Abstract
Background
Sleep problems are a frequent distressing symptom in cancer patients, yet little is known about their treatment. Sleep problems and depression frequently co-occur, leading healthcare professionals to treat depression with the expectation that sleep problems will also improve. The purpose of this study was to compare the effect of paroxetine to placebo on sleep problems via a secondary data analysis of a RCT designed to compare the effects of paroxetine to placebo on fatigue in cancer patients undergoing chemotherapy. A previously published report found a significant effect of paroxetine on depression in this cohort.
Methods
A total of 426 patients were randomized following Cycle 2 of chemotherapy to receive either 20 mg of paroxetine or placebo. Sleep problems were assessed using questions from the Hamilton Depression Inventory three times during chemotherapy.
Results
A total of 217 patients received paroxetine and 209 received placebo. Significantly fewer patients taking paroxetine reported sleep problems compared to patients on placebo (Paroxetine 79% versus Placebo 88%; p < 0.05). These differences remained significant even after controlling for baseline sleep problems and depression (p < 0.05).
Conclusion
Paroxetine had a significant benefit on sleep problems in both depressed and non-depressed cancer patients. However, rates of sleep problems remained high even among those effectively treated for depression with paroxetine. There is a need to develop and deliver sleep-specific interventions to effectively treat sleep-related side effects of cancer treatments. These findings suggest that sleep problems and depression are prevalent and co-morbid. Cancer progression, its response to treatment, and overall patient survival are intricately linked to host factors, such as inflammatory response and circadian rhythms, including sleep/wake cycles. Sleep problems and depression are modifiable host factors that can influence inflammation and impact cancer progression and quality of life. Future research should focus on discovering the pathogenesis of sleep dysregulation and depression in cancer so that better treatment approaches can be developed to ameliorate these symptoms.
1. Introduction
Cancer diagnosis and treatment are stressful; many cancer patients experience sleep problems, sadness, and tiredness. Some patients will develop clinically significant symptoms of sleep problems, depression, and fatigue [1–3]. Sleep problems are reported by at least 30–50 percent of newly diagnosed cancer patients [4]. Sleep problems and pain are the most frequent distressing symptoms during adjuvant chemotherapy in breast cancer patients [5]. Research suggests that sleep problems may be precipitated by cancer diagnosis or tumor biology, worsen during treatment, and continue even after treatment is completed [6]. Very little data are available on sleep problems and their treatment in cancer patients receiving chemotherapy. In the general population, sleep problems are associated with significant impairments in quality of life [7], almost a two-fold increase in accidents [8], increased days spent in bed, and decreased activity [9]. Moreover, some data show that sleep problems and sleep loss are associated with dysregulated immune function [10–15].
Emerging evidence also suggests that sleep problems negatively impact health, quality of life, and psychiatric functioning in cancer patients [4,16,17]. Sleep problems rarely occur in isolation and are frequently co-morbid with other psychiatric and psychological symptoms, especially depression. In fact, many studies report a significant positive correlation between sleep disturbance and depression (ranging from 0.30 to 0.57 depending on cancer diagnosis and treatment), suggesting that sleep problems and depression share approximately 25% of common variance [18–21]. Depression symptoms are also very common among cancer patients. Nearly 25% of cancer patients meet criteria for major depression, with an additional 30% reporting symptoms [22]. Several epidemiological and experimental studies suggest that severe and chronic depression in cancer patients might influence disease progression [23–25].
While sleep disturbance and depression are clearly linked, it is unclear whether depression precedes or follows sleep disturbance. Evidence supports both hypotheses [26,27]. Roth emphasizes that both depression and sleep problems are associated with dysregulation of the hypothalamic–pituitary–adrenal axis (HPA) and increased production of cortisol [7]. Serotonin (5-hydroxytryptamine, or 5-HT), a monoamine neurotransmitter that plays an important role in modulating mood, sleep–wake regulation, metabolism, and body temperature, is low in both depressed patients and patients with sleep problems. In a comprehensive review of the impact of antidepressants on sleep, Mayers and Baldwin [28] reported that, in laboratory studies, patients and clinicians frequently report a positive impact of selective serotonin reuptake inhibitors (SSRIs) on sleep despite a disruption of REM sleep, especially in patients without the C variant in the 3111 T/C polymorphism in CLOCK gene [29]. However, it is important to recognize that sleep problems are a common side effect of SSRIs. To the best of our knowledge, no study has examined the effect of antidepressants on sleep problems in cancer patients. The outcomes of the main study showed no impact of paroxetine on fatigue [30].
