Abstract
Aims
To examine whether tamper-resistant formulations (TRFs) of tapentadol hydrochloride ER 50 mg (TAP50) and tapentadol hydrochloride 250 mg (TAP250) could be converted into forms amenable to intranasal (Study 1) or intravenous abuse (Study 2).
Design
Randomized, repeated-measures study designs were employed. A non-TRF of OxyContin® 40 mg (OXY40) served as a positive control. No drug was taken in either study.
Setting
The studies took place in an outpatient setting in New York, NY.
Participants
25 experienced, healthy extended-release oxycodone abusers participated in each study.
Measurements
The primary outcome for Study 1 was percentage of participants who indicated they would snort the tampered tablets, while the primary outcome for Study 2 was percent yield of active drug in solution. Other descriptive variables such as time spent manipulating the tablets were also examined to better characterize tampering behaviors.
Findings
Tampered TRF tablets were less desirable than the tampered OXY40 tablets. Few individuals were willing to snort the TRF particles (TAP50: 24%, TAP250: 16%; OXY40: 100% p<.001). There was less drug extracted from the TAP50 tablet than from the OXY40 tablet (3.5% vs. 37.0%, p=.008), and no samples from the TAP250 tablets contained analyzable solutions of the drug. It took participants longer to tamper with the TAPs (Study 1: TAP50 vs. OXY40, p<.01; TAP250 vs. OXY40, p<.01; Study 2: TAP250 vs. OXY40, p<05).
Conclusions
Taptentadol TRF tablets were not well-liked by individuals who regularly tampered with extended-release oxycodone tablets. Employing tamper resistant technology may be a promising approach towards reducing the abuse potential of tapentadol ER.
Introduction
Prescription opioid medications relieve acute and chronic pain, as well as malignant and non-malignant pain [1–4]. However, these medications are also abused. National survey data reveal that approximately 2 million people aged 12 and older initiated non-medical use of pain relievers in 2010 [5]. An additional 5.1 million people aged 12 years or older were current, non-medical users of pain relievers during the same period of time. Recent data from the Drug Abuse Warning Network (DAWN) reveal that non-medical use of prescription drugs accounted for approximately a quarter of all drug-related ED visits in 2009 [6]. Of those, opioid analgesics accounted for approximately half of the drugs used [6]. While the clinical benefit of these medications is clear, a considerable degree of risk is also associated with their use.
Extended-release opioid formulations were developed for the treatment of pain conditions requiring long-acting, stable levels of medication [7]. When swallowed whole (i.e., taken intact), these formulations have been hypothesized to have a lower abuse potential than immediate release formulations because there is a longer time to peak drug effect [8–9]. Opioid abusers have attempted to circumvent extended-release formulations by crushing the pills for insufflation (“snorting”) or injection (“shooting”) [10] as opioids with rapid rates of onset have been thought to have greater abuse liability [11–13]. Abuse by these routes is also accompanied by increased health risks, such as overdose [14–15], or the transfer of communicable disease [16–20]. Thus, it has been a public health challenge to determine whether it is possible to disrupt opioid tampering while still maintaining the drug delivery necessary to treat pain [21–24].
One development in this area has been the production of abuse deterrent formulations [25]. Mechanisms of available abuse deterrent formulations have been categorized as deterring agents (e.g., naloxone), chemical barriers (e.g., a prodrug), or physical barriers (e.g., hardened tablets) [26]. An example of the physical barrier category are tablets formulated with INTAC™ technology (developed by Grünenthal GmbH; Aachen, Germany). Tablets containing this matrix are difficult to crush, and will gel when combined with small volumes of fluid. This characteristic has been hypothesized to interfere with the ability of individuals to abuse the medication via intranasal and intravenous routes [27–28].
Tapentadol immediate-release (Nucynta®) was developed for moderate to severe acute pain. It is a non-racemic compound that has been characterized as a μ-opioid agonist and a norepinephrine reuptake inhibitor [29–30], with minimal serotonergic effect [31]. It is less potent than morphine, but more potent than tramadol [32]. In a series of preclinical studies in rodents, it was demonstrated that tapentadol was effective in nociceptive, inflammatory, visceral and neuropathic pain models, and was associated with fewer opioid-related side effects (such as emesis and physical dependence) than typical μ-opioid agonists [29].
Tapentadol HCL extended release (Nucynta® ER), was developed for the treatment of chronic pain. Clinically, tapentadol immediate-release and tapentadol extended-release have been compared to oxycodone and have been shown to provide similar levels of pain relief, yet lower levels of gastrointestinal (GI) distress [33–35; see 36 for a review]. Upon consideration of the μ-opioid agonist component along with preliminary data demonstrating that tapentadol generated a similar abuse potential profile of subjective effects as equianalgesic doses of hydromorphone (submitted as part of the NDA application for tapentadol), the DEA and FDA scheduled tapentadol under Schedule II of the Controlled Substances Act. Given this profile, tapentadol ER was formulated with an INTAC™ matrix.
Whether tapentadol ER tablets are able to withstand the tampering attempts of experienced intravenous and intransasal drug abusers is unknown. Thus, the main goal of the present study was to examine the mechanical stability of the tapentadol 50 mg and 250 mg extended-release tablets (TAP50, TAP250) by determining whether experienced abusers were able to convert the tablets into forms that were amenable to intranasal (Study 1) or intravenous (Study 2) drug administration. An additional aim was to evaluate participants’ impressions of the tablets. The original formulation of OxyContin® 40 mg (OXY40) was used as a comparator. OXY40 was chosen because it is a commonly abused prescription opioid, and has been employed as a comparator in a number of other trials with tapentadol [33–35, 37]. No drug was taken, and there was no participant overlap between the studies.
Materials and Methods
Participants
Healthy research volunteers between 21 and 60 years of age who were able to give informed consent were recruited for study participation. Participants had to be currently abusing OxyContin® (or oxycodone) intranasally (Study 1) or intravenously (Study 2) to participate in the studies. Exclusion criteria were current Major Axis I psychopathology other than opioid abuse that could interfere with study participation (e.g., mood disorder with functional impairment, schizophrenia), as well as a history of significant violence, or a significant suicide risk. Participants were recruited through local newspapers and word of mouth.
Design
These were 1-day outpatient studies employing a randomized, repeated-measures design. Participants were provided with OxyContin® 40 mg (OXY40), tapentadol 50 mg ER (TAP50), and tapentadol 250 mg ER (TAP250) tablets in random order. Tablets were referred to as Tablet A, Tablet B, or Tablet C, respectively; the formulation of the tablets was not revealed to participants. Tools that had been specifically requested by the participant for preparing the tablets for abuse were provided. Participants were able to tamper with the tablets for up to an hour to turn them into a form suitable for snorting (Study 1) or shooting (Study 2). These studies were approved by the Institutional Review Board of the New York State Psychiatric Institute (NYSPI) and conducted in 2009.
