Abstract
The importance of mitochondrial DNA (mtDNA) deletions in the progeroid phenotype of exonuclease-deficient DNA polymerase γ mice has been intensely debated. We show that disruption of Mip1 exonuclease activity increases mtDNA deletions 160-fold, whereas disease-associated polymerase variants were mostly unaffected, suggesting that exonuclease activity is vital to avoid deletions during mtDNA replication.
Keywords: Mip1, mtDNA deletions, Pol Gamma, direct repeats, exonuclease
MOST ATP production in eukaryotes requires functional mitochondria and mitochondrial genome (mtDNA) maintenance. Several examples of genetic and pharmacological inactivation of mtDNA replication have demonstrated the importance of maintaining mtDNA. Chain-terminating nucleotide analogs impair mtDNA replication, thus causing toxicity during therapy (Lewis and Dalakas 1995; Lewis et al. 2007). Also, mutant mitochondrial replisome proteins, including DNA polymerase γ (pol γ), contribute to mitochondrial diseases, characterized by mtDNA depletion, deletions, and point mutations (Stumpf and Copeland 2011). Over 200 mutations in the coding region of the human DNA polymerase γ gene, POLG, have been identified in mitochondrial disease patients (detailed in http://tools.niehs.nih.gov/polg/).
Elevated mtDNA mutagenesis also adversely affects mammalian health, exemplified by the progeroid Pol γ exonuclease-deficient mice (Zhang et al. 2000; Trifunovic et al. 2004; Kujoth et al. 2005; Vermulst et al. 2007; Vermulst et al. 2008b; Safdar et al. 2011). Pol γ has 3′-5′ exonuclease activity (Foury and Vanderstraeten 1992; Longley et al. 1998; Spelbrink et al. 2000; Longley et al. 2001) that excises mismatched bases before polymerase extension (Lehman and Nussbaum 1964). This “proofreading” activity reduces mismatch maintenance 20-fold in vitro and 500- to 2000-fold in various model systems (Foury and Vanderstraeten 1992; Spelbrink et al. 2000; Longley et al. 2001; Vermulst et al. 2007). While the progeroid phenotype of mice lacking pol γ exonuclease activity implied a causative role of mtDNA mutations in aging (Trifunovic et al. 2004; Kujoth et al. 2005), asymptomatic exonuclease deficient heterozygotic mice accumulate 500-fold more point mutations than aged wild-type mice (Vermulst et al. 2007). Although homozygous exonuclease-deficient mice show up to 99-fold increases in mtDNA deletions (Vermulst et al. 2008b) absolute deletion amounts remain indeterminate and vary by technique (Khrapko and Vijg 2007; Vermulst et al. 2008a; Greaves et al. 2009; Kraytsberg et al. 2009). Another model system would be useful for quantifying deletion mutants and understanding mechanisms that affect deletion formation.
