Abstract
OBJECTIVES
To develop a translational mouse model for the study and measurement of non-evoked pain in the orofacial region by establishing markers of nociceptive-specific grooming behaviors in the mouse.
BACKGROUND
Some of the most prevalent and debilitating conditions involve pain in the trigeminal distribution. Although there are current therapies for these pain conditions, for many patients they are far from optimal. Understanding the pathophysiology of pain disorders arising from structures innervated by the trigeminal nerve is still limited and most animal behavioral models focus on the measurement of evoked pain. In patients, spontaneous (non-evoked) pain responses provide a more accurate representation of the pain experience than do responses that are evoked by an artificial stimulus. Therefore, the development of animal models that measure spontaneous nociceptive behaviors may provide a significant translational tool for a better understanding of pain neurobiology.
METHODS
C57BL/6 mice received either an injection of 0.9% Saline solution or complete Freund’s adjuvant (CFA) into the right masseter muscle. Animals were video recorded and then analyzed by an observer blind to the experiment group. The duration of different facial grooming patterns performed in the area of injection were measured. After 2 hrs, mice were euthanized, perfused and the brainstem was removed. Fos protein expression in the trigeminal nucleus caudalis was quantified using immunohistochemistry to investigate nociceptive-specific neuronal activation. A separate group of animals was treated with morphine sulfate, to determine the nociceptive-specific nature of their behaviors.
RESULTS
We characterized and quantified 3 distinct patterns of acute grooming behaviors: fore-paw rubbing, lower lip skin/cheek rubbing against enclosure floor and hind paw scratching. These behaviors occurred with a reproducible frequency and time course, and were inhibited by the analgesic morphine. CFA-injected animals also showed Fos labeling consistent with neuronal activation in nociceptive-specific pathways of the trigeminal nucleus after two hours.
CONCLUSIONS
These behaviors and their correlated cellular responses represent a model of trigeminal pain that can be used to better understand basic mechanisms of orofacial pain and identify new therapeutic approaches to this common and challenging condition.
Keywords: Trigeminal, mouse, orofacial, spontaneous nociception, pain
1. INTRODUCTION
Pain in the orofacial region is a common and disabling problem that may have substantial effects on a patient’s quality of life. Although there are current therapies for these pain conditions, for many patients they are far from optimal. This is in part due to the limited understanding of the pathophysiology of pain disorders that arise from structures that are innervated by the trigeminal nerve. The paucity of animal models and reliable methods of measuring pain behaviors that translate to the clinic has been a significant obstacle to a better understanding of orofacial pain. The development of animal models, or the refinement of existing methods, may provide critical new insight into the complex neurobiology that underlies trigeminal nerve related pain. This may ultimately help in the development of novel therapeutic approaches for orofacial pain disorders.
Several existing methods to studying orofacial pain have been developed in rodents using inflammatory and nociceptive agents such as complete Freund’s adjuvant (CFA) 1–5, carrageenan 6–8, capsaicin 9, 10 and formalin 11. Other models induce neuropathic pain with a constriction injury of the infraorbital nerve 12–15. The quantification of pain in rodent models, be it neuropathic or inflammatory, has traditionally used evoked responses, where the animal withdraws from or avoids a painful stimulus and the threshold stimulus amplitude to evoke the withdrawal or the time to withdrawal is quantified. This approach predominantly measures hypersensitivity (hyperalgesia and allodynia) which accompanies pain 16. The limitation of this method is that quantification of hypersensitivity provides only an indirect measure of the subjective experience of pain, and it has been demonstrated in patients with neuropathic and inflammatory pain that sensory hypersensitivity (hyperalgesia and allodynia) does not consistently occur with the continuous, unavoidable pain associated with these conditions. 17–20 Spontaneous pain is also thought to be a much better predictor as a pain rating 17, and likely has a distinct pathophysiology. Additionally the measurement of evoked pain may require significant habituation and training of the animal, and skill on the part of the investigator 15, 21.
Since most pain conditions involve ongoing discomfort that does not require an inducing sensory stimulus, measures of spontaneous behaviors in response to ongoing pain in the orofacial region may more accurately represent the response to typical pain. Spontaneous (non-evoked) behaviors are considered ones that are non-provoked, in which the animal will do freely in the absence of any direct stimuli such as the application of heat or von-frey pressure (experimenter-evoked pain), and in which the animal responds to pain that it cannot control or avoid. Previous examples include facial grimace or grooming in response to an inflammatory stimuli22–24.Therefore the development of models that measure spontaneous nociceptive (non-evoked) behaviors, or the refinement of existing models may have greater translational validity. Assessment of spontaneous (non-evoked) behaviors in animals represents a significant challenge since the measurement of their frequency and the practicability in data collection and analysis can be problematic 25. More importantly, it can be challenging to measure the specificity of the behavior as a response to pain rather than an indication of anxiety, stress or other behavioral components that could appear as pain behaviors 25, 26. In freely moving rodents, it is known that injury in the facial region results in alteration in the facial grooming patterns. Some spontaneous behaviors become more persistent, and directed to the area of injury 11, 15, 27–29, whereas unilateral non-nociceptive facial stimuli do not produce this change in facial grooming 15, 24, 28., Characterizing and cataloguing markers of nociceptive-specific grooming behaviors in the mouse, related to pain in the orofacial region, would serve as a great tool for recognizing spontaneous responses after nociceptive stimulation in regions innervated by the trigeminal nerve.
In this study, we examined the response to CFA, a commonly used trigger of a local inflammatory response. Although the response to CFA is typically studied over an extended time course (up to days after exposure), it has also been reported to have multiple acute effects (minutes to hours after exposure) 30, in the same time frame as those that we report here. We chose this agent in preference to other commonly used noxious agents (such as mustard oil or capscaicin) because it will also allow us to also observe in the future whether the spontaneous nociceptive specific behaviors described parallel those observed in the chronic states.
The present study had two main aims: first, to characterize markers of nociceptive-specific grooming behaviors in the orofacial region using observation of acute grooming patterns as a marker of spontaneous pain in response to injection of CFA into the masseter muscle. We identified 3 distinct patterns of acute grooming behavior that could be quantified, and we found that these behaviors occurred with a reproducible frequency and time course. The second aim, having characterized the behaviors, was to define them as nociceptive by the effects of the analgesic morphine, and to demonstrate an association of these nociceptive responses to neuronal activation in pain processing areas in the trigeminal nucleus. We found that alterations in grooming behaviors were inhibited by morphine, and were associated with neuronal activation in nociceptive specific laminae of the trigeminal nucleus. These behaviors and their correlated cellular responses represent a model of trigeminal pain that can be used to better understand basic mechanisms of orofacial pain as well as to study new therapeutic approaches to this common and challenging condition.
