Abstract
Using transgenic mice model (i.e., “clock” knockouts), clock transcription factors have been suggested as critical regulators of dopaminergic behaviors induced by drugs of abuse. Moreover, it has been shown that systemic administration of psychostimulants, such as cocaine and methamphetamine regulate the striatal expression of clock genes. However, it is not known whether dopamine receptors mediate these regulatory effects of psychostimulants at the cellular level. Primary striatal neurons in culture express dopamine receptors as well as clock genes and have been successfully used in studying dopamine receptor functioning. Therefore, we investigated the role of dopamine receptors on neuronal clock gene expression in this model using specific receptor agonists. We found an inhibitory effect on the expression of mClock and mPer1 genes with the D2-class (i.e., D2/D3) receptor agonist quinpirole. We also found a generalized stimulatory effect on the expression of clock genes mPer1, mClock, mNPAS2, and mBmal1 with the D1-class (i.e., D1) receptor agonist SKF38393. Further, we tested whether systemic administration of dopamine receptor agonists causes similar changes in striatal clock gene expression in vivo. We found quinpirole-induced alterations in mPER1 protein levels in the mouse striatum (i.e., rhythm shift). Collectively, our results indicate that the DA receptor system may mediate psychostimulant-induced changes in clock gene expression. Using striatal neurons in culture as a model, further research is needed to better understand how dopamine signaling modulates the expression dynamics of clock genes (i.e., intracellular signaling pathways) and thereby influences neuronal gene expression, neuronal transmission, and brain functioning.
Keywords: primary striatal cultures, mouse, transcription factors, mRNA, quinpirole, SKF38393
“Clock” genes are family of transcription factors that can regulate gene expression by binding specific binding sites called “E-box” in their promoter regions (Darlington et al., 1998). Clock gene expression is not limited to the suprachiasmatic nucleus (SCN), where they participate in molecular feedback loops to operate the “circadian clock”; they are strongly expressed throughout the brain including the mesolimbic system (Shieh, 2003; Uz et al., 2005; McClung et al., 2005; Shieh et al., 2005). Our understanding regarding how clock genes are involved in central nervous system (CNS) functioning and pathologies in vertebrates has changed after the availability of the transgenic mice deficient in various members of the clock family (e.g., mClock, mPer1, mNPAS2). It has been shown that the clock gene family is crucial for the development of behavioral responses to drugs of abuse, i.e., by modulating dopaminergic system functioning in the mesolimbic system (Abarca et al., 2002; McClung et al., 2003, 2005; Perreau-Lenz and Spanagel, 2008).
Clock genes are not only involved in the action of drugs of abuse, they are also influenced by these drugs. Psychostimulant (cocaine and amphetamines)-induced changes in extracellular dopamine levels are responsible for the stimulation of dopamine receptor-mediated intracellular signaling and the activation of transcription factors and immediate-early genes in the mesolimbic system (Hyman and Malenka, 2001). In fact, transcriptional alterations of “clock” gene expression in the mesolimbic system (i.e., the striatum) have been reported after both cocaine and methamphetamine treatments (Nikaido et al., 2001; Iijima et al., 2002; Yuferov et al., 2003; Uz et al., 2005; Lynch et al., 2008). Furthermore, using receptor antagonists systemically, it has been suggested that receptor systems, including dopamine receptors (i.e., D1), mediate methamphetamine-induced clock gene expression changes in this brain region (Nikaido et al., 2001). More recently, increased expression and phosphorylation of tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis, has been reported in the ventral tegmental area of Clock mutants and this increase is thought to be responsible for the increased reward to cocaine (McClung et al., 2005).
The development of addictive behaviors in response to systemically administered psychostimulants is complex and requires the involvement of multiple brain regions, such as the ventral tegmental area, nucleus accumbens, and prefrontal cortex, various cell types, and a number of neurotransmitter systems, including the dopaminergic and glutamatergic systems. Therefore, to better understand the role of the DA receptor system in the regulation of clock gene expression, studies focused on the cellular level (i.e., neuronal) are needed.
Primary striatal neurons in culture have been used successfully for mechanistic studies to understand dopaminergic system functioning and drug addiction (Voulalas et al., 2005). Since striatal neurons express both dopamine receptors and clock genes, we chose this culture model to study the role of dopamine receptor-mediated signaling systems on clock gene expression dynamics. We investigated the effects of dopamine receptor agonists that stimulate either D1-class (i.e., D1 with SKF38393) or D2-class (i.e., D2/D3 with quinpirole) dopamine receptors on the expression of clock genes mPer1, mClock, mNPAS2, and mBmal1 in cultured striatal neurons. In addition, we tested D2-class receptor agonist (i.e., quinpirole)-induced clock gene (i.e., mPER1) regulation in vivo.
EXPERIMENTAL PROCEDURES
Preparation of primary cultures of striatal neurons and drug treatments
For the preparation of primary neuronal cultures from the striatum, we used ICR mice (Harlan, Indianapolis, Indiana). Mice were housed in a temperature-controlled room under conditions of 14.light: 10 h dark cycle (lights on at 5 am). The experimental protocols were approved by the Institutional Animal Care and Ethics Committee of the University of Illinois at Chicago. Pups from embryonic day 16 (E16)-timed pregnant female mice were used to obtain striata. Striata were excised under sterile surgical conditions using a dissection microscope and dissociated in serum free Neurobasal medium (Invitrogen-Gibco, Carlsbad, California) by trituration with a fire-polished Pasteur pipette. Cells were plated to 20 μg/ml poly-D-lysine (Sigma-Aldrich, St. Louis, Missouri)-coated 3.5 cm-dishes (for Western blot assays) or 24-well plates with 12 mm round cover glass (for immunohistochemistry assays) in Neurobasal medium supplemented with B27 (Invitrogen-Gibco). The density of cells was 1 million/ml (for the 3.5 cm-dishes) and 200,000/ml (for the 24-well plates). Cultures were maintained for 7–9 days at 37 °C and 5% CO2 before use. Quinpirole and SKF38393 were dissolved in 0.1% dimethyl sulfoxide (DMSO, Sigma-Aldrich) and applied directly into the culture medium at 9 days in vitro (DIV) for 6 h. All drugs were obtained from Sigma-Aldrich. Controls were treated with a corresponding concentration of DMSO.
