Abstract
Cancer gene therapy approaches have benefited greatly from the utilization of molecular-based therapeutics. Of these, adenovirus-based interventions hold much promise as a platform for targeted therapeutic delivery to tumors. However, a barrier to this progression is the lack of native adenovirus receptor expression on a variety of cancer types. As such, any adenovirus-based cancer therapy must take into consideration retargeting the vector to nonnative cellular surface receptors. Predicated upon the knowledge gained in native adenovirus biology, several strategies to transductionally retarget adenovirus have emerged. Herein, we describe the biological hurdles as well as strategies utilized in adenovirus transductional targeting, covering the progress of both adapter-based and genetic manipulation-based targeting. Additionally, we discuss recent translation of these targeting strategies into a clinical setting.
1. INTRODUCTION
The development of rationally designed cancer interventions has followed the progress of the molecular understanding of cancer development and progression. To this end, gene therapy has endeavored multiple strategies for molecular targeted therapeutics. Of these strategies, adenovirus (Ad)-based vectors have been used prevalently, especially in the field of cancer. Ad vectors entail many characteristics that make it an ideal choice compared to other vectors. Biologically, Ad is able to efficiently transduce a variety of both dividing and quiescent cell types in vitro and in vivo. Additionally, the amenability to genetic modification, large genetic payload capacity, and the ability to produce high titers of good manufacturing practice quality are all factors that favor the use of Ad-based vectors as cancer therapeutics. Of importance, Ad-based vectors have shown an impressive safety record in the preclinical and clinical setting. However, despite the safety profile and preclinical efficacy, these vectors have failed to achieve therapeutic efficacy in the clinical setting. Thus, efforts have been refocused on basic vector design, especially on maximizing gene delivery by specifically transducing the target cell population. Achievements in Ad transductional targeting technology have steadily progressed and show great promise as a therapeutic for cancer treatment.
2. AD BIOLOGY
The most common Ad used in gene therapy, human Ad serotype 5, is a member of the Adenoviridae family. This family comprises 51 Ad serotypes originally classified by their ability to be neutralized by animal antisera. These serotypes are then divided into six species based upon hemagglutination properties, oncogenicity, and genomic structure (Davison, Benko, & Harrach, 2003). In addition, there are some correlations between species and tissue target and clinical presentation. Of importance to current Ad-based gene therapy vectors are species B, C, and D. These Ad species comprise the serotypes most commonly used in gene therapy.
3. AD CAPSID STRUCTURE
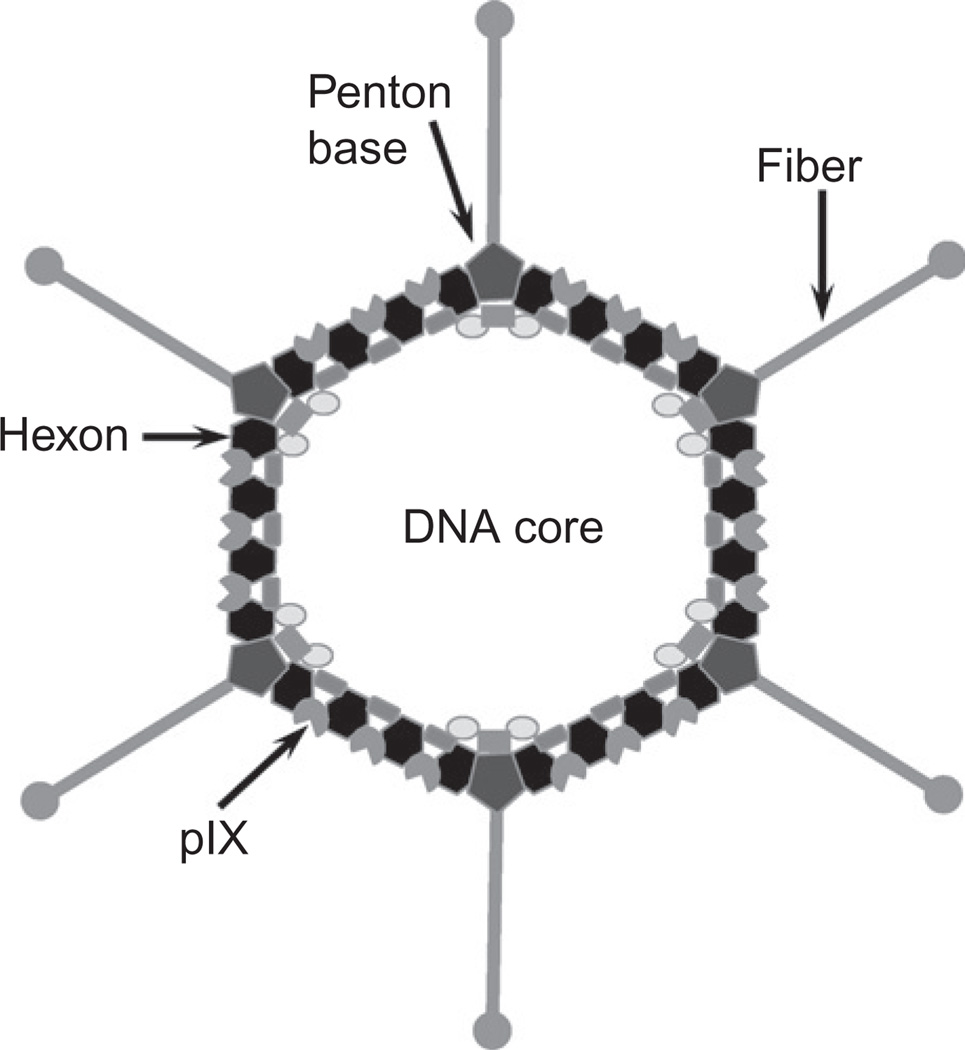
The human Ad is a 36-kb double-stranded DNA virus encapsulated by an icosahedral protein particle (Fig. 2.1). Progress in X-ray crystallography and cryo-electron microscopy has furthered our understanding of the structural components of the Ad capsid (Fabry et al., 2005; Reddy, Natchiar, Stewart, & Nemerow, 2010; Saban, Silvestry, Nemerow, & Stewart, 2006). Three major proteins comprise most of the Ad capsid. Hexon is a homotrimeric protein found in 720 copies and comprises most of the Ad capsid, playing a structural role as the main coating protein. The Ad hexon is highly conserved among human serotypes; however, hexon contains nine surface exposed hypervariable regions (HVR1–HVR9) which contain serotype variation (Burnett, 1985). Penton forms a homopentamer which makes up the penton base. This pentameric structure is located at each of the 12 vertices and plays a structural role, interacting with each of the Ad capsid 12 fibers. In addition, penton contains an Arg-Gly-Asp (RGD) motif