Procedures
Detailed procedures were described previously [38]. Briefly, after an extensive series of screening interviews and consent procedures, participants were provided with test tablets (OXY40, TAP50, TAP250) in a random sequence under direct supervision of the investigators. Approved tools were provided to them. Investigators recorded the time spent manipulating the tablets with stopwatches. After the tampering procedures were completed, participants responded to scripted questions concerning their impression of the formulations. All participants were paid $100 before leaving the laboratory.
Upon the completion of each tampering attempt, the senior investigator packaged the tampered samples into storage vials. Batch orders were shipped to Johnson & Johnson Pharmaceutical Research and Development, L.L.C. (Spring House, PA) for particle size analysis (Study 1), or measurement of the volume and drug concentration in the liquid extracts that had been drawn up into syringes (Study 2). With regard to Study 2, all other extracts other than those in syringes (for instance, gelled extracts in vials) were not analyzed because they could not be injected.
Study 1
Outcome Measures
The primary outcome of the Study 1 was percentage of participants who indicated they were willing to snort the prepared tablets. Secondary outcomes were the particle size distribution of the tampered tablets, the actual time spent tampering with the tablets, and the self-reported maximum time participants would be willing to spend on a routine basis preparing the tablets for intranasal abuse. Additional measures collected included the monetary amounts participants were willing to pay for the tablets. Participants were also asked how often they took measures to prevent unwanted particles from ending up in the powder when they prepared OxyContin® for snorting (to estimate the degree of caution that exists in this population regarding insufflation of particles).
Data Analysis
Three contrasts were planned prior to the conduct of the study for primary and secondary outcomes, namely, OXY40 vs. TAP50, OXY40 vs. TAP250, and TAP50 vs. TAP250. Willingness to snort the powder produced was analyzed with the Cochran’s Q statistic. Particle size distribution was analyzed with a gravimetric sieve analysis, followed by a high-speed image analysis (HSIA). Samples were passed through a #20 ASTM sieve (850 μM square perforations). The fraction that was retained on the sieve (i.e., particles > 850 μM) was considered non-snortable, i.e., not available for snorting. The fraction that passed through the sieve was submitted to the high-speed image analysis with the Sympatec QicPic (Clausthal-Zellerfeld, Germany). These data were summarized with descriptive statistics. Continuous secondary endpoint measures were analyzed with repeated measures ANOVA. Additional data were described with frequency analyses. A degree of variability was expected, thus, it was estimated that a sample size of 25 participants would provide 75% power to detect a 35% difference in a comparison between percentages willing to snort the tampered product. Data analyses were performed with SPSS v. 20.0.0 (IBM), NQuery Advisor v. 4.0 (Statistical Solutions), and SAS v. 9.1.
Results
Twenty-eight participants signed the screening consent form, but 3 did not meet study criteria upon reporting to the laboratory: two for psychiatric reasons, and one who did not meet the inclusion criteria of current OxyContin® use. Table 1 (left panel), presents the demographic characteristics of the 25 participants who completed the study. Fifty-two percent reported using OxyContin® to treat pain before recreational opioid use was initiated. Table 2 (left panel) presents the tools and solvents used by participants to prepare the tablets for insufflation.
Table 1.
Demographics
| Study 1, n=25 (Intranasal) | Study 2, n=25 (Intravenous) | |
|---|---|---|
| Age, M(SD) | 42 (11) | 41 (9) |
| Gender, % Male | 72 | 84 |
| Ethnicity, % | ||
| Black | 44 | 4 |
| Hispanic | 36 | 36 |
| White | 16 | 56 |
| Mixed | 4 | 4 |
| Education, % with some college | 52 | 72 |
| Age of first OxyContin® use, M(SD) | 37 (11) | 34 (10) |
| Length of OxyContin® use in yrs, M(SD) | 5(4) | 6 (5) |
| Current Drug Use, % | ||
| Oxycontin® or generics | 100 | 100 |
| Heroin | 56 | 96 |
| Benzodiazepines | 48 | 16 |
| Cocaine | 36 | 20 |
| Methadone | 36 | 8 |
| Alcohol | 8 | 16 |
| Marijuana | 0 | 16 |
| Ecstasy | 4 | 0 |
| PCP | 4 | 0 |
| Quaaludes | 4 | 0 |
| Percocet™ | 0 | 8 |
| Vicodin™ | 0 | 8 |
| Morphine | 0 | 8 |
| Tylenol with Codeine #3™ | 0 | 4 |
| Crystal Methamphetamine | 0 | 4 |
Table 2.
Tools and Solvents Used for Intranasal (Left) and Intravenous (Right) Drug Preparation
| Intranasal Tools | n | (%) | Intravenous Tools | n | (%) |
|---|---|---|---|---|---|
| Hammer | 22 | (88) | Lighter | 25 | (100) |
| Razor | 21 | (84) | Spoon | 25 | (100) |
| Dollar Bill | 9 | (36) | Syringe | 25 | (100) |
| Paper | 7 | (28) | Hammer | 23 | (92) |
| Lighter | 6 | (24) | Cotton/Cotton pads | 23 | (92) |
| Paper Towels/Napkins | 6 | (24) | Razor | 20 | (80) |
| Credit Card/ID Card/MetroCard | 4 | (16) | Paper Towel/Napkin | 15 | (60) |
| Spoon | 4 | (16) | Paper | 12 | (48) |
| Wax Paper | 4 | (16) | Dollar Bill | 9 | (36) |
| Pill Grinder | 3 | (12) | Knife/Exacto Knife | 7 | (28) |
| Quarter/Coins | 3 | (12) | Wax Paper | 6 | (24) |
| Screwdriver | 3 | (12) | Pliers | 3 | (12) |
| Strainer | 3 | (12) | Quarters/Coins | 3 | (12) |
| Syringe/Plastic Syringe | 3 | (12) | Coffee Grinder | 2 | (8) |
| Pen Knife/Knife/Exacto Knife | 2 | (8) | Screwdriver | 2 | (8) |
| Pill Crusher | 2 | (8) | Strainer | 2 | (8) |
| Pliers | 2 | (8) | Glass vial | 1 | (4) |
| Bottle Opener | 1 | (4) | Mortar & Pestle | 1 | (4) |
| Cotton | 1 | (4) | Paper Clip | 1 | (4) |
| Matchbox | 1 | (4) | Plastic Cap | 1 | (4) |
| Scissors | 1 | (4) | |||
| Straw | 1 | (4) | |||
|
| |||||
| Solvents | n | (%) | Solvents | n | (%) |
|
| |||||
| Water | 6 | (24) | Water/Saline | 25 | (100) |
| Alcohol Pads | 1 | (4) | Lemon Juice | 1 | (4) |
Primary Outcome Measure
All participants (100%) were willing to snort the powder produced from the OXY40 tablet, compared to 24% who were willing to snort the powder produced from the TAP50 tablet, and 16% who were willing to snort the TAP250 tablet. The differences between OXY40 and both doses of TAP were statistically significant (Cochran’s Q(2)= 38.38; p<.001; OXY40 vs. TAP50, p<.01; OXY40 vs. TAP250, p <.01). There were no statistically significant differences between the percentages of participants who were willing to snort TAP50 or TAP250.