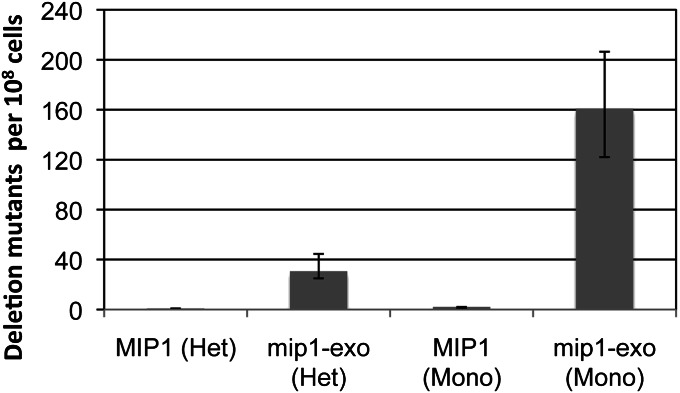
Deletions, mostly between 13-nucleotide direct repeats, accumulate in humans during aging (Cortopassi and Arnheim 1990; Chomyn and Attardi 2003) and Kearns-Sayre syndrome (Shoffner et al. 1989). Contributing genetic mechanisms are poorly understood. In Saccharomyces cerevisiae, deletions occur frequently between 96-nucleotide direct repeat flanks and 47,000-fold less frequently between 21-nucleotide flanks (Phadnis et al. 2005). Unlike in mammals, yeast mtDNA undergoes frequent recombination (Azpiroz and Butow 1993), and the likelihood that 96-nucleotide repeats, not 21-nucleotide repeats, are adequate recombination substrates explains the frequent deletion formation. Short direct repeats are unlikely to promote recombination but may facilitate mispriming events during mtDNA replication. To test whether exonuclease activity attenuates deletions between 21-nucleotide direct repeats, heteroallelic NPY75-derived strains (Phadnis et al. 2005) with a wild-type MIP1 chromosomal allele and an exonuclease-deficient mip1 allele expressed from a centromeric plasmid were assayed. Mitochondrial genomes from NPY75-derived strains contain an ARG8 insertion, flanked by direct repeats, in the mitochondrial-encoded COX2 gene. Deletion between direct repeats simultaneously results in loss of ARG8 and restoration of COX2, allowing for growth on glycerol-containing media. With wild-type MIP1, less than one deletion mutant per 108 cells contained the deletion between 21-nucleotide direct repeats (Figure 1). However, disrupting exonuclease activity increases deletion formation 30-fold and 160-fold in strains with and without chromosomal MIP1, respectively (Figure 1). Wild-type MIP1 prevented over half of the deletions. It is possible that exonuclease-proficient Mip1, the yeast DNA pol γ, replicates more mtDNA genomes per cell division, although in vitro results suggest that exonuclease-deficient human pol γ has similar replication kinetics as wild-type pol γ (Longley et al. 1998; Johnson and Johnson 2001a,b). Alternatively, extrinsic proofreading by wild-type Mip1 could suppress deletions generated by the exonuclease-deficient Mip1 at the replication fork. Extrinsic proofreading has been demonstrated in vitro with Escherichia coli Klenow fragment (Joyce 1989) and in vivo during Saccharomyces cerevisiae lagging strand synthesis (Pavlov et al. 2006). These results indicate that exonuclease activity is important for limiting replication-dependent mtDNA deletions between direct repeats.
Figure 1.
MtDNA deletions between 21-mer direct repeats in strains with Mip1-proficient or -deficient exonuclease. Heteroallelic mip1 strains were created by transforming TRP1-containing centromeric plasmids PFL39, containing wild-type MIP1 (Foury and Vanderstraeten 1992) (MIP1 [Het]; JSY113), or mip1 encoding an exonuclease-deficient mutant variant (Strand et al. 2003) (mip1-exo [Het]; JSY114) into trp1::G418 NPY75 (Phadnis et al. 2005) (JSY77). JSY113 and JSY114 were made monoallelic by transforming a MIP1::HYG cassette, selecting for colonies that grew on minimal plates lacking arginine and tryptophan and were resistant to hygromycin and G418, creating JSY131 (MIP1 [Mono]) and JSY133 (mip1-exo [Mono]), respectively. Monoallelic strains were confirmed by their inability to maintain mtDNA after plasmid loss. All strains were grown independently from at least 20 colonies at 30° in YP (yeast extract 1%, peptone 2%) with 2% glucose and 0.01% adenine sulfate for 2 days. Appropriate dilutions from saturated cultures were plated on synthetic complete media lacking arginine to determine total number of cells with mtDNA. Approximately 108 cells were plated onto YP with 2% glycerol and deletion mutants were counted after 4 days. Deletions were confirmed phenotypically in several mutants from each strain; all rho+ colonies tested were Arg− (data not shown). The mutant frequency was determined as the median number of mutant colonies per 108 Arg+ cells. The 95% confidence levels are represented as error bars and were determined using the method of the median.