Detection of D1, D2, and D3 dopamine receptors and clock gene mRNAs with RT-PCR
Cultured neurons were harvested in TRIzol® extraction system (100μl/million cells; Invitrogen-Gibco), as described by the manufacturer, to isolate total RNA. To avoid DNA contamination, the samples were treated for 30 min at 37°C with DNase I (Ambion, Austin, Texas). RNA absorption was measured photometrically at a wavelength of OD260/280. Amplification primers (see Table 1) were ordered from Integrated DNA Technologies, Inc. (Coralville, Iowa). Each primer contained comparable G+C content to minimize variability in hybridization efficiency at the annealing temperature. The RT-PCR assay is described elsewhere (Uz et al., 2005). Briefly, total RNA from each sample (0.5 μg of starting amount) was denaturated at 80 °C for 6 min and then reverse transcribed with cloned Moloney murine leukemia virus (M-MLV) reverse transcriptase (200U; Invitrogen-Gibco) in a reverse transcription (RT) buffer containing 50 mM Tris-HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2, and 1 mM deoxynucleotide triphosphate (dNTPs; Invitrogen-Gibco) using random hexamers (5 mM; Pharmacia Biotech, Piscataway, New Jersey) and ribonuclease inhibitors (HPRI, 28 U; Amersham, Piscataway, New Jersey) in a volume of 20 μl. The RT mixture was incubated at 37 °C for 60 min to promote cDNA synthesis. The reaction was terminated by heating the samples at 98 °C for 5 min and then rapidly chilling on ice. The cDNA aliquots containing reverse-transcribed material and specific primer pairs for D1, D2, D3, mPer1, mClock, mNPAS2, and mBmal1 were amplified with Hot Tub DNA polymerase (Amersham) in a thermal cycler (9600, Perkin Elmer, Wellesley, Massachusetts) for 28 to 31 cycles of denaturation (94 °C, 15 s), annealing (60 °C, 30 s), and elongation (72 °C, 30 s) steps. The reaction was terminated with a 5-min final elongation step. DNA was visualized by staining the gel with ethidium bromide and UV-light illumination. The fragment sizes were estimated by comparison to calibrated marker bands of 1 Kb plus DNA ladder (Invitrogen-Gibco).
Table 1.
PCR amplification primers
| number | Primer sequence | Accession |
|---|---|---|
| D1 | F: 5′- AAAAGCCTATGCATTCCTTGGGGA R: 5′- GGTATTCCCTAAGAGAGTGGACAG |
NM_010076 |
| D2 | F: 5′- ACCACCACCAACTACCTGATAGTC R: 5′- AGTTGCCCTTGAGTG GTGTCTTCA |
NM_010077 |
| D3 | F: 5′- CATAGACAGGTACACAGCAGTGGT R: 5′- GTAGTAGCGTTTCAGCTCTCCATG |
NM_007877 |
| mClock | F: 5′-CCACAGCAGTTCTTACAGACATCT R: 5′-GACCCACTAAAGCTACCAACACTG |
AF000998 |
| mPer1 | F: 5′-ATGGCTCAAGTGGCAATGAGTCCA R: 5′-CTGGTTAGCCTGAACCTGCTTGAC |
NM_011065 |
| mBmal1 | F: 5′-AAGGATCAAGAATGCAAGGGAGGC R: 5′-TCCAGTCTTGGCATCAATGAGTCG |
NM_007489 |
| mNPAS2 | F: 5′-CTGATGTTGGAGGCATTAGATGGC R: 5′-TTCCTTTAAGAACTGCGGGGTTGC |
U77969 |
| cyclophilin | F: 5′-ATTTGGCTATAAGGGTTCCTC R: 5′-ACGCTCCATGGCTTCCACAAT |
X52803 |
F, forward; R, reverse. For details see Materials and Methods.
Demonstration of neuronal D2 – mCLOCK co-localization with immunofluorescence double-labeling
For immunofluorescence double-labeling, cultured neurons were grown on cover glasses (Fisher, Pittsburgh, Pennsylvania) in 24-well plates for 9 days. The cells were fixed for 10 min in 4% (v/v) formaldehyde (Sigma-Aldrich) in phospate-buffered saline (PBS, Sigma-Aldrich) and washed in PBS (3×10min). After fixation, cells were incubated in 3% (v/v) normal goat serum (NGS, Vector Laboratories, Burlingame, California) in PBS/0.25% Triton X-100 (Sigma-Aldrich) for 30 min to block non specific staining. Cultured neurons were co-labeled with primary antibodies against CLOCK (1:200, Santa Cruz Biotechnology Inc., Santa Cruz, California) and D2 receptor (1:100, Santa Cruz Biotechnology Inc.) for two hours at room temperature. The cultures were washed with PBS (3×5min) and co-incubated for one hour at room temperature with following secondary antibodies: Cy5-linked anti-rabbit IgG (1:1,000; Amersham) to produce red fluorescent staining for mCLOCK protein and Cy2-linked anti-mouse IgG (1:1,000; Amersham) to produce green fluorescent staining for D2 receptor protein. The cells were finally washed in PBS (2×10min) followed by double distilled water (2×10min). Cover glasses with labeled cells were air-dehydrated and mounted onto glass slides using “entellan new” mounting media (Electron Microscopy Science, Washington, Pennsylvania). Fluorescent labeling were visualized and captured applying proper filters for each secondary antibody used under an AxioVision 3.1 fluorescent microscope equipped with a camera (Carl Zeiss Vision, Germany) at a magnification of ×40.