Table 3 summarizes verbatim explanations participants provided when asked why they would (or would not) snort the tampered product. Figure 1, top panel depicts representative photographic samples from OXY40, TAP50 and TAP250. OXY40 tablets could be crushed into a fine powder whereas both TAP formulations could only be cut into large, jagged particles.
Table 3.
“If given the opportunity to snort the powder that you made, would you do it? Why?”
| OXY40 (%) | TAP50 (%) | TAP250 (%) | |
|---|---|---|---|
| Would snort the product | 100 | 24 | 16 |
| ▪ Form: In a form that would snort/nice, fine powder/small enough/powdery form/in form would use | 44 | ||
| ▪ Process: Easy to crush/able to get small enough/easy to grind/takes minimal time | 36 | ||
| ▪ Recognition: Know it’s Oxy/like it/looking for high | 20 | ||
| ▪ Dissolving possibility: Would dissolve for nose drops/would use water to wash down nostrils | 16 | 8 | |
| ▪ Particle size: Particles are small enough | 8 | 8 | |
| Would not snort the product | 0 | 76 | 84 |
| ▪ Form: Cannot snort/too dense/too large/hard as a brick/too sticky/unbreakable/plastic-y/not powdery/rough/pill not real | 36 | 40 | |
| ▪ Process: Not able to make/too difficult to turn into powder/requires too much time/too much work/too process consuming/not crushable | 40 | 44 |
Figure 1.

Top Panel: Intranasal particles produced from the OXY40 (A), TAP50 (B) and TAP250 (C) tablets; Bottom Panel: Intravenous solutions/gels produced from the OXY40 (D), TAP50 (E) and TAP250 (F) tablets.
Secondary Outcome Measures
Particle size
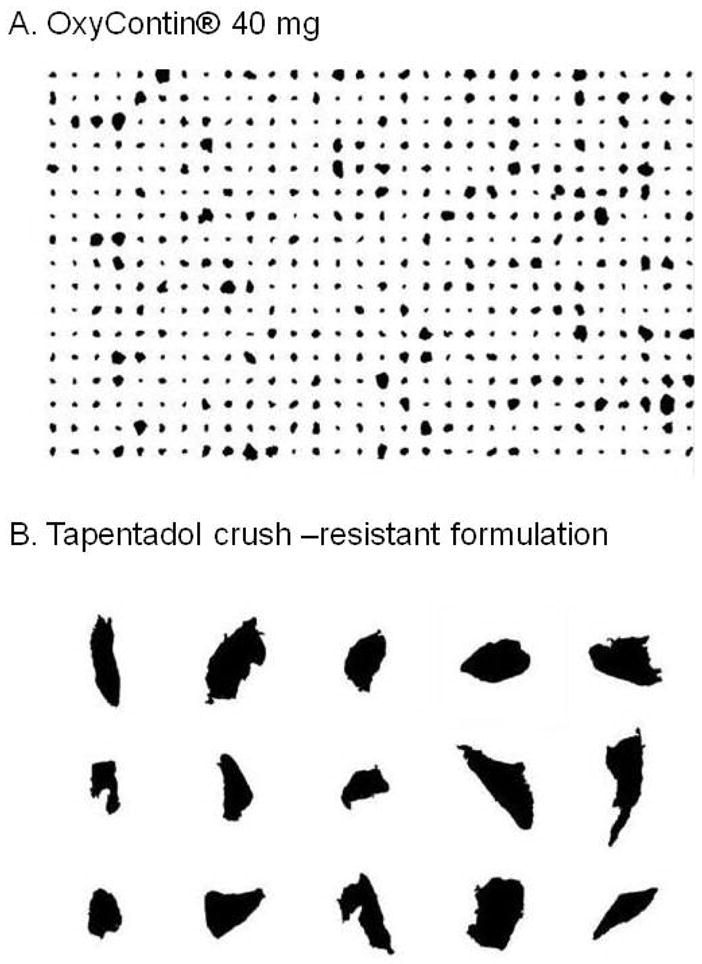
Particle size data are presented in Table 4. Lower percentages of the initial, intact tablet by weight were recovered as tampering products from the OXY40 tablets than from TAP50 or TAP250. This was likely due to the fine powder produced from the OXY40 during the crushing process which stuck to the work surface or potentially blew away during the product transfer. The OXY40 powder that filtered through the #20 ASTM sieve mesh represented an average of 79.2% of the weight of the recovered OXY40, whereas 20.8% was left on the mesh screen. The average number of particles (smaller than 850 μm) available for a dose of OXY40 was 41,595. HSIA analysis revealed that the particles were, in general, uniformly shaped and predominantly spherical. Figure 2, top panel, depicts shapes of the OXY40 tablets from the HSIA.
Table 4.