Given these results and in order to achieve parsimony in statistical analyses, the present study focused on the examination of depression and sleep problems. We hypothesized that an SSRI effective in the treatment of depression might also be effective for the management of sleep disturbance, particularly in those with more severe depression symptoms. The main aim of this study was to evaluate the impact of a commonly prescribed SSRI (paroxetine) on sleep problems in cancer patients receiving chemotherapy. We hypothesized that paroxetine would reduce sleep problems compared to placebo, particularly in those who were depressed, and that a difference would be seen between the two study groups in sleep problems. Further, the group of depressed patients randomized to paroxetine would have fewer sleep problems than those in the placebo group, indicating that paroxetine improved sleep. To test these hypotheses, we used data from a multicenter, double-blind randomized clinical trial (RCT) examining the effects of paroxetine versus placebo on fatigue in cancer patients undergoing chemotherapy [30].
2. Patients and methods
This is a post-hoc analysis of prospectively collected data for an RCT from the University of Rochester Cancer Center Community Clinical Oncology Program [30]. The primary outcome results of the RCT on fatigue and a full description of all participant (N = 704) characteristics have been previously published [30,31]. The enrollment of patients occurred between 6/23/1997 and 4/ 19/1999. Eligible participants were at least 18 years old, were scheduled for cyclical cytotoxic chemotherapy without concurrent radiation therapy or interferon treatment, were able to swallow medication, and had a score of >1 on question 1 of the Multidimensional Assessment of Fatigue (MAF). Question 1 of the MAF asked, “To what degree have you experienced fatigue?” with responses ranging from 1 “not at all” to 10 “a great deal.” Exclusion criteria included concomitant use of psychotropic medications, monoamine oxidase inhibitors or tryptophan, history of mania or seizures, and prior hospitalization for any psychiatric condition. Moreover, to be eligible for the current study, participants had to have completed the Hamilton Depression Index inventory at four cycles of chemotherapy and needed to have been randomized to one of two groups to receive either paroxetine or placebo.
2.1. Patients
Patients with any diagnosis and stage of cancer who were beginning chemotherapy were recruited from 18 oncology private practice groups that were part of the National Cancer Institute’s (NCI) Community Clinical Oncology Program (CCOP) for an RCT designed to examine the impact of paroxetine on fatigue in patients receiving chemotherapy. The Institutional Review Boards of the University of Rochester and of each participating site approved the protocol in accordance with an assurance filed with and approved by the Department of Health and Human Services. All participants provided written informed consent.
2.2. Design and procedures
Eligible patients were randomized to either 20 mg of paroxetine daily or an identical-looking placebo (Fig. 1). Patients were randomized centrally at the University of Rochester site using a computer-generated random numbers table. A 60 day supply of paroxetine or placebo was sent to each eligible patient via mail with directions on how and when to take the medication and when to complete the study assessments. To minimize variability, the patients who were included in the study were scheduled for at least four chemotherapy cycles separated by at least three weeks. The data were always collected seven days post chemotherapy cycle. Chemotherapy cycle is the day of the infusion. The infusions (cycles) are separated by several weeks to give patients a chance to recover. At study baseline, patients have already had one cycle of chemotherapy. Patients were randomized to receive paroxetine or placebo at Cycle 2 of chemotherapy. Five to seven days post chemotherapy Cycle 2, patients completed questionnaires and started taking study medication/placebo no later than 10 days post chemotherapy Cycle 2. Patients were instructed to stop taking study medication after day 7 post chemotherapy Cycle 4.
Fig. 1.
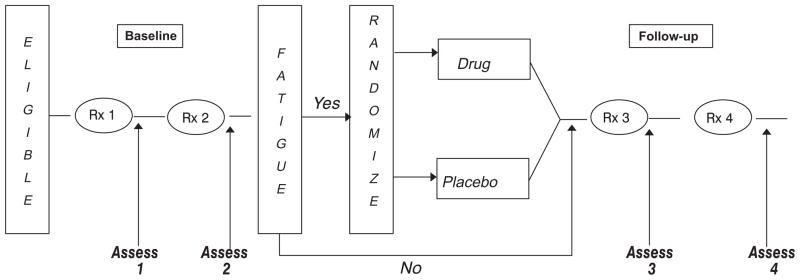
Trial design and randomization schema.
