, whereas in the latter the inner, now separate, vesicle of the autophagosome enters the vacuole lumen, where it is subsequently degraded. This inner vesicle, when present in the vacuole lumen, is termed an autophagic body. It is thus critical to prevent premature fusion between a phagophore and the vacuole. Although the mechanism involved is unknown, there are indications that release of the Atg machinery from the phagophore could be a critical regulatory step (see below). Similarly, it is not understood how the phagophore opening is closed, an event that presumably requires scission or fusion to separate the inner and outer membrane.
Figure 4.
Topology of autophagosome fusion with the vacuole. Fusion of an expanding phagophore (i.e., an incomplete autophagosome) with the vacuole (or lysosome in higher eukaryotes) does not allow delivery of the cytoplasmic content into the interior of the degradative organelle (left side of the drawing). In contrast, the fusion of a sealed autophagosome with the vacuole permits the delivery of its internal vesicle and cargo into the lumen making it accessible for subsequent degradation (right side of the drawing). The mechanism that prevents premature fusion of a phagophore with the vacuole is not known. The electron micrograph shows the presence of autophagic bodies in the vacuole. Scale bar, 1 µm. This image was modified from data previously published in Scott et al. (2000) and is reproduced by permission of the American Society for Biochemistry and Molecular Biology and Elsevier, copyright 2000.
In most situations, sequestration of the cargo is not the end point of macroautophagy. A possible exception is seen with reticulophagy (Klionsky et al. 2007), the selective degradation of the ER that is induced by extreme stress in this organelle due to extensive protein misfolding (Yorimitsu et al. 2006). In this case, sequestration of a portion of the ER is sufficient to restore secretory capacity at a level that allows maintaining cell viability (Bernales et al. 2006), essentially providing additional time for the stress to dissipate and/or be handled by other systems. Under conditions where macroautophagy is induced by nutrient depletion, the final critical step of the process requires lysis of the autophagic body membrane, breakdown of the cargo, and efflux of the resulting metabolites for reuse in the cytosol. Although some vacuole membrane amino acid permeases have been identified or biochemically characterized (Klionsky et al. 1990), there is no information regarding the mechanism by which other types of macromolecules such as carbohydrates, nucleotides or lipids might be transported out of the vacuole.
Selective macroautophagy
The overall morphology of selective macroautophagy is largely the same as that of nonselective macroautophagy with one primary distinction—in the former, the sequestering membrane is in close apposition to the cargo, excluding bulk cytoplasm. Partly for this reason, the completed sequestering double-membrane vesicles are given different names, whereas phagophore is a common term used in all cases. Thus, in the Cvt pathway the initial vesicle is termed a Cvt vesicle rather than an autophagosome (see below), and the resulting single-membrane vesicle in the vacuole lumen is a Cvt body (Baba et al. 1997). The terms mitophagosome and pexophagosome have similarly been used when referring to mitophagy and pexophagy, respectively (Ano et al. 2005b; Kim et al. 2007).
In nonselective macroautophagy the cargo is considered to be random cytoplasm. Thus, there is almost no effective size limit to the cargo, although the cytoskeleton is not sequestered within autophagosomes. In contrast, there appears to be a limit to the size of the sequestering vesicles formed during selective types of macroautophagy, which is dictated in part by the volume of the cargo. It is not clear why there is a size limit to the sequestering vesicle of selective autophagy if the membrane forms by wrapping around the cargo due to interactions between the autophagy receptor and Atg8, but the levels of this latter protein could be the limiting factor; higher amounts of Atg8 are required during nonselective macroautophagy to sustain the formation of large autophagosomes (see below).
Nonselective microautophagy
Nonselective microautophagy has been studied both in vitro and in vivo (Muller et al. 2000; Sattler and Mayer 2000). During this process a portion of the vacuole membrane invaginates to form a long, narrow tube-like structure. The sides and/or tip of the tube bud off to form an intravacuolar vesicle. The budding tip is devoid of membrane proteins and thus the resulting vesicle is similar in size to, and indistinguishable from, an autophagic body. In the last step of this process, as well as in selective microautophagy (see below), the intravacuolar vesicles must be degraded. In contrast to macroautophagy, however, the vesicle membrane is derived from the vacuole. It is not known how these membranes are now distinguished from the vacuole limiting membrane such that they can be degraded without disrupting the integrity of the vacuole.
Selective microautophagy
In microautophagy the sequestration of the cargo occurs directly at the vacuole-limiting membrane. The mechanism through which the vacuole membrane is induced to invaginate or protrude/septate to sequester the cargo is unknown. The closest parallel may be seen in the multivesicular body (MVB) pathway, in which the endosomal membrane invaginates to generate intralumenal vesicles. The MVB pathway requires the function of a series of large protein complexes, but the components of these complexes do not appear to play a role in yeast macroautophagy (Reggiori et al. 2004b), and hence presumably not in microautophagy either. In general, the protein machinery needed for macroautophagy is also needed for microautophagy. The simplest way to view this overlap is that one of the important roles for these proteins is the rearrangement of intracellular membrane to form a sequestering double-membrane structure, whether it involves the de novo generation of the phagophore or utilizes the sequestering arms of the vacuole.
The morphological details of selective microautophagy have been the most thoroughly explored in the case of micropexophagy in methylotrophic yeast such as Pichia pastoris and Hansenula polymorpha. A unique structure, the micropexophagic apparatus (MIPA) (Oku et al. 2003), which does not have an obvious functional equivalent in macropexophagy, characterizes this process. The MIPA is a membranous cistern that may operate as a scaffold for completion of the sequestering membrane and it is located at the open end of the vacuolar sequestering membranes (Oku et al. 2006). After the completion of sequestration, the peroxisomes are enclosed within a single-membrane intralumenal vesicle (Sakai et al. 1998), which is similar to the outcome of macropexophagy. One distinct difference between micro- and macropexophagy, however, is that during the former, multiple peroxisomes are sequestered, compared to a single peroxisome being the target during macropexophagy (Dunn et al. 2005). This difference may reflect the membrane source(s) used in these respective modes of sequestration. In particular, the vacuole is a relatively large organelle, and accordingly it may be possible to use a substantial amount of membrane during sequestration.
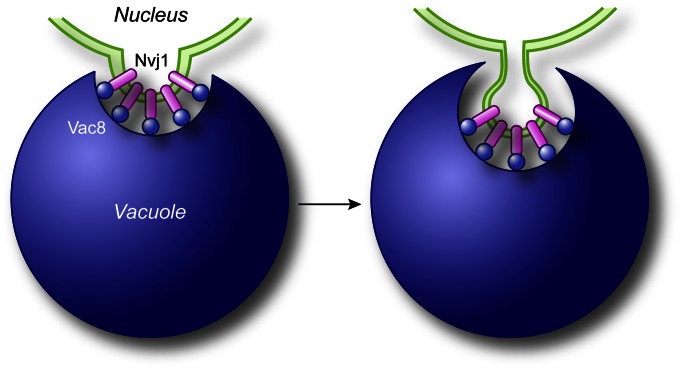
Another example of selective microautophagy is seen with micronucleophagy, also called piecemeal microautophagy of the nucleus (Roberts et al. 2003; Krick et al. 2008b). Small portions of the nucleus, including the nuclear double membrane and part of the nucleoplasm, protrude into the vacuole lumen (Figure 5). A scission event separates the membrane from the nucleus and the vacuole-limiting membrane, again generating a single-membrane intralumenal vesicle. There is no apparent specificity for which part of the nucleus is degraded, but this is still a selective process because it targets only the nucleus.
Figure 5.
Mechanism of micronucleophagy. During micronucleophagy (also called piecemeal microautophagy of the nucleus), small portions of the nucleus, including the nuclear double membrane and part of the nucleoplasm, protrude into the vacuole lumen though a process that requires the association between Nvj1 in the nuclear membrane and Vac8 on the surface of the vacuole. Subsequently, a scission event mediated by Atg proteins leads to the generation of a subvacuolar vesicle that is degraded by resident hydrolases.
There is also evidence for micromitophagy, but this process has been less well characterized. Electron microscopy studies suggest that portions of the vacuole membrane may protrude to sequester mitochondria (Kissova and Camougrand 2009).
Noncanonical autophagy
Cytoplasm-to-vacuole targeting pathway:
The morphology of the Cvt pathway is similar to that of other types of selective autophagy. The primary cargo protein of the Cvt pathway, precursor aminopeptidase I (prApe1), is synthesized in the cytosol and assembles into a dodecamer, which subsequently associate into an oligomer of a supra-order that is termed the Ape1 complex. This complex in combination with the receptor protein Atg19 and smaller oligomers formed by Ams1 (see below) is named the Cvt complex (Shintani et al. 2002). As mentioned above, the double-membrane sequestering vesicle is called a Cvt vesicle, and the intravacuolar single-membrane vesicle is called a Cvt body. The Cvt vesicle has a diameter of ∼140–160 nm, which corresponds with the size of the Cvt complex that is generated under physiological conditions. In contrast, when prApe1 is overproduced, a larger cytosolic complex is formed (Baba et al. 1997). This complex can no longer be sequestered within a Cvt vesicle, and as a consequence the major part of the prApe1 complex accumulates in the cytosol. If nonselective macroautophagy is induced, however, even these large complexes can be sequestered within an autophagosome and efficiently delivered into the vacuole (Scott et al. 1996).
Compartment for unconventional protein secretion:
Studies in Saccharomyces cerevisiae and P. pastoris have revealed that extracellular delivery of the cytosolic acyl coenzyme A-binding protein (Acb1), which occurs under starvation conditions, is not mediated by the secretory pathway (Duran et al. 2010; Manjithaya et al. 2010a). This transport route depends on Atg proteins, leading to the suggestion that autophagosomes could be the hallmark of this type of unconventional secretion (Duran et al. 2010; Manjithaya et al. 2010a). This conclusion, however, is not supported by ultrastructural observations, and therefore, the nature of the carriers transporting Acb1 remains to be deciphered. Nonetheless, electron microscopy work in S. cerevisiae has revealed that the initial precursor structure of this transport route is a cluster of membranes and vesicles, which morphologically resemble the precursor structures involved in autophagy (Mari et al. 2010), and they are positive for both Atg8 and Atg9 (Bruns et al. 2011).
Protein Machinery
Macroautophagy
Most of the machinery used in macroautophagy and selective microautophagy is conserved between these pathways (Kraft et al. 2009; Li et al. 2012). During the initial identification of the genes encoding the Atg proteins, several were classified as being specific for nonselective macroautophagy, the Cvt pathway and/or pexophagy. For example, Atg11 was originally characterized as being a Cvt pathway-specific protein (Harding et al. 1996; Oda et al. 1996). These denotations, however, reflected the different screens used to identify the corresponding genes and the limited analyses available at that time. We now know that Atg11 is involved in most or even all types of selective micro- and macroautophagy (Kim et al. 2001; Kanki and Klionsky 2008; Krick et al. 2008b). Furthermore, Atg11 even plays a role in the transition from the vegetative PAS to a starvation-specific PAS (Cheong et al. 2008). Here, we briefly review the current information on the functions of, and interactions among, the Atg proteins.
Atg1 kinase complex:
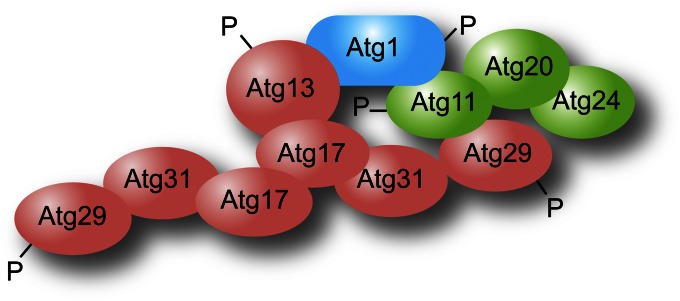
Atg1 is a serine/threonine protein kinase (Matsuura et al. 1997). It carries out autophosphorylation and presumably also phosphorylates other targets. The key substrate(s) of Atg1 with regard to autophagy, however, is unknown. Atg13 is required for optimal Atg1 kinase activity (Kamada et al. 2000), and Atg13 is hyperphosphorylated under nutrient-rich conditions, while being largely dephosphorylated under starvation conditions (Scott et al. 2000). Initial studies suggested that hyperphosphorylated Atg13 interacts with Atg1 with low affinity (Kamada et al. 2000), leading to a model whereby the Atg1 kinase complex functions in part as a switch between the constitutive Cvt pathway and nonselective autophagy. More recent data, however, suggest that Atg13 is always in a complex with Atg1 (Kraft et al. 2012), which would be in agreement with the interactions of the homologous proteins in higher eukaryotes. Atg17, Atg29, and Atg31 form a stable ternary complex, with Atg31 bridging the other two proteins (Kawamata et al. 2008; Cao et al. 2009; Kabeya et al. 2009). Atg17 most likely binds Atg13 directly, but in addition interacts with the complex via Atg29 and Atg11 (Yorimitsu and Klionsky 2005), a scaffold protein that also binds Atg1 (Figure 6). Atg17 (and hence, the Atg17–Atg31–Atg29 subcomplex) is also required for maximal Atg1 kinase activity (Kamada et al. 2000), although the mechanism through which Atg17 or Atg13 regulate Atg1 is not known. Atg17 may also play a role in organizing the recruitment of Atg proteins to the PAS, particularly under autophagy-inducing conditions (Cheong et al. 2008). This possibility is supported by the Atg17 crystal structure, which reveals that this protein assembles into a dimer with an extended coiled-coil domain that could regulate the intrinsic ability of Atg1 to tether membranes, but also acts as a scaffold (Ragusa et al. 2012). In the absence of Atg1 or Atg13 function Atg9–GFP localizes primarily at the PAS (Reggiori et al. 2004a), suggesting a role in regulating the movement of this protein, a step in autophagosome formation and/or completion. Atg13 (and also Atg1), however, are phosphorylated by the target of rapamycin (TOR) and/or protein kinase A (PKA) (Budovskaya et al. 2005; Kamada et al. 2010); post-translational modification by these upstream nutrient sensors suggests that these proteins act as a core regulator that functions at an early step in autophagy induction.