Particle Size Analysis
| OXY40 | TAP50 | TAP250 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| n | M | SD | Med | n | M | SD | Med | n | M | SD | Med | |||||
|
|
||||||||||||||||
| % Tablet weight (wgt) recovered | 24 | 68.9 | 11.2 | 70.6 | 25 | 96.2 | 9.2 | 99.5 | 25 | 93.3 | 14.7 | 98.8 | ||||
| % Recovered tab wgt > 850 μm | 24 | 20.8 | 21.1 | 15.6 | 25 | 97.9 | 7.1 | 100.0 | 25 | 98.7 | 4.6 | 100.0 | ||||
| % Recovered tab wgt < 850 μm | 24 | 79.2 | 21.1 | 84.4 | 25 | 2.1 | 7.1 | 0.0 | 25 | 1.3 | 4.6 | 0.00 | ||||
| # Particles measured by HSIA | 23 | 41,595 | 24,269 | 38,080 | 8 | 1,337 | 1,917 | 440 | 9 | 1,818 | 2,252 | 969 | ||||
| Pairwise Comparisons | TAP50 vs. OXY40 | TAP250 vs. OXY40 | TAP50 vs. TAP250 | |||
|---|---|---|---|---|---|---|
|
| ||||||
| LS Mean Difference | 95% CI | LS Mean Difference | 95% CI | LS Mean Difference | 95% CI | |
| % Tablet wgt recovered | 27.1** | (21.4 to 32.9) | 24.2** | (18.4 to 29.9) | −2.9 | (−8.6 to 2.7) |
| % Recovered tab wgt > 850 μm | 77.0** | (69.8 to 84.2) | 77.8** | (70.6 to 85.0) | 0.8 | (−6.3 to 7.9) |
| % Recovered tab wgt < 850 μm | −77.0** | (−84.2 to 69.8) | −77.8** | (−85.0 to −70.6) | −0.8 | (−7.9 to 6.3) |
| # Particles measured by HSIA | −31,878** | (− 44,041 to −19,725) | −32,878** | (−44,483 to −21,274) | −1000.4 | (−13,679 to 11,678) |
M=Mean, SD=Standard Deviation, Med=Median
p<.001
Figure 2.
HSIA particle sizes to scale
In contrast to the results for OXY40, most of the TAP50 and TAP250 particles did not pass through the #20 ASTM sieve mesh because they were too large. The amounts that passed through represented 2.1% and 1.3% of the weight of the TAP50 and TAP250 tablets, respectively. HSIA analysis revealed that these particles were large, jagged, and irregular Figure 2, lower panel. Thus, particle size analyses revealed that both TAP formulations produced fewer particles that accounted for less of the tablet weight than the OXY40 formulation (all ps <.001), while there were no differences between the two TAP formulations.
Time Preparing Tablets
Table 5 demonstrates that participants spent more time preparing the TAP50 and TAP250 tablets than the OXY40 tablet. Participants reported that they were willing to spend more time preparing the TAP50 and the TAP250 tablets for use than the OXY40 tablets.
Table 5.
Time Participants Spent Preparing Tablets for Intranasal Abuse, and Self-Reported Maximum Time Participants Would Spend Preparing Tablets for Intranasal Abuse
| Actual Time Spent Preparing Tablets (minutes)1 | Maximum Time Would Spend Preparing Tablets (minutes)2 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| M | SD | Range | M | SD | Range | |
| OXY40 | 2.9** | 1.9 | 0.7 to 8.8 | 4.8* | 4.8 | 1.0 to 15.3 |
| TAP50 | 5.9** | 4.9 | 0.9 to 20.9 | 10.8* | 12.7 | 0.0 to 28.7 |
| TAP250 | 7.1** | 4.6 | 0.9 to 16.2 | 11.6** | 12.5 | 0.0 to 48.9 |
M=Mean, SD=Standard deviation
p<.01;
p<.05, TAP50 vs. OXY40, and TAP250 vs. OXY40
F(2, 24)=9.55, p=.0003;
F(2, 24)= 6.60, p=.003.
Relative Perceived Monetary Value and Removing Unwanted Particles
Table 6 presents verbatim responses to the question of whether participants would pay “Less,” “More,” or “The Same” for a tablet that took substantially more time and effort to prepare for snorting than for a tablet with the same amount of drug that was easier to prepare. The majority of participants would pay “Less,” whereas the remainder would pay “The Same” for such a tablet. It was notable that none were willing to pay “More” than for a tablet that was easier to prepare. Table 7 reveals that most participants (68%) “Always” or “Usually” took measures to prevent unwanted particles from ending up in the powder when preparing an OXY40 tablet for abuse.
Table 6.
“For a tablet that takes substantially more time and effort to prepare for snorting, would you pay LESS, MORE, or THE SAME than for a tablet with the same drug in it that is quicker and easier to prepare?”
| Less (64%) | The Same (36%) | More (0%) | |
|---|---|---|---|
| ▪ Could not manipulate tablet: Can’t snort/can’t make into powder/not worth it if in withdrawal | 24 | ||
| ▪ Preparation time: Time matters/takes more time/would take longer | 16 | ||
| ▪ Effort required: Difficult pill/requires more effort/won’t have same effect/harder to prepare | 16 | ||
| ▪ Particle qualities: Particles/too large/texture gummy/would take orally | 8 | ||
| ▪ Buying the drug, not the formulation: High from TRF may be better even though more work/paying for drug/if same effect, same price | 32 | ||
| ▪ New ideas: Would think of new way to prepare pill | 4 |
Cochran’s Q (2) =24.13, p<.01; “Less vs. More,” p<.05
Table 7.
“When you prepare an OxyContin® tablet for snorting, how often do you take measures to prevent unwanted particles from ending up in the powder?”
| % | |
|---|---|
| Always | 52 |
| ▪ Removes coating first: Cracks and removes shell/uses alcohol (before mixing with heroin)/sucks coating off/coating irritates stomach | 28 |
| ▪ Uses filter: Cotton filter/metal strainer | 8 |
| ▪ Particles: Doesn’t want foreign particles in nose/won’t snort with large pieces | 8 |
| ▪ No answer | 8 |
| Usually | 16 |
| ▪ Depends: If has dark shell will remove, otherwise won’t/if coating comes off easily will remove, otherwise won’t/sometimes too sick to bother | 8 |
| ▪ Removes coating first: Cracks and removes shell/uses alcohol (before mixing with heroin)/sucks coating off/coating irritates stomach | 4 |
| ▪ Snorts everything, including coating: Usually snorts all of the remnants | 4 |
| Sometimes | 12 |
| ▪ Snorts everything, including coating: Usually snorts all of the remnants | 4 |
| ▪ Depends: If has dark shell will remove, otherwise won’t/if coating comes off easily will remove, otherwise won’t/sometimes too sick to bother | 4 |
| ▪ No answer | 4 |
| Never | 20 |
| ▪ Snorts everything, including coating: Usually snorts all of the remnants | 20 |
Study 2
Outcome Measures
The primary endpoint was the percent yield of active drug in solution that participants were able to obtain from the OXY40, TAP50 and TAP250 tablets. Secondary outcomes were the self-reported willingness to inject the tampered product, the actual time spent tampering with the tablets, and the self-reported maximum time participants would be willing to spend on a routine basis preparing the tablets for abuse. Additional data collected were similar to Study 1.