2. MATERIALS AND METHODS
2.1 Animals
C57Bl/6J female mice (The Jackson Laboratory, Bar Harbor, Maine, USA), aged 5–8 weeks and weighing 20–27 g were used for all experiments. The mice were housed individually and maintained in a 12:12 light/dark cycle with ad libitum access to food and water. All studies were performed using University of California, Los Angeles Chancellor’s Animal Research Committee (ARC) and NYU IACUC approved procedures.
2.2 Injections
All injections were performed under light isoflurane anesthesia (0.8–2%). The lightly anesthetized mice received either an injection of 15 μl of 0.9% NaCl solution or 15 μl of complete Freund’s adjuvant (CFA, 0.5mg heat killed mycobacterium/ml, cappel, MP Biomedicals, LLC). The injection was performed in the right masseter muscle of the mice with a sterile 1cc insulin syringe (U-100 27g 5/8 with permanently attached needle). In the second study the injection of morphine sulfate (5 mg/kg in a volume of 25 μl, Sigma-Aldrich, Inc) was performed subcutaneously in the scruff of the neck with a sterile 1cc insulin syringe, 5 minutes prior to the masseter injection. This dose has been used previously to produce analgesia, and does not affect motor activity in mice 23, 31. A summary of the animal groupings is below.
Study 1
-
Group 1
Mice receiving CFA injection in masseter (n=8).
-
Group 2
Mice receiving saline injection in masseter (n=8).
Study 2
-
Group 1
Mice receiving CFA injection in masseter + morphine injection (sc, n=4)
-
Group 2
Mice receiving saline solution in masseter + morphine injection (sc, n=4)
2.3 Behavioral assay and analysis
All test sessions took place at approximately 10:00 am. The observation chamber consisted of a mouse basic husbandry box, 78 square inches, with a thin layer of wood chip bedding. Mice were placed in the observation chamber and allowed to habituate for 15 minutes before the masseter and scruff injections were performed, to minimize stress. The habituation was performed without access to food or water. After the 15 minute habituation period, animals were injected under light isoflurane anesthesia. Mice were then placed in the observation chamber and were allowed 10 minutes recuperation after the anesthesia. At this point all the animals were fully awake and the video recording and assessment of nociceptive behaviors were performed. The assay involved the observation and the video recording of the duration of specific facial grooming patterns performed by the animal in the area of injection.
These grooming patterns were video recorded for a 60 minute period. The recording time was divided into 10 blocks of 6 min and a score was determined for each block by measuring the duration of time they spent grooming the injected area. The videotaped observations were analyzed by an observer blinded to the experimental condition. For each observation session of grooming behavior a tabular record of the sequence of observed actions was generated by entering identified actions (forepaw facial rubbing, lower lip skin/cheek rubbing or hindpaw facial scratching) and the time that they occurred (as determined by a calibrated video recorder clock) into a spreadsheet using the Etholog software 32. Values were expressed as mean ± SEM. The data were analysed using a repeated measures ANOVA of mixed design with between subject’s factors of CFA vs saline, CFA-morphine vs saline-morphine, and CFA vs CFA-morphine. If there was significance between groups we conducted an independent t-test for different time points to compare between groups. P < 0.05 was considered significant.
2.4 Slice preparation and Immunohistochemistry
Two hrs after the masseter injection, mice were overdosed with halothane and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde in phosphate buffered saline (pH 7.4). Production and the detection of Fos protein in cells, using immunohistochemical techniques, after a noxious stimulus occurs up to 60 mins after the stimulus 33. Given that without further stimulus Fos protein disappears with a half-life of approximately a further 2 hrs, perfusing the animals 2 hrs after the initial stimulus was chosen as the optimal time to detect neuronal activity using Fos detection 33. After perfusion, brain and brainstem was removed, post-fixed in 4% paraformaldehyde for 24hrs at 4°C and then stored in a solution of 20% sucrose and 0.02M potassium phosphate buffer saline (KPBS, pH 7.4) at 4°C until used for slice preparation. For slice preparation, coronal sections (25μm thick) were cut on a cryostat at −20 °C and collected. The trigeminal nucleus caudalis (Sp5C) region was identified from atlas plates 94–98 34. Four consecutive sections were placed in one well of a 24 well plate, and then the next four sections in the adjacent well, and this process continued until all sections were collected in this region for each animal. We would then choose one section from each well randomly, so in essence only every 4th slice taken was processed for Fos protein expression to verify neuronal activation.
The Fos protein immunohistochemistry procedure is based on previous studies35, briefly sections were first rinsed 3 times with KPBS and incubated with 0.3% hydrogen peroxide in KPBS for 10 minutes at room temperature. The sections were blocked with 10% milk, 5% bovine serum albumin (BSA, Sigma, St. Louis, MO) and 0.5% TritonX (TX, Sigma, St. Louis, MO) for 1 hr to avoid non-specific binding. The sections were processed with a polyclonal rabbit anti-Fos antibody (anti-c-Fos Ab-5, 1:104 dilution, Calbiochem/EMD biochemicals, Gibbstown, NJ) in 2% normal goat serum (NGS), 5% BSA and 0.5% TX in KPBS for 48hrs at 4°C with gentle agitation. After washing several times, a first round of biotinylated anti-rabbit IG (H+L) made in goat (Vector, Burlingame, CA) was applied, and sections were incubated for 1 hr at room temperature. Sections were then incubated using avidin-biotinylated-horseradish peroxidase (ABC Kit, Vector Burlingame, CA) reagent for 1 hr at room temperature. To enhance detection of Fos, a second round of the biotinylated anti-rabbit IG incubation was performed, followed by a second round of the ABC kit, with each incubation lasting 30 minutes at room temperature. The reaction product was visualized by a diaminobenzidine tetrahydrochloride (DAB, Sigma) with Nickel intensification (0.05% DAB, 0.3% Ni, 0.005% H2O2 and 0.02M KPBS). The sections were serially mounted on gelatin coated slides, dehydrated in serial ethyl alcohol, cleared in xyline and coverslipped.
2.5 Fos quantification
Coronal sections were examined with a light microscope (Carl Zeiss) and slices containing the Sp5C region. For a Fos cell to be considered positive it had must be visible at magnifications x5, x10 and x20 objective, plus x10 eyepiece. Laminae I, II, V and X were examined for Fos positive nuclei, and the nuclear and laminae boundaries were based on the Paxinos and Franklin brain atlas plates 34, for the region depicting Sp5C. An observer blind to the experimental conditions was trained to visually identify and count the DAB stained Fos nuclei within the specified region. At least 20 hemi sections were analyzed per treatment group (CFA group and Saline group), and at least 4 slices/animal. Each section was treated individually from each animal for performing statistical analysis and presented as mean ± standard error of the mean (SEM) of Fos-postive cells in each region. The data were tested for normality using the Kolomogorov-Smirnov test and considered normal, therefore parametric statistics were performed. Comparisons of Fos expression between groups was made using independent t-test and when Levene’s test for Equality of variance was violated we used the ‘not assumed’ statistic. A probability of P < 0.05 at the two-tailed level was considered significant, and the effect size r is reported. Analysis was performed using SPSS v16 (SPSS Inc, Chicago, IL) and Field 36 was used as reference for presenting the data.