Measurement of clock gene mRNA levels with quantitative RT-PCR
Total RNAs were isolated from cultured neurons as described above. The RT-PCR assays were run as described above in the presence of specific amplification primers for clock genes as well as cyclophilin (ordered from Integrated DNA Technologies, Inc.; see Table 1). To allow co-amplification of clock genes with the more abundant cyclophilin (cyc) mRNA, pilot studies were conducted to determine optimal relative primer concentration and cycle number whereby the PCR would still be within the exponential phase of amplification for all transcripts. The ratio of specific primer pairs to cyclophilin was kept as 1:5. The PCR mixture was amplified with denaturation (94 °C, 15 s), annealing (60 °C, 30 s), and elongation (72 °C, 30 s) steps for either 28 (for mPer1 and mClock) or 31 (for mBmal1 and mNPAS2) cycles. Trace amounts of [32P]dCTP (0.5 μCi/sample; Amersham) were included during the PCR step for subsequent quantification. To quantify the amount of the product corresponding to the amplified mRNA, the ethidium bromide-stained bands were excised from the electrophoresis gels and the radioactivity was counted as described earlier (Uz et al., 2005).
In vivo studies with drug treatment, sample preparation, and the measurement of mPER1 protein levels with quantitative Western blotting
Male 8 week old C3H/HeJ mice weighing 25–30 g were purchased from Jackson Laboratories (Bar Harbor, Maine). Animals were housed in groups of three and had free access to laboratory chow and water. They were kept in a temperature-controlled room under conditions of 14 h light: 10 h dark cycle (lights on at 5 am). In our previously published studies using this light: dark cycle (Uz et al., 2003), we have demonstrated that daily striatal rhythms of clock genes as well as locomotor activity rhythms in mice are similar to published reports opting the 12h:12h LD cycle. Therefore, for consistency, same light schedule (14h:10h) applied for the studies described here. The experimental protocol was approved by the Institutional Animal Care and Ethics Committee of the University of Illinois at Chicago. Quinpirole (Sigma-Aldrich) was dissolved in sterile physiological saline (0.9% NaCl, Sigma-Aldrich) and administered intraperitoneally (1 mg/kg; i.p.) in an injection volume of 0.05 ml/25 g body weight every third day (10 am) or night (1 am) to two different groups of subjects. Nighttime injections were administered under dim red light. The quinpirole dose (1mg/kg), treatment frequency (every 72 hours), and the length of treatment (7 injections) were adapted from an established quinpirole treatment model (Szechtman et al., 1994; Akhisaroglu et al., 2005).
To quantitate the drug-induced changes in mPER1 protein levels, striatal samples (i.e., caudate-putamen, CPu) were excised from 1-mm-thick coronal brain slices [Bregma 1.54 mm through 0.74 mm (Paxinos and Franklin, 2001)] as described elsewhere (Uz et al., 2003). Sample collection was done 72 h after the last (seventh) daytime or nighttime quinpirole injections, either during the day (10 am) or at night (1 am, under dim red light). Sample size was 12 for both day and nighttime groups. Tissue samples were frozen immediately on dry ice and stored at −80°C until assay. mPER1 protein levels were measured after homogenizing the striatal tissue in a homogenizing buffer described earlier (Uz et al., 2003). After reading the protein content of the tissue homogenate, 40–80 μg samples were loaded onto a 7.5% (w/v) acrylamide gel using the Mini Protean II gel apparatus (Bio-Rad, Hercules, California). The gels were run in a running buffer described earlier (Uz et al., 2003). The proteins were subsequently transferred electrophoretically to an ECL nitrocellulose membrane (Amersham) using the Mini TransBlot transfer unit (Bio-Rad) at 150 mA constant current overnight. The blots were incubated with anti-mouse PER1 antibody (4 μg/ml; Alpha Diagnostic International, Inc., San Antonio, Texas) in a blotting buffer with powdered nonfat milk overnight as described before (Uz et al., 2003). The blots were then incubated with the horseradish-peroxidase-linked secondary antibody (anti-rabbit IgG; 1:1000; Amersham) for 4 h at room temperature and processed with an ECL kit; blots were then exposed to Hyperfilm ECL (Amersham). To normalize the signal for mPER1 protein, the presence of noninducible β-actin protein was measured on the same blot using a mouse monoclonal antibody against the β-actin (1:10,000; Sigma-Aldrich). The optical densities of the mPER1 bands were corrected by the optical density of the corresponding β-actin bands on the film using the Loats Image Analysis System (Loats Associates, Inc., Westminster, Maryland).
Statistical analyses
Statistical analyses were performed using the SPSS software (version 12.0, SPSS Inc., Chicago, Illinois). Quantitative RT-PCR results (mean ± SEM) for clock genes were calculated as the ratio to cyclophilin for each specific gene tested and were evaluated by one-way ANOVA followed by the Dunnett’s multiple comparison test. Results are expressed as the mean ± SEM and are evaluated from at least four independent culture preparations. An average of three samples for each culture preparation is accepted as one n value. Quantitative Western blotting results (mean ± SEM; n=12 per group) for mPER1 were calculated as the ratio to β-actin and were evaluated by independent sample t-test. Significance was accepted as p< .05 for all the statistical analyses performed.