Data Analysis
As in Study 1, three contrasts were planned prior to the conduct of the study for primary and secondary outcomes, namely, OXY40 vs. TAP50, OXY40 vs. TAP250, and TAP50 vs. TAP250. To determine the percent yield of active drug that was recovered, the mg of active drug recovered was divided by the mg of active drug in the tablet label claim. Percent yield was summarized with descriptive statistics, and compared with the non-parametric Wilcoxon signed rank test to reflect the non-normal distribution of the data. It was estimated that a sample size of 25 participants would provide approximately 80% power to detect a 15% difference in yield between the formulations, assuming a standard deviation of 25% yield. Other measures were analyzed and reported as in Study 1.
Results
Table 1 (right panel) presents the demographic characteristics of the 25 participants who completed the study. There were no screen failures. Twenty-eight percent reported using OxyContin® to treat pain before recreational opioid use was initiated. Table 2 (right panel) presents the tools and solvents used by participants to prepare the tablets for intravenous use. Hammers were used to crush the tablets, spoons were used to hold the tablet fragments, lighters were used to heat a solution of tablet fragments and solvent, while cotton was used as a filter through which the extract was drawn up into the syringe.
Primary Outcome Measure
Amount of active drug in solution
The average percent yield of OXY40 was 37.02% (±16.67%), representing a mean of 14.8 mg of active drug (± 6.7 mg) per 40 mg tablet. The average percent yield of TAP50 was 3.52% (± 2.77%), representing a mean of 1.76 mg (± 1.4 mg) of active drug per 50 mg tablet. Both the mean percentage and average amount (in mg) of drug recovered were greater in the OXY40 dataset (Wilcoxon signed-rank test for both comparisons: Z=2.67, p=.008). No active drug was extracted as a liquid into a syringe from the TAP250 tablets by any of the participants. Figure 1, bottom panel depicts the solutions produced from each tablet. As can be seen, approximately 25ccs of clear solution was drawn up from the OXY40 tablet, whereas both TAP tablets primarily produced chunky, partially-gelled solutions.
Secondary Outcome Measures
Time Preparing Tablets
Table 8 presents the analyses of time spent preparing tablets. Planned contrasts revealed that participants spent longer preparing TAP250 than OXY40. There were no differences among the maximum amounts of time that participants would be willing to spend preparing tablets for intravenous use.
Table 8.
Time Spent Preparing Tablets for Intravenous Abuse, and Self-Reported Maximum Time Participants Would Spend Preparing Tablets for Intravenous Abuse
| Actual Time Spent Preparing Tablets (minutes) | Maximum Time Would Spend Preparing Tablets (minutes) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| M | SD | Range | M | SD | Range | |
| OXY40 | 6.57 | 3.16 | 2.75–15.27 | 15.22 | 18.03 | 0.00–60.00 |
| TAP50 | 8.27 | 7.60 | 1.15–28.67 | 17.66 | 20.23 | 0.00–60.00 |
| TAP250 | 10.58* | 9.68 | 1.53–48.85 | 22.52 | 40.53 | 0.00–180.00 |
M= mean, SD = standard deviation.
F(2, 48) = 3.08, p=.055;
p<.05, TAP250 vs. OXY40
Willingness to Inject
Table 9 presents the data describing percentages of participants who would inject the solutions produced from the tampering attempts. All participants were willing to inject the OXY40, yet few were willing to inject the TAP50 solution, and none were willing to inject the TAP250 solution.
Table 9.
“If given the opportunity to inject the extract (the solution) that you made, would you do it? Why?”
| OXY (%) | TAP50 (%) | TAP250 (%) | |
|---|---|---|---|
| Would inject the solution | 100 | 16 | 0 |
| ▪ Form: Acceptable/can inject in current form | 32 | ||
| ▪ Process: No problem breaking up/could crush and cook/easy to draw up/easy to extract | 32 | ||
| ▪ Recognition: It’s Oxy/it’s an opiate/know what it is | 24 | ||
| ▪ Effort: Minimal effort/minimal time required | 12 | ||
| ▪ Solution consistency: Clear/not sticky/has drug in it | 12 | ||
| ▪ Effort invested: Because spent so much time preparing | 4 | ||
| Would not inject the solution | 0 | 84 | 100 |
| ▪ Form: Couldn’t get into form/won’t break up/can’t cook/not breaking down/not melting/not dissolving | 40 | 36 | |
| ▪ Solution consistency: Jelly/rubbery/plastic/gummy/gel-like/gooey/syrupy | 24 | 44 | |
| ▪ Risks: Dangerous/scared about veins/scared about potential abscesses | 12 | 8 | |
| ▪ Recognition: Don’t know what it is/not a pill/unfamiliar/pill is oily/pill is rubbery | 8 | 12 |
Cochran’s Q(2) = 38.17, p<.001; OXY40 vs. TAP50 p<.01; OXY40 vs. TAP250, p<.01; TAP50 vs. TAP250,NS.
Relative Perceived Monetary Value and Removing Unwanted Particles
Table 10 demonstrates that almost all of the sample would pay “Less” for a tablet that that took more time and effort to prepare than one that was quicker and easier to prepare. The remainder (<10%) would pay the same for both tablets. None would pay “More.” Lastly, Table 11 reveals that all of the participants took precautions to avoid unwanted particles from ending up in extractions that they typically prepared for use.
Table 10.
“For a tablet that takes substantially more time and effort to prepare for injection, would you pay LESS, MORE, or THE SAME than for a tablet with the same drug in it that is quicker and easier to prepare?”
| Less (92%) | The Same (8%) | More (0%) | |
|---|---|---|---|
| ▪ Requires more work: Harder to break up/too hard/aggravating | 32 | ||
| ▪ Can’t use: not sure can prepare/can’t break it down/would break needle/can’t use in a syringe/can’t use | 32 | ||
| ▪ Too time consuming: takes too long – especially if desperate/not worth the risk of getting caught | 28 | ||
| ▪ Need: If wanted it bad enough, would pay the same | 4 | ||
| ▪ Economics: wouldn’t pay more, dealer won’t sell for less | 4 |
Cochran’s Q (2) =42.35, p<.001; “Less vs. The Same,” p<.01; “Less vs. More,” p<.01
Table 11.