which is responsible for virion internalization. Fiber is found as a homotrimer at each vertex and noncovalently binds to its corresponding penton base via its N-terminus. Fiber is composed of a shaft domain and a globular knob domain found at the distal tip, which plays a role as the primary cellular attachment site (Zubieta, Schoehn, Chroboczek, & Cusack, 2005). In addition to the major capsid proteins, the Ad capsid comprises of an assortment of minor proteins: IIIa, VI, VIII, and IX. Of note, polypeptide IX (pIX), which has gained favor as an alternative locus for targeting incorporation (discussed later), is a structural protein that is believed to help stabilize the Ad capsid (Vellinga, Van der Heijdt, & Hoeben, 2005). Four monomers of pIX interact to form a four-helix bundle with a surface exposed C-terminus (Marsh et al., 2006; Saban et al., 2006).
Figure 2.1.
Adenovirus capsid structure: The adenovirus capsid structure is composed of both major capsid proteins and minor proteins. Labeled here are capsid proteins hexon, penton base, fiber, and polypeptide IX (pIX). These capsid structures have all been utilized as locales for retargeting Ad strategies.
4. AD ENTRY BIOLOGY
Ad entry biology comprises two distinct steps: attachment of the virus to its primary receptor subsequently followed by molecular interactions that lead to internalization of the virus. Initially, high-affinity interactions occur when the fiber knob domain binds to its cognate primary receptor. In general, for serotypes from species A, C, E, and F, this receptor is the coxsackie and Ad receptor (CAR), while serotypes from species B and D tend to utilize alternative receptors. CAR is a 46-kDa protein that is a member of the immunoglobulin superfamily and is involved in the formation of tight junctions (Coyne & Bergelson, 2006; Philipson & Pettersson, 2004). Once the Ad virion has attached, cellular integrins including αvβ3, αvβ5 (Wickham, Mathias, Cheresh, & Nemerow, 1993), αvβ1 (Li et al., 2001), α3β1, and α5β1 (Davison, Diaz, Hart, Santis, & Marshall, 1997) interact with RGD motifs in the penton base. This interaction induces cellular responses that lead to cytoskeleton alterations which aid in internalization (Li, Stupack, Bokoch, & Nemerow, 1998; Li, Stupack, Klemke, Cheresh, & Nemerow, 1998). Ultimately, virus internalization occurs via clathrin-coated vesicles, and the Ad virion is transported to the endosome (Meier et al., 2002). Upon endosomal acidification, the Ad virion disassembles and is released into the cytoplasm where it ultimately travels to the infected cells nucleus for viral replication.
5. TRANSDUCTIONAL TARGETING OF AD VECTORS
Knowledge gained from studies concerning native Ad entry biology has predicated understanding of findings that non-CAR-expressing cancer cells are refractory to Ad infection and gene delivery. Thus, if target cancer cells exhibit low levels of CAR, we are left with a scenario where by high-CAR-expressing nontarget cells are effectively transduced, while low-CAR cancer cells show poor transduction. Higher expression of CAR also appears to be growth inhibitory in some cancers (Okegawa et al., 2001). In summary, targeting CAR appears to be strategically incompatible with Ad-based cancer therapeutics.
Biodistribution of Ad, although effected by CAR distribution, is not solely determined by expression profiles in vivo (Fechner et al., 1999). Systemic administration of Ad, intravenously, results in the majority of transduction occurring in the liver followed by the spleen, heart, lung, and kidneys of mice. This profile, however, does not correlate with the highest levels of CAR expression (Wood et al., 1999). This is especially true in regard to liver transduction which absorbs the vast majority of systemic Ad vector via hepatic kupffer cell uptake (Tao et al., 2001) and hepatocyte transduction, potentially resulting in liver toxicity. Due to the toxicity issues regarding liver transduction, this biological interaction in vivo has been given great scrutiny.