A follow-up phone call by the study nurse at each site was conducted to answer patients’ questions. Patients also received reminder phone calls. Completed assessments were returned by mail at the completion of Cycle 3 and Cycle 4 of chemotherapy. The study personnel who collected assessments were blind to group assignment. The study did not assess compliance to treatment, and an “intent-to-treat” analysis was employed: patients were analyzed according to their study arm at randomization regardless of whether they took the medication or not. Since the original study by Morrow et al. [30] found no effect of paroxetine on fatigue, we decided not to analyze the correlations between fatigue, depression, and sleep in the present study.
2.3. Assessments
2.3.1. Demographic questionnaire
The Demographic questionnaire was administered to all participants. This measure included questions about the participants’ age, gender, race/ethnicity, level of education, and relationship status, as well as questions about treatment and diagnosis.
2.3.2. Sleep problems
The Hamilton Depression Inventory (HDI) [32] consists of 38 questions designed to evaluate 23 symptom domains. The HDI is a paper and pencil version of the structured clinical interview Hamilton Depression Rating Scale (HDRS) and measures severity of symptoms in the last two weeks. Responses to sleep-related questions that asked about the type of sleep difficulty were used as a primary outcome of the study.
There were three questions that assessed sleep problems frequency: (4a) “Over the past two weeks, how often did you have trouble falling asleep at night?”; (5a) “How often did you have trouble with waking up in the middle of the night during the past two weeks? (Do not count waking up to go the bathroom or waking due to external causes such as a baby crying, phone calls, illness, etc.)”; (6a) “In the past two weeks, how often did you wake up earlier than usual and couldn’t fall back to sleep again?” The response items included “none of the time,” “1–2 nights a week,” “3–5 nights a week,” and “6–7 nights a week.”
In addition, three questions assessed average duration of sleep problems with responses ranging from “less than ½ an hour, ½ an hour to one hour; and over one hour”. The frequency items were as following: (4b) “On those nights that you had trouble falling asleep, on average, how much longer than usual did it take you to fall asleep?; (5b) “On average, how long did it take you to fall back to sleep again on those nights you woke up during the night?” (6b) “On average how much earlier than usual did you wake up?”
2.3.3. Depression
The Center for Epidemiological Studies Depression Scale (CESD) [33] was used. The CES-D consists of 20 items, assesses symptoms in the previous week, and has an anchor scale that ranges from “1” = “rarely or none of the time” to “4” = “most of the time”. To assess the differential effectiveness of paroxetine on sleep disturbance, a dichotomous depression variable was created using a cut-off score of 19 or greater to assign patients to a “depressed” group. Those who had a score of <19 were assigned to a “not depressed” group. Turk and Okifuji [34] reported that a cut-off of 19 on the CES-D had a Sensitivity of 0.82, Specificity of 0.62, Positive Predictive Value of 0.68, and Negative Predictive Value of 0.78 in medically ill patients in diagnosing depression.
2.4. Analyses
2.4.1. Data reduction
For the purposes of this study we classified patients into one of two categories according to their HDI scores: Group with Sleep Problems and Good Sleepers.
2.4.1.1. Group with sleep problems
Patients who reported difficulty falling asleep, difficulty staying asleep, or early morning awakenings at least “1–2 nights a week” on the HDI were coded as having sleep problems.
In addition, for severity analyses, we broke the group with sleep problems into 2 categories: severe and mild sleep problems. Patients who reported difficulty falling asleep, difficulty staying asleep (waking up in the middle of the night), and/or early morning awakenings for at least three days a week for two weeks, with each episode lasting at least 30 min, were coded as having severe sleep problems. Those who endorsed some sleep problems were coded as having mild sleep problems.
2.4.1.2. Good sleepers group
Patients were categorized as having no sleep problems if they answered “none of the time” to all questions on the HDI.
2.5. Statistical analyses
Descriptive statistics were employed for demographic and clinical characteristics at baseline for subjects enrolled in the study and were calculated for the two treatment groups. We used means and standard deviations for continuous data and frequencies and percentages for categorical or dichotomous data. For the normally distributed continuous data, t-tests were conducted to compare means. For dichotomous and categorical data, chi-square analyses were conducted. Any significant imbalances among the groups were reported using student t-tests or chi-squares when appropriate.