“When you prepare an OxyContin® tablet for intravenous injection, how often do you take measures to prevent unwanted particles from ending up in the extraction?”
| % | |
|---|---|
| Always | 100 |
| ▪ Removes shell first: Peels pill/removes coating with damp cloth/shaves shell/sucks shell off) then filters with cotton | 44 |
| ▪ Uses Cotton: cotton balls/cigarette filters to keep particles out/filter out excess particles/doesn’t want foreign material | 32 |
| ▪ To avoid infection: avoid dangerous particles in body/blood/avoid abscess/prevent illness | 16 |
| ▪ Clean solution: to avoid clogging needle/wants clean fluid | 8 |
Discussion
Two studies were conducted to determine whether the TAP formulations were able to be manipulated by experienced intranasal and intravenous OxyContin® abusers. OxyContin® 40 mg was used as a comparator. The results from both studies revealed differences between the TAP and OXY40 formulations across almost all of the measures assessed.
Few individuals were willing to snort the particles or inject the solutions produced from the TAP tablets. With regard to the particle sizes, fewer particles were generated from the TAP tablets and, on average, were larger that those produced from OXY40. A previous study estimated that particles up to 285.9 μm would easily be intranasally administered [38]. Thus, the current data suggest that greater than 96% of the TAP tablets may consist of particles too large to be considered for snorting. This is notable in view of the observation that half of those who indicated they would snort the remnants of their tampering attempts used water to prepare a solution to use as drops. Lastly, there was a 10-fold increase in the percent yield of active drug recovered from OXY40 in comparison with TAP50 while no drug was able to be recovered from the TAP250 samples, primarily due to the fact that none of the TAP250 samples returned from analysis were in a liquid state; all were gelled.
In addition to these differences between formulations, this data set affords a consideration of tampering behaviors. Participants were given an hour to tamper with each tablet in the present studies, yet none of the intranasal abusers approached the 60-minute time frame. The average time spent preparing the OXY40 was 3 minutes compared to 6 minutes for TAP50 and 7 minutes for TAP250. One participant spent a maximum of 21 minutes preparing a TAP50 tablet. More meaningfully, however, the median preparation time for intranasal abuse across the sample was 4.6 minutes (versus a median of 2.5 minutes for OXY40, and 7.3 minutes for TAP250). Similarly, the maximum time employed by an intravenous abuser working with a TAP250 tablet was 48.9 minutes, yet the median preparation time for the TAP250 was 7.3 minutes (versus 5.7 minutes for OXY40 and 6.8 minutes for TAP50). Thus, although they indicated they were willing to spend more time preparing the TAP tablets, most intravenous and intranasal abusers did not spend a great amount of time tampering with any of the tablets [23, 27, 39–40].
As has also been found [38], no particularly unique tools were requested for tampering purposes. Intranasal abusers worked primarily with a hammer, razor, or dollar bill; while intravenous abusers primarily employed a lighter, spoon, syringe, cotton, and water. This is consistent with observations gleaned from the internet regarding tampering, namely, complex procedures are not typically employed by substance abusers to tamper with medications [41–42].
One question that surrounds the TRFs is their effectiveness towards abuse-deterrence in a population known for extraordinary creativity and determination. Taken together with another similar published study to-date [38], these data suggest that approximately 14% of intranasal abusers (range 8%-20%) will attempt to snort them, and 18% of intravenous abusers (range 16%-20%) will attempt to inject them. This implies that up to 86% of intranasal abusers, and up to 82% of intravenous abusers may experience some deterrence from these formulations. Whether or not these estimates bear out in larger samples remains to be seen; however, these data are encouraging towards the employment of this technology.
It is not known what will happen if an individual snorts the powder, or more problematically, injects the extracts from these formulations [see summary in 38]. These risks should not be underestimated [43–50]. It is encouraging that all individuals in this sample indicated that they removed unwanted particles from their drug extractions, and 48% indicated this was due in some part to safety or not wanting foreign materials in their bodies. Whether or not this logic extends to the extracts from the INTAC™ matrix remains to be seen. Future research should locate substance abusers who have abused or attempted to abuse these formulations because they may be more proficient at bypassing the matrix and extracting active drug.
With regard to this study, the findings must be considered in the context of the present limitations. It is possible that the recruitment of OxyContin® or oxycodone abusers confounded the study results, notably because the OXY40 tablet may have been preferred due to familiarity. However, only eight participants in each study reported abusing the OXY40 tablet; the remainder used other doses. This was reflected in the results: while some IN and IV abusers indicated that they preferred the OXY40 due to the product recognition, a larger number indicated that their preference was due to the form, or the production process of the powder or solution (reported in Tables 3 and 9). Further, in a comparable study [38], there were no restrictions placed on the type of prescription opioids being abused by participants, and similar results were generated. This suggests, at minimum, that familiarity was not the only driving force in participants’ preferences. Much of the data collected were self-reported, which leaves open the possibility that when faced with an immediate opportunity to snort or shoot a tampered product, a different decision would actually be made. It is also possible that if participants had some time to think about the products after the sessions, they would have generated strategies. To this end, follow-up phone calls the day after the session might have revealed more information. A direct comparison to another formulation that has been designed to be tamper-resistant would have provided useful information with regard to relative ease of tampering with different products, however, none were available during the conduct of these procedures. This would be an interesting follow-up study to pursue.
To conclude, data from Studies 1 and 2 suggest that the abuse-deterrent formulations of tapentadol reduced the ability of both intranasal and intravenous abusers to quickly and successfully tamper with this medication when compared to the non-tamper-resistant OxyContin® formulation. These findings were captured in the quantitative data that were generated, as well as the participants’ qualitative perceptions of their experiences. It seems reasonable to conclude that even when considering the potential safety concerns, tamper-resistant tablets are a promising technology for reducing the intranasal and intravenous abuse of prescription opioids.
Acknowledgments
The authors gratefully acknowledge Janet Murray, R.N., Claudia Tindall, N.P., Sharifa James, Greta B. Raglan, Joseph Lazar, Phillip Saccone, Elias Dakwar, M.D., Shanthi Mogali, M.D., David Mysels, M.D., and Rebecca Pyle for their assistance with the execution of the study. In addition, two anonymous reviewers provided valuable feedback on an earlier version of this manuscript. This study was supported by Johnson and Johnson PRD, LLC.
Footnotes
Declaration of Interest: Funding for this study was provided by Johnson & Johnson PRD, L.L.C.