Early strategies to retarget Ad to non-CAR pathways were initially thought to also detarget the liver, as the initial hypothesis was that liver transduction was CAR and integrin dependent. However, studies that ablated CAR and integrin binding in the Ad capsid had little effect on biodistribution profiles (Alemany & Curiel, 2001; Smith et al., 2002). Thus, Ad liver tropism was shown to be linked to a novel pathway. Following initial studies implicating motifs in the fiber shaft (Breidenbach et al., 2004; Smith et al., 2003; Vigne et al., 2003), Shayakhmetov, Gaggar, Ni, Li, and Lieber (2005) reported a major role for fiber interactions with blood coagulation factors and complement component C4 binding protein in hepatocyte and kupffer cell transduction. Modification of the Ad5 fiber to ablate this interaction resulted in a 50-fold decrease in liver transduction along with reduced levels of liver toxicity. Analysis determined that this in vivo tropism was due to Ad associating with hepatocellular heparin sulfate proteoglycan and low-density lipoprotein receptor-related protein (Shayakhmetov et al., 2005).
More recent studies, however, have shown that fiber structure and motifs do not play a role in liver sequestration and have elucidated hexon interactions with blood coagulation factors as the major pathway directing hepatocyte transduction by systemic delivery of Ad. Kalyuzhniy et al. (2008) and Waddington et al. (2008) defined the specific interaction between blood coagulation factor X (FX) and hexon. In addition, utilizing structural studies, FX was shown to interact with hypervariable regions 3, 5, and 7 of hexon. Both groups showed that this interaction could be inhibited by mutated forms of hexon or by pharmacological methods involving warfarin or snake venom protein X-bp (Kalyuzhniy et al., 2008; Waddington et al., 2008). Additionally, Waddington et al. elucidated that different serotypes of Ad interact with FX with different affinities, some to the point of not binding at all. Of note, those Ad serotypes that did not bind FX were all from species D. Following this, candidate viruses from low (Ad35) and nonbinding (Ad26 and Ad48) groups were examined in vivo by intravenous injection with or without X-bp protein. These viruses showed a lack of liver transduction (Waddington et al., 2008).
From this work, several groups have reported success with genetic manipulations of hexon to ablate liver sequestration. These strategies fall into two different categories. From the original studies, hexon mutations blocking the FX/hexon interaction have been utilized and shown to drastically reduce liver sequestration and transduction. This strategy was further developed by Alba et al., identifying the exact amino acids in hypervariable regions 5 and 7 responsible for FX binding. Altering either of these points resulted in a drastic decrease in FX binding and FX-mediated gene transduction (Alba et al., 2009). These FX-ablated Ad vectors also possessed altered biodistribution, with decreased liver transduction and greater vector accumulation in the spleen, especially following macrophage depletion (Alba et al., 2010). Additionally, since different serotypes have different affinities for FX, some labs have reported that either whole hexon swaps or hyper-variable region swaps with lesser binding serotypes have also been successful in preventing liver uptake. Short et al. (2010) utilized the swapping of Ad5 hexon with that of Ad3 hexon. This modification was shown to block FX binding to Ad virions by surface plasmon resonance (SPR) analysis and prevent FX-mediated gene transduction in vitro. Interestingly, ablation of FX binding in an oncolytic Ad vector provided increased tumor killing and prolonged viral replication in a skov3.ip1 subcutaneous flank tumor model (Short et al., 2010).
In summary, primary biology determined by in vitro experimentation as well as host interactions outside of this primary pathway plays a role in the overall in vivo biodistribution of Ad. It is clear that alternative targets for cell transduction must be explored as a wide variety of target cells including cancer are not amenable to CAR-targeted Ad vectors. Two distinct approaches have been utilized to transductionally target Ad-based vectors: (1) adapter based and (2) genetically capsid modification. While these strategies show great efficacy in retargeting Ad-based therapeutics, it is also clear that liver detargeting must also be considered in any Ad vector as these external biological forces will effect overall target cell transduction in vivo. Thus, any Ad-based therapeutic must entail both detargeting strategies and retargeting strategies in order to reach its full therapeutic efficacy.