Figure 6.
The interactome of the Atg1 complex. Note that there is no indication that all the depicted interactions occur simultaneously, and not all of the known interactions are shown; the Atg1 complex interactors could vary depending on both the step in the formation of the double-membrane vesicle and the type of autophagy.
Atg20 and Snx4/Atg24, two sorting nexins, were identified on the basis of the Cvt-defective phenotype of the corresponding null strains (Nice et al. 2002). Atg20 interacts with Atg11, and both proteins bind Atg17. Atg20 and Snx4 also bind PtdIns3P via PX domains and localize to the PAS. The function of these proteins in the Cvt pathway, however, is not known.
PtdIns 3-kinase complex:
In yeast there are at least two protein complexes that direct the synthesis of PtdIns3P (Kihara et al. 2001). Both complexes include Vps15 (a regulatory kinase), Vps34 (the PtdIns 3-kinase), and Vps30/Atg6 (Herman et al. 1991; Stack et al. 1993; Stack et al. 1995; Kametaka et al. 1998; Kihara et al. 2001). Complex I also includes Atg14 and functions in autophagy, whereas complex II contains Vps38 and is involved in endosomal trafficking, endocytosis, and the vacuolar protein sorting pathway (Kihara et al. 2001). The role of Vps30 is unknown, but it interacts directly with Atg14 (Kametaka et al. 1998). The latter plays a role in directing the localization of the complex to the PAS (Obara and Ohsumi 2011). A few of the Atg proteins bind PtdIns3P (Nice et al. 2002; Reggiori et al. 2004a; Krick et al. 2006; Nair et al. 2010), suggesting that one function of this phosphoinositide is the recruitment of proteins that function in phagophore and autophagosome formation. However, it cannot be excluded that PtdIns3P is able to regulate the activity of one or more Atg proteins.
Atg9 complex:
Atg9 is the only transmembrane protein that is absolutely required for autophagosome formation (Noda et al. 2000). Atg9 transits through a portion of the secretory pathway and can be detected at the ER and Golgi apparatus as well as the PAS (Mari et al. 2010; Ohashi and Munro 2010; Yamamoto et al. 2012). In wild-type cells, Atg9–GFP is found in multiple puncta, one of these corresponding with the PAS, and others with peripheral sites (Noda et al. 2000; Reggiori et al. 2004a). As noted above, in an atg1Δ (or atg13Δ) strain Atg9–GFP is localized exclusively at the PAS. In an atg1ts mutant shifted from the nonpermissive to the permissive temperature, Atg9–GFP puncta are seen initially at the PAS and then also appear at the peripheral sites. These observations led to an initial model whereby Atg9 transits between these peripheral sites and the PAS, delivering membrane from donor sites to the expanding phagophore. Recent real-time imaging data, however, have indicated that Atg9 is not cycling through the PAS (Yamamoto et al. 2012). Rather, Atg9 appears to act as a regulator of autophagy initiation possibly by providing at least part of the initial membranes essential to recruit Atg proteins and organize the PAS (Mari et al. 2010; Yamamoto et al. 2012). This latter idea of Atg9 being a landmark scaffold is supported by the fact that this protein can self-interact (Reggiori et al. 2005b; He et al. 2008). The possible regulatory function is also underlined by the observation that Atg9, which principally sits on the external surface of the growing phagophore, is retrieved from the autophagosomal membrane just before or after fusion of autophagosomes with the vacuole (Yamamoto et al. 2012).
The current data indicate that shortly after synthesis, Atg9 is translocated into the ER, and from there it reaches the Golgi where it is very likely sorted into vesicles (Geng et al. 2010; Wang et al. 2012). This hypothesis is supported by the observation that in mutants defective in the function of SNARE proteins involved in protein secretion, in particular an sso1Δ sso2ts strain, Atg9 is detected in small vesicles (Nair et al. 2011). High-resolution microscopy has also confirmed the presence of a vesicular pool of Atg9 (Yamamoto et al. 2012). The fate of these vesicles continues to be a controversial issue. One idea is that they remain independent vesicles and one (or a few) will move in close proximity of the vacuole to become the PAS upon autophagy induction (Yamamoto et al. 2012). Another possibility is that the Atg9-positive vesicles assemble in a SNARE-dependent process (Nair et al. 2011) to generate larger structures corresponding to the peripheral sites and at least one of these structures relocates near the vacuole surface to become the PAS (Mari et al. 2010). Nevertheless, Atg9-positive membranes are probably maturing into the PAS/phagophores possibly by fusing together or with other membranes, as suggested by the colocalization of Atg9 with Ypt1 and the TRAPPIII complex (Lynch-Day et al. 2010; Lipatova et al. 2012) and the recruitment of these latter factors onto autophagosomal membranes by Atg9 (Kakuta et al. 2012).
Both Atg18 and Atg2 are peripheral membrane proteins that interact with each other and associate with Atg9 at the PAS (Reggiori et al. 2004a; Obara et al. 2008). The absence of either protein results in a defect in Atg9 localization similar to that seen in the atg1Δ strain (Reggiori et al. 2004a). The precise role of Atg2 and Atg18 in autophagosome biogenesis is unknown, as is the mechanism through which they mediate Atg9 retrograde transport from autophagosomal membranes and/or the vacuole. The recruitment and localization of Atg18 and Atg2 to the PAS depends on each other, Atg9 and the Atg1–Atg13 kinase complex, and also on the presence of PtdIns3P generated by the PtdIns 3-kinase complex I (Shintani et al. 2001; Reggiori et al. 2004a; Suzuki et al. 2007; C.-W. Wang et al. 2001). The main structural feature of Atg18 is that its 7 WD40 repeats fold into a seven-bladed β-propeller (Barth et al. 2001; Dove et al. 2004). Its predicted structure is very similar to the recently published crystal structure of Kluyveromyces lactis Hsv2, a homolog of Atg18 (Baskaran et al. 2012; Krick et al. 2012; Watanabe et al. 2012). Atg18 is also able to bind both PtdIns3P and phosphatidylinositol-3,5-bisphosphate [PtdIns(3,5)P2] through a conserved phenylalanine–arginine–arginine–glycine (FRRG) motif within its β-propeller (Nice et al. 2002; Dove et al. 2004; Krick et al. 2006; Nair et al. 2010). Atg18 binding to PtsInd3P is essential for its localization to the PAS (Krick et al. 2006, 2008a; Obara et al. 2008; Nair et al. 2010). The PAS localization of Atg18 also depends upon Atg2 and vice versa (Guan et al. 2001; Suzuki et al. 2007; Obara et al. 2008), and it has been proposed that these two proteins constitutively form a complex (Obara et al. 2008). The ability of Atg18 to interact with Atg2 does not depend on its PtdIns3P-binding capacity but rather on residues positioned on the opposite surface from the FRRG motif on the β-propeller (Watanabe et al. 2012; Rieter et al. 2013), whereas the binding of Atg18 to PtdIns3P seems necessary for the appropriate targeting of the Atg18–Atg2 complex to the PAS (Obara et al. 2008).
Atg23 and Atg27 are nonconserved peripheral and integral membrane proteins (Tucker et al. 2003; Yen et al. 2007), respectively, which bind Atg9 and are needed for the efficient movement of this protein to the PAS (Legakis et al. 2007). In particular, they appear to play a key role in this trafficking step by mediating Atg9 sorting from the Golgi (Yamamoto et al. 2012).
Ubiquitin-like protein conjugation complexes:
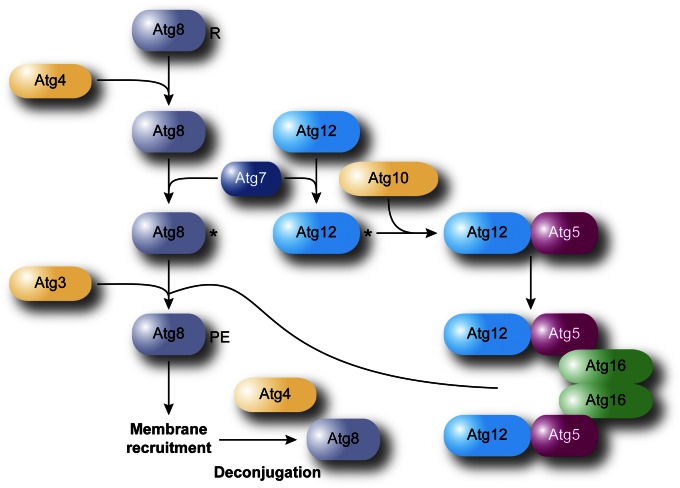
There are two unique ubiquitin-like protein conjugation complexes that participate in autophagy, involving Atg8 and Atg12 (Geng and Klionsky 2008). Based on the crystal structure of the mammalian Atg8 homolog MAP1LC3 (LC3), Atg8 has structural similarities with ubiquitin (Sugawara et al. 2004). It is initially synthesized with a C-terminal arginine that is removed by the Atg4 cysteine protease (Kirisako et al. 1999; Huang et al. 2000) (Figure 7). The processed Atg8 is next activated in an ATP-dependent reaction by the ubiquitin-activating enzyme homolog Atg7 and then transferred to Atg3, a ubiquitin-conjugating enzyme analog. Atg3 forms a covalent bond between the now-exposed C-terminal glycine residue of Atg8 and PE (Ichimura et al. 2000). Atg8 is initially located on both sides of the phagophore. Atg4 can subsequently cleave the amide bond to PE in a deconjugation step, liberating Atg8, particularly the population of the protein that is on the external surface of the autophagosome, from the membrane, and allowing it to cycle through the conjugation process again. Analysis of human ATG4B alone and in combination with LC3 indicates that the protease undergoes a substantial conformational change, which may be critical in gaining access to the lipidated (and hence membrane bound) LC3-II (Atg8—PE) molecule (Sugawara et al. 2005; Kumanomidou et al. 2006; Satoo et al. 2009).
Figure 7.
Schematic of the two ubiquitin-like conjugation systems involved in autophagy. Atg12, a ubiquitin-like molecule, is covalently conjugated to Atg5 through the activity of Atg7 and Atg10, an E1- and an E2-like enzyme, respectively. The Atg12—Atg5 complex subsequently associates with Atg16, and dimerization leads to the formation of a large complex. Atg8 is a second ubiquitin-like protein participating in autophagy. Atg8 is post-translationally processed by the specific cysteine protease Atg4, which removes the C-terminal amino acid (an arginine residue in yeast) exposing a glycine. Through another ubiquitination-like reaction mediated by Atg7 and the E2-like enzyme Atg3, Atg8 is covalently conjugated to PE. While it has been proposed that the Atg12—Atg5–Atg16 complex could be the E3 ligase catalyzing the formation of Atg8—PE, these proteins promote the linkage of Atg8 to PE, but they are not essential for it.