References
- 1.Portenoy RK. Treatment of cancer pain. Lancet. 2011;377:2236–2247. doi: 10.1016/S0140-6736(11)60236-5. [DOI] [PubMed] [Google Scholar]
- 2.Richarz U, Waechter S, Sabatowski R, Szczupanski L, Binsfeld H. Sustained safety and efficacy of once-daily hydromorphone extended-release (OROS hydromorphone ER) compared with twice-daily oxycodone controlled-release over 52 weeks in patients with moderate to severe chronic noncancer pain. Pain Pract. 2012 doi: 10.1111/j.1533-2500.2012.00553.x. (Epub, in press) [DOI] [PubMed] [Google Scholar]
- 3.Taylor R, Raffa RB, Pergolizzi JV. Controlled release formulation of oxycodone in patients with moderate to severe chronic osteoarthritis: A critical reivew of the literature. J Pain Res. 2012;5:77–87. doi: 10.2147/JPR.S21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webster L. Efficacy and safety of dual-opioid therapy in acute pain. Pain Med. 2012;13:S12–S20. doi: 10.1111/j.1526-4637.2012.01330.x. [DOI] [PubMed] [Google Scholar]
- 5.Substance Abuse and Mental Health Services Administration. NSDUH Series H-41, HHS Publication No (SMA) 11-4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011a. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- 6.Substance Abuse and Mental Health Services Administration. HHS Publication No (SMA) 11-4659, DAWN Series D-35. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011b. Drug Abuse Warning Network, 2009: National Estimates of Drug-Related Emergency Department Visits. [Google Scholar]
- 7.Balch RJ, Trescot A. Extended-release morphine sulfate in treatment of severe acute and chronic pain. J Pain Res. 2010;3:191–200. doi: 10.2147/JPR.S6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schram MJ, Sathyan G, Khanna S, Tudor J, Nath R. Evaluation of the abuse potential of extended release hydromorphone versus immediate release hydromorphone. J Clin Psychopharmacol. 2010;30:25–33. doi: 10.1097/JCP.0b013e3181c8f088. [DOI] [PubMed] [Google Scholar]
- 9.Setnik B, Roland CL, Cleveland JM, Webster L. The abuse potential of Remoxy®, an extended-release formulation of oxycodone, compared with immediate– and extended-release oxycodone. Pain Med. 2011;12:618–631. doi: 10.1111/j.1526-4637.2011.01093.x. [DOI] [PubMed] [Google Scholar]
- 10.Raffa RB, Pergolizzi JV., Jr Opioid formulations designed to resist/deter abuse. Drugs. 2010;70:1657–1675. doi: 10.2165/11537940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology. 2001;154:76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- 12.Comer SD, Ashworth JB, Sullivan MA, Vosburg SK, Saccone PA, Foltin RW. Relationship between rate of infusion and reinforcing strength of oxycodone in humans. J Opioid Manag. 2009;5:203–212. doi: 10.5055/jom.2009.0022. [DOI] [PubMed] [Google Scholar]
- 13.Marsch LA, Bickel WK, Badger GJ, Rathmell JP, Swedberg MD, Jonzon B, Norsten-Hoog C. Effects of infusion rate of intravenously administered morphine on physiological, psychomotor, and self-reported measures in humans. J Pharmacol Exp Ther. 2001;299:1056–1065. [PubMed] [Google Scholar]
- 14.Darke S, Ross J. Fatal heroin overdoses resulting from non-injecting routes of administration, NSW, Australia, 1992–1996. Addiction. 2000;95:569–573. doi: 10.1046/j.1360-0443.2000.9545698.x. [DOI] [PubMed] [Google Scholar]
- 15.Thiblin I, Eksborg S, Petersson A, Fugelstad A, Rajs J. Fatal intoxication as a consequence of intranasal administration (snorting) or pulmonary inhalation (smoking) of heroin. Foren Sci Int. 2004;139:241–247. doi: 10.1016/j.forsciint.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Green TC, Black R, Grimes Serrano JM, Budman SH, Butler SF. Typologies of prescription opioid use in a large sample of adults assessed for substance abuse treatment. PloS one. 2011;6:e27244. doi: 10.1371/journal.pone.0027244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez A, Talal AH. Noninjection drug use: An under-appreciated risk factor for hepatitis c virus transmission. Liver Int. 2008;28:757–760. doi: 10.1111/j.1478-3231.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- 18.Macias J, Palacios RB, Claro E, Vargas J, Vergara S, Mira JA, Merchante N, Corzo JE, Pineda JA. High prevalence of hepatitis c virus infection among noninjecting drug users: Association with sharing the inhalation implements of crack. Liver Int. 2008;28:781–786. doi: 10.1111/j.1478-3231.2008.01688.x. [DOI] [PubMed] [Google Scholar]
- 19.Scheinmann R, Hagan H, Lelutiu-Weinberger C, Stern R, Des Jarlais DC, Flom PL, Strauss S. Non-injection drug use and hepatitis c virus: A systematic review. Drug Alcohol Depend. 2007;89:1–12. doi: 10.1016/j.drugalcdep.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surratt H, Kurtz SP, Cicero TJ. Alternate routes of administration and risk for hiv among prescription opioid abusers. J Addict Dis. 2011;30:334–341. doi: 10.1080/10550887.2011.609805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz N. Abuse-deterrent opioid formulations: Are they a pipe dream? Curr Rheum Rep. 2008;10:11–18. doi: 10.1007/s11926-008-0003-z. [DOI] [PubMed] [Google Scholar]
- 22.Katz N, Dart RC, Bailey E, Trudeau J, Osgood E, Paillard F. Tampering with prescription opioids: Nature and extent of the problem, health consequences, and solutions. Am J Drug Alcohol Abuse. 2011;37:205–217. doi: 10.3109/00952990.2011.569623. [DOI] [PubMed] [Google Scholar]
- 23.Webster L. Update on abuse-resistant and abuse-deterrent approaches to opioid formulations. Pain Med. 2009;10 (Suppl 2):S124–S133. doi: 10.1111/j.1526-4637.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- 24.Webster LR, Fine PG. Approaches to improve pain relief while minimizing opioid abuse liability. J Pain. 2010;11:602–611. doi: 10.1016/j.jpain.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Mastropietro DJ, Omidian H. Current approaches in tamper-resistant and abuse-deterrent formulations. Drug Dev Ind Pharm. 2012 doi: 10.3109/03639045.2012.680468. in press. [DOI] [PubMed] [Google Scholar]