RESULTS
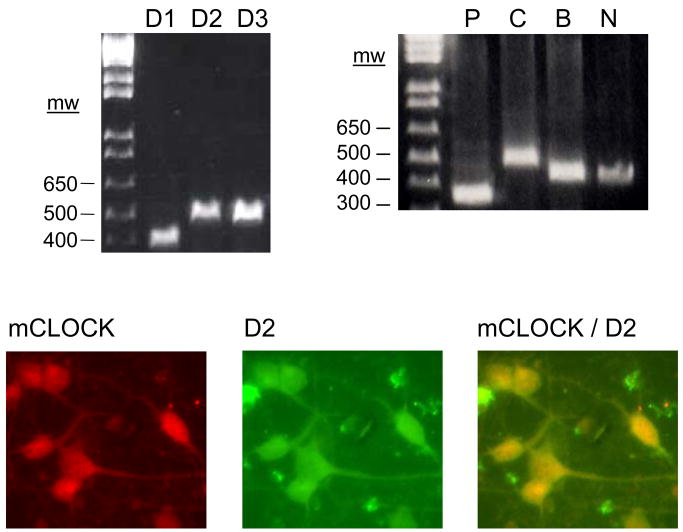
Dopamine receptors and clock genes are expressed in striatal neurons in culture (Fig. 1). Gene specific primer pairs (see Table 1 for primer sequences) detected the expected size PCR products in gel electrophoresis for D1 (404 bp), D2 (532 bp), and D3 (536 bp) dopamine receptors and the clock genes, mPer1 (387 bp), mClock (534 bp), mBmal1 (471 bp), and mNPAS2 (458 bp) (Fig. 1). Moreover, mCLOCK and D2 dopamine receptor proteins co-exist in the same neurons in culture (Fig. 1). Since co-labeling studies revealed that vast majority of the cultured neurons are immunopositive for both dopamine receptors and clock genes the number of the neurons expressing both genes was not analyzed quantitatively.
Fig. 1.
The presence of dopamine receptors and clock genes in striatal neurons. Total RNA and protein were extracted from striatal neurons in culture (9 DIV). The presence of receptor mRNAs was demonstrated by applying RT-PCR (upper panel) and co-localization of mRNA and proteins was demonstrated by applying immunofluorescence double-labeling (bottom panel). A representative gel electrophoresis picture of the PCR products on the upper panel is used to demonstrate the presence of the D1 (404 bp), D2 (532 bp), and D3 (536 bp) dopamine receptors as well as clock genes, mPer1 (P, 387 bp), mClock (C, 534 bp), mBmal1 (B, 471 bp), and mNPAS2 (N, 458 bp) by using specific primers for each gene (see Table 1 for primer sequences). Co-expression of mCLOCK and D2 dopamine receptor in striatal neurons in culture was demonstrated by co-labeling each protein with corresponding antibodies (for CLOCK, 1:200, and for D2 receptor, 1:100, both from Santa Cruz Biotechnology Inc.). Proteins were visualized with fluorescent labeled secondary antibodies, applying filters for each antibody under a fluorescent microscope for CLOCK alone (bottom panel left hand image, red), for D2 dopamine receptor alone (middle image, green), and for both (co-applying dual filters, right hand image, yellow).
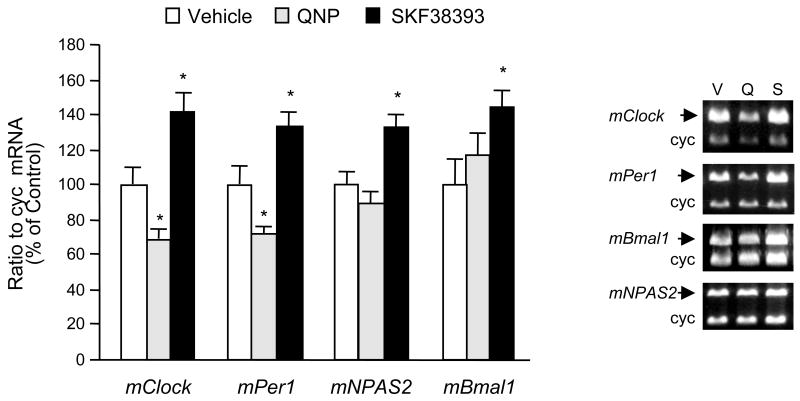
Treatments of dopamine receptor agonists quinpirole (1 μM) and SKF38393 (1 μM) differentially regulated the expression of all clock genes studied (Fig. 2). The D2/D3 dopamine receptor agonist quinpirole decreased mClock and mPer1 mRNA levels significantly while mNPAS2 and mBmal1 mRNA expressions were not affected by this treatment (Fig. 2). On the other hand, the D1 receptor agonist SKF38393 caused a general increase in mRNA levels for all clock genes studied (Fig. 2).
Fig. 2.
The differential regulatory effect of dopamine receptor agonists on clock gene expression in striatal neurons. Quinpirole (1μM) or SKF38393 (1 μM) were applied to the striatal neurons in culture (9 DIV) for 6 h. After treatment, cells were harvested in TRIzol® reagent for mRNA extraction. Specific primers for each clock gene measured were co-amplified with cyclophilin (cyc) primers (see Table 1 for primer sequences) using RT-PCR to quantify mRNA levels for each gene. Radioactivity counts of RT-PCR products (mean ± SEM; n=4 to 6) were calculated as the ratio of each clock gene to cyclophilin and expressed as the ratio of control. *p < .05 in comparison to control group (one-way ANOVA followed by the Dunnett’s multiple comparison test). Quinpirole decreased mRNA levels for both mClock and mPer1 but did not affect mNPAS2 and mBmal1 mRNA levels. On the other hand, SKF38393 increased mRNA levels for all clock genes studied. DMSO was used as the vehicle in control groups in a final concentration of 0.1%. The sample gel electrophoresis pictures in the right panel demonstrate the co-amplification of clock genes and cyclophilin for quantitation assays after the vehicle (V) and dopamine agonist (quinpirole, Q and SKF38393, S) treatments in striatal neurons. The sizes for the individual products are: 534 bp for mClock, 387 bp for mPer1, 471 bp for mBmal1, 458 bp for mNPAS2, and 298 bp for cyc. Please note the drug-induced changes in the density of clock genes except the housekeeping gene cyc.