The effect of paroxetine on sleep problems at treatment Cycle 4 was assessed with the Cochran–Mantel–Haenszel (CMH) [35] procedure for adjusting the two-by-two contingency table analysis (Sleep problems versus Treatment Arm) by baseline sleep problems and depression strata. We also evaluated the effect of the drug on unadjusted sleep problem risk and on risk adjusted by baseline sleep problems. Estimates of the common relative risk (drug versus placebo) across baseline sleep problems and depression strata were calculated with the logit method. The Breslow–Day test [36] was used to assess whether the drug effect (expressed as the odds ratio) was consistent across strata. All p values reported are two-sided; p < 0.05 is considered statistically significant. All computations were conducted using SPSS Version 16.0 and SAS Version 9.2.
3. Results
A total of 902 patients at the 18 CCOP study sites met initial eligibility criteria. The full details of the sample have been reported by Morrow et al. [30]; demographic and medical variables are reported in Table 1. In brief, a total of 704 patients (78%) completed questionnaires at Cycle 2 of chemotherapy, which was considered the baseline for this study. The remaining patients (n = 198) were excluded from the study because of one of the following reasons: they were no longer medically eligible, or did not complete questionnaires (n = 155), or refused to be randomly assigned to the treatment arm (n = 43). Out of 704 eligible patients, 549 (78%) reported fatigue and were randomized to receive either paroxetine (n = 277) or placebo (n = 272). Of these, 426 (61%, 217 in the paroxetine group and 209 in the placebo group) provided complete data on sleep items at Cycle 3, Cycle 4, or both. Patients included in this study were significantly younger (t = 3.24, p < 0.001) and more likely to be female (X2 = 5.51, p = 0.02) than the patients excluded from the study due to missing data, medical eligibility, or refusal to participate in the study. There were no differences in race and marital status. Table 1 shows distributions of demographics by treatment arm.
Table 1.
Demographic and medical characteristics by treatment arm (N = 426).
| Paroxetine N = 217 | Placebo N = 209 | |
|---|---|---|
| Age | ||
| Mean (SD) | 56.5 (12.6) | 56.3 (12.3) |
| Range | 27–87 | 23–84 |
| Sex | ||
| Male | 45 (21%) | 57 (27%) |
| Female | 172 (79%) | 152 (73%) |
| Race | ||
| White | 197 (91%) | 184 (88%) |
| Black | 12 (6%) | 16 (8%) |
| Other | 8 (3%) | 9 (4%) |
| Education | ||
| Some College | 121 (56%) | 112 (54%) |
| High School or Less | 96 (44%) | 97 (46%) |
| Marital Status | ||
| Married | 153 (71%) | 150 (72%) |
| Not Married | 64 (29%) | 59 (28%) |
| Diagnosis | ||
| Breast | 128 (59%) | 108 (52%) |
| Lung | 30 (14%) | 22 (11%) |
| Hematologic | 24 (11%) | 33 (16%) |
| Gynecologic | 15 (7%) | 18 (9%) |
| Gastrointestinal | 8 (4%) | 19 (9%) |
| Other | 12 (6%) | 9 (4%) |
| Treatment Indication | ||
| Adjuvant or Neo-adjuvant | 153 (71%) | 145 (69%) |
| Metastatic, recurrent, other | 64 (29%) | 64 (31%) |
| Previous surgery | 150 (69%) | 146 (69%) |
| Previous chemotherapy | 24 (12%) | 24 (14%) |
| Previous radiotherapy | 20 (9%) | 14 (7%) |
| Depression: Mean CES-D (SD) | 14.7 (10.6) | 15.4 (9.8) |
| Fatigue: Mean Question 1 MAF (SD) | 5.6 (2.2) | 5.6 (2.1) |
3.1. Description of sleep problems
A total of 311 (73.2%) patients reported difficulty falling asleep (44% of the sample had difficulty falling asleep three or more nights per week), 238 (56%) reported waking in the middle of the night (31.6% of the sample reported that they were waking in the middle of the night three or more nights per week), and 277 (65%) reported waking earlier than intended in the morning (37% of the total sample reported that they had awakened earlier than intended three or more nights per week). Table 2 shows the distribution of symptoms by group and by chemotherapy cycle. There were no significant differences between the paroxetine and placebo groups at baseline in proportion of sleep problems (Paroxetine arm: 81% with sleep problems; Placebo arm: 81.8% with sleep problems; X2(1) = 0.10, p = 0.77). t-Tests comparisons at baseline failed to find significant differences between levels of fatigue on MAF question 1 (paroxetine mean 5.60 versus placebo 5.64 p = 0.88) and depression (paroxetine 14.67 versus 15.39, p = 0.46). Nearly 30% (n = 127) met criteria for Major Depression using a CES-D cutoff score of 19 [34]. Sleep problems and depression were moderately positively correlated (r = 0.41, p < 0.001) at baseline.