Comparison to the crystal structure of Arabidopsis thaliana ATG12b (Suzuki et al. 2005) suggests that the yeast Atg12 homolog also contains ubiquitin folds and participates in a parallel conjugation pathway. This protein is also activated by Atg7, and structural studies have provided insight into the mechanism by which Atg7 can act as a common E1 for two different conjugating enzymes (Hong et al. 2011; Noda et al. 2011; Taherbhoy et al. 2011; Yamaguchi et al. 2012a). The activated Atg12 is then transferred to the Atg10-conjugating enzyme (Shintani et al. 1999), which catalyzes the formation of a covalent bond between the C-terminal glycine of Atg12 and an internal lysine of Atg5 (Mizushima et al. 1998), a protein that also contains two ubiquitin-like structural domains (Matsushita et al. 2007). The conjugation of Atg12 to Atg5 occurs independently of an E3 ligase, and structural studies may provide information on this unique aspect of Atg10-conjugating activity (Yamaguchi et al. 2012b). Atg5, and preferentially the Atg12—Atg5 conjugate, noncovalently binds Atg16, promoting Atg16 self-interaction (Mizushima et al. 1999), generating a dimer of the Atg12—Atg5–Atg16 complex (Kuma et al. 2002; Fujioka et al. 2010). This complex is proposed to function as an E3-like enzyme for Atg8 conjugation (Hanada et al. 2007; Noda et al. 2013; Otomo et al. 2013), but Atg8—PE can be generated in the absence of these proteins (Cao et al. 2008). Thus, the function of the Atg12—Atg5–Atg16 complex remains unclear, but recent structural studies have revealed that it is probably acting as a platform to bring into close proximity the activated Atg8 in the Atg8–Atg3 conjugate to the acceptor PE (Kaiser et al. 2012; Noda et al. 2013; Otomo et al. 2013). In agreement with this model, recent data suggest that Atg5 contains a membrane-binding domain that is negatively regulated by Atg12, and mutations that interfere with Atg5 membrane binding inhibit macroautophagy (Romanov et al. 2012). Recruitment of the components of the Atg8 conjugation system, i.e., Atg7 and Atg3, onto membranes depends on the Atg12—Atg5–Atg16 complex being able to associate with lipid bilayers (Romanov et al. 2012).
Atg8 shows the greatest change in synthesis of any of the Atg proteins upon autophagy induction in yeast (Kirisako et al. 1999; Huang et al. 2000). Experiments in which the amount of Atg8 is clamped at levels lower than that normally generated during autophagy induction indicate that the amount of this protein correlates with the size of the autophagosome (Xie et al. 2008). Atg8 that lines the concave side of the phagophore also plays a role in cargo recognition during selective types of autophagy by binding the receptors used in the Cvt pathway, pexophagy, and mitophagy (Shintani et al. 2002; Chang and Huang 2007; Mijaljica et al. 2012; Motley et al. 2012). X-ray crystallography, combined with NMR, has revealed that a hydrophobic pocket in Atg8 interacts with the Atg8-interacting motif (AIM, or, with regard to the mammalian homolog, an LC3-interacting region, LIR) in Atg19, providing insight into the mechanism of selective cargo recognition (Noda et al. 2008).
Cargo recognition during selective macroautophagy:
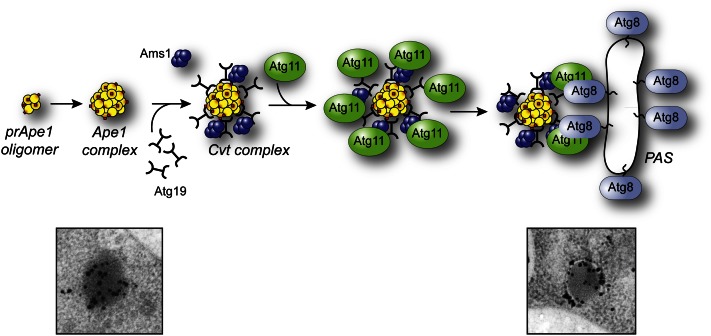
The best-characterized process of selective macroautophagy is the Cvt pathway. Precursor Ape1 contains an N-terminal propeptide that may keep the enzyme inactive in the cytosol. In addition, this amino acid sequence binds a soluble receptor, Atg19 (Scott et al. 2001). The C terminus of Atg19 contains binding sites for Atg11 and Atg8 (Shintani et al. 2002). Localization and affinity isolation experiments suggest that Atg11 binds Atg19 after the latter interacts with the prApe1 propeptide. The extreme C terminus of Atg19 contains a WXXL motif (equivalent to the AIM) that allows binding to Atg8, thus linking the cargo complex with the phagophore (Figure 8) and its subsequent selective sequestration (Kraft et al. 2010). Atg19 also functions as a receptor for Ape4 (Yuga et al. 2011) and Ams1 (Hutchins and Klionsky 2001), two other resident vacuolar hydrolases that are part of the Cvt complex. In addition, Atg34 can substitute for Atg19 as an Ams1 receptor under starvation conditions (Watanabe et al. 2010).
Figure 8.
Mechanism of cargo recruitment during the Cvt pathway. Shortly after synthesis, prApe1 forms dodecamers that subsequently self-assemble in a larger oligomer that has been called the prApe1 complex. Association with the Atg19 autophagy receptor and oligomers of Ams1 (and additional cargo proteins) leads to the generation of the Cvt complex. The subsequent interaction between Atg19 and the autophagy adaptor Atg11 allows the movement of the Cvt complex within proximity of the vacuole through a mechanism that requires actin filaments and the Arp2/3 complex. This relocalization, which probably also coordinates the trafficking of Atg9-positive membranes, participates in the formation of the PAS. At this site, the interaction between Atg19 and Atg8 plays a key role in the sequestration of the Cvt complex into Cvt vesicles. One of the primary differences between selective and nonselective macroautophagy is that the sequestering vesicles of the former exclude bulk cytoplasm and contain primarily the targeted cargo. The electron micrographs depict the electron dense Cvt complex detected with antiserum to Ape1 (left) and a phagophore sequestering a Cvt complex marked with an antibody that detects GFP–Atg8 (right). The electron micrographs in this figure were modified from data previously published in Yen et al. (2010) and are reproduced by permission of the American Society for Cell Biology, copyright 2010.
Similar to Atg19, the mitochondria autophagy receptor Atg32 also interacts first with Atg11 (Kanki et al. 2009b; Okamoto et al. 2009) and then with Atg8 (Okamoto et al. 2009) via an AIM. Atg32 is a transmembrane protein residing in the outer mitochondrial membrane. Atg32 is phosphorylated (Aoki et al. 2011) and the interaction with Atg11 occurs only under conditions that induce mitophagy. Thus, an unknown alteration, perhaps in Atg32 conformation or the phosphorylation-dependent generation of a binding motif, presumably leads to its activation. Mitophagy induced at the post-log phase, but not that induced by starvation, also requires Atg33, a transmembrane protein residing in the outer membrane of the mitochondria (Kanki et al. 2009a). Similar to Atg32, P. pastoris Atg30 (PpAtg30) and S. cerevisiae Atg36 act as peroxisome autophagy receptors during pexophagy (Farre et al. 2008; Motley et al. 2012). Both proteins bind Atg11 and Atg8. Thus, Atg11 is a scaffold protein that is common to many types of selective autophagy. Atg11 interacts with receptors, the Atg1 kinase complex, and itself, thus playing a central role in organization of the Atg proteins at the PAS. In this regard, Atg11 interacts with Atg9 and mediates the relocation of part of the membranes positive for this protein from the peripheral pool to the perivacuolar site that will become the PAS (He et al. 2006; Mari et al. 2010). The movement of Atg9–Atg11 (and probably the cargo that must be sequestered into the nascent double membrane vesicles associated with them) is guided by actin filaments (Reggiori et al. 2005a) via a direct interaction between Atg11 and the Arp2/3 complex (Monastyrska et al. 2008). The cargo and ultimately Atg11, probably through its interaction with the Atg1 complex, could also dictate the kinetics of the autophagic process. The maturation time of precursor Ape1 is ∼90 min, and this time presumably reflects the transport rate of the Cvt pathway (Klionsky et al. 1992). In contrast, monitoring GFP–Atg8 fluorescence at the PAS suggests that a cycle of autophagosome formation and fusion with the vacuole occurs in ∼10 min (Xie et al. 2008), which is somewhat faster than the proposed 10 min half-life of mammalian autophagosomes (Mizushima et al. 2001).
Studies in methylotrophic yeast have identified additional ATG genes that are essential for micro- and/or macropexophagy in these organisms, i.e., ATG25, ATG26, ATG28, and ATG35. Most of them do not have homologs in other yeast, and their precise molecular role has not yet been unveiled. Atg25 is a coiled-coil protein that localizes to the PAS (Monastyrska et al. 2005). This protein appears to be a regulator modulating the switch between selective types of pexophagy, because in its absence peroxisome turnover during glucose-induced macrophexophagy is abolished, whereas the cells constitutively degrade these organelles by micropexophagy. Atg28 and Atg35 form a complex that interacts with Atg17 and is essential for efficient MIPA formation during micropexophagy (Stasyk et al. 2006; Nazarko et al. 2011). As a result, these two proteins are required for this selective process, with Atg28 also being essential for macropexophagy, whereas Atg35 is not. The sterol glucosyltransferase Atg26 is also crucial for the generation of the MIPA (Oku et al. 2003). Upon micropexophagy induction, the synthesis of phosphatidylinositol-4-phosphate at the site where the MIPA will emerge leads to the recruitment of Atg26 through its GRAM domain, which specifically binds to this lipid, and permits the local production of ergosterol glucoside (Oku et al. 2003; Yamashita et al. 2006). The function of this molecule in micropexophagy, however, is unclear and it appears to be essential only for glucose-induced micropexophagy, but not when the same pathway is triggered by oleate or amine (Nazarko et al. 2007).
Late stages of macroautophagy:
Completion of phagophore expansion and sealing of the autophagosome need to be regulated and also must provide some type of signal to allow subsequent fusion with the vacuole (Figure 4). One event that occurs at this time is the turnover of PtdIns3P, which is carried out mostly by the PtsIns3P-specific phosphatase Ymr1 (Cebollero et al. 2012b). The hydrolysis of this lipid is critical in releasing the Atg machinery from the completed autophagosome, and it appears to be requisite for the fusion of these vesicles with the vacuole (Cebollero et al. 2012b). Accordingly, autophagosomes with Atg8 on their surface fuse inefficiently with the vacuole (Nair et al. 2012; Nakatogawa et al. 2012). This fusion step involves components that are common to other transport processes that terminate at the vacuole. Thus, the Rab protein Ypt7, its GDP exchange factor complex Ccz1–Mon1, and SNARE proteins including Vam3, Vam7, Vti1, and Ykt6, along with the class C Vps/HOPS complex, are needed for tethering and fusion (Darsow et al. 1997; Rieder and Emr 1997; Ishihara et al. 2001; Meiling-Wesse et al. 2002; Wang et al. 2002; Nair et al. 2011; Polupanov et al. 2011). The fusion of the autophagosome outer membrane with the vacuole-limiting membrane releases the inner vesicle into the lumen (Figure 1). The membrane of the resulting autophagic bodies is lysed through the action of Atg15, a putative vacuolar lipase (Epple et al. 2001; Teter et al. 2001). While resident hydrolases such as prApe1 are activated in the vacuole lumen by removal of a propeptide, other cargoes are degraded through the action of the various hydrolases. The breakdown products are subsequently transported into the cytosol through vacuole membrane permeases including the amino acid transporter Atg22 (Yang et al. 2006).
Micronucleophagy
The key molecular machinery of micronucleophagy includes two proteins, Nvj1 and Vac8, which are localized in the nuclear and vacuolar membranes, respectively (Roberts et al. 2003). Sequestration occurs at the nucleus–vacuole junction (Figure 5) that is formed through the interaction of these two proteins (Pan et al. 2000). The core Atg machinery is also needed for micronucleophagy; however, it is unknown what role they play in this process or how they become localized to the contact area between the nuclear and vacuolar membranes. In the absence of these core components, micronucleophagy is blocked at a very late step, after protrusion of a part of the nucleus into the vacuole, but prior to scission (Roberts et al. 2003; Krick et al. 2008b). Thus, micronucleophagy appears to differ from micropexophagy because protrusion of the vacuole membrane in the latter requires the Atg proteins. This lack of a requirement for the core machinery in the initial sequestration event actually suggests a fundamental difference between micronucleophagy and other types of autophagy. Micronucleophagy also requires the oxysterol-binding proteins (Kvam and Goldfarb 2004), as well as components involved in very-long-chain fatty-acid formation (Kvam et al. 2005; Dawaliby and Mayer 2010). Two separate stages of micronucleophagy are dependent on activity of the vacuolar ATPase and an electrochemical potential across the vacuole membrane: the initial invagination of the membrane at the nucleus-–vacuole junctions, and the late step of vesicle scission (Dawaliby and Mayer 2010).