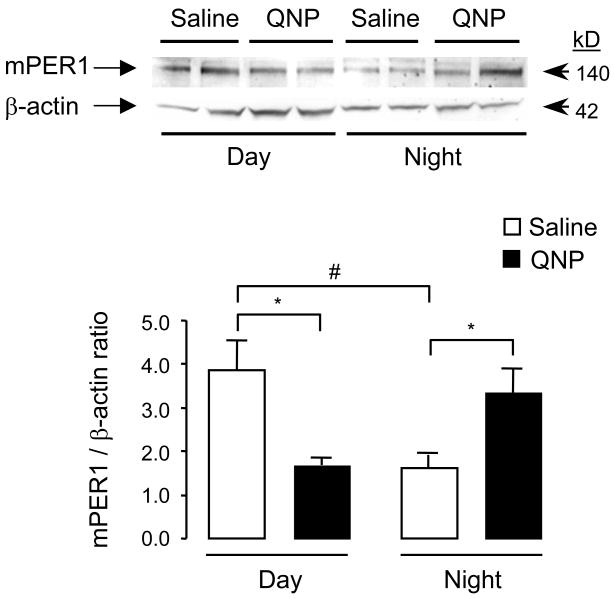
In vivo, protracted systemic treatment of the D2/D3 receptor agonist quinpirole caused differential changes in mPER1 protein levels in the CPu of striatum depending on the time of injection (Fig. 3). Prolonged changes induced by quinpirole were measured 72 h after the last (i.e., seventh) injections either during the day (after repeated daytime only injections) or at night (after repeated nighttime only injections). While quinpirole decreased mPER1 protein levels after daytime injections, it increased mPER1 levels after nighttime injections (Fig. 3).
Fig. 3.
The effect of quinpirole on striatal mPER1 protein levels. Western blots using an antibody against mPER1 (4 μg/ml; Alpha Diagnostic International, Inc.) on striatal samples after vehicle, saline and quinpirole treatments are shown on the top of the figure. Three days after the last injections of protracted quinpirole treatment (1 mg/kg, i.p.; every three days either during the day or at night), animals were sacrificed and striatal (i.e., CPu) brain samples were collected for mPER1 Western blotting either during the day (10 am) or at night (1 am, under dim red light). Repeated daytime injections of quinpirole decreased striatal mPER1 protein levels whereas nighttime injections resulted in increase in mPER1 levels. For the quantitative assay, mPER1 protein levels were normalized by the corresponding β-actin signal. The results of the quantitative assay are the mean ± SEM, obtained from 12 animals from each time point. The results are shown as ratios of PER1/β-actin. *p < .05 compared to corresponding control, saline groups and #p <.05 compared to daytime control, saline group (independent sample t-test).
DISCUSSION
In this study using dopamine receptor specific drugs (i.e., agonists), we demonstrated that dopamine receptor-mediated intracellular signaling differentially regulates neuronal clock gene expression at the cellular level (i.e., striatal neurons in culture). Further, we found that when given systemically, the dopamine receptor agonist quinpirole regulates the striatal expression of the clock gene mPer1 depending on the time of injection.
As a part of the mesolimbic system, the striatum is primarily involved in the development of dopaminergic behaviors such as locomotor sensitization and reward in response to addictive drugs. Primary striatal neurons in culture have been used successfully for mechanistic studies to understand dopaminergic system functioning and drug addiction (Chartoff et al., 2003; Baker et al., 2003; Voulalas et al., 2005). We demonstrated that striatal neurons in culture express dopamine receptors including D1, D2, and D3 and clock genes (Figures 1 and 2). Considering their expression in mesocorticolimbic system as well as published reports demonstrating dopaminergic system/clock gene relations, including our previous report (Uz et al., 2005), we chose to test the following four clock genes; mClock, mPer1, mNPAS2, and mBmal1 in the studies reported here. Moreover, dopamine receptors and clock genes are co-expressed in the same neurons in this culture (see an example of mCLOCK and D2 dopamine receptor in Fig. 1). Therefore, we chose this culture model to study the role of the dopamine/dopamine receptor system on clock gene expression dynamics at the cellular level (i.e., neuronal).
Striatal neurons in culture were treated with the dopamine receptor agonists quinpirole and SKF38393. We found an inhibitory effect on gene expression for mClock and mPer1 with the D2/D3 agonist quinpirole. In contrast, the D1 agonist SKF 38393 produced a generalized stimulatory effect on all clock genes studied (Fig. 2).