3. Results
3.1 Nociceptive behavioral assessment
CFA injection into the right masseter muscle induced three different nociceptive-specific grooming behaviors directed to the affected facial area or area of injection when compared to the control group injected with saline solution in their right masseter (n=8 in each group), figure 1. These behaviors were completely inhibited by the prior administration of morphine (5 mg/kg) but not by prior administration of saline (n=4 in each group).
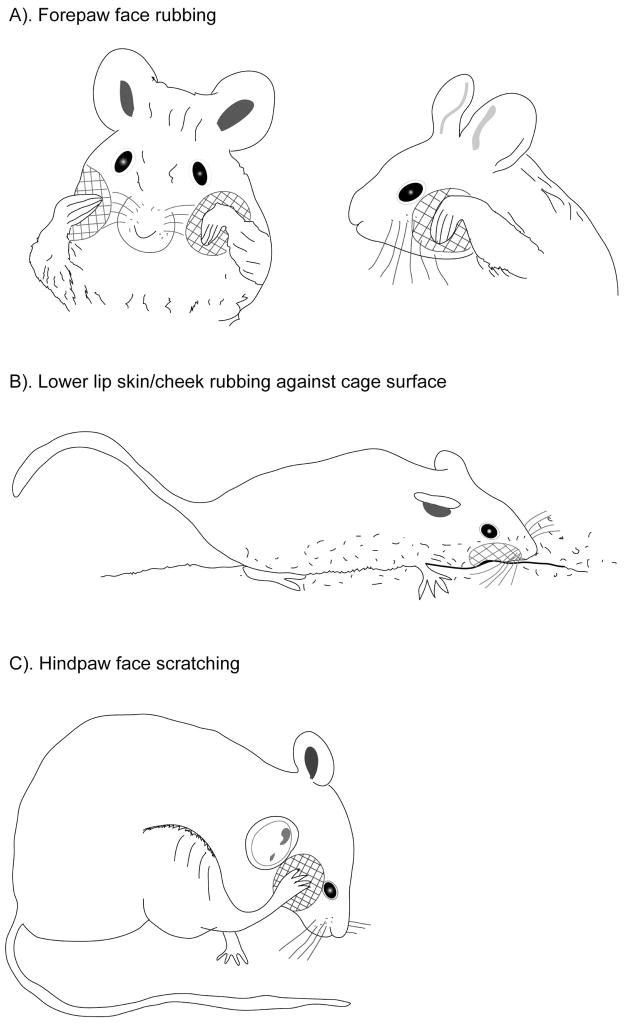
Figure 1. Line drawings of mouse behaviors following injection of CFA in the masseter.
A). Mouse exhibiting bilateral forepaw face rubbing, which consists of repetitive rubbing or washing strokes over the face and snout with one or both forepaws. These actions do not include strokes to the ears or long passes over the whole face. B). Mouse demonstrating lower lip skin/cheek rubbing against enclosure floor. This behavior consisted of repetitive rubbing of the inferior (lower lip skin) and lateral mandibular regions (cheek) against the bottom or side of the enclosure. C). Mouse performing hindpaw face scratching. The behavior consisted of repetitive rapid short scraping motions with the claws of the ipsilateral hindpaw directed to the area of injection. The cross-hatched area on each mouse face is the specific area of the face where the particular nociceptive behavior is directed.
3.1.1 Forepaw face rubbing
Forepaw face rubbing consisted of short vigorous and repetitive rubbing or washing strokes over the face and snout with one forepaw or bilaterally with both forepaws (see figure 1A). The bilateral nature of forepaw rubbing has been reported previously, using formalin injection, in both mice and rats 24, 28. The duration of one forepaw facial rubbing was quantified as the lifting of the forepaws directed to the area of injection and finalized when the animal stopped the washing motion, regardless of how many persistent rubbing or washing strokes took place between the starting of the motion and its cessation. These actions do not include strokes to the ears or long passes over the whole face, the head and body (Please see link of video 1) https://files.nyu.edu/mrr7/public/Video1Forepawfacialgrooming.avi Mice injected with CFA alone showed significantly different responses to those injected with just saline (F1,14 = 63.25, P < 0.001) and this presented as an increase in the duration of forepaw face rubbing behavior compared to control animals. While the behavior showed a steady decrease in duration over the 60 minutes observation period, the CFA and saline group remained significantly different throughout the behavioral assessment. When morphine injection preceded the injection of CFA or saline into the masseter muscle there was no significant difference between these groups (F1,6 = 3.05, P = 0.13), but there was a significant difference between the CFA alone and CFA with morphine groups (F1,10 = 29.91, P < 0.001). This indicates that morphine inhibited this nociceptive grooming pattern in the CFA animals (Fig. 2A).
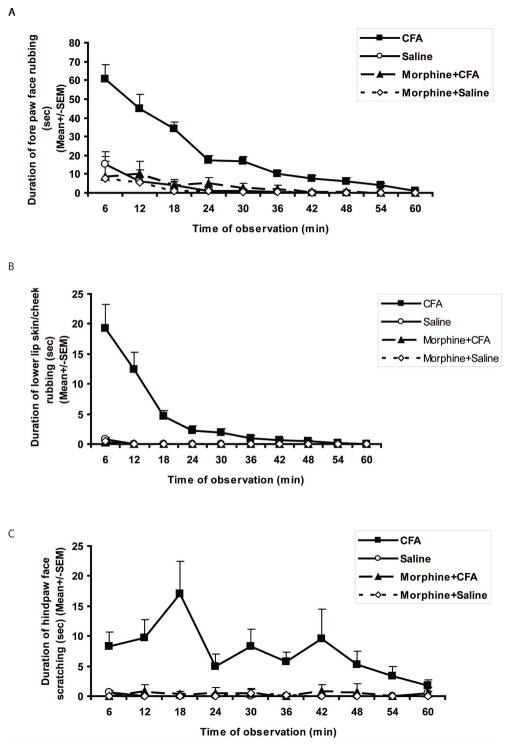
Figure 2. The effects of complete Freund’s adjuvant (CFA) injection in the masseter muscle on nociceptive behaviors.
