Abstract
Bortezomib (V) was combined with thalidomide (T) and dexamethasone (D) in a phase I/II trial to determine dose-limiting toxicities (DLT’s) and clinical activity of the VTD regimen in 85 patients with advanced and refractory myeloma. The starting dose of V was 1.0 mg/m2 (days 1, 4, 8, 11, every 21 day) with T added from cycle 2 at 50 mg/day, with 50 mg increments per 10 patient cohorts, to a maximum dose of 200 mg. In the absence of DLT’s, the same reiteration of T dose increases was applied with a higher dose of V=1.3 mg/m2. D was added with cycle 4 in the absence of partial response (PR). Ninety-two percent had prior autotransplants, 74% had prior T and 76% abnormal cytogenetics. MTD was reached at V=1.3 mg/m2 and T=150 mg. Minor response (MR) was recorded in 79%, and 63% achieved PR including 22% who qualified for near-complete remission. At 4 years, 6% remain event-free and 23% alive. Both OS and EFS were significantly longer in the absence of prior T exposure and when at least MR status was attained. The MMSET/FGFR3 molecular subtype was prognostically favorable, a finding since reported for a VTD-incorporating tandem transplant trial (Total Therapy 3) for untreated patients with myeloma (BJH 2008).
Keywords: refractory myeloma, bortezomib, thalidomide
Introduction
Novel effective therapies targeting both tumor cells and their microenvironment may overcome resistance of multiple myeloma (MM) to conventional treatment. Bortezomib (Velcade, V) is a potent and reversible inhibitor of the proteasome system, a key step in the catabolic pathway for many intracellular regulatory proteins including inhibitor of κ B (IkB), p53 and the cyclin-dependent kinase inhibitors p21 and p27.1 Following demonstration of activity in a dose-finding phase I trial by Orlowski et al.,2 a multi-institutional phase II trial established bortezomib’s activity in advanced and refractory MM,3 which was followed by a phase III study revealing superiority of V over D salvage treatment in terms of response rate, time to progression and survival.4 We had previously identified thalidomide (T) as the first independently active agent in MM since the introduction of melphalan and prednisone in the 1960s.5–7 Its role in combination with dexamethasone in the management of patients with both refractory and newly diagnosed MM has been firmly established.8,9 T may act by interfering with adhesive interactions between MM cells and bone marrow stromal cells,10 altering cytokine secretion,11 and interfering with secretion of vascular endothelial growth factor and basic fibroblast growth factor-2.12,13
A phase II study conducted at the University of Arkansas for Medical Sciences (UAMS) of T had shown that 30% of 169 heavily pretreated patients with advanced and refractory MM achieved a partial response (PR).6 As V and T target different molecular pathways, we conducted a phase I/II trial combining V with escalating doses of T and added D for patients failing to achieve PR status, with the objectives of defining dose-limiting toxicities (DLT’s) and the clinical activity of the VTD regimen in terms of response rate and event-free survival (EFS) and overall survival (OS). Although early results of this trial have been previously reported,14,15 we now provide a long-term follow-up account of 85 patients enrolled into this phase I-II trial.
Patients and methods
Eligibility
Patients over the age of 17 years with MM that had relapsed after or had proven refractory to at least 2 lines of prior therapy were candidates for this study. Serum M-protein level >1.0 g/100 ml measured by serum protein electrophoresis or urinary M-protein excretion >200 mg/24 h or bone marrow plasmacytosis of >30% was required for enrollment. Patients had to have a performance status of ≤2 as per Southwest Oncology Group (SWOG) criteria, unless a higher scale was due to MM-related bone destruction. Except for adequately treated basal cell or squamous cell skin cancer, in situ cervical cancer, patients had to be cancer-free for at least 3 years. Hematopoietic function had to be preserved as defined by levels of neutrophils exceeding 750/mm3 and platelets exceeding 25 000/mm3. Preexisting >2 grade neurotoxicity as well as active infection requiring antibiotics, serious and interfering medical or psychiatric conditions, pregnancy or breast-feeding were exclusion criteria. This study had been approved by the UAMS Institutional Review Board, and written informed consent was obtained from each patient prior to protocol enrollment in keeping with Food and Drug Administration and institutional guidelines.
Study design
The overall design called for single agent V administration with the first cycle followed by the addition of T with the second cycle, whereas D was added with the fourth cycle in the absence of having attained PR status at that time. Given the relatively early evidence of V responsiveness, we hoped to obtain information, through at least weekly M-protein analyses, on whether the slope of M-protein decline was steepened after the addition of T.
Patients were enrolled in 2 dosing groups of V: group A received V at a dose of 1.0 mg/m2 on days 1, 4, 8 and 11 on a 21 day cycle; T was added with the second cycle at a starting dose of 50 mg/day with increments to 100, 150 and 200 mg/day in cohorts of at least 10 patients. Group B received V at 1.3 mg/m2 with the same schedule of administration, and T was added at the incremental dosing schedule delineated for group A. D was added with the fourth cycle at a dose of 20 mg on the day of and after V administration if PR status was not achieved by that time. Those achieving at least PR status after 8 cycles of induction therapy received maintenance therapy with VT or VTD every 3 months at the originally assigned doses.
Toxicities
Toxicities were assessed using the National Cancer Institute-Common Toxicity Criteria version 2.0. Within a single cohort, DLT was defined as the dose level at which at least four patients experienced ≥ grade 3 neuropathy or other non-hematologic toxicity, or grade 4 hematologic toxicity after completion of 2 cycles of treatment (one cycle of T added to V). The maximum tolerated dose of the V and T was defined as the dose level below that resulting in DLT.