Table 2.
Sleep problems at Cycle 2 (Baseline), Cycle 3 and Cycle 4, by Study Group Assignment (Paroxetine versus Placebo) (N = 426).
| Cycle 2 N (%) | Cycle 3 N (%) | Cycle 4 N (%) | |
|---|---|---|---|
| Paroxetine (N = 217) | |||
| Sleep problems | 175 (80.6%) | 177 (81.6%) | 172 (79.3%) |
| Good sleepers | 42 (19.4%) | 40 (18.4%) | 45 (20.7%) |
| Placebo (N = 209) | |||
| Sleep problems | 171 (81.8%) | 175 (83.7%) | 184 (88%) |
| Good sleepers | 38 (18.2%) | 34 (16.3%) | 25 (12%) |
3.1.1. Impact of paroxetine on sleep
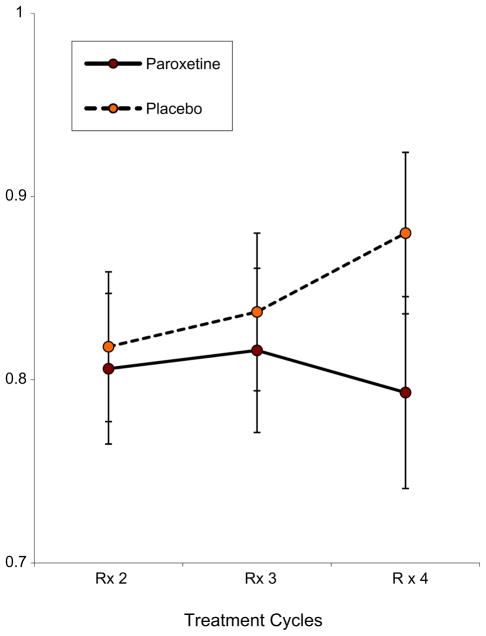
At the end of the study (seven days post chemotherapy Cycle 4), there were significant differences between the paroxetine and placebo groups in the presence of sleep problems (X2(1) = 5.97, p = 0.01, Cohen d = 0.23) (Fig. 2). There was no early effect of paroxetine at Cycle 3 (X2(1) = 0.52, p = 0.46). The differences between arms remained at Cycle 4 after controlling for baseline sleep problems levels (X2 = 6.05, p = 0.01; Cohen d = 0.24) and after controlling for baseline sleep problems and depression levels (X2 = 6.49, p = 0.01; Cohen d = 0.25) (Table 3). Again, both baseline sleep problems and depression were strongly predictive of sleep problems at Cycle 4 (Table 4). The relative risk of experiencing sleep problems at Cycle 4 for patients with sleep problems at baseline was 1.48 (p < 0.001), suggesting that patients who presented with sleep problems at baseline were 48% more likely to experience sleep problems than those who did not. The effect of baseline depression on sleep problems was smaller, but still significant, all p < 0.001. In fact, the risk of sleep problems for patients who met criteria for depression at baseline was 20% higher than for those who were not depressed, independent of whether or not they had sleep problems (both those with and without disturbed sleep were included in the analysis).
Fig. 2.
Effects of paroxetine on sleep problems incidence as measured by the Hamilton Depression Index (HDI). Error bars show confidence intervals. N = 426 (N = 217 on paroxetine and N = 209 on placebo).
Table 3.
Relative Risks of experiencing sleep problems at Cycle 3 and Cycle 4 of chemotherapy according to the treatment arm (Paroxetine versus Placebo): unadjusted, corrected for baseline sleep problems and adjusted for both baseline sleep problems and depression.
| Time | Proportion with sleep problems
|
Unadjusted Relative Risk (95% CI), p-value | Relative Risk adjusted for baseline sleep problems (95% CI), p-value | Relative Risk adjusted for baseline sleep problems and depression (95% CI), p-value | |
|---|---|---|---|---|---|
| Placebo | Drug | ||||
| Cycle 3 | 0.84 N:176/209 | 0.82 N:177/217 | 0.97 (0.88–1.1), p = 0.47 | 0.97 (0.90–1.06), p = 0.51 | 0.97 (0.90–1.05), p = 0.47 |
| Cycle 4 | 0.88 N:184/209 | 0.79 N:172/217 | 0.90 (0.83–0.98), p = 0.02 | 0.90 (0.83–0.98), p = 0.01 | 0.90 (0.83–0.98), p = 0.01 |
Table 4.