A). There was a significantly higher duration of persistent and sustained forepaw facial rubbing actions directed to the site of injection in mice injected with CFA compared with saline, that lasted over 54 minutes. Subcutaneous injection of morphine in the scruff of the neck prior to CFA injection, prevented this nociceptive forepaw facial rubbing behavior, which was significant compared to the CFA alone group (P < 0.001).
B). There was a significantly higher duration of lower lip skin/cheek rubbing against the enclosure floor on the side of injection in mice injected with CFA compared with saline that lasted over 45 minutes, and this nociceptive behavior returned to control levels at after 50 minutes. There was no difference between the CFA and saline groups when the injection was preceded by subcutaneous morphine injection to the scruff of the neck. This lower lip skin/cheek rubbing nociceptive behavior was prevented by the prior injection of morphine.
C). There was a significantly higher duration of hindpaw facial scratching performed by the ipsilateral hind paw in the area of the injection with CFA, compared with saline (P < 0.001). When the injection of CFA or saline into the masseter muscle was preceded by morphine there was no longer a significant difference between these groups. Prior subcutaneous morphine injection in the scruff of the neck prevented the scratching action induced by the CFA injection in the right masseter. Values are expressed as mean ± SEM throughout. P < 0.05 was considered significant.
The fact that the lines for saline, morphine-CFA and morphine-saline are superimposed is an indicator that there was very little difference between these groups as illustrated by the statistics.
3.1.2 Lower lip skin/cheek rubbing against enclosure floor
The lower lip skin/cheek behavior consisted of repetitive rubbing of the inferior (lower lip skin) and lateral mandibular regions (cheek) against the bottom or side of the enclosure (see figure 1B). The duration of one lower lip skin/cheek rubbing behavior was quantified as the placement or contact of the lower lip skin and/or cheek of the side of the injection against the bottom of the enclosure and finalized by the cessation of the rubbing motion with the lifting of the face (Please see link of video 2) https://files.nyu.edu/mrr7/public/Video2Lowerlipskin-cheekgrooming.avi
Mice injected with CFA exhibited a significant increase in duration of this behavior in comparison with controls (F1,14 = 30.68, P < 0.001), across the 60 minute observation period. The nociceptive behavior after CFA did decrease over the observation period, and was no different to saline injection at the 54 (t7=1.7, P = 0.132) and 60 (t7= 1.0, P = 0.351) minutes time points. However, the data were significantly different from 6–48 minutes with the duration of behavior being greater during the first 18 minutes of observation. Again, the injection of morphine prior to CFA injection inhibited this behavior, as highlighted by that fact there was no difference between the CFA-morphine and saline-morphine groups when injection was preceded by morphine (F1,6 = 0.22, P = 0.654), but there was a significant difference between the CFA alone and CFA with morphine groups (F1,10 = 15.0, P < 0.005) (Fig. 2B).
3.1.3 Hindpaw face scratching
Hindpaw face scratching consisted of repetitive rapid short scraping motions with the claws of the ipsilateral hindpaw directed to the area of injection (see figure 1C and link of video 3) https://files.nyu.edu/mrr7/public/Video3Hindpawfacialscratching.avi
The duration of the hindpaw face scratching behavior was quantified as the lifting of the ipsilateral hindpaw towards the area of injection and finalized by replacing the hindpaw back to the floor regardless of how many persistent short scraping motions were performed between lifting and replacing of the hind paw. Mice injected with CFA consistently exhibited this behavior, whereas the control group receiving the saline injection in the right masseter did not, (F1,14 = 45.6, P < 0.001). This difference between groups was significant for up to 48 mins as the nociceptive responses decreased. When the injection of CFA or saline was preceded by morphine there was no longer a significant difference between these groups (F1,6 = 4.63, P = 0.075) but there was a significant difference between the CFA alone and CFA with morphine groups (F1,10 = 21.11, P < 0.005). These data again indicate that injection of morphine markedly inhibits the hindpaw scratching behavior triggered by CFA injection in the masseter muscle (Fig. 2C).
In all behaviors studied morphine had no direct effects in control animals.
3.2 Histological assessment of neuronal activation after masseter injection of CFA
3.2.1. Comparison between CFA and saline
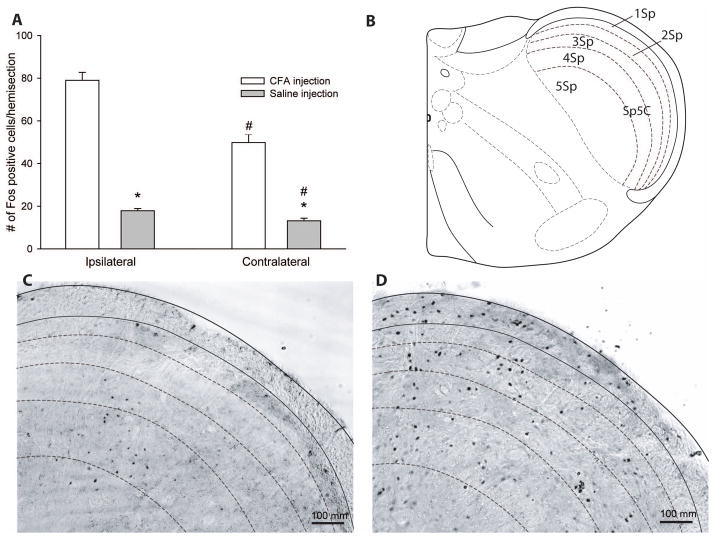
We also examined Fos protein expression in the region of the trigeminal nucleus caudalis (Sp5C, an area known to be involved in trigeminal nociceptive processing2, 3) 2 hours after injection of CFA in the right masseter muscle. All the data were considered normal using the Kolmogorov-Smirnov test. The expression of Fos protein was found predominantly in the dorsomedial region of Sp5C, which is specific to the mandibular (V3) region of the trigeminal nerve37, and mainly in laminae I and II (see figure 3B), which receives nociceptive specific inputs, similar to the spinal dorsal horn 38, 39. Fos protein expression was significantly higher in Sp5C in CFA mice after 2 hrs (79 ± 4 cells, t24.3 = 15.9, P < 0.001) compared to saline animals (18 ±1 cell, figure 3A) and with a large effect size (r = 0.96). These studies provide histological confirmation of neuronal activation in brainstem regions involved in nociceptive processing that is associated with the nociceptive grooming behaviors in mice injected with CFA. Interestingly there was also a significantly higher expression of Fos protein in the CFA group with a large effect size (49.8 ± 4 cells, t24.9 = 8.8, P < 0.001, r = 0.87) compared to the saline (13.2 ± 1 cell) when the contralateral side to the injection sites were compared. Example micrographs of Fos immunoreactivity in the ipsilateral trigeminal nucleus can be found in figures 3C and D.
Figure 3. Fos immunoreactivity in the trigeminal nucleus after CFA or saline injection in the masseter muscle.