6. AD TRANSDUCTIONAL TARGETING: ADAPTER-BASED STRATEGIES
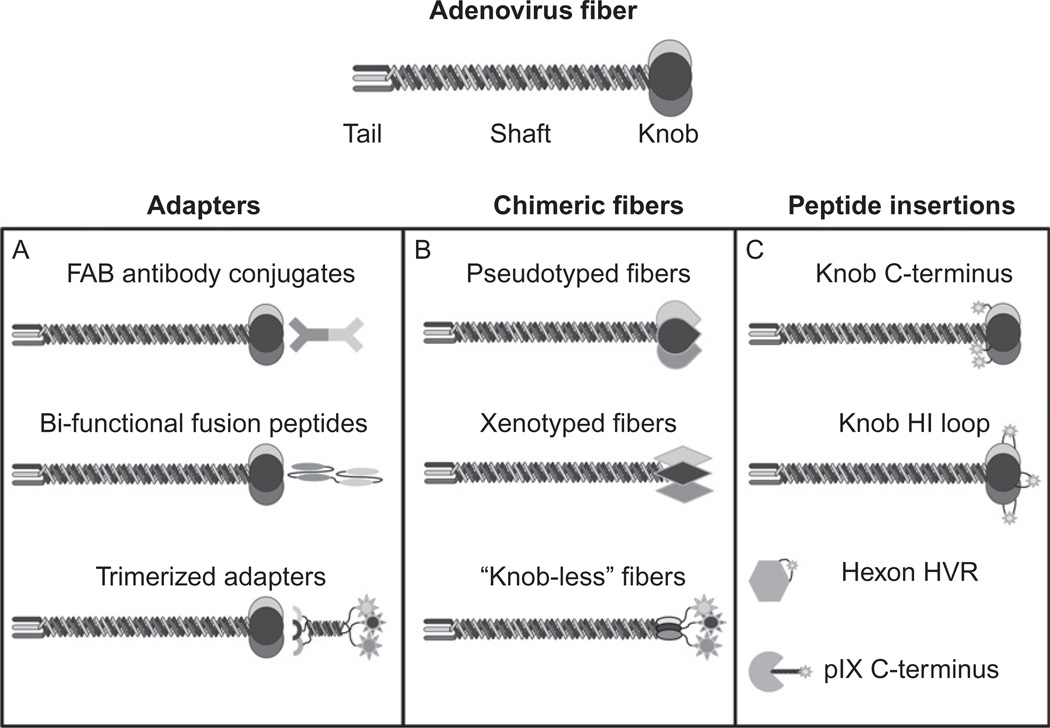
The adapter-based strategy (Fig. 2.2A) involves the use of a molecular bridge to retarget the Ad from its native primary receptor to a different cell surface receptor. This function is performed due to the bispecific nature of adapter molecules. One end of the adapter binds specifically to the Ad capsid, while the alternative end redirects Ad away from its native CAR binding and interacts with the alternative cellular receptor. Of note, this process does not impede with Ad infection as entry biology is determined by a two-step process, with binding being a separate mechanism from internalization. Conceptually, the most elegant location for conjugating an adapter molecule to the Ad virion is the fiber knob domain. This interaction allows for the retargeting of Ad to alternative receptors while also detargeting Ad from its primary receptor CAR. As such, within this retargeting strategy, the majority of adapters interact with the Ad knob domain. However, alternative capsid sites have been used as adapter interaction locales and have shown to be effective in retargeting Ad.
Figure 2.2.
Transductional retargeting modifications: Adenoviral vectors can be retargeted to specific cell surface receptors using a variety of strategies. (A) Heterologous retargeting ligands (adapters) composed of a variety of ligands including FAB antibodies, single-chain antibodies, and biological ligands. (B) Chimeric Ads composed of fiber/knob domains from alternative serotypes and “knob-less” fiber modifications. (C) Genetic incorporation of peptides into fiber, hexon, and pIX.
7. FAB ANTIBODY ADAPTERS
The first in vitro manifestations of the adapter strategy were accomplished by conjugating an anti-knob FAB antibody to a retargeting ligand. This strategy was first shown by chemically conjugating an anti-knob FAB antibody to folate. Conjugated to Ad virions, this adapter showed CAR-independent targeting to cancer cells overexpressing the folate receptor (Douglas et al., 1996). FAB antibody adapters against fibroblast growth factor 2 (FGF2) receptor have also been widely used and shown to retarget Ad-based vectors to a variety of cancers including pancreatic cancer (Huch et al., 2006), melanoma (Gu et al., 1999), Kaposi’s sarcoma (Goldman et al., 1997), ovarian cancer (Printz et al., 2000), and head and neck cancer (Araki et al., 2010; Figures et al., 2009). In all, a wide variety of alternative cancer-relevant targeting ligands have been conjugated to anti-knob FAB antibodies and used to retarget Ad vectors to cellular receptors such as CD40 (Tillman et al., 1999), epidermal growth factor (EGF) receptor (Miller et al., 1998), epithelial cell adhesion molecule (EPCAM) (Haisma et al., 1999; Kraaij, van Rijswijk, Oomen, Haisma, & Bangma, 2005), prostate-specific membrane antigen (PSMA) (Kraaij et al., 2005), and Tag-72 (Kelly et al., 2000), resulting in similar transductional gains over untargeted Ad. Full utility of this strategy was progressed when Reynolds et al. (2000) employed an anti-knob FAB antibody chemically conjugated to monoclonal antibody (9B9) against angiotensin-converting enzyme (ACE). This cellular marker is found in the pulmonary epithelium and upregulated in various pulmonary diseases. Of note, this study showed a 20-fold increase in lung gene expression while also reducing liver expression by 83% when compared to an untargeted Ad vector. More recently, an anti-knob FAB antibody fused to FGF2 showed increased transduction efficiency in a mouse model of head and neck carcinoma. The targeted Ad, expressing a mutant Rad50 protein, also demonstrated an increase in tumor suppression compared to untargeted Ad when combined with cisplatinum, resulting in greater DNA double-strand breaks and reduced angiogenesis (Araki et al., 2010).