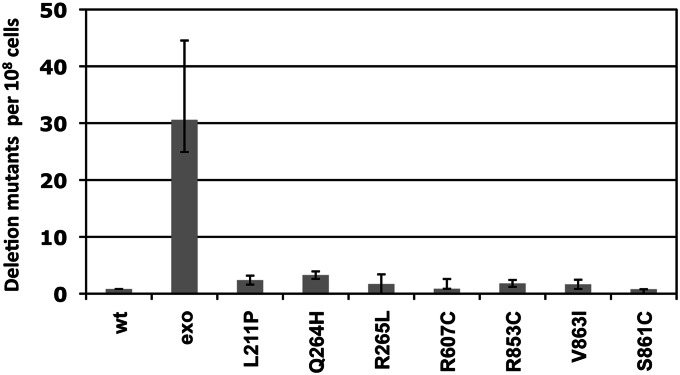
The observation of mtDNA deletions in pol γ-related diseases, such as progressive external ophthalmoplegia (PEO), suggests that mtDNA polymerase impairment may influence deletion formation (Van Goethem et al. 2001). Previously, mip1 mutations that alter amino acids in conserved domains were used to show that disease mutations cause mtDNA depletion and mutagenesis (Baruffini et al. 2006, 2007, 2011; Stuart et al. 2006; Stumpf et al. 2010; Szczepanowska and Foury 2010). To identify disease mutations that cause increased deletion formation, we assayed several heteroallelic mip1 mutants for deletion formation frequency that were either located within the exonuclease domain (L211P, Q264H, and R265L) or shown to increase point mutagenesis (R607C, R853C, V863I, and S861C) (Stumpf et al. 2010). Analysis of mip1 mutants was limited to heteroallelic strains that did not exhibit high petite frequencies, because growth of deletion mutants requires mitochondrial function and most of these mip1 mutations caused 100% petites as monoallelic strains (Stumpf et al. 2010). Even though all of these amino acid substitutions in Mip1 cause mtDNA depletion and/or mutagenesis (Stumpf et al. 2010; Szczepanowska and Foury 2010), none of these mutations increased deletion formation as much as disrupting exonuclease activity (Figure 2). These data suggest that sporadic mtDNA deletion formation between direct repeats is not predominantly caused by inefficient mtDNA replication. While point mutagenesis is also affected by mip1 polymerase activity (Baruffini et al. 2006; Stuart et al. 2006; Stumpf et al. 2010), these results suggest that increased deletion mutagenesis is greatly enhanced by impaired exonuclease activity, making deletion mutagenesis an ideal method of testing genetic and environmental changes that alter exonuclease activity.
Figure 2.
Deletion mutagenesis of mip1 mutants with disease-associated POLG mutations. All strains are NPY75-derived heteroallelic strains with wild-type chromosomal MIP1 and a centromeric plasmid containing either wild-type MIP1 (Foury and Vanderstraeten 1992), the exonuclease-deficient mip1 allele (mip-exo), or a disease-associated variant denoted by the amino acid change in Mip1 (Stumpf et al. 2010). All strains were grown independently from at least 20 colonies at 30° in YP (yeast extract 1%, peptone 2%) with 2% glucose and 0.01% adenine sulfate for 2 days. Appropriate dilutions of samples from the saturated cultures were plated on synthetic complete media lacking arginine to determine total number of cells with mtDNA. The cultures were plated onto YP with 2% glycerol and deletion mutants were counted after 4 days. Deletions were confirmed phenotypically in several mutants from each strain; all rho+ colonies tested were Arg− (data not shown). The mutant frequency was determined as the median number of mutant colonies per 108 Arg+ cells. Error bars represent 95% confidence levels and were determined using the method of the median. P-values relative to wild type were 6 × 10−5 (Exo−), 0.9 (L211P), 0.7 (Q264H), 0.3 (R265L), 0.6 (R607C), 0.5 (R853C), 0.6 (V863I), and 0.5 (S861C).