The nociceptive behaviors that mice presented after the CFA injection in the right masseter muscle were correlated with an increase of Fos expression at the level of the trigeminal nucleus caudalis (Sp5C), specifically in laminae I and II, which received input from nociceptive fibers, when compared to the group that received a saline injection in their right masseter muscle.
A). Fos protein expression was significantly higher in the ipsilateral Sp5C in mice that received CFA injection compared to saline. Contralateral Fos protein expression in Sp5C was also significantly higher in the CFA group compared to the contralateral Fos expression of the saline group. In mice injected with CFA, Fos protein expression in Sp5C on the ipsilateral side was significantly higher compared to the contralateral side to injection, with a major effect size. Likewise Fos expression on the ipsilateral side was also significantly higher compared to the contralateral side after saline injection, but with a small effect size. Data are presented as mean ± SEM of Fos positive cells/hemisection. *P < 0.05, significance of CFA compared to the saline injection group. # P < 0.05, significance of ipsilateral compared to contralateral to injection site.
B) Schematic of the lamina organization of the mouse trigeminal nucleus caudalis at −8.24mm caudal to bregma. Adapted from Paxinos and Franklin 34, 71. Representative microphotographs of Fos expression in the ipsilateral trigeminal nucleus 2 hrs after C). saline or D). CFA injection in the masseter muscle, shown using x10 objective and scale bar = 100 μm.
3.2.2 Comparison between ipsilateral and contralateral injection sites
As indicated above, there was also an increase in the expression of Fos protein as a consequence of CFA injection compared to saline on the contralateral side to injection, as well as with the ipsilateral comparison. We therefore compared ipsilateral and contralateral Fos immunoreactivity within each injection group, (Fig. 3A). In the CFA group at 2 hrs, there was significantly higher expression of Fos protein and large effect size (t42 = 5.4, P < 0.001, r = 0.64) on the ipsilateral side (79 ± 4 cells) compared to the contralateral side (18 ±1 cell). In the saline group, the ipsilateral side to injection (49.8 ± 4 cells) also expressed significantly higher Fos protein (t48 = 3.0, P < 0.01) compared to the contralateral side (13.2 ± 1 cell) but the effect size was only small (r = 0.40)
4. DISCUSSION
There are several rodent models for the study of different types of acute and chronic pain. While these models have been developed primarily in rats, the development of transgenic mice technology has necessitated adaptation of these models for their use in mice. Most current well characterized behavioral assays are significantly limited by the fact that they generally measure responses to evoked sensory modalities, such as mechanical or thermal stimuli, and animals have control over the duration of these stimuli 15, 21, 40–42. These evoked responses typically measure hyperalgesia or allodynia in both neuropathic and inflammatory pain. Moreover, it has been shown that spontaneous pain both of neuropathic and inflammatory origin is related to increase in frequency in intact C fibers in contrast to allodynia 43. Patients with chronic pain may not always experience allodynia and hyperalgesia while they consistently report spontaneous pain. In patients with neuropathic pain measuring spontaneous pain is a much more reliable pain rating than is the measurement of hypersensitivity 17.Spontaneous (non-evoked) pain behavior is not being measured routinely in animal models of neuropathic or inflammatory pain 44. Animal models that quantify spontaneous (non-evoked) pain behavior to neuropathic and inflammatory pain induction may have significant advantages over those that primarily measure hyperalgesia.
At the bedside, the pain response comprises a range of different sensations resulting in a range of different symptoms 45, 46. Each patient’s description of their pain experience can be very subjective and therefore challenging to interpret. At the bench the measurement of spontaneous behaviors in response to nociception, in a small mammal such as the mouse, is an even greater challenge. We cannot ask a mouse where and how it hurts. Therefore, new methods of assessing non-evoked pain have the potential to be of great translational research value.
Normal grooming behaviors in freely moving animals have been well-described previously and behaviors such as scratching, nibbling, rubbing are defined by ethologists as a primary biological function concerned with the care of the body surface 47. These behaviors serve multiple functions including maintenance of the pelage,47, 48, pheromone secretion used for social signalling47, 49 and thermoregulation49. These behaviors are described as very prolonged episodes of attention to the pelage 24, 48, typically showing a rostrocaudal progression with long symmetrical and stereotyped face grooming sequences at the transition from one body part to the other and not only directed to one part of the body 24, 50, 51. Grooming behaviors are altered by painful stimulation, such that they present a different organization pattern, occurring in short episodes directed strictly to the area that is painful 11, 24, 27. Such alterations in behavior have been observed after formalin injection in the face 11, 24, 28 and paw 52, and noxious thermal stimulation of the paw 52, 53.
In this study we describe a set of nociceptive-specific, non-evoked grooming behaviors that occur when pain is present in the orofacial region. Previous studies have demonstrated that nociceptive input arising from structures innervated by the trigeminal nerve causes persistent facial rubbing or washing strokes directed to the area of lesion in the orofacial region 15, 21, 24, 28. Our observations show two additional patterns of grooming behavior directed to the facial region: lower lip skin/cheek rubbing against the enclosure floor and hindpaw face scratching.
There is anecdotal evidence that spontaneous pain such as sensations of burning and lancinating spontaneous dysesthesia developed within minutes at the site of an accidental CFA injection in a human 30. If we take into consideration this human pain experience in response to CFA injection the grooming patterns that we observed in mice can be used as a correlate to a pain experience in these animals in the orofacial region. We found that the 3 distinct grooming behaviors described in this study occurred with a reproducible pattern following CFA injection. These behaviors were quantifiable at the first time point and reduced to baseline levels by 60 mins. These nociceptive grooming behaviors were all inhibited by prior administration of morphine.
Forepaw face rubbing, consisted of sustained and persistent, washing strokes executed with one or both forepaws and directed to the site of injection. This behavior is similar to that which has been previously described 28 (video 1/Fig. 1A).
Mice also presented with another form of behavior directed to the area of injection. This behavior involved rubbing the lower lip skin and cheek of the area of the injection site against the enclosure floor or lower corners (video 2/Fig. 1B). This was also significantly increased in duration when compared with the saline injected group across the 60 minute observation period, and showed a consistent time course with decreasing frequency over time. The rubbing action presents itself in a series of short, sometimes repetitive episodes, directed exclusively to the affected area (masseter muscle) and sometimes it can be present before and after, or after, the persistent facial rubbing or washing strokes. (Please see link of video 4) https://files.nyu.edu/mrr7/public/Video4Lowerlipskin-cheek&facialgroomingsequence.avi
It is well known that gentle stroking or rubbing of an injured area may help to alleviate pain in humans 54. It was observed that lower lip skin/cheek and face rubbing behaviors were most intense during the 15 minutes. It is possible that these rubbing behaviors in mice are a response intended to reduce pain. Studies have reported that another afferent unmyelinated C-fiber system called C tactile afferents (CT) are activated by gentle touch 55, 56. Interestingly, activation of these CT fibers has in turn been reported to reduce nociceptive signaling and consequently may reduce pain perception 56–59. In our model, the action of rubbing the affected area could be an indication that even small mammals such as the mouse possess a more elaborated tactile sensitivity.