Dopamine receptors belong to a G-protein-coupled receptor family. They have been divided into two major subtypes, D1- and D2-class receptors. D1-class receptors (D1/D5) activate the enzyme adenylyl cyclase and increase intracellular levels of cAMP, whereas D2-class receptors (D2/D3/D4) exert an inhibitory influence on this system (Andersen et al., 1990). In fact, we found a generalized stimulatory effect on clock gene expression with the D1-class receptor agonist while the D2-class receptor agonist caused an inhibitory effect on the expression activity of these genes in our neuronal culture model (Fig. 2). A functional role for a cAMP responsive element (CRE) that is present on the promoter of the mPer1 gene has been demonstrated in the regulation of the expression of this gene (Yamaguchi et al., 2000; Yagita and Okamura, 2000; von Gall et al., 2001). For example, it has been shown that melatonin inhibits both the phosphorylation of the cAMP responsive element binding protein (CREB, a transcriptional activator of cAMP-sensitive genes) and the forskolin-induced Per1 mRNA levels (von Gall et al., 2000; Morgan et al., 1998). Along the same line, we found a decrease in mPer1 mRNA levels after quinpirole treatment while SKF38393 treatment caused an increase in the neuronal expression of this gene. Although the involvement of CRE activity has been suggested in the transcriptional regulation of mClock and mBmal1 genes (Takahata et al., 2000), further promoter analyses are needed to explain the differential gene regulation expression of these genes: i.e., decreased mRNA levels after quinpirole and increased mRNA levels after SKF38393 for mClock.
We also tested in vivo correlates of dopaminergic drug-induced changes in clock gene expression observed in vitro. We measured quinpirole-induced changes in mPER1 protein levels at the time points when its striatal levels are highest (during the day, 10 am) and lowest (at night, 1 am) as we reported earlier (Uz et al., 2003). A quinpirole treatment protocol that had been used successfully earlier was employed for this purpose (Szechtman et al., 1994; Akhisaroglu et al., 2005). We treated mice with quinpirole (1 mg/kg) either during the day or at night in two separate groups and measured mPER1 protein levels both during the day and at night 72 h after the last quinpirole injections in corresponding groups. We found that in vehicle-treated groups, similar to our previously published results (Uz et al., 2003), mPER1 protein levels were lower at night and higher during the day, indicating their daily rhythm (Fig. 3). Moreover, repeated daytime quinpirole treatment caused a decrease whereas nighttime administration caused an increase in mPER1 protein levels, indicating a rhythm shift mediated by dopaminergic receptor-mediated signaling (Fig. 3).
The interaction between the D2/D3 receptor agonist quinpirole and the Period gene has been demonstrated earlier in fruit flies (Andretic and Hirsh, 2000). Since we found significant changes after quinpirole treatment in clock gene expression in vitro, we wanted to test the effect of quinpirole on clock gene expression levels in vivo. Clock genes maintain their rhythms in cultured cells only few days (depending on the type of cultured cells up to 4 days). At the time our in vitro drug treatment performed (i.e., 9 days in vitro), clock genes are expressed at their basal levels without demonstrating any rhythm. Since in vivo PER1 levels demonstrate daily rhythms in the striatum, we administered quinpirole at two different time points according to the daily rhythms of this protein. Further studies with both receptor agonists and antagonists targeting other dopamine receptors such as D1 are needed fully understand the interactions between these two systems.
Studies with [3H]-spiroperidol binding (for D2 receptors) as well as with Western immunoblotting with specific antibodies against dopamine receptors demonstrated that dopamine receptors present circadian rhythms (Naber et al., 1980; Wirz-Justice, 1987; Viyoch et al., 2001; Akhisaroglu et al., 2005). In parallel, dosing time-dependent behavioral effects were observed with dopaminergic drugs, such as D1 and D2 agonists and antagonists (Nagayama et al., 1978; Tirelli & Jodogne, 1992; Martin-Iverson and Yamada, 1992; Viyoch et al., 2001). We recently reported that protracted quinpirole treatment causes dosing time-dependent behavioral changes in accordance with the changes in D2/D3 dopamine ratio caused by this drug (Akhisaroglu et al., 2005). Thus, our results support the possibility that diurnal changes in the content of striatal dopamine receptors may determine not only the dosing time-dependent behavioral actions of dopaminergic drugs but also molecular changes caused by these drugs including differential regulation of clock gene expressions (i.e., shift in daily mPER1 protein levels). In fact, using Per mutant flies, it has been demonstrated that the modulation of responsiveness to the dopamine receptor agonist quinpirole is under Per-dependent circadian control and that the modulation of quinpirole-responsive dopamine receptors is likely downstream of Per (Andretic and Hirsh, 2000).
Since clock proteins contain a basic region-helix-loop-helix domain and an E-box (a regulatory element that confers transcriptional cycling) in their promoters, they are recognized as transcription factors (Darlington et al., 1998). They can regulate the expression of the non-clock genes at the promoter level (i.e., clock-controlled genes). Numbers of genes important for the development of dopaminergic behaviors in response to psychostimulants contain E-box sites in their promoters and they are putative targets for clock genes (Manev and Uz, 2006). For example, Hampp et al. (2008) reported that the clock proteins BMAL1, NPAS2, and PER2 mediate the transcriptional regulation of monoamine oxidase A (an enzyme that catalyzes the oxidation of the monoamines dopamine, noradreanline and serotonin) by binding E-box sites at the promoter level.
In rodents, the expression of clock genes, including Per1, Clock, Bmal1, and NPAS2, has been cloned and their expression has been demonstrated throughout the brain (Masubuchi et al. 2000; Nikaido et al., 2001; Abe et al., 2001; Yamamoto et al., 2001; Shieh, 2003; Uz et al., 2003, 2005; McClung et al., 2005; Shieh et al., 2005). Even though clock genes have been studied primarily in the suprachiasmatic nucleus (SCN) (i.e., circadian/clock function), based on their SCN-independent expression in the brain and their involvement on the development of region-specific behavioral responses (e.g., drug-induced locomotor sensitization and reward, alcohol consumption, drug self administration), studies focusing on the new concept of a “non-clock” function of clock genes are emerging.