Relative Risks of experiencing sleep problems at Cycle 3 and Cycle 4 of chemotherapy according to the presence or not at baseline of sleep problems or depression. All p values are less than 0.0001.
| Time | Proportion with sleep problems
|
Relative Risk (Sleep prob./No sleep prob.) (95% CI) | Proportion with depression
|
Relative Risk (dep./no dep.) (95% CI) | ||
|---|---|---|---|---|---|---|
| No baseline sleep problems | Baseline sleep problems | No baseline depression | Baseline depression | |||
| Cycle 3 | 0.56 N:45/80 | 0.89 N:308/346 | 1.58 (1.30–1.93) | 0.78 N:232/299 | 0.95 N:121/127 | 1.23 (1.14–1.32) |
| Cycle 4 | 0.60 N:48/80 | 0.89 N:308/346 | 1.48 (1.24–1.78) | 0.79 N:236/299 | 0.94 N:120/127 | 1.20 (1.11–1.29) |
3.1.2. Impact on sleep severity
According to the severity criteria described above, at study baseline (chemotherapy Cycle 2), the paroxetine arm had the following breakdown of sleep problems: slightly more than half (57.7%) had severe sleep problems and 42.3% had mild sleep problems. Rates for severity were similar in the placebo arm (severe sleep problems 54.4%; mild sleep problems 45.6%). At the end of the study, the severity of sleep problems changed in the following way: In the paroxetine arm, 44% of subjects had severe sleep problems, 42.3% had mild sleep problems, and 13.7% had none, and in the placebo arm 44.4% of subjects had severe problems, 47.4% had mild sleep problems, and 8.2% had none.
4. Discussion
This is the first study to evaluate the impact of a commonly used antidepressant on sleep problems in cancer patients. We found that paroxetine produced a statistically significant improvement in sleep problems. The medication had a delayed effect on sleep problems similar to the delayed (2–3 weeks) onset of antidepressant benefit seen in treatment of depression with all available antidepressants [37]. This suggests that the improvement in sleep may be mediated by improvement in depression.
Dunbar et al. (1993) [38] showed that, in depressed patients without cancer, paroxetine improves sleep. They evaluated the efficacy of paroxetine in 336 patients with major depression. Patients receiving paroxetine reported improvements in sleep at the end of week one as measured by the Hamilton Rating Scale of Depression Sleep items. These significant improvements in sleep were maintained throughout six weeks of treatment. In that study, the dosage of paroxetine was originally 20 mg but was adjusted throughout treatment as clinically necessary (in our study paroxetine dosage was kept constant throughout the treatment), suggesting that a difference in findings might be due to the difference in methodology and population.
Unexpectedly, we found no significant interactions between initial levels of sleep problems and depression and the subsequent effects of paroxetine on sleep problems at the completion of study treatment, suggesting that paroxetine worked equally well for improving sleep problems among both depressed and non-depressed cancer patients. Perhaps, in cancer patients, sleep problems stem from biopsychosocial mechanisms (other than depression) related to the presence and treatment of cancer (e.g., prolonged time in bed, disease rumination while trying to sleep, side effects of treatment such as nausea and pain, circadian disruption). Interestingly, we found that, for non-depressed individuals, sleep problems at the end of the study (Cycle 4) were best predicted by sleep problems levels at baseline, not level of depression. However, for those who were depressed, depression at baseline was a predictor for developing sleep problems. This suggests that among cancer patients, as is the case for other disorders (e.g., pain conditions), depression is a risk factor for the development of sleep problems.
There are several limitations associated with conducting secondary data analyses. This study was not specifically designed to test the impact of paroxetine on sleep problems. Although participants were not randomized based on their sleep problems, we found no significant differences between groups in sleep problems at baseline. Another important limitation of this study has to do with eligibility criteria: all the patients in the study were experiencing fatigue symptoms; whether similar results would hold with non-fatigued patients is not known. Patients who experience fatigue might be psychologically and physiologically different from patients who have no cancer-related fatigue. However, some degree of fatigue is an extremely frequent subjective complaint of cancer patients. Indeed, its incidence has been reported to be as high as 95% in patients on anticancer treatment [39].
In addition, SSRIs might not be the best antidepressant class for sleep complaints in cancer patients. SSRIs are known to stimulate serotonin type 2 (5-HT2) receptors that are associated with sleep disturbance. There is a class of antidepressants that are serotonin antagonists (e.g., nefazodone, mirtazapine) that sometimes improve sleep; these medications might produce a larger effect in improving patients’ sleep.