Mice injected with CFA in the right masseter muscle also showed a distinctive hindpaw face scratching behavior when compared with the control group that received the saline injection. The scratching motion involves persistent scratching actions directed toward the area of injection and performed by the ipsilateral hindpaw (video 3/Fig. 2C). Scratching is generally considered to be a reflex response to sensing an itch, and while itch is an unpleasant sensory experience it is not considered the same as pain 60. It is known that opioids can enhance itch perception 61–63, particularly when they are administered spinally 64. However, we did not observe an increase in scratching response with animals injected with morphine SC, and indeed the scratching response was inhibited by morphine and therefore we consider the behavior to be nociceptive specific.
Each of the three behaviors in response to CFA injection in the masseter was completely inhibited by prior injection of morphine 5 mg/kg SC. This dose of morphine did not result in any change in grooming behavior in control animals in which saline rather than CFA was injected in the masseter. The inhibition of these behaviors by morphine indicates that each is inhibited by analgesia mediated primarily by the μ-opioid receptor.
The nociceptive behaviors that animals presented after the CFA injection were correlated with an increase of Fos expression at the level of the trigeminal nucleus caudalis (Sp5C) predominantly in the region which receives nociceptive-specific inputs, laminae I and II 39 compared to the saline group and also compared to the contralateral side of injection (Fig. 3). While some Fos cells were also found in laminae III and IV which receives innocuous inputs, this is understandable given the non-noxious grooming stimulation to the orofacial region, but the majority were in the nociceptive-specific region. This neuronal activation has been reported in similar studies in rats with inflammation in the temporomandibular joint as well as the masseter muscle 1–5, 65, 66. The pattern of Fos expression is therefore consistent with the conclusion that the non-evoked behaviors observed in the animals injected with CFA were mediated by trigeminal neuronal nociceptive activation as a result of an inflammatory stimulus.
Fos protein expression was also observed in the contralateral Sp5C region as reported previously 1–5, 65, 66. Ipsilateral Fos expression at the level Sp5C in the CFA group was significantly higher compared to the contralateral side to injection (Fig 3C). Sp5C may have an important role in the neural pathways underlying bilateral activation of muscles of mastication 67. Nociceptive stimulation of the masseter and the TMJ has been shown to reflexively activate electromyographic (EMG) activity of the ipsilateral and contralateral masseter muscle in rats 68. The Sp5C may have a role in reflex control of oral motor activities in the presence of masseteric pain65 The development of not only ipsilateral but contralateral hyperalgesia after masseteric injection of an inflammatory substance has been shown to be in response of innocuous mandibular movements 65. This may also explain why on some occasions there were bilateral facial rubbing strokes. This could be of interesting clinical relevance since very frequently patients with unilateral masseter muscle pain report bilateral pain and tenderness to palpation.
We chose an inflammatory mediator because acute and chronic inflammation is known to be responsible for the sensitization of peripheral sensory neurons, giving rise to spontaneous pain and hypersensitivity. Spontaneous pain is the result of activation of nociceptors by inflammatory mediators 18–20 and also inflammatory pain may be an important component of the response to orofacial injury, infection, and surgery, and may also be a component of migraine. We chose to study the effects of CFA over other inflammatory agents because the major aim of the study was to characterize markers of nociceptive behaviors in response to an inflammatory nociceptive stimulus, which could ultimately be used to test both acute and chronic models of spontaneous nociceptive behavior in the head and facial region. While the effects of CFA are more typically studied over longer time periods (days) during which a chronic inflammatory response occurs, there is evidence of Fos immunoreactivity in the TNC after only 2 hrs 3, 5 that allows direct comparison. Moreover, the case report of a single individual accidentally injected with CFA described above illustrates pain beginning minutes after the injection, and lasting for 2 hours 30. Additionally, while the acute effects of inflammatory mediators such as capsaicin and mustard oil may have been described previously 65, 69 these agents are not commonly used in chronic models of pain. The above studies and observations regarding CFA support our own observations of an acute response to CFA. Ongoing studies will also evaluate the specific mechanisms underlying this acute response to CFA, as well as the relationship of this acute response to the more chronic effects of CFA that follows.
5. CONCLUSION
This study describes a set of quantifiable markers of mouse orofacial nociceptive non-evoked behaviors. These behaviors are elicited by inflammatory pain in the trigeminal distribution and are correlated with neuronal activation as represented by Fos expression in the Sp5C.
These behavioral markers represent a translational tool that can take advantage of transgenic technology 70, and can be used to characterize the effects of treatments for spontaneous nociception (pain) in the orofacial region as well as to increase our understanding of the neurobiology of trigeminal pain.
Supplementary Material
Acknowledgments
FUNDING SOURCE: This research was supported by funding from the NIH National Institute of Drug and Abuse Grant Number DA-05010, American Academy of Orofacial Pain research grant award 2008 (MRR), 2009 AHS/ Migraine Research Foundation Thomas E. Heftler Migraine Research Award and NYUCD Romero-Reyes startup fund incentive. Funding source had no involvement in the experimental design or interpretation of the results.
The authors would like to thank Jonathan Biag, Ivan Lukachynetz and Daniel S. Romero for assistance and technical support.
Footnotes
CONFLICT OF INTEREST: There are no conflicts of interest to report for any of the authors.