Laboratory monitoring for toxicities and response
Baseline evaluation included a careful clinical examination with special emphasis on the presence of preexisting peripheral or autonomous system neuropathy. MM-relevant investigations included electropheretic analyses of serum and urine to determine the M-protein concentration in the serum and the daily urinary M-protein excretion; in addition, serum levels of albumin, C-reactive protein, β-2-microglobulin and immunoglobulins were determined. Bone marrow examinations called for the procurement of aspirates for morphologic, DNA-cytoplasmic immunoglobulin flow cytometric16 and cytogenetic analyses to determine the presence of metaphase-based cytogenetic abnormalities (CA),17 whereas bone marrow biopsies were evaluated for hematopoietic cellularity, plasmacytic involvement and evidence of myelodysplastic changes. In consenting patients, bone marrow aspirate and biopsy specimens were procured for gene expression profiling (GEP) studies prior to initiation of protocol therapy to define a risk score and the molecular subtype.18,19 We wished to determine whether the GEP-derived risk score and molecular subgroup designation were associated with clinical response and survival. Imaging studies included metastatic bone surveys and magnetic resonance imaging evaluations of the axial bone marrow-containing bone sites to document the presence of focal lesions.20
Standard laboratory monitoring included baseline and serial follow-up determinations of peripheral hemogram and multichemical scan to document hematological and other organ toxicities. Clinical history and physical examinations were repeated prior to each cycle to document symptoms especially of peripheral neuropathy.
Response and relapse criteria
Complete response (CR) was defined as the absence of urine and serum M-component by immunofixation analysis similar to the criteria established by Blade et al.21 and, in addition, included the requirement of normal bone marrow aspirate and biopsy without DNA aneuploidy or cytoplasmic light chain excess on flow cytometry.16 Near-CR (n-CR) required the same criteria except that immunofixation was positive, however, with negative eletrophoresis. Very good partial response was defined as ≥90% reduction in both serum M-concentration and urinary M-protein excretion; response (R) was defined as ≥75% reduction in serum M-protein or ≥90% reduction of urine M-protein; partial response (PR) was defined as ≥50% reduction in serum M-protein or ≥75% reduction of urine M-protein; for all three response levels, the bone marrow had to contain fewer than 5% plasma cells. Minor response (MR) was defined as a 25% reduction in serum M-protein production and/or a 75% reduction in Bence Jones protein excretion. Responses had to last for a minimum of 2 months and required the absence of new or enlarging bone or extramedullary lesions. All responses were evaluated on an intent-to-treat basis.
Progression was defined as an increase by at least 25% in M-protein serum level or 24-h urinary excretion above the baseline levels. Relapse from CR required the consistent re-appearance of monoclonal protein on immunofixation analysis; relapse from n-CR required increases in serum-M levels to at least 0.5 g/100 ml or in urinary M-protein excretion to more than 200 mg/day. Relapse from both PR or MR status required an increase in the aforementioned parameters by at least 25% beyond the lowest levels recorded. In addition, the reappearance of monoclonal bone marrow plasmacytosis constituted relapse from CR, n-CR, R and PR status when documented on at least two occasions 2 months apart. In the case of MR, only protein criteria applied. Additionally, the documentation of new bone lesions or extramedullary disease constituted relapse.
Definitions of event-free survival and overall survival
EFS and OS were calculated by the Kaplan–Meier method.22 EFS was calculated from the beginning of treatment until relapse, disease progression, death, or the date of last contact whereas OS was calculated from enrollment until the time of death from any cause or the date of last contact.23 Multivariate analyses applied stepwise Cox proportional hazards regression models.24
Results
Patient characteristics
Between March 2002 and December 2004, 85 patients were enrolled and all could be evaluated for toxicities. The median age was 60 years, and 27% were older than 65 years; 61% were male subjects, 92% had received one and 65% two prior autotransplants; 74% had received and most were deemed to have become refractory to prior T (Table 1). Seventy-six percent had CA, 42% had GEP-defined high-risk disease, and 27% had FGFR3-type MM. As per protocol none had been treated with prior V. Preexisting peripheral neuropathy was present in 23% of all patients. The median time from start of initial MM therapy was 41 months and was similar in the V and T dosing groups and unaffected by prior T exposure (data not shown). This was also true for most other potentially relevant baseline features. The only imbalances observed between the four dosing groups (V=1.0 or 1.3 mg/m2 and T <150 mg or T ≥150 mg) were C-reactive protein ≥4 mg/l (P=0.028) and hemoglobin <10 g/100 ml (P=0.047). The presence of κ-light chain (38 vs 79%, P=0.001) and β-2-microglobulin levels ≥4 mg/100 ml (23 vs 58%, P=0.004) were more frequent in the patients with prior T exposure. GEP data on CD138-purified plasma cells were available in 45 patients, whose characteristics were similar to the remainder without such information and comparable among the four treatment cohorts. As is apparent, 42% of subjects with GEP data were deemed to have high-risk MM, which is approximately threefold higher than in newly diagnosed patients enrolled on our Total Therapy trials;19 27% belonged to the MMSET/FGFR3 molecular subgroup, recently noted to benefit from the addition of bortezomib in Total Therapy 3 when compared to outcomes reported for Total Therapy 2.25,26
Table 1.