This study has many important strengths, including the use of randomized design and of placebo control in a very large diverse population of cancer patients. This research works towards disentangling a complicated relationship between depression and sleep problems and the management of both symptoms. In the general population, sleep problems frequently predict development of depression, but sometimes depression precipitates dysregulated sleep. This study shows that, while paroxetine is effective for both depression and sleep problems, the response rate of sleep problems to paroxetine is rather small, with an absolute reduction in incidence of 8.7% for sleep problems (Table 2). Indeed, the vast majority of patients treated with paroxetine still reported sleep problems at the end of the study (Table 2). Additionally, we observed that sleep problems improved in patients with and without depression symptoms, suggesting that perhaps depression is not the main explanation for sleep problems in cancer patients.
Given the limited improvements obtained with paroxetine in sleep and recent data showing that paroxetine might in fact affect disease progression in women taking tamoxifen, through modification of its metabolism [40–42], we propose that tailored sleep-specific treatments be used for patients with sleep problems. In fact, RCTs in healthy people with sleep problems show that providing treatments for depression (antidepressant or cognitive behavioral treatment [CBT]) and sleep (hypnotics or CBT for insomnia [CBTI]) creates the best outcome [43–45]. Recent trials in cancer patients support the efficacy of CBT-I for sleep problems in cancer patients [46,47]. Treating depression in hopes of improving sleep is never the best approach for symptom management, since these are co-morbid complaints and both should be treated.
Although more RCTs specifically designed to treat sleep problems are needed, our findings suggest that reduction in sleep problems might be greater with sleep specific interventions (e.g., hypnotics, CBT-I) rather than with antidepressants.
The results of this study improve our understanding of sleep problems and depression management in cancer patients who are undergoing chemotherapy. We found that sleep problems are prevalent and persistent in cancer patients and partially respond to an antidepressant. Given highly prevalent levels of sleep disturbance in this population, targeted interventions are needed to address the problem early in order to prevent acute problems in sleep from becoming chronic and debilitating in cancer patients.
Acknowledgments
We want to thank Claire Conley for her help in submission of the manuscript.
Footnotes
Conflict of interest
The ICMJE Uniform Disclosure Form for Potential Conflict of Interest associated with this article can be viewed by clicking on the following link: doi:10.1016/j.sleep.2012.06.001.
References
- 1.Parker KP, Bliwise DL, Ribeiro M, et al. Sleep/wake patterns of individuals with advanced cancer measured by ambulatory polysomnography. J Clin Oncol. 2008;26:2464–72. doi: 10.1200/JCO.2007.12.2135. [DOI] [PubMed] [Google Scholar]
- 2.Esther Kim JE, Dodd MJ, Aouizerat BE, Jahan T, Miaskowski C. A review of the prevalence and impact of multiple symptoms in oncology patients. J Pain Symptom Manage. 2009;37:715–36. doi: 10.1016/j.jpainsymman.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14:201–9. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savard J, Morin CM. Insomnia in the context of cancer: A review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 5.Byar KL, Berger AM, Bakken SL, Cetak MA. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol Nurs Forum. 2006;33:E18–26. doi: 10.1188/06.ONF.E18-E26. [DOI] [PubMed] [Google Scholar]
- 6.Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–77. doi: 10.1200/JCO.2007.14.3248. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3:S7–10. [PMC free article] [PubMed] [Google Scholar]
- 8.Balter MB, Uhlenhuth EH. New epidemiologic findings about insomnia and its treatment. J Clin Psychiatry. 1992;53(Suppl):34–9. [PubMed] [Google Scholar]
- 9.Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154:1417–23. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 10.Redwine L, Hauger RL, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85:3597–603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- 11.Vgontzas AN, Zoumakis M, Bixler EO, et al. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab. 2003;88:2087–95. doi: 10.1210/jc.2002-021176. [DOI] [PubMed] [Google Scholar]
- 12.Vgontzas AN, Vhrousos GP. Sleep, the hypothalamic–pituitary–adrenal axis, and cytokines: Multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31:15–36. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 13.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 14.Cover H, Irwin M. Immunity and depression: insomnia, retardation, and reduction of natural killer cell activity. J Behav Med. 1994;17:217–23. doi: 10.1007/BF01858106. [DOI] [PubMed] [Google Scholar]