References
- 1.Ikeda T, Terayama R, Jue SS, Sugiyo S, Dubner R, Ren K. Differential rostral projections of caudal brainstem neurons receiving trigeminal input after masseter inflammation. J Comp Neurol. 2003;465:220–233. doi: 10.1002/cne.10836. [DOI] [PubMed] [Google Scholar]
- 2.Imbe H, Dubner R, Ren K. Masseteric inflammation-induced Fos protein expression in the trigeminal interpolaris/caudalis transition zone: contribution of somatosensory-vagal-adrenal integration. Brain Res. 1999;845:165–175. doi: 10.1016/s0006-8993(99)01913-7. [DOI] [PubMed] [Google Scholar]
- 3.Imbe H, Iwata K, Zhou QQ, Zou S, Dubner R, Ren K. Orofacial deep and cutaneous tissue inflammation and trigeminal neuronal activation. Implications for persistent temporomandibular pain. Cells Tissues Organs. 2001;169:238–247. doi: 10.1159/000047887. [DOI] [PubMed] [Google Scholar]
- 4.Sugiyo S, Takemura M, Dubner R, Ren K. Trigeminal transition zone/rostral ventromedial medulla connections and facilitation of orofacial hyperalgesia after masseter inflammation in rats. J Comp Neurol. 2005;493:510–523. doi: 10.1002/cne.20797. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Q, Imbe H, Dubner R, Ren K. Persistent Fos protein expression after orofacial deep or cutaneous tissue inflammation in rats: implications for persistent orofacial pain. J Comp Neurol. 1999;412:276–291. doi: 10.1002/(sici)1096-9861(19990920)412:2<276::aid-cne7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Tang N, Ong WY, Yeo JF, Farooqui AA. Anti-allodynic effect of intracerebroventricularly administered antioxidant and free radical scavenger in a mouse model of orofacial pain. J Orofac Pain. 2009;23:167–173. [PubMed] [Google Scholar]
- 7.Yeo J-F, Ling S-F, Tang N, Ong W-Y. Antinociceptive effect of CNS peroxynitrite scavenger in a mouse model of orofacial pain. Experimental Brain Research. 2008;184:435. doi: 10.1007/s00221-007-1211-x. [DOI] [PubMed] [Google Scholar]
- 8.Yeo J-F, Ong W-Y, Ling S-F, Farooqui AA. Intracerebroventricular injection of phospholipases A2 inhibitors modulates allodynia after facial carrageenan injection in mice. Pain. 2004;112:148. doi: 10.1016/j.pain.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez P, Saavedra G, Hernández A, Paeile C, Pelissier T. Synergistic Antinociceptive Effects of Ketamine and Morphine in the Orofacial Capsaicin Test in the Rat. Anesthesiology. 2003;99:969–975. doi: 10.1097/00000542-200310000-00033. [DOI] [PubMed] [Google Scholar]
- 10.Pelissier T, Pajot J, Dallel R. The orofacial capsaicin test in rats: effects of different capsaicin concentrations and morphine. Pain. 2002;96:81. doi: 10.1016/s0304-3959(01)00432-8. [DOI] [PubMed] [Google Scholar]
- 11.Clavelou P, Dallel R, Orliaguet T, Woda A, Raboisson P. The orofacial formalin test in rats: effects of different formalin concentrations. Pain. 1995;62:295. doi: 10.1016/0304-3959(94)00273-H. [DOI] [PubMed] [Google Scholar]
- 12.Gregg JM. A surgical approach to the ophthalmic-maxillary nerve trunks in the rat. J Dent Res. 1973;52:392. doi: 10.1177/00220345730520024001. [DOI] [PubMed] [Google Scholar]
- 13.Kernisant M, Gear RW, Jasmin L, Vit J-P, Ohara PT. Chronic constriction injury of the infraorbital nerve in the rat using modified syringe needle. Journal of Neuroscience Methods. 2008;172:43. doi: 10.1016/j.jneumeth.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim EJ, Jeon HJ, Yang GY, et al. Intracisternal administration of mitogen-activated protein kinase inhibitors reduced mechanical allodynia following chronic constriction injury of infraorbital nerve in rats. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31:1322. doi: 10.1016/j.pnpbp.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. J Neurosci. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 17.Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. J Pain. 2004;5:491–497. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 19.Woolf CJaCM. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci. 1999;96:7723–7730. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lolignier S, Amsalem M, Maingret F, et al. Nav1.9 channel contributes to mechanical and heat pain hypersensitivity induced by subacute and chronic inflammation. PLoS One. 6:e23083. doi: 10.1371/journal.pone.0023083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav. 1999;67:711–716. doi: 10.1016/s0031-9384(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 22.Sotocinal SG, Sorge RE, Zaloum A, et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langford DJ, Bailey AL, Chanda ML, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 24.Vos BP, Hans G, Adriaensen H. Behavioral assessment of facial pain in rats: face grooming patterns after painful and non-painful sensory disturbances in the territory of the rat’s infraorbital nerve. Pain. 1998;76:173–178. doi: 10.1016/s0304-3959(98)00039-6. [DOI] [PubMed] [Google Scholar]
- 25.Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 151:12–17. doi: 10.1016/j.pain.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Mogil JS, Graham AC, Ritchie J, et al. Hypolocomotion, asymmetrically directed behaviors (licking, lifting, flinching, and shaking) and dynamic weight bearing (gait) changes are not measures of neuropathic pain in mice. Mol Pain. 6:34. doi: 10.1186/1744-8069-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen JA, Price EO. Grooming in the Norway rat: Displacement activity or “boundary-shift”? Behavioral and Neural Biology. 1979;26:177. doi: 10.1016/s0163-1047(79)92563-9. [DOI] [PubMed] [Google Scholar]
- 28.Luccarini P, Childeric A, Gaydier AM, Voisin D, Dallel R. The orofacial formalin test in the mouse: a behavioral model for studying physiology and modulation of trigeminal nociception. J Pain. 2006;7:908–914. doi: 10.1016/j.jpain.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Bennett GJ. An animal model of neuropathic pain: a review. Muscle Nerve. 1993;16:1040–1048. doi: 10.1002/mus.880161007. [DOI] [PubMed] [Google Scholar]
- 30.Gould HJ., 3rd Complete Freund’s adjuvant-induced hyperalgesia: a human perception. Pain. 2000;85:301–303. doi: 10.1016/s0304-3959(99)00289-4. [DOI] [PubMed] [Google Scholar]
- 31.Zarrindast MR, Heidari-Darvishani A, Rezayof A, Fathi-Azarbaijani F, Jafari-Sabet M, Hajizadeh-Moghaddam A. Morphine-induced sensitization in mice: changes in locomotor activity by prior scheduled exposure to GABAA receptor agents. Behav Pharmacol. 2007;18:303–310. doi: 10.1097/FBP.0b013e3282186baa. [DOI] [PubMed] [Google Scholar]
- 32.Ottoni EB. EthoLog 2.2: a tool for the transcription and timing of behavior observation sessions. Behav Res Methods Instrum Comput. 2000;32:446–449. doi: 10.3758/bf03200814. [DOI] [PubMed] [Google Scholar]
- 33.Svendsen OLK. Neuronal c-fos immunoreactivity as a quantitative measure of stress or pain. Acta Agriculturae Scandinavica, A. 2001;51:131–134. 134. [Google Scholar]