These results provide further evidence that three of the most conserved disease mutations, (L211P, Q264H, and R265L), in the exonuclease domain do not inhibit exonuclease activity. Many POLG disease mutations alter amino acids near the exonuclease active site (http://tools.niehs.nih.gov/polg/) (Szczepanowska and Foury 2010; Stumpf and Copeland 2011), suggesting that exonuclease activity and mutation avoidance is important for preventing mitochondrial diseases. However, genetic and biochemical characterizations in yeast have shown that the most conserved naturally occurring exonuclease domain disease mutations have no significant effect on exonuclease activity (Stumpf et al. 2010; Szczepanowska and Foury 2010). These unexpected results illustrate the difficulty of determining the role of each POLG mutation in human mitochondrial disease. Many mutations are published only once with no information on familial genetic history and are present with one or more mutations in POLG in cis, in trans, or both (references outlined in http://tools.niehs.nih.gov/polg/). Also, age of disease onset is varied even among patients with similar POLG genotypes (Cohen and Naviaux 2010). While it is possible that naturally occurring exonuclease-deficient POLG mutations are linked to mitochondrial disease, exonuclease-deficient mutations may be too rare to have been identified to date. Furthermore, extrinsic proofreading by a functional allele may be sufficient to prevent disease. Indeed, mouse models indicate that only homozygous exonuclease-deficient individuals are symptomatic (Trifunovic et al. 2004; Kujoth et al. 2005), a prohibitively rare event in human populations. Additionally, mutations that inactivate exonuclease activity may exacerbate diseases not yet associated with POLG.
Using a yeast model system previously employed to study POLG-associated disease mutations, this report addresses two key consequences of exonuclease activity. First, exonuclease activity suppresses mtDNA deletions flanked by 21-nucleotide direct repeats, in agreement with the increased deletion observed in exonuclease-deficient mice (Vermulst et al. 2008b). These observations support the model that frequent misinsertion events from a proofreading-deficient polymerase cause replication pausing that favors strand slippage between direct repeats (Ponamarev et al. 2002). Also, mutations homologous to disease mutations in POLG did not significantly increase deletion formation, suggesting that defective exonuclease activity results in replication-mediated deletions. Combined with previous work describing the very modest increase in point mutation in exonuclease-domain mutations, this report further supports the hypothesis that the exonuclease disease mutations are not devoid of exonuclease activity.
Acknowledgments
We thank Elaine Sia for generously providing NPY75; Dmitry Gordenin for providing technical assistance; the National Institute of Environmental Health Sciences (NIEHS) Sequencing Core Facility for providing nucleotide sequences for the plasmids used; and Matthew Young, Matthew Longley, and Scott Lujan for critical reading of the manuscript. This work was supported by intramural funds from the NIEHS and National Institutes of Health (ES-065078).
