Abstract
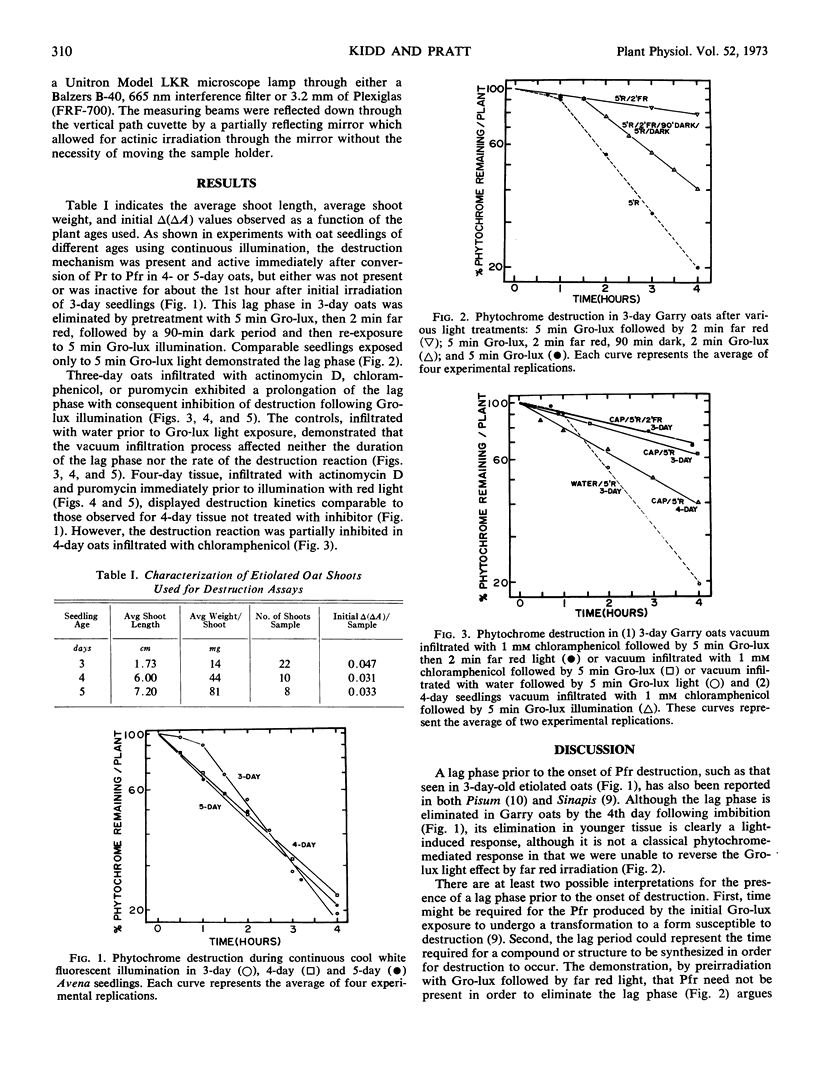
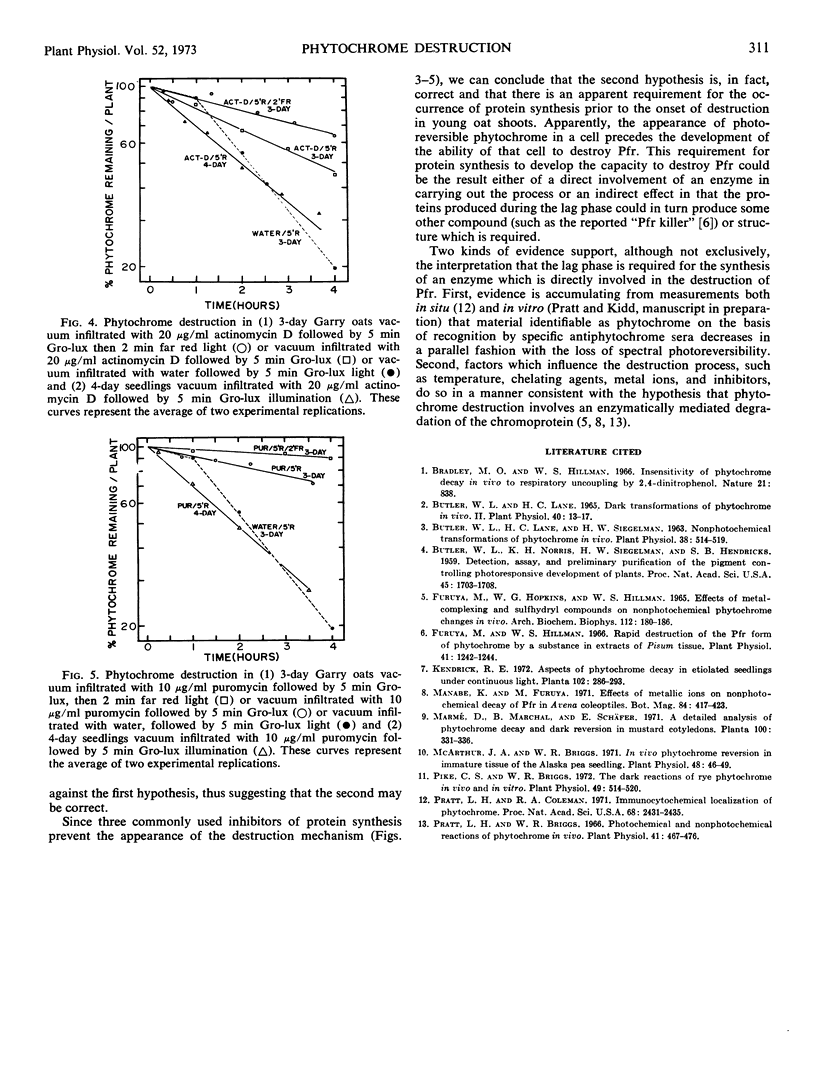
Examination of the phytochrome destruction reaction as a function of age in etiolated oat (Avena sativa L. cv. Garry) seedlings demonstrates that following illumination of 3-day-old shoots there is a lag, not observed in 4- or 5-day-old oats, prior to the onset of destruction. This light-mediated induction of the phytochrome destruction mechanism in 3-day-old shoots is inhibited by chloramphenicol, actinomycin D, and puromycin suggesting that protein synthesis is required. In 4-day-old shoots, actinomycin D and puromycin do not alter the kinetics of destruction while chloramphenicol partially inhibits the process. Thus, the inhibitors have a specific effect on the induction of the destruction mechanism but not its subsequent operation.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler W. L., Lane H. C. Dark Transformations of Phytochrome in vivo. II. Plant Physiol. 1965 Jan;40(1):13–17. doi: 10.1104/pp.40.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Lane H. C., Siegelman H. W. Nonphotochemical Transformations of Phytochrome in Vivo. Plant Physiol. 1963 Sep;38(5):514–519. doi: 10.1104/pp.38.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Norris K. H., Siegelman H. W., Hendricks S. B. DETECTION, ASSAY, AND PRELIMINARY PURIFICATION OF THE PIGMENT CONTROLLING PHOTORESPONSIVE DEVELOPMENT OF PLANTS. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1703–1708. doi: 10.1073/pnas.45.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M., Hillman W. S. Rapid Destruction of the P(FS) Form of Phytochrome by a Substance in Extracts of Pisum Tissue. Plant Physiol. 1966 Sep;41(7):1242–1244. doi: 10.1104/pp.41.7.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M., Hopkins W. G., Hillman W. S. Effects of metal-complexing and sulfhydryl compounds on nonphotochemical phytochrome changes in vivo. Arch Biochem Biophys. 1965 Oct;112(1):180–186. doi: 10.1016/0003-9861(65)90026-3. [DOI] [PubMed] [Google Scholar]
- McArthur J. A., Briggs W. R. In vivo phytochrome reversion in immature tissue of the alaska pea seedling. Plant Physiol. 1971 Jul;48(1):46–49. doi: 10.1104/pp.48.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C. S., Briggs W. R. The dark reactions of rye phytochrome in vivo and in vitro. Plant Physiol. 1972 Apr;49(4):514–520. doi: 10.1104/pp.49.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H., Briggs W. R. Photochemical and Nonphotochemical Reactions of Phytochrome in vivo. Plant Physiol. 1966 Mar;41(3):467–474. doi: 10.1104/pp.41.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H., Coleman R. A. Immunocytochemical localization of phytochrome. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2431–2435. doi: 10.1073/pnas.68.10.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]