Baseline characteristics by bortezomib (V) and thalidomide (T) dose
| Factor | All patients | Cohort
|
P-value | |||
|---|---|---|---|---|---|---|
| V 1.0, T <150 | V 1.0, T ≥150 | V 1.3, T <150 | V 1.3, T ≥150 | |||
| Age >65 years | 23/85 (27%) | 7/21 (33%) | 7/24 (29%) | 4/21 (19%) | 5/19 (26%) | 0.762 |
| Caucasian | 79/85 (93%) | 18/21 (86%) | 24/24 (100%) | 19/21 (90%) | 18/19 (95%) | 0.286* |
| Female | 33/85 (39%) | 8/21 (38%) | 6/24 (25%) | 12/21 (57%) | 7/19 (37%) | 0.177 |
| κ-light chain | 52/78 (67%) | 15/20 (75%) | 15/22 (68%) | 12/20 (60%) | 10/16 (63%) | 0.760 |
| IgA isotype | 18/80 (23%) | 4/21 (19%) | 3/22 (14%) | 5/21 (24%) | 6/16 (38%) | 0.359* |
| Creatinine >2.0 mg/100 ml | 6/85 (7%) | 2/21 (10%) | 1/24 (4%) | 2/21 (10%) | 1/19 (5%) | 0.852* |
| Hb < 10 g/100 ml | 30/85 (35%) | 11/21 (52%) | 11/24 (46%) | 4/21 (19%) | 4/19 (21%) | 0.047 |
| Platelet count >150 × 109/l | 59/85 (69%) | 18/21 (86%) | 18/24 (75%) | 12/21 (57%) | 11/19 (58%) | 0.130 |
| CRP > 4 mg/l | 51/82 (62%) | 9/21 (43%) | 19/23 (83%) | 14/20 (70%) | 9/18 (50%) | 0.028 |
| Albumin < 3.5 g/100 ml | 14/85 (16%) | 3/21 (14%) | 8/24 (33%) | 2/21 (10%) | 1/19 (5%) | 0.057* |
| B2 M >4 mg/l | 41/83 (49%) | 12/21 (57%) | 14/23 (61%) | 9/20 (45%) | 6/19 (32%) | 0.232 |
| ISS Stage 1 | 29/81 (36%) | 6/21 (29%) | 6/23 (26%) | 8/19 (42%) | 9/18 (50%) | 0.344 |
| ISS Stage 2 | 38/81 (47%) | 8/21 (38%) | 13/23 (57%) | 9/19 (47%) | 8/18 (44%) | 0.670 |
| ISS Stage 3 | 14/81 (17%) | 7/21 (33%) | 4/23 (17%) | 2/19 (11%) | 1/18 (6%) | 0.106* |
| LDH ≥190 U/l | 35/85 (41%) | 7/21 (33%) | 13/24 (54%) | 11/21 (52%) | 4/19 (21%) | 0.091 |
| Cytogenetic abnormalities | 63/83 (76%) | 16/21 (76%) | 21/23 (91%) | 14/20 (70%) | 12/19 (63%) | 0.168 |
| GEP high-risk | 19/45 (42%) | 6/16 (38%) | 6/10 (60%) | 4/12 (33%) | 3/7 (43%) | 0.608* |
| GEP MS subgroup | 12/45 (27%) | 4/16 (25%) | 1/10 (10%) | 6/12 (50%) | 1/7 (14%) | 0.149* |
| ≥60 months prior therapy | 32/85 (38%) | 8/21 (38%) | 7/24 (29%) | 9/21 (43%) | 8/19 (42%) | 0.767 |
| Prior thalidomide | 63/85 (74%) | 18/21 (86%) | 18/24 (75%) | 16/21 (76%) | 11/19 (58%) | 0.247 |
| Baseline neuropathy | 36/83 (23%) | 9/21 (38%) | 9/24 (12%) | 12/21 (28%) | 6/17 (12%) | 0.491 |
| Grade 1 | 14/83 (17%) | 8/21 (38%) | 2/24 (8%) | 3/21 (14%) | 1/17 (6%) | 0.023* |
| Grade 2 | 5/83 (6%) | 0/21 (0%) | 1/24 (4%) | 3/21 (14%) | 1/17 (6%) | 0.259* |
| Prior transplant | 78/85 (92%) | 21/21 (100%) | 22/24 (92%) | 17/21 (81%) | 18/19 (95%) | 0.148* |
| >1 prior transplant | 55/85 (65%) | 16/21 (76%) | 13/24 (54%) | 11/21 (52%) | 15/19 (79%) | 0.141 |
n/N (%), n-number with factor; N-number with valid data for factor.
Sample size assumption for Chi-Square Test is suspect.
Dose-limiting toxicities according to bortezomib and thalidomide dose levels
In group A (V=1.0 mg/m2), the first two patients at the T=50 mg/day dose level developed DLT including grade 4 neutropenia and grade 3 renal failure (Table 2). Both conditions were considered possibly related to the treatment and the two patients were subsequently taken off protocol, one for orthopedic surgery and the other due to progressive disease. No further DLTs were observed in the subsequent eight patients enrolled. At the T=100 mg/day dose level, one patient developed grade 3 anemia and renal failure; these events were determined to be due to disease progression rather that treatment-related, and the patient was removed from protocol. Another patient developed a fungal pneumonia, which was not considered to be treatment-related. One of the 10 patients entered at this dose level was not evaluable for toxicity. At the 150 mg dose level, one patient was hospitalized for seizures, and a subdural hematoma was detected on radiological evaluation; this patient also developed grade 4 leukopenia, possibly related to treatment. The patient was subsequently taken off protocol for disease progression. At the 200 mg dose, three patients experienced DLT: one developed grade 3 renal failure and grade 4 neutropenia, as a result of progressive disease; the other two patients experienced grade 4 neutropenia, with disease progression in one.
Table 2.
Toxicities by bortezomib (V) and thalidomide (T) dose in cycle 2
| Thalidomide dose | Bortezomib dose
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1.0 mg/m2 | 1.3 mg/m2 | |||||||
|
| ||||||||
| Toxicity | 50 mg (N =12) n |
100 mg (N =9)a n |
150 mg (N =10) n |
200 mg (N =14) n |
50 mg (N =11) n |
100 mg (N =10) n |
150 mg (N =13) n |
200 mg (N =6) n |
| Hematological | ||||||||
| Anemia Grade 3 | 1 | |||||||
| Thrombocytopenia Grade 4 | 6 | |||||||
| Neutropenia Grade 4 | 2 | 1 | 3 | 6 | ||||
| Renal failure Grade 3 | 2 | 1 | 1 | |||||
| Pneumonia/sepsis | 1 | 1 | ||||||
| Subdural hematoma | 1 | |||||||
| Fatigue Grade 3 | 3 | 3 | ||||||
| Hypophosphatemia Grade 3 | 2 | 1 | ||||||
| Peripheral neuropathy Grade 3 | 2 | 1 | ||||||
One patient could not be evaluated for toxicity.
In group B (V=1.3 mg/m2), at the T=50mg/day dose, one patient died from pneumonia and sepsis, and this event was not considered drug-related. Two patients experienced grade 3 hypophosphatemia, one of whom had a normal phosphate level on re-testing on the same day whereas the other had stopped taking phosphate supplements prior to this event; normal phosphate values were reported once the patient resumed the phosphate supplement intake. At the T=100 mg/day dose, two patients experienced grade 3 peripheral neuropathy; these toxicities did not resolve upon discontinuation of treatment and the patients were, therefore, removed from the study. Another patient developed grade 3 hypophosphatemia, deemed unlikely related to the treatment. Three other patients experienced grade 3 fatigue, related to disease progression. After dose escalation of thalidomide to 150 mg, grade 3 sensory neuropathy was seen in a single patient whereas another patient experienced grade 3 fatigue, nausea, diarrhea, hypokalemia, renal failure and mood alteration (depression and confusion). Two other patients in this cohort experienced grade 3 gastrointestinal toxicity, fatigue, syncope and atrial fibrillation, considered not to be related to the study treatment. Grade 4 hematological toxicities were seen in all six patients enrolled in the fourth cohort receiving 200 mg of T. According to protocol design, DLT was reached at V=1.3 mg/m2 and at T=200 mg, so that V=1.3 mg/m2 and T=150mg was defined as the maximum tolerated dose. Dose de-escalation was not required for any of the patients in group A and for cohorts 1 through 4 of group B.