Footnotes
Communicating editor: J. Sekelsky
Literature Cited
- Azpiroz R., Butow R. A., 1993. Patterns of mitochondrial sorting in yeast zygotes. Mol. Biol. Cell 4: 21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruffini E., Ferrero I., Foury F., 2007. Mitochondrial DNA defects in Saccharomyces cerevisiae caused by functional interactions between DNA polymerase gamma mutations associated with disease in human. Biochim. Biophys. Acta 1772: 1225–1235. [DOI] [PubMed] [Google Scholar]
- Baruffini E., Lodi T., Dallabona C., Puglisi A., Zeviani M., et al. , 2006. Genetic and chemical rescue of the Saccharomyces cerevisiae phenotype induced by mitochondrial DNA polymerase mutations associated with progressive external ophthalmoplegia in humans. Hum. Mol. Genet. 15: 2846–2855. [DOI] [PubMed] [Google Scholar]
- Baruffini E., Horvath R., Dallabona C., Czermin B., Lamantea E., et al. , 2011. Predicting the contribution of novel POLG mutations to human disease through analysis in yeast model. Mitochondrion 11: 182–190. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Attardi G., 2003. MtDNA mutations in aging and apoptosis. Biochem. Biophys. Res. Commun. 304: 519–529. [DOI] [PubMed] [Google Scholar]
- Cohen B. H., Naviaux R. K., 2010. The clinical diagnosis of POLG disease and other mitochondrial DNA depletion disorders. Methods 51: 364–373. [DOI] [PubMed] [Google Scholar]
- Cortopassi G. A., Arnheim N., 1990. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 18: 6927–6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foury F., Vanderstraeten S., 1992. Yeast mitochondrial DNA mutators with deficient proofreading exonucleolytic activity. EMBO J. 11: 2717–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves L. C., Beadle N. E., Taylor G. A., Commane D., Mathers J. C., et al. , 2009. Quantification of mitochondrial DNA mutation load. Aging Cell 8: 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. A., Johnson K. A., 2001a Exonuclease proofreading by human mitochondrial dna polymerase. J. Biol. Chem. 276: 38097–38107. [DOI] [PubMed] [Google Scholar]
- Johnson A. A., Johnson K. A., 2001b Fidelity of nucleotide incorporation by human mitochondrial DNA polymerase. J. Biol. Chem. 276: 38090–38096. [DOI] [PubMed] [Google Scholar]
- Joyce C. M., 1989. How DNA travels between the separate polymerase and 3′-5′-exonuclease sites of DNA polymerase I (Klenow fragment). J. Biol. Chem. 264: 10858–10866. [PubMed] [Google Scholar]
- Khrapko K., Vijg J., 2007. Mitochondrial DNA mutations and aging: a case closed? Nat. Genet. 39: 445–446. [DOI] [PubMed] [Google Scholar]
- Kraytsberg Y., Simon D. K., Turnbull D. M., Khrapko K., 2009. Do mtDNA deletions drive premature aging in mtDNA mutator mice? Aging Cell 8: 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujoth G. C., Hiona A., Pugh T. D., Someya S., Panzer K., et al. , 2005. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309: 481–484. [DOI] [PubMed] [Google Scholar]
- Lehman I. R., Nussbaum A. L., 1964. The deoxyribonucleases of Escherichia coli V. On the specificity of exonuclease I (phosphodiesterase). J. Biol. Chem. 239: 2628–2636. [PubMed] [Google Scholar]
- Lewis W., Dalakas M. C., 1995. Mitochondrial toxicity of antiviral drugs. Nat. Med. 1: 417–422. [DOI] [PubMed] [Google Scholar]
- Lewis W., Day B. J., Kohler J. J., Hosseini S. H., Chan S. S. L., et al. , 2007. MtDNA depletion, oxidative stress, cardiomyopathy, and death from transgenic cardiac targeted human mutant polymerase gamma. Lab. Invest. 87: 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley M. J., Ropp P. A., Lim S. E., Copeland W. C., 1998. Characterization of the native and recombinant catalytic subunit of human DNA polymerase gamma: identification of residues critical for exonuclease activity and dideoxynucleotide sensitivity. Biochemistry 37: 10529–10539. [DOI] [PubMed] [Google Scholar]
- Longley M. J., Nguyen D., Kunkel T. A., Copeland W. C., 2001. The fidelity of human DNA polymerase gamma with and without exonucleolytic proofreading and the p55 accessory subunit. J. Biol. Chem. 276: 38555–38562. [DOI] [PubMed] [Google Scholar]