8. RECOMBINANT FUSION ADAPTERS
Studies have shown FAB antibody-based adapters to be effective in providing a proof-of-principle strategy for retargeting Ad vectors. However, the production of these molecules has been hampered by the randomness of chemical conjugation, producing heterogeneous populations of molecules. As such, strategies that utilize single-component molecules could be advantageous. Thus, more recent efforts have focused on the development of single-component fusion proteins that can be easily expressed and whose population is genetically homogenous. Building on the use of anti-knob antibodies, Haisma et al. (2000) developed a recombinant fusion protein composed of a single-chain antibody (scFv) against Ad fiber knob domain fused to an scFv against EGF receptor. This adapter could be easily purified without loss of function and increased Ad gene transfer compared to untargeted Ad in EGF receptor expressing cell lines (Haisma et al., 2000). Later, Haisma et al. showed selective targeting to a variety of angiogenesis-related markers including αvβ3 integrins, vascular endothelial growth factor receptor 2, and the angiopoietin receptor TIE-2. These adapters retargeted Ad to both mouse and human endothelial cell lines. In addition, they showed in vivo tumor-specific targeting to a subcutaneous C26 colon carcinoma (Haisma et al., 2010). These scFv diabodies have also shown efficacy in a variety of studies retargeting Ad to cellular targets such as EGF receptor (Carette et al., 2007; Grill et al., 2001), EPCAM (Heideman et al., 2002), human epidermal growth factor receptor 2 (HER2/neu), carcinoembryonic antigen (CEA), endoglin (CD105) (Nettelbeck et al., 2001), and high molecular weight melanoma antigen (Nettelbeck et al., 2004).
In addition to scFv diabody-based adapters, a novel adapter utilizing a truncated, soluble portion of CAR (sCAR) fused to a targeting molecule was developed. Utilizing the first sCAR-based adapters, Dmitriev, Kashentseva, Rogers, Krasnykh, and Curiel (2000) created sCAR adapters fused to either an anti-CD40 antibody or EGF. With this, they demonstrated in several EGF-overexpressing cancer cell lines a ninefold increase in gene expression when compared to nontargeted Ad vectors. In addition to Ad-targeting specificity, Dmitriev et al. questioned the stability of these adapters when complexed with Advirions. They showed through comparing Ad/sCAR-EGF and Ad/sCAR-EGF purified through gel filtration that there was no difference in targeting profile, confirming the stability of these complexes. Similar to scFv diabody-based adapters, studies have shown this strategy to be efficacious in retargeting Ad vectors utilizing a wide variety of targeting ligands against EGF receptor (Harvey et al., 2010), urokinase-type plasminogen activator receptor (Harvey et al., 2010), CEA (Everts et al., 2005; Li, Everts, Yamamoto, Curiel, & Herschman, 2009), HER2/neu (Barker et al., 2003), CD40 (Hakkarainen et al., 2003), and high-affinity Fcgamma receptor I (CD64) (Ebbinghaus et al., 2001).
Kashentseva, Seki, Curiel, and Dmitriev (2002) furthered this strategy by incorporating a novel trimerization domain. This trimeric adapter contained sCAR fused to a HER2/neu-specific scFv and displayed increased affinity for the Ad fiber knob. In addition, the adapter augmented gene transduction 17-fold in HER2/neu positive breast and ovarian cancer cell lines. Kim et al. (2002) additionally reported that adapter trimerization provided drastic increase (100-fold) in gene transduction over its identical monomeric adapter. These studies have proven the targeting efficacy and stability of adapter virus complexes and have paved the way for these complexes to be utilized in a clinical setting, where complex stability could affect targeting efficacy and safety.
9. ALTERNATIVE ADAPTER BINDING LOCALES
In addition to bispecific adapters that utilize the Ad fiber knob domain for presentation, penton base and hexon have also been explored as sites for adapter-based retargeting. Li, Brown, Von Seggern, Brown, and Nemerow (2000) constructed a bispecific adapter molecule composed of an antipenton monoclonal antibody fused to tumor necrosis factor α, insulin-like growth factor 1, and EGF. These adapters when complexed with Ad provided increased gene transduction in M21-L12 melanoma cells. The use of hexon as a retargeting local is predicated on newly identified in vivo biology regarding Ad interaction with blood coagulation factors. As previously described, upon entering circulation, Ad hexon interacts with FX. Chen, May, and Barry (2010) utilized this interaction to create a novel retargeting ligand by fusing the GLA domain of FX to an scFv against HER2/neu. When conjugated to Ad, this novel adapter promoted increased transduction of HER2/neu positive cells versus cells lacking HER2/neu expression. They additionally showed that this strategy could be used to target other cell surface receptors such as EGF receptor and ATP-binding cassette protein G2. Since this technology utilizes the same binding interaction that leads to liver transduction, gains in retargeting Ad to an alternative receptor also yield gains in detargeting the liver.
10. ADAPTERS UTILIZING GENETICALLY MODIFIED AD CAPSID
Alternative to bispecific adapters, approaches that genetically modify the Ad virion to bind retargeting ligands have been developed. The benefit of this strategy is that a variety of off the shelf, commercially available ligands can be used without any additional modification. This strategy is best portrayed by incorporation of the immunoglobulin (Ig)-binding domain of Staphylococcus aureus protein A into the fiber HI loop or C-terminus. This fiber-modified Ad vector is thus able to bind a wide variety of targeting moieties that contain the Fc region of Ig. Most recently, Takahashi et al. (2011) utilized this technology to screen antibody libraries against the prostate cancer cell line LNCaP. Identifying an antibody against neural adhesion molecule 2, they showed that this antibody when conjugated to Ad increases gene transduction in prostate and breast cancer cell lines. A variety of other targets including CD40 (Korokhov et al., 2005), mesothelin (Breidenbach et al., 2005), EGF2 receptor, HER2/neu, CA242 antigen, and PSMA (Henning et al., 2005) have been explored. Utilizing the same concept, a biotin acceptor peptide (BAP) has been inserted into fiber (Parrott et al., 2003; Pereboeva, Komarova, Roth, Ponnazhagan, & Curiel, 2007). During virus production, BAP is biotinylated by the endogenous biotin ligase in 239 cells. Once purified, this virus can be conjugated to biotin-labeled ligand. pIX has also been explored as a site for BAP insertion. Campos et al. fused BAP to the terminus of pIX and compared targeting to BAP inserted into fiber. Of interest, using an anti-CD71 (transferin receptor) antibody as ligand, BAP fused to pIX failed to retarget Ad vectors unlike BAP inserted into fiber. However, when pIX-BAP Ad was retargeting with biotinylated transferrin, transduction was successful (Campos, Parrott, & Barry, 2004).