Ribophagy
Because ribosomes are very abundant in the cytoplasm of a yeast cell and readily detectable in the interior of autophagosomes by electron microscopy during bulk macroautophagy (Takeshige et al. 1992; Baba et al. 1994), it has been assumed for a long time that they were randomly sequestered into autophagosomes. It has been revealed, however, that ribosomes are degraded through a selective type of macroautophagy, named ribophagy (Kraft et al. 2008), which could also be important for the disposal of defective or incorrectly assembled ribosomes (Cebollero et al. 2012a). Analysis of ribosome half-life under nutrient starvation conditions in S. cerevisiae has shown that these multiprotein complexes are more rapidly turned over compared to other cytoplasmic components, supporting the notion of a selective degradation process (Kraft et al. 2008). While it is clear that ribophagy depends on core components of the autophagy machinery such as Atg1 and Atg7, the molecular principles underlying the selectivity of this pathway remain to be elucidated. Several lines of evidence have indicated that ubiquitination/deubiquitination reactions are probably involved in determining the fate of ribosomes. In particular, the ubiquitin protease Ubp3 and its cofactor Bre5 are required for ribophagy, but not for bulk macroautophagy (Kraft et al. 2008). Interestingly, Ubp3 interacts with Atg19 and influences its ubiquitination status (Baxter et al. 2005), but it is still unclear whether Atg19 participates in ribophagy. Additional evidence for the possible involvement of ubiquitin modifications in ribophagy comes from the observation that a decrease of the cytoplasmic levels of the ubiquitin ligase Rsp5 together with the deletion of UBP3 results in a defect in the turnover of ribosomes greater than that seen in ubp3Δ cells (Kraft and Peter 2008).
Reticulophagy
There are not many studies on reticulophagy. Furthermore, this process has been studied in various contexts. As a result, the data cannot be assembled into a single model because there may be significant differences in the process reflecting the way in which reticulophagy is stimulated. This selective type of macroautophagy has been investigated as a response to chemically induced ER stress, the accumulation of protein aggregates in the ER, starvation, and ER size recovery upon termination of an ER stress. How the ER is targeted for degradation and specifically sequestered into autophagosomes remains to be elucidated. In analogy with mitochondria and mitophagy, one possibility could be that fragments of the ER are fissioned off from the main ER body and are transported to the site where autophagosomes are generated. It is clear that the core Atg components are required for reticulophagy induced by both starvation and ER stress caused by treatment with dithiothreitol or tunicamycin (Hamasaki et al. 2005; Bernales et al. 2006; Yorimitsu et al. 2006), The fact that Atg19, Atg20, and the actin cytoskeleton are essential (Hamasaki et al. 2005; Bernales et al. 2006), however, supports the notion that reticulophagy is a selective type of macroautophagy, but also that the ER could be recruited to the site where it will then be incorporated into nascent autophagosomes. Interestingly, Atg proteins are necessary for cell survival, while vacuolar proteases are dispensable, under conditions of ER stress, indicating that the sequestration of the ER without degradation is sufficient to mitigate the effects of this type of stress (Bernales et al. 2006).
Vacuole import and degradation pathway
The Vid pathway involves the translocation of substrate proteins into 30-nm vesicles. The formation of these Vid vesicles is blocked in the absence of the ubiquitin-conjugating enzyme Ubc1 (Shieh et al. 2001), whereas import of Fbp1 into Vid vesicles requires the plasma membrane protein Vid22, the cyclophilin Cpr1, and the heat-shock protein Ssa2 (Brown et al. 2000, 2001, 2002). In contrast, the peripheral vesicle membrane protein Vid24 acts after the import step, because the vid24-1 mutant accumulates Fbp1 within completed vesicles (Chiang and Chiang 1998). Association of Vid24 with these vesicles is dependent on the coatomer subunit Sec28 (Brown et al. 2008). Vid vesicles merge/cluster with endosomes at actin patches in a process requiring Vid30 (Alibhoy et al. 2012), and subsequent transport to and/or fusion with the vacuole is dependent on Vph1 (Liu et al. 2005), a subunit of the vacuolar H+–ATPase, and both Rab and SNARE components that participate in most vacuolar fusion events including Ypt7, Ykt6, Vti1, and the class C Vps/HOPS complex (Brown et al. 2003).
Unconventional protein secretion
Genetic screens in S. cerevisiae and P. pastoris have revealed that genes involved in autophagy and endosomal trafficking, as well as the phospholipase D Spo14, are essential for the unconventional secretion of Acb1 into the extracellular milieu (Duran et al. 2010; Manjithaya et al. 2010a). Another factor required for this process is Grh1, the yeast homolog of the mammalian Golgi reassembly and stacking protein (GRASP), which has been implicated in various types of unconventional secretion (Nickel and Rabouille 2009). As discussed above, while the nature of the carrier transporting Acb1 remains to be determined, a precursor structure named the compartment for unconventional protein secretion (CUPS) has been characterized (Bruns et al. 2011). This organelle, in close proximity to the ER exit sites and onto which Acb1 is recruited upon nitrogen starvation, is positive for Grh1, Atg8, Atg9, and Vps23, one of the components of the endosomal sorting complex required for transport (ESCRT), as well as PtdIns3P. The formation of the CUPS, however, does not depend on the Atg proteins or Vps23. Consequently it remains to be established how these structures are generated.
Regulation
One of the main differences between autophagy in yeast relative to other eukaryotes concerns the signals that induce the process beyond its basal level. In yeast, nutrient withdrawal is the primary stimulus that induces autophagy, whereas in mammals, numerous cues can regulate this pathway. One point to consider is that regulation is likely complex, in part because excessive—as well as insufficient—autophagy would be deleterious for the cell. In addition, multiple types of signals depending on the nature of the limiting nutrients need to be coordinated, suggesting an intricate network of interactions among the regulatory components.
Nitrogen-dependent regulation
The TOR kinase is considered to be the primary sensor of nitrogen (and amino acids), and the main negative regulator of macroautophagy (Noda and Ohsumi 1998; Cutler et al. 1999). TOR can directly regulate macroautophagy through the phosphorylation of Atg proteins including Atg13. However, TOR also acts through a signaling cascade. Tap42 is a TOR effector that is in a complex with the type 2A protein phosphatase Pph21/Pph22. Overexpression of either Pph21 or Pph22 inhibits macroautophagy, whereas inactivation of a temperature-sensitive tap42 mutant or overexpression of the Tap42 interacting protein Tip41 results in macroautophagy induction under nutrient-rich conditions (Yorimitsu et al. 2009). The target(s) of Tap42–Pph21/Pph22 with regard to macroautophagy regulation is not known. One example of the complexity of the regulatory network is seen with the Ksp1 kinase. Ksp1 appears to positively regulate TOR (Umekawa and Klionsky 2012), but it is also a target of TOR phosphorylation (Huber et al. 2009), which suggests either a feedback or stimulatory feed-forward type of regulation. Furthermore, PKA, which is considered to be primarily a glucose sensor, could act upstream of TOR by regulating Ksp1 activity (Umekawa and Klionsky 2012).
Glucose depletion
Yeasts have a complex pathway for sensing and responding to glucose levels (Zaman et al. 2008). Here, we highlight the information known about the glucose response as it pertains to macroautophagy. High levels of glucose result in the production of cAMP, which binds to, and inactivates, Bcy1, the regulatory subunit of PKA. As a consequence, PKA is activated and inhibits macroautophagy (Budovskaya et al. 2004). PKA directly phosphorylates Atg1 and Atg13, at sites that are distinct from those targeted by TOR, and this post-translational modification regulates the association of these proteins with the PAS (Budovskaya et al. 2005; Stephan et al. 2009). Sch9 is a second glucose sensor that acts in parallel with PKA. Sch9 kinase activity is partly dependent on phosphorylation by TOR, but this is independent of the Sch9 phosphorylation that occurs in the presence of glucose. Similar to PKA, inactivation of Sch9 induces macroautophagy (Yorimitsu et al. 2007; Stephan et al. 2009). This regulation is mediated in part through the Rim15 kinase (a positive regulator of macroautophagy) and the Msn2/Msn4 transcription factors. As in the absence of nitrogen, the depletion of glucose serves as a positive signal for macroautophagy induction. In this case, the Snf1 kinase is involved in regulation (Z. Wang et al. 2001), although the details have not yet been elucidated.
Amino acid and phosphate starvation
Macroautophagy can be induced by nitrogen depletion, and one source of nitrogen is amino acids. Indeed, amino acid depletion is another stress that triggers macroautophagy. The general control of nutrient (GCN) pathway regulates amino acid biosynthesis and also modulates macroautophagy. The Gcn2 kinase is involved in sensing the level of intracellular amino acids and, when activated, initiates a cascade resulting in the activation of the Gcn4 transcription factor (although Gcn4 may also be able to sense amino acid levels through a Gcn2-independent mechanism). One outcome of this signal transduction is the activation of genes involved in amino acid synthesis, but there may also be an increase in the transcription of specific ATG genes (Natarajan et al. 2001). Thus, active Gcn2 stimulates macroautophagy, as does Gcn4. Negative regulation occurs through the degradation of Gcn4, which is mediated by Pho85-dependent phosphorylation when Pho85 is in a complex with the Pcl5 cyclin.
Pho85 is a cyclin-dependent kinase that has both inhibitory and stimulatory roles in macroautophagy regulation, depending on the particular cyclin to which it is bound (e.g., Pho80 or Clg1). Under conditions of high phosphate, the Pho85–Pho80 complex inhibits the Pho4 transcription factor that is needed to induce genes involved in the generation, uptake, and storage of phosphate. Pho85–Pho80 also inhibits the Rim15 kinase (Yang et al. 2010). Conversely, the Pho85–Clg1 complex inhibits the cyclin-dependent kinase inhibitor Sic1, resulting in an activation of Rim15.
Mitophagy
Organelles that are involved in degradative processes such as peroxisomes, which carry out β-oxidation, or mitochondria, which utilize an electron transport chain, are prone to generating reactive oxygen species. Accordingly, these compartments need to be constantly repaired or degraded to prevent additional damage to the organelle or to the remainder of the cell. Maintaining organelle homeostasis is costly, and as a result, organelles that are superfluous, as well as those that are damaged, are subjected to selective degradation. When yeast grow on nonfermentable carbon sources such as glycerol or lactate, the mitochondria proliferate. A subsequent shift to glucose, particularly in medium lacking nitrogen, results in the selective degradation of a portion of the mitochondrial population through mitophagy. Growth of a yeast culture in a nonfermentable carbon source past the logarithmic phase can also induce mitophagy, through a mechanism that does not completely overlap with that stimulated by glucose in combination with nitrogen starvation. In contrast to the autophagic machinery where many components have been identified, however, relatively little is known about the proteins involved in regulating selective autophagy.
Both starvation-dependent and post-logarithmic phase-induced mitophagy are controlled in part through two separate mitogen-activated protein kinase (MAPK) pathways. The bck1Δ mutant was identified in a screen for mitophagy-defective strains (Kanki et al. 2009a). Bck1 is a MAPK kinase kinase, and analysis of both upstream and downstream kinases demonstrated that Pkc1, Bck1, Mkk1/Mkk2, and Slt2 are all required for mitophagy, along with the cell surface sensor Wsc1 (Mao et al. 2011). Slt2 acts at an early stage of mitophagy induction, relative to the second MAPK, Hog1 (Mao et al. 2011). Mitophagy is also regulated by a cell surface sensor, Sln1, along with a two-component signal transducer that includes Ssk1 and the MAPK kinase Pbs2, both of which act upstream of Hog1 (Mao et al. 2011). Downstream targets for Slt2 and Hog1 that are involved in mitophagy have not been identified. Although these MAPKs target certain transcription factors, they may have other targets for controlling mitophagy, since both proteins appear to remain in the cytosol under mitophagy-inducing conditions (Mao et al. 2011). Finally, the regulation of mitophagy in yeasts is likely to be somewhat distinct from the mechanism(s) used in higher eukaryotes. For example, CCCP or poisons that interfere with the electron transport chain do not appear to be strong inducers of mitophagy in yeast, compared to mammalian cells. Such differences may reflect the fact that yeast have evolved to prefer fermentation to respiration, and unlike some mammalian cells, they can dilute out damaged or superfluous organelles by division.
Other factors also control selective mitochondria degradation. For example, starvation-dependent mitophagy is delayed in the absence of Uth1 (Kissova et al. 2004). In addition to Atg33, post-log-phase mitophagy is regulated by Aup1, a phosphatase that localizes to the mitochondrial intermembrane space (Tal et al. 2007). Aup1 function is mediated at least in part through its effect on the phosphorylation of Rtg3, a transcription factor that is a component of the retrograde signaling pathway, which is also required for post-log phase mitophagy (Journo et al. 2009).