The median treatment cycle numbers per T cohort in group A were 6, 10, 7 and 2 compared with 12, 6, 11 and 3, respectively, in group B. T was administered to 42 patients at a dose ≤100 mg/day and to 43 patients at a dose ≥150 mg/day. Twenty patients in group A and 15 in group B received D.
Reasons for trial discontinuation
Sixty patients went off-study due to progressive disease (PD), 8 due to toxicities (neuropathy being the most common), and 3 patients died on-study (one due to progressive disease, another to progressive disease with pneumonia/ARDS as a secondary cause and the third due to sepsis). Six patients chose to go off-study, and the remaining patients discontinued due to insurance, 3 non-compliance,2 inter-current illness1 and physician’s choice.1 One patient went off-study prior to being treated.
Rates and duration of response
Eighty-two of the 85 enrolled patients could be evaluated for response; 3 patients went off-study before response could be evaluated. When examined according to best response level achieved through cycle 8 on an intent-to-treat basis, 6% achieved stringently defined CR, 16% qualified for n-CR and 41% for PR status, for a total percentage of patients achieving at least PR of 63% (Table 3). An additional 16% attained MR, 21% were deemed to have not responded or progressed (NR). Although the study was not designed to evaluate whether clinical outcomes were V and T dose-related, an exploratory analysis failed to show such association with frequency and levels of response (Table 3).
Table 3.
Best response through cycle 8 by bortezomib (V) and thalidomide (T) dose
| Response level | All patients | V =1.0 mg/m2, T <150 mg | V =1.0 mg/m2, T >=150 mg | V =1.3 mg/m2, T <150 mg | V =1.3 mg/m2, T >=150 mg |
|---|---|---|---|---|---|
| CR (100%) | 5/82 (6%) | 1/21 (5%) | 0/24 (0%) | 3/21 (14%) | 1/16 (6%) |
| n-CR (99%) | 13/82 (16%) | 6/21 (29%) | 3/24 (13%) | 3/21 (14%) | 1/16 (6%) |
| PR (50–90%) | 34/82 (41%) | 7/21 (33%) | 9/24 (38%) | 10/21 (48%) | 8/16 (50%) |
| MR (20–25%) | 13/82 (16%) | 4/21 (19%) | 3/24 (13%) | 3/21 (14%) | 3/16 (19%) |
| NR | 17/82 (21%) | 3/21 (14%) | 9/24 (38%) | 2/21 (10%) | 3/16 (19%) |
Abbreviations: CR, complete response; MR, minor response; PR, partial response; n/N (%), n-number with factor; N, number with valid data for factor.
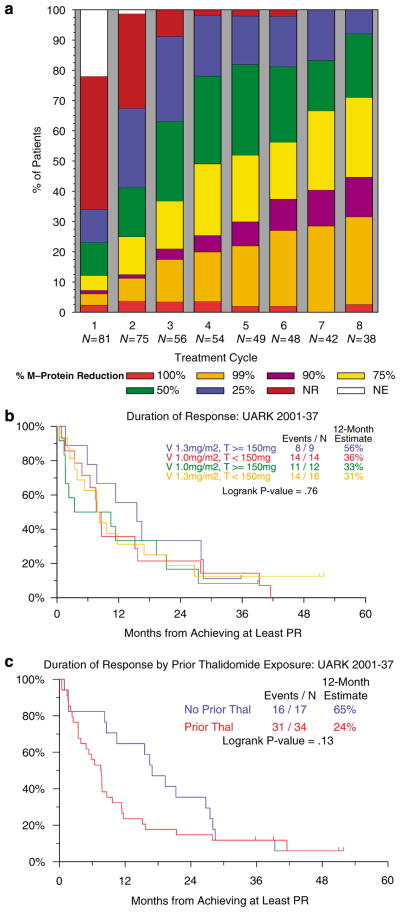
Cumulative rates of response levels, per cycle, are portrayed in Figure 1a on an intent-to-treat basis for all 82 evaluable patients. There was a steep increase in the frequencies of all response levels during the first 3 cycles of therapy; maximum MR and PR rates were reached after 5 cycles although higher levels of response continued to increase through cycle 8. Altogether 26 patients received D at cycle 4 or later. These 26 patients included 12 who received V at a dose of 1.0 mg/m2 and 14 at the higher dose of 1.3 mg/m2. There was no significant difference in highest response rates achieved by patients receiving D vs those who did not receive D. As was the case for response frequency (Table 3), the duration of PR measured from its onset did not appear to be affected by V and T dosing (Figure 1b), but tended to be longer in the absence of prior exposure to T (Figure 1c). Although significantly associated with lower PR and n-CR rates on univariate analysis, prior T exposure did not enter the multivariate model (data not shown).
Figure 1.
Response to the VTD regimen. (a) Cumulative response rates by treatment cycle on an-intent-to-treat basis. Portrayed are the highest frequencies of the different response levels achieved during successive cycles of therapy. Per protocol, thalidomide was added with the second cycle whereas dexamethasone was added with the fourth cycle in case partial response status had not been achieved. (b) Duration of partial response by bortezomib (V) and thalidomide (T) dose. The median overall duration of response was 8.6 months. Differences in duration of response between V and T doses were not significant. (c) Duration of partial response according to prior exposure to thalidomide (T). Patients who had not received prior T achieved slightly greater durations of response than patients who had received prior T (P=0.13).