- 26.Hamed E, Moe D. Development of tamper deterrent formulations: State of the pharmaceutical industry. Curr Drug Abuse Rev. 2010;3:139–146. doi: 10.2174/1874473711003030139. [DOI] [PubMed] [Google Scholar]
- 27.Budman SH, Grimes Serrano JM, Butler SF. Can abuse deterrent formulations make a difference? Expectation and speculation. Harm Reduct J. 2009;6:8–15. doi: 10.1186/1477-7517-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wick JY. Drug-abuse deterrent formulations. Consult Pharm. 2009;24:356–365. doi: 10.4140/tcp.n.2009.356. [DOI] [PubMed] [Google Scholar]
- 29.Tzschentke TM, Cristoph T, Kögel B, Schiene K, Hennies H-H, Englberger W, Haurand M, Jahnel U, Cremers TIFH, Friderichs E, de Vry J. (−)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (Tapentadol HCl): A novel μ-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther. 2007;323:265–276. doi: 10.1124/jpet.107.126052. [DOI] [PubMed] [Google Scholar]
- 30.Tzschentke TM, Jahnel U, Kögel B, Cristoph T, Englberger W, de Vry J, Schiene K, Okamoto A, Upmalis D, Weber J, Lange C, Stegmann J-U, Kleinert R. Tapentadol hydrochloride: A next-generation, centrally active analgesic with two mechanisms of action in a single molecule. Drugs Today. 2009;45:483–496. doi: 10.1358/dot.2009.45.7.1395291. [DOI] [PubMed] [Google Scholar]
- 31.Vadivelu N, Timchenko A, Huang Y, Sinatra R. Tapentadol extended-release for treament of chronic pain: A review. J Pain Res. 2011;4:211–218. doi: 10.2147/JPR.S14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tzschentke TM, de Vry J, Terlinden R, Hennies H-H, Lange C, Strassburger W, Haurand M, Kolb J, Schneider J, Buschmann H, Finkam M, Jahnel U, Friedrichs E. Tapentadol hydrochloride. Drugs Future. 2006;31:1053. [Google Scholar]
- 33.Afilalo M, Stegmann JU, Upmalis D. Tapentadol immediate release: a new treatment option for acute pain management. J Pain Res. 2010;8:1–9. doi: 10.2147/jpr.s4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buynak R, Shapiro DY, Okamoto A, van Hove I, Rauschkolb C, Steup A, Lange B, Lange C, Etropolski M. Efficacy and safety of tapentadol extended release for the management of chronic low back pain: Results of a prospective, randomized, double-blind, placebo-and active-controlled Phase III study. Exp Opin Pharmacother. 2010;11:1787–1804. doi: 10.1517/14656566.2010.497720. [DOI] [PubMed] [Google Scholar]
- 35.Etropolski M, Kelly K, Okamoto A, Rauschkolb C. Comparable efficacy and superior gastrointestinal tolerability (nausea, vomiting, constipation) of tapentadol compared with oxycodone hydrochloride. Adv Ther. 2011;28:401–417. doi: 10.1007/s12325-011-0018-0. [DOI] [PubMed] [Google Scholar]
- 36.Wade WE, Spruill WJ. Tapentadol hydrochloride: A centrally acting oral analgesic. Clin Ther. 2009;31:2804–2818. doi: 10.1016/j.clinthera.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Cepeda MS, Sutton A, Weinstein R, Kim M. Effect of tapentadol extended release on productivity: Results from an analysis combining evidence from multiple sources. Clin J Pain. 2012;28:8–13. doi: 10.1097/AJP.0b013e3182201983. [DOI] [PubMed] [Google Scholar]
- 38.Vosburg SK, Jones JD, Manubay JM, Ashworth JB, Benedek IH, Comer SD. Assessment of a formulation designed to be crush-resistant in prescription opioid abusers. Drug Alc Depend. 2012 doi: 10.1016/j.drugalcdep.2012.05.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler SF, Fernandez KC, Chang A, Benoit C, Morey LC, Black R, Katz N. 2010b. Measuring attractiveness for abuse of prescription opioids. Pain Med. 2010;11:67–80. doi: 10.1111/j.1526-4637.2009.00736.x. [DOI] [PubMed] [Google Scholar]
- 40.Coleman JJ, Bensinger PB, Gold MS, Smith DE, Bianchi RP, Dupont RL. Can drug design inhibit abuse? J Psychoactive Drugs. 2005;37:343–362. doi: 10.1080/02791072.2005.10399808. [DOI] [PubMed] [Google Scholar]
- 41.Cone EJ. Ephemeral profiles of prescription drug and formulation tampering: Evolving pseudoscience on the internet. Drug Alcohol Depend. 2006;83:S31–S39. doi: 10.1016/j.drugalcdep.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 42.Katz NP, Buse DC, Budman SH, Wing Venuti S, Fernandez KC, Benoit C, Bianchi R, Cooper D, Jasinski DR, Smith DE, Butler SF. Development and preliminary experience with an ease of extractability rating system for prescription opioids. Drug Dev Ind Pharm. 2006;32:727–746. doi: 10.1080/03639040500529093. [DOI] [PubMed] [Google Scholar]
- 43.Crompton B. Misuse of benzodiazepines. Voluntary ban on prescribing is effective. BMJ. 1994;308:1709. [PMC free article] [PubMed] [Google Scholar]
- 44.Moley FM, Dawkes M, Berry JP. Misuse of benzodiazepines. Avoid benzodiazepines whenever possible. BMJ. 1994;308:1709–1710. [PMC free article] [PubMed] [Google Scholar]
- 45.Ruben SM, Morrison CL. Temazepam misuse in a group of injecting drug users. Br J Addict. 1992;87:1387–1392. doi: 10.1111/j.1360-0443.1992.tb01918.x. [DOI] [PubMed] [Google Scholar]
- 46.Russell ID, Kane GG, Royle CA, Jackson DS. Aggressive management of intra-arterial temazepam injection. J R Army Med Corps. 1994;140:93–94. doi: 10.1136/jramc-140-02-09. [DOI] [PubMed] [Google Scholar]
- 47.Adiseshiah M, Jones DA, Round JM. Intra-arterial temazepam. BMJ. 1992;304:1630. doi: 10.1136/bmj.304.6842.1630-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhabra MS, Meshikhes AN, Thomson GJ, Craig P, Parrott NR. Intraarterial temazepam: An important cause of limb ischaemia in intravenous drug abusers. Eur J Vas Surg. 1994;8:240–242. doi: 10.1016/s0950-821x(05)80470-0. [DOI] [PubMed] [Google Scholar]
- 49.Blair SD, Holcombe C, Coombes EN, O’Malley MK. Leg ischaemia secondary to non-medical injection of temazepam. Lancet. 1991;338:1393–1394. doi: 10.1016/0140-6736(91)92269-8. [DOI] [PubMed] [Google Scholar]
- 50.Scott RN, Going J, Woodburn KR, Gilmour DG, Reid DB, Leiberman DP, Maraj B, Pollock JG. Intra-arterial temazepam. BMJ. 1992;304:1630. doi: 10.1136/bmj.304.6842.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]