Pexophagy
S. cerevisiae has evolved to grow on glucose as its preferred carbon source. Under standard laboratory conditions, peroxisomes are not abundant in this yeast. If forced to grow on oleic acid, however, peroxisomes proliferate because this is the only organelle in this organism that can carry out β-oxidation. A subsequent shift to glucose or to a medium lacking nitrogen results in the rapid and selective degradation of peroxisomes (Hutchins et al. 1999). Methylotrophic yeasts including P. pastoris, H. polymorpha, and Yarrowia lipolytica also synthesize a peroxisomal alcohol oxidase that is able to utilize methanol. When P. pastoris cells are shifted from methanol to glucose, micropexophagy is induced, whereas growth on ethanol results in elimination of the excess organelles through macropexophagy (Tuttle and Dunn 1995). This response, however, appears to directly correlate with ATP levels, rather than the actual carbon source, with higher ATP leading to micropexophagy (Ano et al. 2005a). The nutritional control of pexophagy is complex and varies depending on the specific organism and carbon source. For example, in contrast to P. pastoris, H. polymorpha induces macropexophagy when shifted from methanol to glucose (Till et al. 2012; van Zutphen et al. 2008).
Similar to mitophagy, pexophagy is regulated by the Slt2 pathway (Manjithaya et al. 2010b; Mao et al. 2011). In contrast, the Hog1 pathway does not appear to be involved in controlling this process (Mao et al. 2011).
Transcriptional control
Considering that the amount of Atg8 protein changes substantially upon autophagy induction, displaying as much as a 40-fold increase, and that ATG8 mRNA shows a similar rapid upregulation within 30 min after shifting to starvation conditions (Kirisako et al. 1999), transcriptional control is an obvious component of autophagy regulation. The Ume6 transcription factor is part of a large complex that includes the Sin3 corepressor and the Rpd3 histone deacetylase. Deletion of any of the corresponding genes results in a dramatic increase in the amount of Atg8 prior to macroautophagy induction (Bartholomew et al. 2012). The ATG8 promoter contains a consensus DNA binding sequence for Ume6. This protein is phosphorylated under nitrogen starvation conditions, and this modification is largely blocked in the absence of Rim15. As noted above, the Rim15 kinase appears to be regulated by several kinase sensors that act upstream primarily in inhibitory pathways. These findings support a model in which the nutrient-sensing kinases such as PKA inactivate Rim15 during nutrient-rich conditions, allowing active Ume6 to downregulate the synthesis of Atg8. The lower level of this protein is sufficient to facilitate the formation of the smaller Cvt vesicles under growing conditions. When nutrients are depleted, the sensing kinases are no longer active, alleviating the suppression of Rim15 function, which in turn results in the phosphorylation and inhibition of Ume6. The subsequent increase in transcription of ATG8 results in an increase in the Atg8 protein, allowing the formation of the larger autophagosome.
It is likely that transcriptional control is involved in the regulation of many additional ATG genes, but this has not yet been extensively explored. For example, ATG14 transcription is regulated by the Gln3 transcription factor (Chan et al. 2001). Under conditions of nitrogen starvation, ATG14 transcript levels increase more than 20-fold in a Gln3-dependent manner. Similarly, deletion of URE2, which encodes a negative regulator of Gln3, leads to constitutive expression of ATG14 at a level similar to that seen with rapamycin treatment (Chan et al. 2001). Thus, the TOR pathway, which regulates the phosphorylation of Ure2 and the nuclear localization of Gln3, is also involved in regulation of macroautophagy via transcriptional control.
Inositols
Phosphoinositides, such as PtdIns3P, play a role in recruiting Atg proteins to the PAS and possibly modulating the activity of some of them, and thus might be considered to have a regulatory function in macroautophagy. Another type of inositol-containing macromolecule, the inositol polyphosphates, are also involved in controlling this process, although the mechanism remains to be elucidated. A screen of enzymes involved in inositol polyphosphate synthesis revealed a role for Ipk2 and Kcs1 in macroautophagy (Taylor et al. 2012). The phenotype of the kcs1Δ strain is consistent with a defect in autophagosome formation, which may reflect a failure to correctly localize PtdIns3P, and consequently Atg18, and/or generate PtdIns4P under macroautophagy-inducing conditions.
Conclusions
Autophagy, in all of its various modes, is a complex process devoted mostly to intracellular degradation. Considering that a characterization of the first ATG gene was published in 1997, our molecular understanding of autophagy has expanded tremendously in a relatively short period of time. Nonetheless, many fundamental questions remain to be answered. These include the identification of the membrane(s) used to generate the phagophore (along with a characterization of the machinery used to target the membrane into the macroautophagy pathway), the mechanism of phagophore formation and expansion (including the role of the PAS), the function of most of the Atg proteins, and the identification and characterization of additional regulatory elements that modulate the process, and the enzymes and permeases involved in the degradation and efflux of the vacuolar breakdown products. A more complete understanding of autophagy in yeast is likely to lead to additional breakthroughs in the analysis of this process in other organisms and holds the promise for advances that can lead to therapeutic uses for manipulating autophagy to treat disease.
Acknowledgments
F.R. is supported by Chemical Science (CW) ECHO (700.59.003), Earth and Life Sciences (ALW) Open Program (821.02.017), Deutsche Forschungsgemeinschaft–Netherlands Organization for Scientific Research (NWO) cooperation (DN 82-303), and Netherlands Organization for Health Research and Development (ZonMW) VICI (016.130.606) grants. D.J.K. is supported by National Institutes of Health grant GM053396.
Footnotes
Communicating editor: T. N. Davis
Literature Cited
- Alibhoy A. A., Giardina B. J., Dunton D. D., Chiang H.-L., 2012. Vid30 is required for the association of Vid vesicles and actin patches in the vacuole import and degradation pathway. Autophagy 8: 29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ano Y., Hattori T., Kato N., Sakai Y., 2005a Intracellular ATP correlates with mode of pexophagy in Pichia pastoris. Biosci. Biotechnol. Biochem. 69: 1527–1533. [DOI] [PubMed] [Google Scholar]
- Ano Y., Hattori T., Oku M., Mukaiyama H., Baba M., et al. , 2005b A sorting nexin PpAtg24 regulates vacuolar membrane dynamics during pexophagy via binding to phosphatidylinositol-3-phosphate. Mol. Biol. Cell 16: 446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y., Kanki T., Hirota Y., Kurihara Y., Saigusa T., et al. , 2011. Phosphorylation of serine 114 on Atg32 mediates mitophagy. Mol. Biol. Cell 22: 3206–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M., Takeshige K., Baba N., Ohsumi Y., 1994. Ultrastructural analysis of the autophagic process in yeast: detection of autophagosomes and their characterization. J. Cell Biol. 124: 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M., Osumi M., Scott S. V., Klionsky D. J., Ohsumi Y., 1997. Two distinct pathways for targeting proteins from the cytoplasm to the vacuole/lysosome. J. Cell Biol. 139: 1687–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth H., Meiling-Wesse K., Epple U. D., Thumm M., 2001. Autophagy and the cytoplasm to vacuole targeting pathway both require Aut10p. FEBS Lett. 508: 23–28. [DOI] [PubMed] [Google Scholar]
- Bartholomew C. R., Suzuki T., Du Z., Backues S. K., Jin M., et al. , 2012. Ume6 transcription factor is part of a signaling cascade that regulates autophagy. Proc. Natl. Acad. Sci. USA 109: 11206–11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaran S., Ragusa M. J., Boura E., Hurley J. H., 2012. Two-site recognition of phosphatidylinositol 3-phosphate by PROPPINs in autophagy. Mol. Cell 47: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter B. K., Abeliovich H., Zhang X., Stirling A. G., Burlingame A. L., et al. , 2005. Atg19p ubiquitination and the cytoplasm to vacuole trafficking pathway in yeast. J. Biol. Chem. 280: 39067–39076. [DOI] [PubMed] [Google Scholar]
- Bernales S., McDonald K. L., Walter P., 2006. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 4: e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. R., McCann J. A., Chiang H.-L., 2000. The heat shock protein Ssa2p is required for import of fructose-1, 6-bisphosphatase into Vid vesicles. J. Cell Biol. 150: 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. R., Cui D.-Y., Hung G. G.-C., Chiang H.-L., 2001. Cyclophilin A mediates Vid22p function in the import of fructose-1,6-bisphosphatase into Vid vesicles. J. Biol. Chem. 276: 48017–48026. [DOI] [PubMed] [Google Scholar]
- Brown C. R., McCann J. A., Hung G. G.-C., Elco C. P., Chiang H.-L., 2002. Vid22p, a novel plasma membrane protein, is required for the fructose-1,6-bisphosphatase degradation pathway. J. Cell Sci. 115: 655–666. [DOI] [PubMed] [Google Scholar]
- Brown C. R., Liu J., Hung G. C., Carter D., Cui D., et al. , 2003. The Vid vesicle to vacuole trafficking event requires components of the SNARE membrane fusion machinery. J. Biol. Chem. 278: 25688–25699. [DOI] [PubMed] [Google Scholar]
- Brown C. R., Wolfe A. B., Cui D., Chiang H.-L., 2008. The vacuolar import and degradation pathway merges with the endocytic pathway to deliver fructose-1,6-bisphosphatase to the vacuole for degradation. J. Biol. Chem. 283: 26116–26127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. R., Hung G. C.-C., Dunton D., Chiang H.-L., 2010. The TOR complex 1 is distributed in endosomes and in retrograde vesicles that form from the vacuole membrane and plays an important role in the vacuole import and degradation pathway. J. Biol. Chem. 285: 23359–23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns C., McCaffery J. M., Curwin A. J., Duran J. M., Malhotra V., 2011. Biogenesis of a novel compartment for autophagosome-mediated unconventional protein secretion. J. Cell Biol. 195: 979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budovskaya Y. V., Stephan J. S., Reggiori F., Klionsky D. J., Herman P. K., 2004. The Ras/cAMP-dependent protein kinase signaling pathway regulates an early step of the autophagy process in Saccharomyces cerevisiae. J. Biol. Chem. 279: 20663–20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budovskaya Y. V., Stephan J. S., Deminoff S. J., Herman P. K., 2005. An evolutionary proteomics approach identifies substrates of the cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 102: 13933–13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Cheong H., Song H., Klionsky D. J., 2008. In vivo reconstitution of autophagy in Saccharomyces cerevisiae. J. Cell Biol. 182: 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Nair U., Yasumura-Yorimitsu K., Klionsky D. J., 2009. A multiple ATG gene knockout strain for yeast two-hybrid analysis. Autophagy 5: 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebollero E., Reggiori F., Kraft C., 2012a Reticulophagy and ribophagy: regulated degradation of protein production factories. Int. J. Cell Biol. 2012: 182834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebollero E., van der Vaart A., Zhao M., Rieter E., Klionsky D. J., et al. , 2012b Phosphatidylinositol-3-phosphate clearance plays a key role in autophagosome completion. Curr. Biol. 22: 1545–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. F., Bertram P. G., Ai W., Zheng X. F., 2001. Regulation of APG14 expression by the GATA-type transcription factor Gln3p. J. Biol. Chem. 276: 6463–6467. [DOI] [PubMed] [Google Scholar]
- Chang C. Y., Huang W.-P., 2007. Atg19 mediates a dual interaction cargo sorting mechanism in selective autophagy. Mol. Biol. Cell 18: 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong H., Nair U., Geng J., Klionsky D. J., 2008. The Atg1 kinase complex is involved in the regulation of protein recruitment to initiate sequestering vesicle formation for nonspecific autophagy in Saccharomyces cerevisiae. Mol. Biol. Cell 19: 668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang M.-C., Chiang H.-L., 1998. Vid24p, a novel protein localized to the fructose-1, 6-bisphosphatase-containing vesicles, regulates targeting of fructose-1,6-bisphosphatase from the vesicles to the vacuole for degradation. J. Cell Biol. 140: 1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler N. S., Heitman J., Cardenas M. E., 1999. TOR kinase homologs function in a signal transduction pathway that is conserved from yeast to mammals. Mol. Cell. Endocrinol. 155: 135–142. [DOI] [PubMed] [Google Scholar]