Event-free survival and overall survival
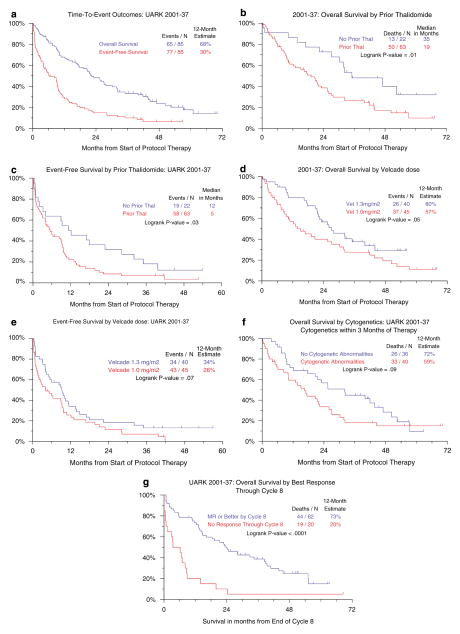
With a median follow-up of live patients of 46 months, the median durations of OS and EFS for all 85 patients were 22 and 6 months, respectively, and the 12-month survival rates were 68 and 30% (Figure 2a). Both OS and EFS of the 22 patients without prior T exposure were significantly longer than outcomes in the 63 subjects with prior T (Figures 2b and c). OS and EFS were also slightly longer among patients receiving the higher V dose of 1.3 vs 1.0 mg/m2 (P=0.05 and P=0.07, respectively) (Figures 2d and e). The presence of CA documented in 76% imparted borderline inferior OS (P=0.09) (Figure 2f). Finally, patients achieving at least MR status had a superior subsequent survival than those with a lesser degree of response (P<0.001) (Figure 2g). There was no significant difference in survival among greater levels of response (MR vs PR vs n-CR vs CR) (data not shown).
Figure 2.
Survival outcomes. (a) Overall and event-free survival of all patients. Among 85 patients enrolled, overall and event-free 12-month survival rates were 68 and 30%, respectively. (b) Overall survival according to prior thalidomide (T) exposure. Prior thalidomide therapy was associated with inferior survival. (c) Event-free survival according to prior thalidomide (T) exposure. Prior thalidomide therapy was associated with inferior event-free survival. (d) Overall survival according to bortezomib (V) dose. Overall survival tended to be superior among patients receiving the higher Velcade (V) dose of 1.3 vs 1.0 mg/m2. (e) Event-free survival according to bortezomib (V) dose. Event-free survival tended to be superior among patients receiving the higher Velcade (V) dose of 1.3 vs 1.0 mg/m2. (f) Survival according to the presence of cytogenetic abnormalities (CA). The presence of CA was borderline significant for inferior outcome. (g) Survival according to quality of response (MR v<MR). Patients achieving at least MR status by cycle 8 had significantly superior survival than those with no response. There was no significant difference in survival among further divisions of response levels (CR, n-CR, PR and MR) (P=0.76, data not shown).
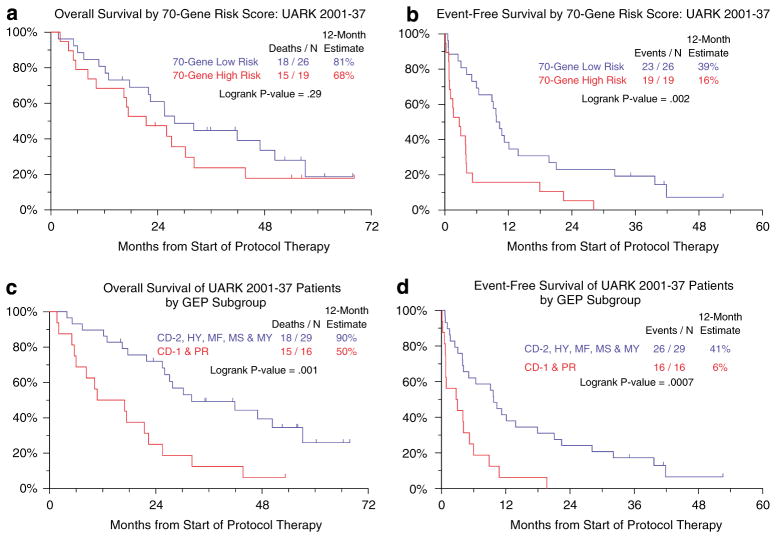
Among the subset of 45 patients with GEP data, EFS but not OS was significantly inferior among the 19 with high-risk MM compared to the 26 with low-risk MM (Figures 3a and b).19 Given the limited sample size, a representative account of patients’ outcome according to the 8 molecular subgroups could not be given;25 combining subgroups revealed that patients with CCND1-1 (CD1) and proliferation designations fared significantly worse than the remaining six other groups both in terms of OS and EFS (Figures 3c and d).
Figure 3.
Survival outcomes according to gene expression profiling (GEP) data. (a) Overall survival according to GEP-defined risk group. Patients in the 70-gene-defined low-risk group experienced slightly greater overall survival than patients in the high-risk group (P=0.29). (b) Event-free survival according to GEP-defined risk group. Patients in the 70-gene-defined low-risk group enjoyed significantly longer event-free survival than patients in the high-risk group. (c) Overall survival according to GEP-defined molecular subgroups.18 These included CCND1-1 (CD-1), CCND1-2 (CD-2), myeloid (MY), hyperdiploid (HY), MMSET/FGFR3 (MS), MAF/MAFB (MF), low bone disease (LB) and proliferation (PR) subgroups. Patients in the CD-1 and PR subgroups had significantly inferior survival compared to those in the remaining subgroups. (d) Event-free survival according to GEP-defined molecular subgroups. Patients in the CD-1 and PR subgroups also fared significantly worse in terms of event-free survival compared to patients in the remaining subgroups.
On multivariate analysis of the 77 patients with complete data sets, both OS and EFS were independently adversely affected by prior T exposure (Table 4). Using response as a time-dependent variable, patients achieving MR status enjoyed superior EFS and OS compared to those not responding. OS was also superior when the higher V dose was applied, while EFS was superior for patients presenting without CA and with κ-light chain disease.
Table 4.