- 15.Savard J, Miller SM, Mills M, et al. Association between subjective sleep quality and depression on immunocompetence in low-income women at risk for cervical cancer. Psychosom Med. 1999;61:496–507. doi: 10.1097/00006842-199907000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28:292–8. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24:583–90. doi: 10.1093/sleep/24.5.583. [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Fiorentino L, Natarajan L, et al. Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psychooncology. 2009;18:187–94. doi: 10.1002/pon.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrykowski MA, Curran SL, Lightner R. Off-treatment fatigue in breast cancer survivors: a controlled comparison. J Behav Med. 1998;21:1–18. doi: 10.1023/a:1018700303959. [DOI] [PubMed] [Google Scholar]
- 20.Stepanski EJ, Walker MS, Schwartzberg LS, Blakely LJ, Ong JC, Houts AC. The relation of trouble sleeping, depressed mood, pain, and fatigue in patients with cancer. J Clin Sleep Med. 2009;5(2):132–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Redeker NS, Lev EL, Ruggiero J. Insomnia, fatigue, anxiety, depression, and quality of life of cancer patients undergoing chemotherapy. Sch Inq Nurs Pract. 2000;14:275–90. [PubMed] [Google Scholar]
- 22.Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry. 2003;54:269–82. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 23.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–75. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 24.Spiegel D, Sephton S. Re: Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2002;94:530–3. doi: 10.1093/jnci/94.7.530. [DOI] [PubMed] [Google Scholar]
- 25.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–8. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4:104–13. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- 27.Van Moffaert MM. Sleep disorders and depression: the ‘chicken and egg’ situation. J Psychosom Res. 1994;38(Suppl 1):9–13. doi: 10.1016/0022-3999(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 28.Mayers AG, Baldwin DS. Antidepressants and their effect on sleep. Hum Psychopharmacol. 2005;20:533–59. doi: 10.1002/hup.726. [DOI] [PubMed] [Google Scholar]
- 29.Serretti A, Cusin C, Benedetti F, et al. Insomnia improvement during antidepressant treatment and CLOCK gene polymorphism. Am J Med Genet B Neuropsychiatr Genet. 2005;137B:36–9. doi: 10.1002/ajmg.b.30130. [DOI] [PubMed] [Google Scholar]
- 30.Morrow GR, Hickok JT, Roscoe JA, et al. Differential effects of paroxetine on fatigue and depression: A randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol. 2003;21:4635–41. doi: 10.1200/JCO.2003.04.070. [DOI] [PubMed] [Google Scholar]
- 31.Roscoe JA, Morrow GR, Hickok JT, et al. Effect of paroxetine hydrochloride (Paxil(R)) on fatigue and depression in breast cancer patients receiving chemotherapy. Breast Cancer Res Treat. 2005;89:243–9. doi: 10.1007/s10549-004-2175-1. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 34.Turk DC, Okifuji A. Detecting depression in chronic pain patients. Adequacy of self-reports. Behav Res Ther. 1994;32(1):9–16. doi: 10.1016/0005-7967(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 35.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 36.Breslow NE, Day NE. Statistical methods in cancer research. Volume I – The analysis of case-control studies. IARC Sci Publ. 1980:5–338. [PubMed]
- 37.Blier P, De MC. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15:220–6. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 38.Dunbar GC, Claghorn JL, Kiev A, Rickels K, Smith WT. A comparison of paroxetine and placebo in depressed outpatients. Acta Psychiatr Scand. 1993;87:302–5. doi: 10.1111/j.1600-0447.1993.tb03376.x. [DOI] [PubMed] [Google Scholar]
- 39.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: The scale of the problem. Oncologist. 2007;12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 40.Kelly CM, Juurlink DN, Gomes T, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. BMJ. 2010;340:c693. doi: 10.1136/bmj.c693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–64. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 42.Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97:30–9. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 43.Morin CM. Combined therapeutics for insomnia: should our first approach be behavioral or pharmacological? Sleep Med. 2006;7(Suppl 1):S15–9. doi: 10.1016/j.sleep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine review. Sleep. 1999;22:1134–56. doi: 10.1093/sleep/22.8.1134. [DOI] [PubMed] [Google Scholar]
- 45.Morin CM, Vallieres A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301:2005–15. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berger AM, Kuhn BR, Farr LA, et al. One-year outcomes of a behavioral therapy intervention trial on sleep quality and cancer-related fatigue. J Clin Oncol. 2009;27:6033–40. doi: 10.1200/JCO.2008.20.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: Sleep and psychological effects. J Clin Oncol. 2005;23(25):6083–96. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]