- Darsow T., Rieder S. E., Emr S. D., 1997. A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J. Cell Biol. 138: 517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawaliby R., Mayer A., 2010. Microautophagy of the nucleus coincides with a vacuolar diffusion barrier at nuclear-vacuolar junctions. Mol. Biol. Cell 21: 4173–4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffieu M., Bhatia-Kissova I., Salin B., Galinier A., Manon S., et al. , 2009. Glutathione participates in the regulation of mitophagy in yeast. J. Biol. Chem. 284: 14828–14837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove S. K., Piper R. C., McEwen R. K., Yu J. W., King M. C., et al. , 2004. Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors. EMBO J. 23: 1922–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. A., Jr, Cregg J. M., Kiel J. A. K. W., van der Klei I. J., Oku M., et al. , 2005. Pexophagy: the selective autophagy of peroxisomes. Autophagy 1: 75–83. [DOI] [PubMed] [Google Scholar]
- Duran J. M., Anjard C., Stefan C., Loomis W. F., Malhotra V., 2010. Unconventional secretion of Acb1 is mediated by autophagosomes. J. Cell Biol. 188: 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epple U. D., Suriapranata I., Eskelinen E.-L., Thumm M., 2001. Aut5/Cvt17p, a putative lipase essential for disintegration of autophagic bodies inside the vacuole. J. Bacteriol. 183: 5942–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen E.-L., Reggiori F., Baba M., Kovacs A. L., Seglen P. O., 2011. Seeing is believing: the impact of electron microscopy on autophagy research. Autophagy 7: 935–956. [DOI] [PubMed] [Google Scholar]
- Farre J. C., Manjithaya R., Mathewson R. D., Subramani S., 2008. PpAtg30 tags peroxisomes for turnover by selective autophagy. Dev. Cell 14: 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka Y., Noda N. N., Nakatogawa H., Ohsumi Y., Inagaki F., 2010. Dimeric coiled-coil structure of Saccharomyces cerevisiae Atg16 and its functional significance in autophagy. J. Biol. Chem. 285: 1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J., Klionsky D. J., 2008. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. EMBO Rep. 9: 859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J., Nair U., Yasumura-Yorimitsu K., Klionsky D. J., 2010. Post-Golgi Sec proteins are required for autophagy in Saccharomyces cerevisiae. Mol. Biol. Cell 21: 2257–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J., Stromhaug P. E., George M. D., Habibzadegah-Tari P., Bevan A., et al. , 2001. Cvt18/Gsa12 is required for cytoplasm-to-vacuole transport, pexophagy, and autophagy in Saccharomyces cerevisiae and Pichia pastoris. Mol. Biol. Cell 12: 3821–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki M., Noda T., Baba M., Ohsumi Y., 2005. Starvation triggers the delivery of the endoplasmic reticulum to the vacuole via autophagy in yeast. Traffic 6: 56–65. [DOI] [PubMed] [Google Scholar]
- Hanada T., Noda N. N., Satomi Y., Ichimura Y., Fujioka Y., et al. , 2007. The Atg12—Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J. Biol. Chem. 282: 37298–37302. [DOI] [PubMed] [Google Scholar]
- Harding T. M., Hefner-Gravink A., Thumm M., Klionsky D. J., 1996. Genetic and phenotypic overlap between autophagy and the cytoplasm to vacuole protein targeting pathway. J. Biol. Chem. 271: 17621–17624. [DOI] [PubMed] [Google Scholar]
- He C., Song H., Yorimitsu T., Monastyrska I., Yen W.-L., et al. , 2006. Recruitment of Atg9 to the preautophagosomal structure by Atg11 is essential for selective autophagy in budding yeast. J. Cell Biol. 175: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Baba M., Cao Y., Klionsky D. J., 2008. Self-interaction is critical for Atg9 transport and function at the phagophore assembly site during autophagy. Mol. Biol. Cell 19: 5506–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman P. K., Stack J. H., Emr S. D., 1991. A genetic and structural analysis of the yeast Vps15 protein kinase: evidence for a direct role of Vps15p in vacuolar protein delivery. EMBO J. 10: 4049–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman M., Chiang H.-L., 1996. Isolation of degradation-deficient mutants defective in the targeting of fructose-1,6-bisphosphatase into the vacuole for degradation in Saccharomyces cerevisiae. Genetics 143: 1555–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. B., Kim B. W., Lee K. E., Kim S. W., Jeon H., et al. , 2011. Insights into noncanonical E1 enzyme activation from the structure of autophagic E1 Atg7 with Atg8. Nat. Struct. Mol. Biol. 18: 1323–1330. [DOI] [PubMed] [Google Scholar]
- Horak J., Regelmann J., Wolf D. H., 2002. Two distinct proteolytic systems responsible for glucose-induced degradation of fructose-1,6-bisphosphatase and the Gal2p transporter in the yeast Saccharomyces cerevisiae share the same protein components of the glucose signaling pathway. J. Biol. Chem. 277: 8248–8254. [DOI] [PubMed] [Google Scholar]
- Huang P.-H., Chiang H.-L., 1997. Identification of novel vesicles in the cytosol to vacuole protein degradation pathway. J. Cell Biol. 136: 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.-P., Scott S. V., Kim J., Klionsky D. J., 2000. The itinerary of a vesicle component, Aut7p/Cvt5p, terminates in the yeast vacuole via the autophagy/Cvt pathways. J. Biol. Chem. 275: 5845–5851. [DOI] [PubMed] [Google Scholar]
- Huber A., Bodenmiller B., Uotila A., Stahl M., Wanka S., et al. , 2009. Characterization of the rapamycin-sensitive phosphoproteome reveals that Sch9 is a central coordinator of protein synthesis. Genes Dev. 23: 1929–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung G.-C., Brown C. R., Wolfe A. B., Liu J., Chiang H.-L., 2004. Degradation of the gluconeogenic enzymes fructose-1,6-bisphosphatase and malate dehydrogenase is mediated by distinct proteolytic pathways and signaling events. J. Biol. Chem. 279: 49138–49150. [DOI] [PubMed] [Google Scholar]
- Hutchins M. U., Klionsky D. J., 2001. Vacuolar localization of oligomeric α-mannosidase requires the cytoplasm to vacuole targeting and autophagy pathway components in Saccharomyces cerevisiae. J. Biol. Chem. 276: 20491–20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins M. U., Veenhuis M., Klionsky D. J., 1999. Peroxisome degradation in Saccharomyces cerevisiae is dependent on machinery of macroautophagy and the Cvt pathway. J. Cell Sci. 112: 4079–4087. [DOI] [PubMed] [Google Scholar]
- Ichimura Y., Kirisako T., Takao T., Satomi Y., Shimonishi Y., et al. , 2000. A ubiquitin-like system mediates protein lipidation. Nature 408: 488–492. [DOI] [PubMed] [Google Scholar]
- Ishihara N., Hamasaki M., Yokota S., Suzuki K., Kamada Y., et al. , 2001. Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion. Mol. Biol. Cell 12: 3690–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journo D., Mor A., Abeliovich H., 2009. Aup1-mediated regulation of Rtg3 during mitophagy. J. Biol. Chem. 284: 35885–35895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y., Noda N. N., Fujioka Y., Suzuki K., Inagaki F., et al. , 2009. Characterization of the Atg17-Atg29-Atg31 complex specifically required for starvation-induced autophagy in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 389: 612–615. [DOI] [PubMed] [Google Scholar]
- Kaiser S. E., Mao K., Taherbhoy A. M., Yu S., Olszewski J. L., et al. , 2012. Noncanonical E2 recruitment by the autophagy E1 revealed by Atg7-Atg3 and Atg7-Atg10 structures. Nat. Struct. Mol. Biol. 19: 1242–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuta S., Yamamoto H., Negishi L., Kondo-Kakuta C., Hayashi N., et al. , 2012. Atg9 vesicles recruit vesicle-tethering proteins, Trs85 and Ypt1, to the autophagosome formation site. J. Biol. Chem. 287: 44261–44269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y., Funakoshi T., Shintani T., Nagano K., Ohsumi M., et al. , 2000. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J. Cell Biol. 150: 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y., Yoshino K., Kondo C., Kawamata T., Oshiro N., et al. , 2010. Tor directly controls the Atg1 kinase complex to regulate autophagy. Mol. Cell. Biol. 30: 1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kametaka S., Okano T., Ohsumi M., Ohsumi Y., 1998. Apg14p and Apg6/Vps30p form a protein complex essential for autophagy in the yeast, Saccharomyces cerevisiae. J. Biol. Chem. 273: 22284–22291. [DOI] [PubMed] [Google Scholar]
- Kanki T., Klionsky D. J., 2008. Mitophagy in yeast occurs through a selective mechanism. J. Biol. Chem. 283: 32386–32393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki T., Wang K., Baba M., Bartholomew C. R., Lynch-Day M. A., et al. , 2009a A genomic screen for yeast mutants defective in selective mitochondria autophagy. Mol. Biol. Cell 20: 4730–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki T., Wang K., Cao Y., Baba M., Klionsky D. J., 2009b Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev. Cell 17: 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata T., Kamada Y., Kabeya Y., Sekito T., Ohsumi Y., 2008. Organization of the pre-autophagosomal structure responsible for autophagosome formation. Mol. Biol. Cell 19: 2039–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A., Noda T., Ishihara N., Ohsumi Y., 2001. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J. Cell Biol. 152: 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I., Rodriguez-Enriquez S., Lemasters J. J., 2007. Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 462: 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kamada Y., Stromhaug P. E., Guan J., Hefner-Gravink A., et al. , 2001. Cvt9/Gsa9 functions in sequestering selective cytosolic cargo destined for the vacuole. J. Cell Biol. 153: 381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Huang W.-P., Stromhaug P. E., Klionsky D. J., 2002. Convergence of multiple autophagy and cytoplasm to vacuole targeting components to a perivacuolar membrane compartment prior to de novo vesicle formation. J. Biol. Chem. 277: 763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisako T., Baba M., Ishihara N., Miyazawa K., Ohsumi M., et al. , 1999. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J. Cell Biol. 147: 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissova I. B., Camougrand N., 2009. Glutathione participates in the regulation of mitophagy in yeast. Autophagy 5: 872–873. [DOI] [PubMed] [Google Scholar]
- Kissova I., Deffieu M., Manon S., Camougrand N., 2004. Uth1p is involved in the autophagic degradation of mitochondria. J. Biol. Chem. 279: 39068–39074. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Herman P. K., Emr S. D., 1990. The fungal vacuole: composition, function, and biogenesis. Microbiol. Rev. 54: 266–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Cueva R., Yaver D. S., 1992. Aminopeptidase I of Saccharomyces cerevisiae is localized to the vacuole independent of the secretory pathway. J. Cell Biol. 119: 287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Cregg J. M., Dunn W. A., Jr, Emr S. D., Sakai Y., et al. , 2003. A unified nomenclature for yeast autophagy-related genes. Dev. Cell 5: 539–545. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Cuervo A. M., Dunn W. A., Jr, Levine B., van der Klei I., et al. , 2007. How shall I eat thee? Autophagy 3: 413–416. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Baehrecke E. H., Brumell J. H., Chu C. T., Codogno P., et al. , 2011. A comprehensive glossary of autophagy-related molecules and processes. Autophagy 7: 1273–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs A. L., Palfia Z., Rez G., Vellai T., Kovacs J., 2007. Sequestration revisited: integrating traditional electron microscopy, de novo assembly and new results. Autophagy 3: 655–662. [DOI] [PubMed] [Google Scholar]
- Kraft C., Peter M., 2008. Is the Rsp5 ubiquitin ligase involved in the regulation of ribophagy? Autophagy 4: 838–840. [DOI] [PubMed] [Google Scholar]
- Kraft C., Deplazes A., Sohrmann M., Peter M., 2008. Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat. Cell Biol. 10: 602–610. [DOI] [PubMed] [Google Scholar]
- Kraft C., Reggiori F., Peter M., 2009. Selective types of autophagy in yeast. Biochim. Biophys. Acta 1793: 1404–1412. [DOI] [PubMed] [Google Scholar]
- Kraft C., Peter M., Hofmann K., 2010. Selective autophagy: ubiquitin-mediated recognition and beyond. Nat. Cell Biol. 12: 836–841. [DOI] [PubMed] [Google Scholar]
- Kraft C., Kijanska M., Kalie E., Siergiejuk E., Lee S. S., et al. , 2012. Binding of the Atg1/ULK1 kinase to the ubiquitin-like protein Atg8 regulates autophagy. EMBO J. 31: 3691–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krick R., Tolstrup J., Appelles A., Henke S., Thumm M., 2006. The relevance of the phosphatidylinositolphosphat-binding motif FRRGT of Atg18 and Atg21 for the Cvt pathway and autophagy. FEBS Lett. 580: 4632–4638. [DOI] [PubMed] [Google Scholar]
- Krick R., Henke S., Tolstrup J., Thumm M., 2008a Dissecting the localization and function of Atg18, Atg21 and Ygr223c. Autophagy 4: 896–910. [DOI] [PubMed] [Google Scholar]