Univariate and multivariate analyses of parameters associated with overall survival and event-free survival
| Variable | n/N (%) | Overall survival
|
Event-free survival
|
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |||
| Univariate | Age ≥65 years | 23/85 (27%) | 1.02 (0.59, 1.76) | 0.948 | 0.64 (0.38, 1.07) | 0.087 |
| Caucasiana | 79/85 (93%) | 1.11 (0.35, 3.55) | 0.864 | 1.30 (0.47, 3.55) | 0.614 | |
| Femalea | 33/85 (39%) | 0.76 (0.45, 1.27) | 0.297 | 1.07 (0.68, 1.69) | 0.781 | |
| κ-light chain | 52/78 (67%) | 1.07 (0.63, 1.83) | 0.794 | 0.68 (0.42, 1.11) | 0.124 | |
| IgA Isotype | 18/80 (23%) | 0.71 (0.38, 1.35) | 0.299 | 0.65 (0.38, 1.14) | 0.136 | |
| Albumin <3.5 g/100 ml | 14/85 (16%) | 1.57 (0.82, 3.03) | 0.175 | 1.12 (0.60, 2.08) | 0.721 | |
| B2M ≥4 mg/l | 41/83 (49%) | 0.97 (0.59, 1.60) | 0.901 | 1.04 (0.66, 1.63) | 0.879 | |
| LDH ≥190 U/l | 35/85 (41%) | 1.42 (0.86, 2.33) | 0.170 | 1.73 (1.09, 2.72) | 0.019 | |
| Platelet count >150 × 109/l | 59/85 (69%) | 1.40 (0.80, 2.45) | 0.234 | 1.26 (0.77, 2.06) | 0.350 | |
| Creatinine ≥2.0 mg/100 mla | 6/85 (7%) | 0.83 (0.33, 2.07) | 0.689 | 0.87 (0.38, 2.01) | 0.742 | |
| CRP ≥4 mg/l | 51/82 (62%) | 1.10 (0.65, 1.86) | 0.721 | 0.93 (0.58, 1.48) | 0.747 | |
| Cytogenetic abnormalities | 63/83 (76%) | 1.65 (0.88, 3.11) | 0.121 | 2.22 (1.23, 4.00) | 0.008 | |
| ≥60 months prior therapya | 32/85 (38%) | 0.52 (0.31, 0.87) | 0.014 | 0.65 (0.41, 1.03) | 0.066 | |
| Prior thalidomide (T) | 63/85 (74%) | 2.12 (1.15, 3.92) | 0.017 | 1.78 (1.04, 3.02) | 0.034 | |
| Prior transplanta | 78/85 (92%) | 1.77 (0.55, 5.66) | 0.337 | 1.54 (0.62, 3.83) | 0.349 | |
| >2 transplantsa | 55/85 (65%) | 1.16 (0.68, 1.98) | 0.581 | 1.17 (0.73, 1.88) | 0.520 | |
| Bortezomib (V) 1.3 mg/m2 | 40/85 (47%) | 0.61 (0.37, 1.01) | 0.056 | 0.66 (0.42, 1.04) | 0.073 | |
| Thalidomide (T) ≥150 mg | 43/85 (51%) | 1.19 (0.72, 1.96) | 0.504 | 0.91 (0.58, 1.42) | 0.678 | |
| Achieved at least MRb | 0.42 (0.24, 0.75) | 0.003 | 0.45 (0.27, 0.75) | 0.002 | ||
| Multivariate | Achieved at least MRb | 0.28 (0.14, 0.54) | <.001 | 0.37 (0.21, 0.65) | <.001 | |
| κ-light chain | 51/77 (66%) | N/A | N/A | 0.36 (0.21, 0.64) | <.001 | |
| Cytogenetic abnormalities | 57/77 (74%) | N/A | N/A | 2.87 (1.55, 5.31) | <.001 | |
| Prior thalidomide (T) | 56/77 (73%) | 2.43 (1.27, 4.65) | 0.007 | 3.82 (1.95, 7.46) | <.001 | |
| Bortezomib (V) 1.3 mg/m2 | 35/77 (45%) | 0.44 (0.25, 0.76) | 0.003 | N/A | N/A | |
Not included in multivariate analysis.
Treated as a time-dependent variable.
Discussion
The VTD combination was well tolerated in all cohorts receiving bortezomib at the 1.0 mg/m2 dose and in those receiving 1.3 mg/m2 up to T 150 mg. Although 23% of trial participants had grade ≤2 peripheral neuropathy at trial onset, aggravation of this toxicity was infrequent with the VTD regimen. Myelosuppression in this heavily pretreated population (92% had one and 65% two transplants) became dose-limiting, but infectious complications were manageable. The VTD regimen had remarkable activity so that 63% achieved PR and 22% n-CR, despite the advanced, refractory and high-risk features of the trial participants: 76% had CA, LDH levels were elevated in 41% and GEP-defined high-risk status was present in 42%. OS and EFS both were shorter in the presence of CA and in case of prior T exposure. Although not designed to determine whether there was a dose effect of T or V on outcome, OS appeared superior when the higher dose of V (1.3 mg/m2) was employed. MR imparted superior EFS and OS, whereas the level of response did not appear to affect survival.
Recently, we have reported on the dramatically different outcomes of newly diagnosed patients when GEP-defined risk was considered.19 These observations have since been confirmed by the Mayo Clinic group in an up-front transplant regimen,27 in a multi-institutional salvage trial (APEX) comparing dexamethasone and bortezomib28,29 and, for EFS, in the present study. Based on GEP comparisons, especially of paired samples procured at diagnosis and relapse reported for our Total Therapy trials, a higher frequency of high-risk disease reported here (42%) was expected, compared to 13% in Total Therapy 2 and 15% in Total Therapy 3.19 Such high-risk designation applied to only 16% of patients accrued to the APEX study,28,29 a frequency similar to that observed in newly diagnosed cases. One can speculate that, in the case of accrual to the APEX trial from a more general practice population, those with high-risk had succumbed early, a reasoning supported also by a lower frequency of CA in the APEX compared to the current VTD trial (68 vs 76%, P=0.072). In view of the superior outcomes with Total Therapies in the 85% of patients with low-risk MM, a higher proportion of those with high-risk was available at any given time for VTD trial participation at our institution.
Superior outcomes with Total Therapy 3 vs Total Therapy 2 were extended to patients with MMSET/FGFR3-type MM, which could be attributed to the incorporation of bortezomib in the more recent trial.26,30 Although the sample size with GEP information in the current study was small, patients with MMSET/FGFR3 MM seemed to benefit from VTD in our trial.
VTD has since been applied as up-front therapy in newly diagnosed patients with MM effecting high rates of CR and n-CR.31 Our Total Therapy 3 regimen has incorporated VTD (V at 1 mg/m2, T at 200 mg, D at 40 mg) into PACE (cisplatin, doxorubicin, cyclophosphamide, etoposide), referred to as VTD-PACE regimen, for induction prior to and consolidation after melphalan-based tandem transplants.32,33 CR rates were similar to those observed on the T arm of Total Therapy 2; yet CR duration and EFS were significant and OS borderline superior in TT3. The adverse implications of the FGFR3-type MM in TT2 were no longer observed in TT3.