11. AD SECRETION OF ADAPTER
Although the adapter strategy for retargeting Ad vectors allows for efficient and specific retargeting, it is reliant upon a two-part system. The Ad vector and the adapter molecule are produced separately and are conjugated before being introduced to their cellular target. However, genetic incorporation of targeting ligands is biologically incompatible with a variety of ligands utilized in adapter-based strategies, such as scFvs. This is due to the fact that most scFv molecules must be processed through the endoplasmic reticulum (ER) in order to fold and function correctly, while Ad assembly takes place in the cytosol. Bridging this gap between cytosolically unstable targeting ligands and single-component Ad vector retargeting has been reports of Ad vectors that secrete their own adapter. With this, single-component Ad vectors can utilize targeting ligands which are not biologically compatible with viral assembly. In addition, while adapter-conjugated Ad vectors provide efficient targeting, this strategy only allows for a single round of targeting. Any viral progeny will regress to their native cellular receptor CAR. As a result, Ads that secrete their own adapter have targeting advantages over traditional adapter strategies in regard to replicating Ad vectors.
A novel method for achieving this was explored by Glasgow et al. This strategy utilizes a unique leucine zipper-based binding motif derived from vitellogenin gene-binding protein to allow for adapter conjugation. One zipper domain was incorporated into a knob-less fiber, while its corresponding zipper domain was fused to the retargeting ligand and secreted. Utilizing an scFv against CD40, Glasgow et al. showed that these novel structures could be incorporated into the Ad capsid and corresponding secreted adapter. Most importantly, upon completion of the Ad-native life cycle and Ad release, crude viral lysate was shown to have CD40-specific targeting, thus showing that the virus could interact and bind with its cognate adapter in the intercellular space (Glasgow, Mikheeva, Krasnykh, & Curiel, 2009).
Overall, adapter-based Ad-targeting studies have provided vast evidence that native Ad tropism can be retargeted to alternative cellular receptors and increase gene transfer in non-CAR-expressing cells in vitro. Adapter-targeted vectors have also shown to be efficacious in in vivo studies, showing great stability and transductional efficacy. With further development of expression systems and rigorous analysis of the stability and kinetics of vector adapter complexes, adapter-based strategies could progress to clinical translation.
12. AD TRANSDUCTIONAL TARGETING: GENETICALLY INCORPORATED STRATEGIES
With greater understanding of Ad virion structure, genetic manipulation of capsid proteins has yielded great strides in Ad targeting. Conceptually, genetic manipulation of the Ad capsid to incorporate novel targeting ligands could yield a multitude of targeting strategies. However, any genetic manipulation must function within the structural and biological constraints of the native Ad capsid. Based on this, most genetic manipulation of the Ad capsid has focused on the fiber, as this domain is the primary determinant of native Ad tropism and can be modified without interfering with capsid assembly.
13. CHIMERIC AD
Although Ad5’s primary receptor is CAR and thus nonamenable to a variety of cancer therapeutic strategies, other serotypes of Ad do not use CAR as their primary binding receptor. As such, one genetic strategy for retargeting Ad, termed pseudotyping, developed as a mechanism to utilize non-CAR-targeting serotypes while not abandoning the vast knowledge of Ad5 biology (Fig. 2.2B). These pseudotyped virions have shown great transductional efficacy in a variety of Ad5 refractory cell types such as ovarian carcinoma (Rein et al., 2011; Rocconi et al., 2007), prostate cancer (Murakami, Ugai, Belousova, et al., 2010), breast cancer (Stoff-Khalili et al., 2007), colon carcinoma (Silver & Mei, 2011), glioblastoma (Hoffmann, Meyer, & Wildner, 2007), and others. With pseudotyped Ad vectors, the fiber knob domain or the entire fiber is genetically replaced with its structural counterpart from a different human serotype that recognizes an alternative cellular surface receptor. These alternative serotypes are primarily developed from species B and species D Ads. Species B viruses have been shown to interact with a variety of non-CAR receptors including CD46 (Gaggar, Shayakhmetov, & Lieber, 2003), CD80, and CD86 (Short et al., 2004). Additionally, subgroup D serotypes have been shown to interact with CD46 and the glycoprotein component α(2–3)-linked sialic acid (Arnberg, Edlund, Kidd, & Wadell, 2000). With the great progress developed in pseudotyped Ad vectors, the strategy was also expanded to include the insertion of fiber elements from nonhuman Ad serotypes. This strategy, termed xenotyping, has yielded a variety of non-CAR-targeted Ad vectors including vectors with fiber elements from avian, bovine, canine, murine, and porcine Ad vectors (Bangari & Mittal, 2005; Bangari, Shukla, & Mittal, 2005; Glasgow et al., 2004; Nakayama et al., 2006; Renaut, Colin, Leite, Benko, & D’Halluin, 2004). Of note, most of the receptor targets for these vectors are undetermined as of today. However, the fiber element from porcine Ad serotype 4 has recently been shown to interact with glycan chains containing repeats of N-acetyllactosamine through evidence that this interaction leads to cellular uptake is unknown (Guardado-Calvo et al., 2010). Bovine Ad serotype 4 fiber has also been elucidated recently. This nonhuman Ad was shown to interact with two different immunoevasion molecules of the B7 family of proteins, B7-1 and B7-H1, in murine leukemia cells. Of interest, this fiber requires both interactions in order to result in cellular uptake (Grellier et al., 2011). In addition to nonhuman Ads, structurally similar binding domains from other virus species have also been incorporated into the Ad fiber. This was first shown by the incorporation of the fiber-like σ1 reovirus attachment protein into the Ad fiber. This allowed for effective transduction of target cells expressing junctional adhesion molecule (Tsuruta et al., 2005).