- Krick R., Muehe Y., Prick T., Bremer S., Schlotterhose P., et al. , 2008b Piecemeal microautophagy of the nucleus requires the core macroautophagy genes. Mol. Biol. Cell 19: 4492–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krick R., Busse R. A., Scacioc A., Stephan M., Janshoff A., et al. , 2012. Structural and functional characterization of the two phosphoinositide binding sites of PROPPINs, a β-propeller protein family. Proc. Natl. Acad. Sci. USA 109: E2042–E2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A., Mizushima N., Ishihara N., Ohsumi Y., 2002. Formation of the approximately 350-kDa Apg12-Apg5·Apg16 multimeric complex, mediated by Apg16 oligomerization, is essential for autophagy in yeast. J. Biol. Chem. 277: 18619–18625. [DOI] [PubMed] [Google Scholar]
- Kumanomidou T., Mizushima T., Komatsu M., Suzuki A., Tanida I., et al. , 2006. The crystal structure of human Atg4b, a processing and de-conjugating enzyme for autophagosome-forming modifiers. J. Mol. Biol. 355: 612–618. [DOI] [PubMed] [Google Scholar]
- Kunz J. B., Schwarz H., Mayer A., 2004. Determination of four sequential stages during microautophagy in vitro. J. Biol. Chem. 279: 9987–9996. [DOI] [PubMed] [Google Scholar]
- Kvam E., Goldfarb D. S., 2004. Nvj1p is the outer-nuclear-membrane receptor for oxysterol-binding protein homolog Osh1p in Saccharomyces cerevisiae. J. Cell Sci. 117: 4959–4968. [DOI] [PubMed] [Google Scholar]
- Kvam E., Gable K., Dunn T. M., Goldfarb D. S., 2005. Targeting of Tsc13p to nucleus-vacuole junctions: a role for very-long-chain fatty acids in the biogenesis of microautophagic vesicles. Mol. Biol. Cell 16: 3987–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legakis J. E., Yen W.-L., Klionsky D. J., 2007. A cycling protein complex required for selective autophagy. Autophagy 3: 422–432. [DOI] [PubMed] [Google Scholar]
- Li W. W., Li J., Bao J. K., 2012. Microautophagy: lesser-known self-eating. Cell. Mol. Life Sci. 69: 1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatova Z., Belogortseva N., Zhang X. Q., Kim J., Taussig D., et al. , 2012. Regulation of selective autophagy onset by a Ypt/Rab GTPase module. Proc. Natl. Acad. Sci. USA 109: 6981–6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Brown C. R., Chiang H.-L., 2005. Degradation of the gluconeogenic enzyme fructose-1, 6-bisphosphatase is dependent on the vacuolar ATPase. Autophagy 1: 146–156. [DOI] [PubMed] [Google Scholar]
- Lynch-Day M. A., Klionsky D. J., 2010. The Cvt pathway as a model for selective autophagy. FEBS Lett. 584: 1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch-Day M. A., Bhandari D., Menon S., Huang J., Cai H., et al. , 2010. Trs85 directs a Ypt1 GEF, TRAPPIII, to the phagophore to promote autophagy. Proc. Natl. Acad. Sci. USA 107: 7811–7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjithaya R., Anjard C., Loomis W. F., Subramani S., 2010a Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J. Cell Biol. 188: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjithaya R., Jain S., Farre J. C., Subramani S., 2010b A yeast MAPK cascade regulates pexophagy but not other autophagy pathways. J. Cell Biol. 189: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao K., Wang K., Zhao M., Xu T., Klionsky D. J., 2011. Two MAPK-signaling pathways are required for mitophagy in Saccharomyces cerevisiae. J. Cell Biol. 193: 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari M., Griffith J., Rieter E., Krishnappa L., Klionsky D. J., et al. , 2010. An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J. Cell Biol. 190: 1005–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M., Suzuki N. N., Obara K., Fujioka Y., Ohsumi Y., et al. , 2007. Structure of Atg5·Atg16, a complex essential for autophagy. J. Biol. Chem. 282: 6763–6772. [DOI] [PubMed] [Google Scholar]
- Matsuura A., Tsukada M., Wada Y., Ohsumi Y., 1997. Apg1p, a novel protein kinase required for the autophagic process in Saccharomyces cerevisiae. Gene 192: 245–250. [DOI] [PubMed] [Google Scholar]
- Meiling-Wesse K., Barth H., Thumm M., 2002. Ccz1p/Aut11p/Cvt16p is essential for autophagy and the cvt pathway. FEBS Lett. 526: 71–76. [DOI] [PubMed] [Google Scholar]
- Mijaljica D., Nazarko T. Y., Brumell J. H., Huang W.-P., Komatsu M., et al. , 2012. Receptor protein complexes are in control of autophagy. Autophagy 8: 1701–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N., Klionsky D. J., 2007. Protein turnover via autophagy: implications for metabolism. Annu. Rev. Nutr. 27: 19–40. [DOI] [PubMed] [Google Scholar]
- Mizushima N., Noda T., Yoshimori T., Tanaka Y., Ishii T., et al. , 1998. A protein conjugation system essential for autophagy. Nature 395: 395–398. [DOI] [PubMed] [Google Scholar]
- Mizushima N., Noda T., Ohsumi Y., 1999. Apg16p is required for the function of the Apg12p-Apg5p conjugate in the yeast autophagy pathway. EMBO J. 18: 3888–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N., Yamamoto A., Hatano M., Kobayashi Y., Kabeya Y., et al. , 2001. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J. Cell Biol. 152: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastyrska I., Kiel J. A., Krikken A. M., Komduur J. A., Veenhuis M., et al. , 2005. The Hansenula polymorpha ATG25 gene encodes a novel coiled-coil protein that is required for macropexophagy. Autophagy 1: 92–100. [DOI] [PubMed] [Google Scholar]
- Monastyrska I., He C., Geng J., Hoppe A. D., Li Z., et al. , 2008. Arp2 links autophagic machinery with the actin cytoskeleton. Mol. Biol. Cell 19: 1962–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley A. M., Nuttall J. M., Hettema E. H., 2012. Pex3-anchored Atg36 tags peroxisomes for degradation in Saccharomyces cerevisiae. EMBO J. 31: 2852–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller O., Sattler T., Flotenmeyer M., Schwarz H., Plattner H., et al. , 2000. Autophagic tubes: vacuolar invaginations involved in lateral membrane sorting and inverse vesicle budding. J. Cell Biol. 151: 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair U., Cao Y., Xie Z., Klionsky D. J., 2010. Roles of the lipid-binding motifs of Atg18 and Atg21 in the cytoplasm to vacuole targeting pathway and autophagy. J. Biol. Chem. 285: 11476–11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair U., Jotwani A., Geng J., Gammoh N., Richerson D., et al. , 2011. SNARE proteins are required for macroautophagy. Cell 146: 290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair U., Yen W.-L., Mari M., Cao Y., Xie Z., et al. , 2012. A role for Atg8—PE deconjugation in autophagosome biogenesis. Autophagy 8: 780–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatogawa H., Ishii J., Asai E., Ohsumi Y., 2012. Atg4 recycles inappropriately lipidated Atg8 to promote autophagosome biogenesis. Autophagy 8: 177–186. [DOI] [PubMed] [Google Scholar]
- Natarajan K., Meyer M. R., Jackson B. M., Slade D., Roberts C., et al. , 2001. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol. Cell. Biol. 21: 4347–4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarko T. Y., Polupanov A. S., Manjithaya R. R., Subramani S., Sibirny A. A., 2007. The requirement of sterol glucoside for pexophagy in yeast is dependent on the species and nature of peroxisome inducers. Mol. Biol. Cell 18: 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarko V. Y., Nazarko T. Y., Farre J. C., Stasyk O. V., Warnecke D., et al. , 2011. Atg35, a micropexophagy-specific protein that regulates micropexophagic apparatus formation in Pichia pastoris. Autophagy 7: 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nice D. C., Sato T. K., Strømhaug P. E., Emr S. D., Klionsky D. J., 2002. Cooperative binding of the cytoplasm to vacuole targeting pathway proteins, Cvt13 and Cvt20, to phosphatidylinositol 3-phosphate at the pre-autophagosomal structure is required for selective autophagy. J. Biol. Chem. 277: 30198–30207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W., Rabouille C., 2009. Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 10: 148–155. [DOI] [PubMed] [Google Scholar]
- Noda N. N., Kumeta H., Nakatogawa H., Satoo K., Adachi W., et al. , 2008. Structural basis of target recognition by Atg8/LC3 during selective autophagy. Genes Cells 13: 1211–1218. [DOI] [PubMed] [Google Scholar]
- Noda N. N., Satoo K., Fujioka Y., Kumeta H., Ogura K., et al. , 2011. Structural basis of Atg8 activation by a homodimeric E1, Atg7. Mol. Cell 44: 462–475. [DOI] [PubMed] [Google Scholar]
- Noda N. N., Fujioka Y., Hanada T., Ohsumi Y., Inagaki F., 2013. Structure of the Atg12—Atg5 conjugate reveals a platform for stimulating Atg8—PE conjugation. EMBO Rep. 14: 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T., Ohsumi Y., 1998. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J. Biol. Chem. 273: 3963–3966. [DOI] [PubMed] [Google Scholar]
- Noda T., Kim J., Huang W.-P., Baba M., Tokunaga C., et al. , 2000. Apg9p/Cvt7p is an integral membrane protein required for transport vesicle formation in the Cvt and autophagy pathways. J. Cell Biol. 148: 465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T., Suzuki K., Ohsumi Y., 2002. Yeast autophagosomes: de novo formation of a membrane structure. Trends Cell Biol. 12: 231–235. [DOI] [PubMed] [Google Scholar]
- Obara K., Ohsumi Y., 2011. Atg14: a key player in orchestrating autophagy. Int. J. Cell Biol. 2011: 713435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara K., Sekito T., Niimi K., Ohsumi Y., 2008. The Atg18-Atg2 complex is recruited to autophagic membranes via phosphatidylinositol 3-phosphate and exerts an essential function. J. Biol. Chem. 283: 23972–23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda M. N., Scott S. V., Hefner-Gravink A., Caffarelli A. D., Klionsky D. J., 1996. Identification of a cytoplasm to vacuole targeting determinant in aminopeptidase I. J. Cell Biol. 132: 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y., Munro S., 2010. Membrane delivery to the yeast autophagosome from the Golgi-endosomal system. Mol. Biol. Cell 21: 3998–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Kondo-Okamoto N., Ohsumi Y., 2009. Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev. Cell 17: 87–97. [DOI] [PubMed] [Google Scholar]
- Oku M., Warnecke D., Noda T., Muller F., Heinz E., et al. , 2003. Peroxisome degradation requires catalytically active sterol glucosyltransferase with a GRAM domain. EMBO J. 22: 3231–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku M., Nishimura T., Hattori T., Ano Y., Yamashita S., et al. , 2006. Role of Vac8 in formation of the vacuolar sequestering membrane during micropexophagy. Autophagy 2: 272–279. [DOI] [PubMed] [Google Scholar]
- Otomo C., Metlagel Z., Takaesu G., Otomo T., 2013. Structure of the human ATG12∼ATG5 conjugate required for LC3 lipidation in autophagy. Nat. Struct. Mol. Biol. 20: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Roberts P., Chen Y., Kvam E., Shulga N., et al. , 2000. Nucleus-vacuole junctions in Saccharomyces cerevisiae are formed through the direct interaction of Vac8p with Nvj1p. Mol. Biol. Cell 11: 2445–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polupanov A. S., Nazarko V. Y., Sibirny A. A., 2011. CCZ1, MON1 and YPT7 genes are involved in pexophagy, the Cvt pathway and non-specific macroautophagy in the methylotrophic yeast Pichia pastoris. Cell Biol. Int. 35: 311–319. [DOI] [PubMed] [Google Scholar]
- Ragusa M. J., Stanley R. E., Hurley J. H., 2012. Architecture of the Atg17 complex as a scaffold for autophagosome biogenesis. Cell 151: 1501–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravid T., Hochstrasser M., 2008. Diversity of degradation signals in the ubiquitin-proteasome system. Nat. Rev. Mol. Cell Biol. 9: 679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regelmann J., Schule T., Josupeit F. S., Horak J., Rose M., et al. , 2003. Catabolite degradation of fructose-1,6-bisphosphatase in the yeast Saccharomyces cerevisiae: a genome-wide screen identifies eight novel GID genes and indicates the existence of two degradation pathways. Mol. Biol. Cell 14: 1652–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F., 2006. Membrane origin for autophagy. Curr. Top. Dev. Biol. 74: 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F., Tucker K. A., Stromhaug P. E., Klionsky D. J., 2004a The Atg1-Atg13 complex regulates Atg9 and Atg23 retrieval transport from the pre-autophagosomal structure. Dev. Cell 6: 79–90. [DOI] [PubMed] [Google Scholar]
- Reggiori F., Wang C.-W., Nair U., Shintani T., Abeliovich H., et al. , 2004b Early stages of the secretory pathway, but not endosomes, are required for Cvt vesicle and autophagosome assembly in Saccharomyces cerevisiae. Mol. Biol. Cell 15: 2189–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F., Monastyrska I., Shintani T., Klionsky D. J., 2005a The actin cytoskeleton is required for selective types of autophagy, but not nonspecific autophagy, in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 16: 5843–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F., Shintani T., Nair U., Klionsky D. J., 2005b Atg9 cycles between mitochondria and the pre-autophagosomal structure in yeasts. Autophagy 1: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder S. E., Emr S. D., 1997. A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol. Biol. Cell 8: 2307–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieter E., Vinke F., Bakula D., Cebollero E., Ungermann C., et al. , 2013. Atg18 function in autophagy is regulated by specific sites within its β-propeller. J. Cell Sci. (in press). [DOI] [PubMed] [Google Scholar]