VTD or similar combinations, such as VRD (bortezomib (Velcade), lenalidomide (Revlimid), dexamethasone)34 have since been employed together with melphalan and yielded remarkable activity in both previously treated and especially newly diagnosed patients with MM.35–37 Many trials are currently in progress or in planning stages to sort out the most effective and least toxic combinations of new agents in combination with melphalan toward maximizing CR rates and thereby further improving survival in MM.
Because of its rapid onset of response, we see a role for VTD as front-line therapy for both transplant and non-transplant candidates and in the setting of renal failure up to the level of hemodialysis-dependence.38 Due to its mild myelosuppression, VTD, but not VRD, can be given safely to patients with pancytopenia resulting from cumulative bone marrow damage inflicted by prior therapies. In this setting, we have also combined VTD with metronomically scheduled doxorubicin at a low daily of 5 mg/m2 administered by continuous daily infusions for 14 days.39,40 Results indicated excellent tolerance without aggravation of thrombocytopenia and astonishing anti-myeloma activity, which could be further enhanced by the addition of cisplatin at 1 mg/m2 likewise administered as continuous intravenous infusion for 14 days and of rapamycin (Barlogie, unpublished data). We and others are also exploring VTD and VRD in the treatment of primary AL amyloidosis. In a setting of intolerance of D, VT alone appears to be quite effective as well.
VTD-PACE is also highly effective in the setting of high-risk refractory myeloma with extramedullary disease presentations including central nervous system involvement (Barlogie, unpublished data). VTD could be safely added to dose-escalated and fractionated melphalan in the context of autotransplants to take advantage of the well-documented synergism between melphalan and the components of VTD,1 which results from inhibition of double-stranded DNA repair.41 Thus, we were able to administer melphalan at 100 mg/m2 after V at 1.0 or 1.3 mg/m2 on days 1, 4 and 7 (total dose, 300 mg/m2) together with T at 200 mg/day for 7 days and D at 40 mg on the day of and after V plus melphalan (stem cell infusion on day 8) in the far-advanced disease setting with encouraging results.42 Finally, we were able to combine VTD-PACE with melphalan at 25 mg/m2/day for 4 successive days and autologous stem cell support on day 6 (Barlogie unpublished). These combinations are all currently being explored in the refractory disease setting, especially for patients with high-risk GEP features.
Acknowledgments
This work was supported in part by National Institutes of Health Grant CA55819
References
- 1.Hideshima T, Chauhan D, Richardson P, Anderson KC. Identification and validation of novel therapeutic targets for multiple myeloma. J Clin Oncol. 2005;23:6345–6350. doi: 10.1200/JCO.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 2.Orlowski RZ, Voorhees PM, Garcia RA, Hall MD, Kudrik FJ, Allred T, et al. Phase 1 trial of the proteasome inhibitor bortezomib and pegylated liposomal doxorubicin in patients with advanced hematologic malignancies. Blood. 2005;105:3058–3065. doi: 10.1182/blood-2004-07-2911. [DOI] [PubMed] [Google Scholar]
- 3.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 4.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 5.Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 6.Barlogie B, Desikan R, Eddlemon P, Spencer T, Zeldis J, Munshi N, et al. Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: identification of prognostic factors in a phase 2 study of 169 patients. Blood. 2001;98:492–494. doi: 10.1182/blood.v98.2.492. [DOI] [PubMed] [Google Scholar]
- 7.Alexanian R, Haut A, Khan AU, Lane M, McKelvey EM, Migliore PJ, et al. Treatment for multiple myeloma. Combination chemotherapy with different melphalan dose regimens. JAMA. 1969;208:1680–1685. doi: 10.1001/jama.208.9.1680. [DOI] [PubMed] [Google Scholar]
- 8.Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 9.Weber D, Rankin K, Gavino M, Delasalle K, Alexanian R. Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol. 2003;21:16–19. doi: 10.1200/JCO.2003.03.139. [DOI] [PubMed] [Google Scholar]
- 10.Geitz H, Handt S, Zwingenberger K. Thalidomide selectively modulates the density of cell surface molecules involved in the adhesion cascade. Immunopharmacology. 1996;31:213–221. doi: 10.1016/0162-3109(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 11.Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163:380–386. [PubMed] [Google Scholar]
- 12.Bellamy WT, Richter L, Frutiger Y, Grogan TM. Expression of vascular endothelial growth factor and its receptors in hematopoietic malignancies. Cancer Res. 1999;59:728–733. [PubMed] [Google Scholar]
- 13.Vacca A, Ribatti D, Presta M, Minischetti M, Iurlaro M, Ria R, et al. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood. 1999;93:3064–3073. [PubMed] [Google Scholar]
- 14.Zangari M, Barlogie B, Jacobson J, Rasmussen E, Burns M, Kordsmeier B, et al. VTD regimen comprising velcade (V) + thalidomide (T) and added DEX (D) for non-responders to V + T effects a 57% PR rate among 56 patients with myeloma (M) relapsing after autologous transplant. Blood. 2003;102:830. (abstract) [Google Scholar]
- 15.Barlogie B, Shaughnessy J, Tricot G, Jacobson J, Zangari M, Anaissie E, et al. Treatment of multiple myeloma. Blood. 2004;103:20–32. doi: 10.1182/blood-2003-04-1045. [DOI] [PubMed] [Google Scholar]
- 16.Barlogie B, Alexanian R, Pershouse M, Smallwood L, Smith L. Cytoplasmic immunoglobulin content in multiple myeloma. J Clin Invest. 1985;76:765–769. doi: 10.1172/JCI112033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawyer JR, Waldron JA, Jagannath S, Barlogie B. Cytogenetic findings in 200 patients with multiple myeloma. Cancer Genet Cytogenet. 1995;82:41–49. doi: 10.1016/0165-4608(94)00284-i. [DOI] [PubMed] [Google Scholar]