- Pavlov Y. I., Frahm C., Nick McElhinny S. A., Niimi A., Suzuki M., et al. , 2006. Evidence that errors made by DNA polymerase alpha are corrected by DNA polymerase delta. Curr. Biol. 16: 202–207. [DOI] [PubMed] [Google Scholar]
- Phadnis N., Sia R. A., Sia E. A., 2005. Analysis of repeat-mediated deletions in the mitochondrial genome of Saccharomyces cerevisiae. Genetics 171: 1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponamarev M. V., Longley M. J., Nguyen D., Kunkel T. A., Copeland W. C., 2002. Active site mutation in DNA polymerase gamma associated with progressive external ophthalmoplegia causes error-prone DNA synthesis. J. Biol. Chem. 277: 15225–15228. [DOI] [PubMed] [Google Scholar]
- Safdar A., Bourgeois J. M., Ogborn D. I., Little J. P., Hettinga B. P., et al. , 2011. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc. Natl. Acad. Sci. USA 108: 4135–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Voljavec A. S., Soueidan S. A., Costigan D. A., et al. , 1989. Spontaneous Kearns-Sayre/chronic external ophthalmoplegia plus syndrome associated with a mitochondrial DNA deletion: a slip-replication model and metabolic therapy. Proc. Natl. Acad. Sci. USA 86: 7952–7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelbrink J. N., Toivonen J. M., Hakkaart G. A., Kurkela J. M., Cooper H. M., et al. , 2000. In vivo functional analysis of the human mitochondrial DNA polymerase POLG expressed in cultured human cells. J. Biol. Chem. 275: 24818–24828. [DOI] [PubMed] [Google Scholar]
- Strand M. K., Stuart G. R., Longley M. J., Graziewicz M. A., Dominick O. C., et al. , 2003. POS5 gene of Saccharomyces cerevisiae encodes a mitochondrial NADH kinase required for stability of mitochondrial DNA. Eukaryot. Cell 2: 809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G. R., Santos J. H., Strand M. K., Van Houten B., Copeland W. C., 2006. Mitochondrial DNA defects in S. cerevisiae with mutations in DNA polymerase gamma associated with progressive external ophthalmolplegia. Hum. Mol. Genet. 15: 363–374. [DOI] [PubMed] [Google Scholar]
- Stumpf J. D., Copeland W. C., 2011. Mitochondrial DNA replication and disease: insights from DNA polymerase gamma mutations. Cell. Mol. Life Sci. 68: 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf J. D., Bailey C. M., Spell D., Stillwagon M., Anderson K. S., et al. , 2010. mip1 Containing mutations associated with mitochondrial disease causes mutagenesis and depletion of mtDNA in Saccharomyces cerevisiae. Hum. Mol. Genet. 19: 2123–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanowska K., Foury F., 2010. A cluster of pathogenic mutations in the 3′-5′ exonuclease domain of DNA polymerase gamma defines a novel module coupling DNA synthesis and degradation. Hum. Mol. Genet. 19: 3516–3529. [DOI] [PubMed] [Google Scholar]
- Trifunovic A., Wredenberg A., Falkenberg M., Spelbrink J. N., Rovio A. T., et al. , 2004. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417–423. [DOI] [PubMed] [Google Scholar]
- Van Goethem G., Dermaut B., Lofgren A., Martin J. J., Van Broeckhoven C., 2001. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat. Genet. 28: 211–212. [DOI] [PubMed] [Google Scholar]
- Vermulst M., Bielas J. H., Kujoth G. C., Ladiges W. C., Rabinovitch P. S., et al. , 2007. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat. Genet. 39: 540–543. [DOI] [PubMed] [Google Scholar]
- Vermulst M., Bielas J. H., Loeb L. A., 2008a Quantification of random mutations in the mitochondrial genome. Methods 46: 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermulst M., Wanagat J., Kujoth G. C., Bielas J. H., Rabinovitch P. S., et al. , 2008b DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat. Genet. 40: 392–394. [DOI] [PubMed] [Google Scholar]
- Zhang D., Mott J. L., Chang S. W., Denniger G., Feng Z., et al. , 2000. Construction of transgenic mice with tissue-specific acceleration of mitochondrial DNA mutagenesis. Genomics 69: 151–161. [DOI] [PubMed] [Google Scholar]