In conclusion, using a neuronal culture model (i.e., primary striatal cultures) in vitro as well as a protracted dopamine receptor-specific drug treatment protocol in vivo, we have demonstrated that dopamine receptors mediate neuronal clock gene expression. Our results clarify at least in part the underlying mechanisms of the regulatory role of the psychostimulant/dopamine system on clock gene expression dynamics. Future studies using neuronal culture models are needed to understand the underlying mechanisms of dopamine receptor-mediated clock gene expression, such as the intracellular signaling pathways induced by these receptors. We expect this line of research to provide new mechanistic and treatment targets at the cellular (i.e., receptor/signaling pathway molecules) and molecular (i.e., transcriptional) levels to study the long-term changes caused by the psychostimulant/DA receptor system in the development of addictions.
Acknowledgments
We acknowledge support from the Psychiatric Institute, UIC and by NIH grants R01 DA15072 (T.U.), R01 MH61572 (H.M.), and K01 MH069839 (R.S.).
Comprehensive list of abbreviations
- NPAS2
Neuronal PAS domain protein 2
- cAMP
cyclic adenosine 3′,5′-monophosphate
- CNS
central nervous system
- DMSO
dimethyl sulfoxide
- MMLV
Moloney murine leukemia virus
- dNTP
deoxynucleotide triphosphate
- CREB
cAMP response element binding protein
- SCN
suprachiasmatic nucleus
- PTX
Pertussis toxin
- DA
dopamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci USA. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Honma S, Namihira M, Masubuchi S, Honma K. Behavioural rhythm splitting in the CS mouse is related to clock gene expression outside the suprachiasmatic nucleus. Eur J Neurosci. 2001;14:1121–1128. doi: 10.1046/j.0953-816x.2001.01732.x. [DOI] [PubMed] [Google Scholar]
- Akhisaroglu M, Kurtuncu M, Manev H, Uz T. Diurnal rhythms in quinpirole-induced locomotor behaviors and striatal D2/D3 receptor levels in mice. Pharmacol Biochem Behav. 2005;80:371–377. doi: 10.1016/j.pbb.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Andersen PH, Gingrich JA, Bates MD, Dearry A, Falardeau P, Senogles SE, Caron MG. Dopamine receptor subtypes: beyond the D1/D2 classification. Trends Pharmacol Sci. 1990;11:231–236. doi: 10.1016/0165-6147(90)90249-8. [DOI] [PubMed] [Google Scholar]
- Andretic R, Hirsh J. Circadian modulation of dopamine receptor responsiveness in Drosophila melanogaster. Proc Natl Acad Sci USA. 2000;97:1873–1878. doi: 10.1073/pnas.97.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H, Kobayashi K, Okano H, Saino-Saito S. Cortical and striatal expression of tyrosine hydroxylase mRNA in neonatal and adult mice. Cell Mol Neurobiol. 2003;23:507–518. doi: 10.1023/A:1025015928129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Papadopoulou M, Konradi C, Carlezon WA. Dopamine-dependent increases in phosphorylation of cAMP response element binding protein (CREB) during precipitated morphine withdrawal in primary cultures of rat striatum. J Neurochem. 2003;87:107–118. doi: 10.1046/j.1471-4159.2003.01992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington TK, Wager-Smith K, Ceriani MF, Staknis D, Gekakis N, Steeves TD, Weitz CJ, Takahashi JS, Kay SA. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science. 1998;280:1599–1603. doi: 10.1126/science.280.5369.1599. [DOI] [PubMed] [Google Scholar]
- Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, Brunk I, Spanagel R, Ahnert-Hilger G, Meijer JH, Albrecht U. Regulation of monoamine oxidase a by circadian-clock components implies clock influence on mood. Curr Biol. 2008;18:678–683. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Iijima M, Nikaido T, Akiyama M, Moriya T, Shibata S. Methamphetamine-induced, suprachiasmatic nucleus-independent circadian rhythms of activity and mPer gene expression in the striatum of the mouse. Eur J Neurosci. 2002;16:921–929. doi: 10.1046/j.1460-9568.2002.02140.x. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Girgenti MJ, Breslin FJ, Newton SS, Taylor JR. Gene profiling the response to repeated cocaine self-administration in dorsal striatum: A focus on circadian genes. Brain Res. 2008;1213:166–177. doi: 10.1016/j.brainres.2008.02.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H, Uz T. Clock genes: influencing and being influenced by psychoactive drugs. Trends Pharmacol Sci. 2006;27:186–189. doi: 10.1016/j.tips.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Yamada N. Synergistic behavioural effects of dopamine D1 and D2 receptor agonists are determined by circadian rhythms. Eur J Pharmacol. 1992;215:119–125. doi: 10.1016/0014-2999(92)90616-c. [DOI] [PubMed] [Google Scholar]
- Masubuchi S, Honma S, Abe H, Ishizaki K, Namihira M, Ikeda M, Honma K. Clock genes outside the suprachiasmatic nucleus involved in manifestation of locomotor activity rhythm in rats. Eur J Neurosci. 2000;12:4206–4214. [PubMed] [Google Scholar]
- McClung CA, Cooper DC, Sidiropoulou K, Young QL, Sanchez N, Vitaterna M, Garcia JA, Takahashi JS, White FJ, Nestler EJ. Clock and NPAS2 differentially regulate cocaine reward. Program No. 112.17; Abstract Viewer/Itinerary Planner; Washington, DC: Society for Neuroscience; 2003. Online. [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PJ, Ross AW, Graham ES, Adam C, Messager S, Barrett P. oPer1 is an early response gene under photoperiodic regulation in the ovine pars tuberalis. J Neuroendocrinol. 1998;10:319–323. doi: 10.1046/j.1365-2826.1998.00232.x. [DOI] [PubMed] [Google Scholar]