- 18.Zhan F, Hardin J, Kordsmeier B, Bumm K, Zheng M, Tian E, et al. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood. 2002;99:1745–1757. doi: 10.1182/blood.v99.5.1745. [DOI] [PubMed] [Google Scholar]
- 19.Shaughnessy JD, Jr, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- 20.Walker R, Barlogie B, Haessler J, Tricot G, Anaissie E, Shaughnessy JD, Jr, et al. Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. J Clin Oncol. 2007;25:1121–1128. doi: 10.1200/JCO.2006.08.5803. [DOI] [PubMed] [Google Scholar]
- 21.Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. Am Stat Association. 1958;53:457–481. [Google Scholar]
- 23.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 24.Cox DR. Regression tables and life tables. J R Stat Soc. 1972;B34:187–202. [Google Scholar]
- 25.Zhan F, Huang Y, Colla S, Stewart JP, Hanamura I, Gupta S, et al. The molecular classification of multiple myeloma. Blood. 2006;108:2020–2028. doi: 10.1182/blood-2005-11-013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pineda-Roman M, Zangari M, Haessler J, Anaissie E, Tricot G, van Rhee F, et al. Sustained complete remissions in multiple myeloma linked to bortezomib in total therapy 3: comparison with total therapy 2. Br J Haematol. 2008;140:625–634. doi: 10.1111/j.1365-2141.2007.06921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng W, Kuehl W, Bergsagel P, Fonseca R. Translocation t(4;14) retains prognostic significance even in the setting of high-risk molecular signature. Leukemia. 2007;22:459–461. doi: 10.1038/sj.leu.2404934. [DOI] [PubMed] [Google Scholar]
- 28.Mulligan G, Mitsiades C, Bryant B, Zhan F, Chng WJ, Roels S, et al. Gene expression profiling and correlation with outcome in clinical trials of the proteasome inhibitor bortezomib. Blood. 2007;109:3177–3188. doi: 10.1182/blood-2006-09-044974. [DOI] [PubMed] [Google Scholar]
- 29.Zhan F, Barlogie B, Mulligan G, Shaughnessy JD, Jr, Bryant B. High-risk myeloma: a gene expression based risk-stratification model for newly diagnosed multiple myeloma treated with high-dose therapy is predictive of outcome in relapsed disease treated with single-agent bortezomib or high-dose dexamethasone. Blood. 2008;111:968–969. doi: 10.1182/blood-2007-10-119321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barlogie B, Haessler J, Pineda-Roman M, Zangari M, Anaissie E, van Rhee F, et al. Completion of pre-maintenance phases in total therapies 2 and 3 improves clinical outcomes in multiple myeloma—an important variable to be considered in clinical trial designs. Cancer. 2008 doi: 10.1002/cncr.23487. In press. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Delasalle K, Giralt S, Alexanian R. Rapid control of previously untreated multiple myeloma with bortezomib-thalidomide- dexamethasone followed by early intensive therapy. Blood. 2005;106:784. (abstract) [Google Scholar]
- 32.van Rhee F, Bolejack V, Hollmig K, Pineda-Roman M, Anaissie E, Epstein J, et al. High serum-free light chain levels and their rapid reduction in response to therapy define an aggressive multiple myeloma subtype with poor prognosis. Blood. 2007;110:827–832. doi: 10.1182/blood-2007-01-067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barlogie B, Anaissie E, van Rhee F, Haessler J, Hollmig K, Pineda-Roman M, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Haematol. 2007;138:176–185. doi: 10.1111/j.1365-2141.2007.06639.x. [DOI] [PubMed] [Google Scholar]
- 34.Richardson P, Jagannath S, Avigan D, Alsina M, Schlossman R, Mazumder A, et al. Lenalidomide plus bortezomib (Rev-Vel) in relapsed and/or refractory multiple myeloma (MM): final results of a multicenter phase 1 trial. Blood. 2006;108:405. (abstract) [Google Scholar]
- 35.Mateos MV, Hernandez JM, Hernandez MT, Gutierrez NC, Palomera L, Fuertes M, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood. 2006;108:2165–2172. doi: 10.1182/blood-2006-04-019778. [DOI] [PubMed] [Google Scholar]
- 36.Terpos E, Anagnostopoulos A, Kastritis E, Zomas A, Poziopoulo C, Anagnostopoulos N, et al. The combination of bortezomib, melphalan, dexamethasone and intermittent thalidomide (VMDT) is an effective treatment for relapsed/refractory myeloma: results of a phase II clinical trial. Blood. 2005;106:363. (abstract) [Google Scholar]
- 37.Palumbo A, Ambrosini MT, Benevolo G, Pregno P, Pescosta N, Callea V, et al. Bortezomib, melphalan, prednisone, and thalidomide for relapsed multiple myeloma. Blood. 2007;109:2767–2772. doi: 10.1182/blood-2006-08-042275. [DOI] [PubMed] [Google Scholar]
- 38.Jagannath S, Barlogie B, Berenson JR, Singhal S, Alexanian R, Srkalovic G, et al. Bortezomib in recurrent and/or refractory multiple myeloma. Initial clinical experience in patients with impared renal function. Cancer. 2005;103:1195–1200. doi: 10.1002/cncr.20888. [DOI] [PubMed] [Google Scholar]
- 39.Hollmig K, Stover J, Talamo G, Fassas A, Lee C, Anaissie E. Bortezomib (VelcadeTM) + AdriamycinTM + thalidomide + dexamethasone (VATD) as an effective regimen in patients with refractory or relapsed multiple myeloma (MM) Blood. 2004;104:2399. (abstract) [Google Scholar]
- 40.Barlogie B, Anaissie E, van Rhee F, Pineda-Roman M, Zangari M, Shaughnessy J, et al. The Arkansas approach to therapy of patients with multiple myeloma. Best Pract Res. 2007;20:761–781. doi: 10.1016/j.beha.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murakawa Y, Sonoda E, Barber LJ, Zeng W, Yokomori K, Kimura H, et al. Inhibitors of the proteasome suppress homologous DNA recombination in mammalian cells. Cancer Res. 2007;67:8536–8543. doi: 10.1158/0008-5472.CAN-07-1166. [DOI] [PubMed] [Google Scholar]
- 42.Pineda-Roman M, Fox M, Hollmig K, Anaissie E, van Rhee F, Tricot G, et al. Retrospective analysis of fractionated high-dose melphalan (F-MEL) and bortezomib-thalidomide-dexamethasone (VTD) with autotransplant (AT) support for advanced and refractory multiple myeloma (AR-MM) Blood. 2006;108:3102. (abstract) [Google Scholar]