- 34.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. London: Academic Press; 2003. [Google Scholar]
- 35.Poulos AM, Ponnusamy R, Dong HW, Fanselow MS. Compensation in the neural circuitry of fear conditioning awakens learning circuits in the bed nuclei of the stria terminalis. Proc Natl Acad Sci U S A. 107:14881–14886. doi: 10.1073/pnas.1005754107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Field A. Discovering Statistics Using SPSS. 2. London: Sage Publishing; 2005. [Google Scholar]
- 37.FitzGerald F-C. Clinical neuroanatomy and related neuroscience. 4. London: W.B. Saunders; 2002. [Google Scholar]
- 38.Dubner R, Bennett GJ. Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci. 1983;6:381–418. doi: 10.1146/annurev.ne.06.030183.002121. [DOI] [PubMed] [Google Scholar]
- 39.Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 40.Neubert J, King C, Malphurs W, et al. Characterization of mouse orofacial pain and the effects of lesioning TRPV1-expressing neurons on operant behavior. Molecular Pain. 2008;4:43. doi: 10.1186/1744-8069-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolan JC, Lam DK, Achdjian SH, Schmidt BL. The dolognawmeter: A novel instrument and assay to quantify nociception in rodent models of orofacial pain. Journal of Neuroscience Methods. 2010;187:207. doi: 10.1016/j.jneumeth.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prisca H. Behavioral assessment of neuropathic pain in preclinical models. Drug Development Research. 2006;67:302–307. [Google Scholar]
- 43.Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26:1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals? Pain. 2004;112:12–15. doi: 10.1016/j.pain.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 45.Goadsby PJ. Pathophysiology of migraine. Neurol Clin. 2009;27:335–360. doi: 10.1016/j.ncl.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005;257:139–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 47.Spruijt BM, van Hooff JA, Gispen WH. Ethology and neurobiology of grooming behavior. Physiol Rev. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- 48.Griswold JG, Borchelt PL, Brancher RS, Bensko JA. Condition of the pelage regulates sandbathing and grooming behaviour in the kangaroo rat (Dipodomys merriami) Animal Behaviour. 1977;25(Part 3):602. doi: 10.1016/s0003-3472(76)80042-5. [DOI] [PubMed] [Google Scholar]
- 49.Morrison RR, Ludvigson HW. Discrimination by rats of conspecific odors of reward and nonreward. Science. 1970;167:904–905. doi: 10.1126/science.167.3919.904. [DOI] [PubMed] [Google Scholar]
- 50.Gispen WH, Isaacson RL. ACTH-induced excessive grooming in the rat. Pharmacol Ther. 1981;12:209–246. doi: 10.1016/0163-7258(81)90081-4. [DOI] [PubMed] [Google Scholar]
- 51.Berridge KC, Fentress JC. Deafferentation does not disrupt natural rules of action syntax. Behav Brain Res. 1987;23:69–76. doi: 10.1016/0166-4328(87)90243-9. [DOI] [PubMed] [Google Scholar]
- 52.Koepp J, Lindsey CJ, Motta EM, Rae GA. Role of the paratrigeminal nucleus in nocifensive responses of rats to chemical, thermal and mechanical stimuli applied to the hind paw. Pain. 2006;122:235–244. doi: 10.1016/j.pain.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 53.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 54.Wall PD. The gate control theory of pain mechanisms. Brain. 1978;101:1–18. doi: 10.1093/brain/101.1.1. [DOI] [PubMed] [Google Scholar]
- 55.Nordin M. Low-threshold mechanoreceptive and nociceptive units with unmyelinated (C) fibres in the human supraorbital nerve. J Physiol. 1990;426:229–240. doi: 10.1113/jphysiol.1990.sp018135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olausson H, Wessberg J, Morrison I, McGlone F, Vallbo A. The neurophysiology of unmyelinated tactile afferents. Neurosci Biobehav Rev. 34:185–191. doi: 10.1016/j.neubiorev.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 57.Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci. 2003;23:8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kramer HH, Lundblad L, Birklein F, et al. Activation of the cortical pain network by soft tactile stimulation after injection of sumatriptan. Pain. 2007;133:72–78. doi: 10.1016/j.pain.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 59.McGlone F, Reilly D. The cutaneous sensory system. Neuroscience & Biobehavioral Reviews. 34:148. doi: 10.1016/j.neubiorev.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 60.Yosipovitch G, Carstens E, McGlone F. Chronic itch and chronic pain: Analogous mechanisms. Pain. 2007;131:4–7. doi: 10.1016/j.pain.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 61.Schmelz M. Itch and pain. Neurosci Biobehav Rev. 34:171–176. doi: 10.1016/j.neubiorev.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 62.Katcher J, Walsh D. Opioid-induced itching: morphine sulfate and hydromorphone hydrochloride. J Pain Symptom Manage. 1999;17:70–72. doi: 10.1016/s0885-3924(98)00115-8. [DOI] [PubMed] [Google Scholar]
- 63.Atanassoff PG, Brull SJ, Zhang J, Greenquist K, Silverman DG, Lamotte RH. Enhancement of experimental pruritus and mechanically evoked dysesthesiae with local anesthesia. Somatosens Mot Res. 1999;16:291–298. doi: 10.1080/08990229970357. [DOI] [PubMed] [Google Scholar]
- 64.Andrew DSM, Ballantyne JC. Itch-mechanisms and mediators. Seattle: IASP Press; 2003. [Google Scholar]
- 65.Ro JY, Harriott A, Crouse U, Capra NF. Innocuous jaw movements increase c-fos expression in trigeminal sensory nuclei produced by masseter muscle inflammation. Pain. 2003;104:539–548. doi: 10.1016/S0304-3959(03)00093-9. [DOI] [PubMed] [Google Scholar]
- 66.Shimizu K, Guo W, Wang H, et al. Differential involvement of trigeminal transition zone and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia: the effects of interleukin-10 and glial inhibitors. Molecular Pain. 2009;5:75. doi: 10.1186/1744-8069-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsai CM, Chiang CY, Yu XM, Sessle BJ. Involvement of trigeminal subnucleus caudalis (medullary dorsal horn) in craniofacial nociceptive reflex activity. Pain. 1999;81:115–128. doi: 10.1016/s0304-3959(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 68.Ro JY, Svensson P, Capra N. Effects of experimental muscle pain on electromyographic activity of masticatory muscles in the rat. Muscle Nerve. 2002;25:576–584. doi: 10.1002/mus.10072. [DOI] [PubMed] [Google Scholar]
- 69.Pelissier T, Pajot J, Dallel R. The orofacial capsaicin test in rats: effects of different capsaicin concentrations and morphine. Pain. 2002;96:81–87. doi: 10.1016/s0304-3959(01)00432-8. [DOI] [PubMed] [Google Scholar]
- 70.Wilson SG, Mogil JS. Measuring pain in the (knockout) mouse: big challenges in a small mammal. Behavioural Brain Research. 2001;125:65. doi: 10.1016/s0166-4328(01)00281-9. [DOI] [PubMed] [Google Scholar]
- 71.Watson C, Paxinos G, Kayalioglu G. The Spinal Cord. London: Academic Press; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.