- Naber D, Wirz-Justice A, Kafka MS, Wehr TA. Dopamine receptor binding in rat striatum: Ultradian rhythm and its modification by chronic imipramine. Psychopharmacology. 1980;68:1–5. doi: 10.1007/BF00426642. [DOI] [PubMed] [Google Scholar]
- Nagayama H, Takagi A, Nakamura E, Yoshida H, Takahashi R. Circadian susceptibility rhythm to apomorphine in the brain. Commun Psychopharmacol. 1978;2:301–310. [PubMed] [Google Scholar]
- Nikaido T, Akiyama M, Moriya T, Shibata S. Sensitized increase of period gene expression in the mouse caudate/putamen caused by repeated injection of methamphetamine. Mol Pharmacol. 2001;59:894–900. doi: 10.1124/mol.59.4.894. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- Perreau-Lenz S, Spanagel R. The effects of drugs of abuse on clock genes. Drug News Perspect. 2008;21:211–217. doi: 10.1358/dnp.2008.21.4.1213350. [DOI] [PubMed] [Google Scholar]
- Shieh KR. Distribution of the rhythm-related genes rPERIOD1, rPERIOD2, and rCLOCK, in the rat brain. Neuroscience. 2003;118:831–843. doi: 10.1016/s0306-4522(03)00004-6. [DOI] [PubMed] [Google Scholar]
- Shieh KR, Yang SC, Lu XY, Akil H, Watson SJ. Diurnal rhythmic expression of the rhythm-related genes, rPeriod1, rPeriod2, and rClock, in the rat brain. J Biomed Sci. 2005;12:209–217. doi: 10.1007/s11373-004-8176-6. [DOI] [PubMed] [Google Scholar]
- Szechtman H, Dai H, Mustafa S, Einat H, Sullivan RM. Effects of dose and interdose interval on locomotor sensitization to the dopamine agonist quinpirole. Pharmacol Biochem Behav. 1994;48:921–928. doi: 10.1016/0091-3057(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Takahata S, Ozaki T, Mimura J, Kikuchi Y, Sogawa K, Fujii-Kuriyama Y. Transactivation mechanisms of mouse clock transcription factors, mClock and mArnt3. Genes Cells. 2000;5:739–747. doi: 10.1046/j.1365-2443.2000.00363.x. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Jodogne C. Behavioral sensitization and tolerance to the D2 agonist RU 24213: dissociation between several behavior patterns in mice. Pharmacol Biochem Behav. 1993;44:627–632. doi: 10.1016/0091-3057(93)90178-v. [DOI] [PubMed] [Google Scholar]
- Uz T, Akhisaroglu M, Ahmed R, Manev H. The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology. 2003;28:2117–2123. doi: 10.1038/sj.npp.1300254. [DOI] [PubMed] [Google Scholar]
- Uz T, Ahmed R, Akhisaroglu M, Kurtuncu M, Imbesi M, Arslan AD, Manev H. Effect of fluoxetine and cocaine on the expression of clock genes in the mouse hippocampus and striatum. Neuroscience. 2005;134:1309–1316. doi: 10.1016/j.neuroscience.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Viyoch J, Ohdo S, Yukawa E, Higuchi S. Dosing time-dependent tolerance of catalepsy by repetitive administration of haloperidol in mice. J Pharmacol Exp Ther. 2001;298:964–969. [PubMed] [Google Scholar]
- von Gall C, Weaver DR, Kock M, Korf HW, Stehle JH. Melatonin limits transcriptional impact of phosphoCREB in the mouse SCN via the Mel1a receptor. Neuroreport. 2000;11:1803–1807. doi: 10.1097/00001756-200006260-00002. [DOI] [PubMed] [Google Scholar]
- von Gall C, Schneider-Hüther I, Pfeffer M, Dehghani F, Korf HW, Stehle JH. Clock gene protein mPER1 is rhythmically synthesized and under cAMP control in the mouse pineal organ. J Neuroendocrinol. 2001;13:313–316. doi: 10.1046/j.1365-2826.2001.00643.x. [DOI] [PubMed] [Google Scholar]
- Voulalas PJ, Holtzclaw L, Wolstenholme J, Russell JT, Hyman SE. Metabotropic glutamate receptors and dopamine receptors cooperate to enhance extracellular signal-regulated kinase phosphorylation in striatal neurons. J Neurosci. 2005;25:3763–3773. doi: 10.1523/JNEUROSCI.4574-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A. Circadian rhythms in mammalian neurotransmitter receptors. Progress in Neurobiol. 1987;29:219–259. doi: 10.1016/0301-0082(87)90022-0. [DOI] [PubMed] [Google Scholar]
- Yagita K, Okamura H. Forskolin induces circadian gene expression of rPer1, rPer2 and dbp in mammalian rat-1 fibroblasts. FEBS Lett. 2000;465:79–82. doi: 10.1016/s0014-5793(99)01724-x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Mitsui S, Miyake S, Yan L, Onishi H, Yagita K, Suzuki M, Shibata S, Kobayashi M, Okamura H. The 5′ upstream region of mPer1 gene contains two promoters and is responsible for circadian oscillation. Curr Biol. 2000;10:873–876. doi: 10.1016/s0960-9822(00)00602-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Shigeyoshi Y, Ishida Y, Fukuyama T, Yamaguchi S, Yagita K, Moriya T, Shibata S, Takashima N, Okamura H. Expression of the Per1 gene in the hamster: brain atlas and circadian characteristics in the suprachiasmatic nucleus. J Comp Neurol. 2001;430:518–532. [PubMed] [Google Scholar]
- Yuferov V, Kroslak T, Laforge KS, Zhou Y, Ho A, Kreek MJ. Differential gene expression in the rat caudate putamen after “binge” cocaine administration: advantage of triplicate microarray analysis. Synapse. 2003;48:157–169. doi: 10.1002/syn.10198. [DOI] [PubMed] [Google Scholar]