14. PEPTIDE-TARGETED AD
Although Ad pseudotyping has shown great success, it is predicated by the discovery of novel non-CAR targeting Ads. As such, development of retargeting Ad vectors has progressed into rationally designed targeted Ads. Meticulous structural studies of the knob domain of fiber have yielded two separate locations within the knob that can be exploited for genetic peptide presentation without disrupting fiber function, the C-terminus, and a region termed the HI loop (Fig. 2.2C). Conceptually, the C-terminus is an ideal location for peptide insertion. Successful genetic insertions of an integrin-binding RGD motif or polylysine peptides have yielded positive in vitro and in vivo results (Wickham et al., 1997). However, other peptide insertions have shown no effect possibly due to steric hindrances. Structural studies using a genetically inserted FLAG tag into the HI loop, an exposed loop structure connecting β-sheets H and I in the Ad knob domain, showed proof-of-principle evidence that this location is structurally amenable to peptide insertion (Krasnykh et al., 1998). In fact, further studies have shown that this location can handle peptide insertions of up to 100 amino acids without detriment to fiber function (Belousova, Krendelchtchikova, Curiel, & Krasnykh, 2002). As such, Dmitriev et al. inserted an integrin-binding RGD motif into this location and showed that this virus, AdlucRGD, has enhanced transductional efficacy and gene delivery in ovarian cancer cell lines and primary tumors versus nontargeted Ad (Dmitriev et al., 1998; Hemminki et al., 2001). This tropism-expanded Ad has been utilized widely in the field and shown to be efficacious in gene delivery to a wide variety of cancers including ovarian (Murugesan, Akiyama, Einfeld, Wickham, & King, 2007), cervical (Rein et al., 2004), colon (Lavilla-Alonso et al., 2010), melanoma (Okada et al., 2005), and others.
Progressing beyond the tropism expansion seen in RGD and polylysine motif insertions, several groups have inserted cellular-specific targeting peptides into the HI loop. These peptides developed by traditional phage display biopanning or more novel strategies utilizing peptide incorporated Ad libraries (Bockmann, Drosten, & Putzer, 2005; Miura et al., 2007; Nishimoto et al., 2009) have been proven to be highly specific and generally amenable to Ad insertion. Nicklin et al. (2003) showed that the vascular endothelial cell-targeting peptide, SY-GYLPLP, provided increased transduction in a variety of cancer cell lines. In addition, peptides have been inserted to target a variety of cancers including head and neck (Li et al., 2008), medullary thyroid carcinoma (Schmidt et al., 2011), glioma (Piao et al., 2009), and renal cell carcinoma (Diaconu et al., 2009). In addition to classical target-specific short peptides, Myhre et al. (2009) inserted an Affibody, a small antibody mimetic, into the HI loop and showed HER2/neu and Taq polymerase-specific targeting. They also showed that HER2/neu-specific, Affibody-targeted oncolytic Ad provided increased transduction and killing in prostate cancer cells in vitro and increased survival time while decreasing serum prostate-specific antigen in an orthotopic mouse model of prostate cancer (Magnusson et al., 2011).