- Roberts P., Moshitch-Moshkovitz S., Kvam E., O’Toole E., Winey M., et al. , 2003. Piecemeal microautophagy of nucleus in Saccharomyces cerevisiae. Mol. Biol. Cell 14: 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov J., Walczak M., Ibiricu I., Schuchner S., Ogris E., et al. , 2012. Mechanism and functions of membrane binding by the Atg5-Atg12/Atg16 complex during autophagosome formation. EMBO J. 31: 4304–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y., Koller A., Rangell L. K., Keller G. A., Subramani S., 1998. Peroxisome degradation by microautophagy in Pichia pastoris: identification of specific steps and morphological intermediates. J. Cell Biol. 141: 625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoo K., Noda N. N., Kumeta H., Fujioka Y., Mizushima N., et al. , 2009. The structure of Atg4B–LC3 complex reveals the mechanism of LC3 processing and delipidation during autophagy. EMBO J. 28: 1341–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler T., Mayer A., 2000. Cell-free reconstitution of microautophagic vacuole invagination and vesicle formation. J. Cell Biol. 151: 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S. V., Hefner-Gravink A., Morano K. A., Noda T., Ohsumi Y., et al. , 1996. Cytoplasm-to-vacuole targeting and autophagy employ the same machinery to deliver proteins to the yeast vacuole. Proc. Natl. Acad. Sci. USA 93: 12304–12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S. V., Nice D. C., III, Nau J. J., Weisman L. S., Kamada Y., et al. , 2000. Apg13p and Vac8p are part of a complex of phosphoproteins that are required for cytoplasm to vacuole targeting. J. Biol. Chem. 275: 25840–25849. [DOI] [PubMed] [Google Scholar]
- Scott S. V., Guan J., Hutchins M. U., Kim J., Klionsky D. J., 2001. Cvt19 is a receptor for the cytoplasm-to-vacuole targeting pathway. Mol. Cell 7: 1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh H.-L., Chen Y., Brown C. R., Chiang H.-L., 2001. Biochemical analysis of fructose-1,6-bisphosphatase import into vacuole import and degradation vesicles reveals a role for UBC1 in vesicle biogenesis. J. Biol. Chem. 276: 10398–10406. [DOI] [PubMed] [Google Scholar]
- Shintani T., Klionsky D. J., 2004a Autophagy in health and disease: a double-edged sword. Science 306: 990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T., Klionsky D. J., 2004b Cargo proteins facilitate the formation of transport vesicles in the cytoplasm to vacuole targeting pathway. J. Biol. Chem. 279: 29889–29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T., Mizushima N., Ogawa Y., Matsuura A., Noda T., et al. , 1999. Apg10p, a novel protein-conjugating enzyme essential for autophagy in yeast. EMBO J. 18: 5234–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T., Suzuki K., Kamada Y., Noda T., Ohsumi Y., 2001. Apg2p functions in autophagosome formation on the perivacuolar structure. J. Biol. Chem. 276: 30452–30460. [DOI] [PubMed] [Google Scholar]
- Shintani T., Huang W.-P., Stromhaug P. E., Klionsky D. J., 2002. Mechanism of cargo selection in the cytoplasm to vacuole targeting pathway. Dev. Cell 3: 825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack J. H., Herman P. K., Schu P. V., Emr S. D., 1993. A membrane-associated complex containing the Vps15 protein kinase and the Vps34 PI 3-kinase is essential for protein sorting to the yeast lysosome-like vacuole. EMBO J. 12: 2195–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack J. H., DeWald D. B., Takegawa K., Emr S. D., 1995. Vesicle-mediated protein transport: regulatory interactions between the Vps15 protein kinase and the Vps34 PtdIns 3-kinase essential for protein sorting to the vacuole in yeast. J. Cell Biol. 129: 321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasyk O. V., Stasyk O. G., Mathewson R. D., Farre J. C., Nazarko V. Y., et al. , 2006. Atg28, a novel coiled-coil protein involved in autophagic degradation of peroxisomes in the methylotrophic yeast Pichia pastoris. Autophagy 2: 30–38. [DOI] [PubMed] [Google Scholar]
- Stephan J. S., Yeh Y. Y., Ramachandran V., Deminoff S. J., Herman P. K., 2009. The Tor and PKA signaling pathways independently target the Atg1/Atg13 protein kinase complex to control autophagy. Proc. Natl. Acad. Sci. USA 106: 17049–17054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara K., Suzuki N. N., Fujioka Y., Mizushima N., Ohsumi Y., et al. , 2004. The crystal structure of microtubule-associated protein light chain 3, a mammalian homologue of Saccharomyces cerevisiae Atg8. Genes Cells 9: 611–618. [DOI] [PubMed] [Google Scholar]
- Sugawara K., Suzuki N. N., Fujioka Y., Mizushima N., Ohsumi Y., et al. , 2005. Structural basis for the specificity and catalysis of human Atg4B responsible for mammalian autophagy. J. Biol. Chem. 280: 40058–40065. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kirisako T., Kamada Y., Mizushima N., Noda T., et al. , 2001. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 20: 5971–5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kubota Y., Sekito T., Ohsumi Y., 2007. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells 12: 209–218. [DOI] [PubMed] [Google Scholar]
- Suzuki N. N., Yoshimoto K., Fujioka Y., Ohsumi Y., Inagaki F., 2005. The crystal structure of plant ATG12 and its biological implication in autophagy. Autophagy 1: 119–126. [DOI] [PubMed] [Google Scholar]
- Taherbhoy A. M., Tait S. W., Kaiser S. E., Williams A. H., Deng A., et al. , 2011. Atg8 transfer from Atg7 to Atg3: a distinctive E1–E2 architecture and mechanism in the autophagy pathway. Mol. Cell 44: 451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshige K., Baba M., Tsuboi S., Noda T., Ohsumi Y., 1992. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 119: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal R., Winter G., Ecker N., Klionsky D. J., Abeliovich H., 2007. Aup1p, a yeast mitochondrial protein phosphatase homolog, is required for efficient stationary phase mitophagy and cell survival. J. Biol. Chem. 282: 5617–5624. [DOI] [PubMed] [Google Scholar]
- Taylor R., Jr, Chen P. H., Chou C. C., Patel J., Jin S. V., 2012. KCS1 deletion in Saccharomyces cerevisiae leads to a defect in translocation of autophagic proteins and reduces autophagosome formation. Autophagy 8: 1300–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter S. A., Eggerton K. P., Scott S. V., Kim J., Fischer A. M., et al. , 2001. Degradation of lipid vesicles in the yeast vacuole requires function of Cvt17, a putative lipase. J. Biol. Chem. 276: 2083–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till A., Lakhani R., Burnett S. F., Subramani S., 2012. Pexophagy: the selective degradation of peroxisomes. Int. J. Cell Biol. 2012: 512721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titorenko V. I., Keizer I., Harder W., Veenhuis M., 1995. Isolation and characterization of mutants impaired in the selective degradation of peroxisomes in the yeast Hansenula polymorpha. J. Bacteriol. 177: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker K. A., Reggiori F., Dunn W. A., Jr, Klionsky D. J., 2003. Atg23 is essential for the cytoplasm to vacuole targeting pathway and efficient autophagy but not pexophagy. J. Biol. Chem. 278: 48445–48452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle D. L., Dunn W. A., Jr, 1995. Divergent modes of autophagy in the methylotrophic yeast Pichia pastoris. J. Cell Sci. 108: 25–35. [DOI] [PubMed] [Google Scholar]
- Tuttle D. L., Lewin A. S., Dunn W. A., Jr, 1993. Selective autophagy of peroxisomes in methylotrophic yeasts. Eur. J. Cell Biol. 60: 283–290. [PubMed] [Google Scholar]
- Umekawa M., Klionsky D. J., 2012. Ksp1 kinase regulates autophagy via the target of rapamycin complex 1 (TORC1) pathway. J. Biol. Chem. 287: 16300–16310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttenweiler A., Mayer A., 2008. Microautophagy in the yeast Saccharomyces cerevisiae. Methods Mol. Biol. 445: 245–259. [DOI] [PubMed] [Google Scholar]
- van der Vaart A., Griffith J., Reggiori F., 2010. Exit from the Golgi is required for the expansion of the autophagosomal phagophore in yeast Saccharomyces cerevisiae. Mol. Biol. Cell 21: 2270–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zutphen T., Veenhuis M., van der Klei I. J., 2008. Pex14 is the sole component of the peroxisomal translocon that is required for pexophagy. Autophagy 4: 63–66. [DOI] [PubMed] [Google Scholar]
- Wang C.-W., Stromhaug P. E., Shima J., Klionsky D. J., 2002. The Ccz1-Mon1 protein complex is required for the late step of multiple vacuole delivery pathways. J. Biol. Chem. 277: 47917–47927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Yang Z., Liu X., Mao K., Nair U., et al. , 2012. Phosphatidylinositol 4-kinases are required for autophagic membrane trafficking. J. Biol. Chem. 287: 37964–37972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wilson W. A., Fujino M. A., Roach P. J., 2001. Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol. Cell. Biol. 21: 5742–5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Noda N. N., Kumeta H., Suzuki K., Ohsumi Y., et al. , 2010. Selective transport of α-mannosidase by autophagic pathways: structural basis for cargo recognition by Atg19 and Atg34. J. Biol. Chem. 285: 30026–30033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Kobayashi T., Yamamoto H., Hoshida H., Akada R., et al. , 2012. Structure-based analyses reveal distinct binding sites for Atg2 and phosphoinositides in Atg18. J. Biol. Chem. 287: 31681–31690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Nair U., Klionsky D. J., 2008. Atg8 controls phagophore expansion during autophagosome formation. Mol. Biol. Cell 19: 3290–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Matoba K., Sawada R., Fujioka Y., Nakatogawa H., et al. , 2012a Noncanonical recognition and UBL loading of distinct E2s by autophagy-essential Atg7. Nat. Struct. Mol. Biol. 19: 1250–1256. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Noda N. N., Yamamoto H., Shima T., Kumeta H., et al. , 2012b Structural insights into Atg10-mediated formation of the autophagy-essential Atg12—Atg5 conjugate. Structure 20: 1244–1254. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Kakuta S., Watanabe T. M., Kitamura A., Sekito T., et al. , 2012. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J. Cell Biol. 198: 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S., Oku M., Wasada Y., Ano Y., Sakai Y., 2006. PI4P-signaling pathway for the synthesis of a nascent membrane structure in selective autophagy. J. Cell Biol. 173: 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Huang J., Geng J., Nair U., Klionsky D. J., 2006. Atg22 recycles amino acids to link the degradative and recycling functions of autophagy. Mol. Biol. Cell 17: 5094–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Geng J., Yen W.-L., Wang K., Klionsky D. J., 2010. Positive or negative roles of different cyclin-dependent kinase Pho85-cyclin complexes orchestrate induction of autophagy in Saccharomyces cerevisiae. Mol. Cell 38: 250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen W.-L., Legakis J. E., Nair U., Klionsky D. J., 2007. Atg27 is required for autophagy-dependent cycling of Atg9. Mol. Biol. Cell 18: 581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen W.-L., Shintani T., Nair U., Cao Y., Richardson B. C., et al. , 2010. The conserved oligomeric Golgi complex is involved in double-membrane vesicle formation during autophagy. J. Cell Biol. 188: 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu T., Klionsky D. J., 2005. Atg11 links cargo to the vesicle-forming machinery in the cytoplasm to vacuole targeting pathway. Mol. Biol. Cell 16: 1593–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu T., Nair U., Yang Z., Klionsky D. J., 2006. Endoplasmic reticulum stress triggers autophagy. J. Biol. Chem. 281: 30299–30304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu T., Zaman S., Broach J. R., Klionsky D. J., 2007. Protein kinase A and Sch9 cooperatively regulate induction of autophagy in Saccharomyces cerevisiae. Mol. Biol. Cell 18: 4180–4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu T., He C., Wang K., Klionsky D. J., 2009. Tap42-associated protein phosphatase type 2A negatively regulates induction of autophagy. Autophagy 5: 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuga M., Gomi K., Klionsky D. J., Shintani T., 2011. Aspartyl aminopeptidase is imported from the cytoplasm to the vacuole by selective autophagy in Saccharomyces cerevisiae. J. Biol. Chem. 286: 13704–13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman S., Lippman S. I., Zhao X., Broach J. R., 2008. How Saccharomyces responds to nutrients. Annu. Rev. Genet. 42: 27–81. [DOI] [PubMed] [Google Scholar]