15. “KNOB-LESS”-TARGETED AD
While peptide insertion has shown to be a successful strategy in retargeting Ad, structural conflicts have emerged from fiber knob modifications. As such, a platform by which a wider variety of targeting ligands could be utilized would be a rational goal in further progressing Ad retargeting. The observation that Ads lacking various portions of their knob domain could be rescued leads to the concept of utilizing a knob-less fiber as a platform for ligand presentation (Fig. 2.2B). Limiting this concept, however, was the fact that the knob domain contained the trimerization domain for the fiber that is required for fiber function and insertion into the Ad capsid. Overcoming this structural problem, a foreign trimerization domain, the foldon domain of T4 fibritin, was fused to the native Ad fiber shaft to replace that which was lost by deletion of the knob domain (Papanikolopoulou, Forge, Goeltz, & Mitraki, 2004). Krasnykh, Belousova, Korokhov, Mikheeva, and Curiel (2001) replaced the fiber and knob with the bacteriophage T4 fibritin and showed that this platform could present a 6-histidine (6-His) motif inserted into the C-terminus. This vector showed a 100-fold increase in gene expression in cells expressing an artificial 6-His binding receptor. Variant “deknobing” strategies have also been explored by Magnusson, Hong, Boulanger, and Lindholm (2001), demonstrating that an RGD motif could target integrin-expressing cells. Further, labs have progressed toward larger peptide displays such as small peptides and Affibodies. Belousova, Mikheeva, Gelovani, and Krasnykh (2008) incorporated a HER2/neu-specific Affibody into a knob-less fiber, showing that the novel Affibody technology was compatible with knob-less fiber platforms. This HER2/neu-targeted vector also showed increased gene delivery in HER2/neu-expressing cancer cells (Belousova et al., 2008). The knob-less Ad platform provides the ability to move beyond small ligands and into the use of proteins as targeting ligands. Previously, this would be a very problematic strategy as large protein insertions are much more likely to interfere with native Ad assembly and function. Notably, trimeric CD40 has been fused to this fiber providing evidence that this platform can be amenable to large protein ligands. The CD40 incorporated Ad provided CD40-specific gene delivery in vivo following systemic delivery (Izumi et al., 2005).
16. ALTERNATIVE CAPSID LOCATIONS
Although fiber is the most developed capsid protein for retargeting Ad, the difficulty of incorporating ligands into the Ad capsid has furthered the development of alternative locales. Potentially, alternative sites could provide increased presentation of the targeting ligand through increased copy number per virion and could also allow for multiple targeting ligands to be utilized on the same capsid. To date, a variety of alternative sites have been proposed and explored including hexon, pIX, and pIIIa (Fig. 2.2C). The first two have been shown to be compatible with ligand presentation, while the latter was shown incompatible due to its current structural location within the capsid (San Martin et al., 2008).
Hexon is the most abundant protein in the Ad capsid and as such is an ideal candidate for ligand incorporation. The potential 720 copies of hexon could allow for a “coating” of the Ad capsid in any incorporated ligand. Although most of the hexon sequence is highly conserved among serotypes, nine hypervariable regions are found within the hexon and have solvent-exposed loops. As such, these loops lay in an ideal location for modification. Vigne et al. (1999) genetically modified hypervariable region 5 (HVR5) and inserted an integrin-binding RGD domain. This RGD motif had no effect on hexon structure or capsid stability but increased CAR-independent transduction of vascular smooth muscle cells. Further HVRs 2, 3, and 5–7 were found to be amenable to insertion of a 6-His motif (Wu et al., 2005). In addition, anti-6-His antibodies recognized Ad vectors with 6-His inserted into HVRs 2 and 5. The rescue of Ad vectors with peptides inserted in various hypervariable regions provides us with a potential platform for various downstream targeting applications.
More recently, pIX has developed as a practical platform for the presentation of targeting ligands. pIX is a small protein that plays the role of a cement protein, helping stabilize hexon interactions. Found in 240 copies within each virion, pIX provides drastically increased ligand presentation over fiber modifications. Structural studies and the observation that the C-terminus of pIX may be solvent exposed lead to several groups, exploring the concept of pIX presented ligands. The first reported targeting ligand incorporated into the terminus of pIX was presented by Dmitriev, Kashentseva, and Curiel (2002). By incorporating polylysine or FLAG motifs, they showed CAR-independent transduction via interactions with heparin sulfate chains on the target cell surface (Dmitriev et al., 2002). Furthering development of pIX as a targeting local, Vellinga et al. (2004) fused varying sized α-helical linkers to the terminus of pIX and used these linkers to present integrin-binding RGD motifs. Of note, longer linker length corresponded with increased gene delivery in CAR negative endothelial cells. Relatively large proteins fused to pIX have also been explored. Incorporation of hyperstable scFv against β-galactosidase fused to pIX showed that the scFv retained its binding affinity to β-galactosidase (Vellinga et al., 2007). However, this antibody has no targeting applications and the availability of hyperstable scFvs is limiting. Poulin et al. attempted to incorporate an scFv against a mutant form of the EGF receptor (EGFRvIII) fused to pIX, but the scFv failed to fold properly, resulting in a lack of targeting (Poulin et al., 2010). As a consequence, they attempted to route the pIX-scFv through the ER, but biological incompatibility between the cytoplasmically assembled Ad and the ER routed pIX resulted in low levels of incorporation and thus a lack of targeting. However, they were able to incorporate a single domain antibody (AFAI) against CD66c (CEA-related cell adhesion molecule family 6). This ligand provided CD66c-specific binding and transduction of A549 non-small-cell lung carcinoma cell line. de Vrij et al. (2008) showed that large single-chain T-cell receptors could also be attached to pIX. They fused pIX to a single-chain T-cell receptor against the CT antigen melanoma-associated antigen A1 (MAGE-A1). This vector specifically transduced melanoma cell lines. In addition, transduction was shown to correlate with the levels of MAGE-A1 peptide within the cells. However, lack of or downregulation of HLA-A1 molecules can drastically reduce the transduction efficiency of this vector. As a whole, studies have shown that pIX is a flexible platform for the display of both small and large targeting ligands.
17. NOVEL TRANSDUCTIONAL STRATEGIES
Although Ad-based therapeutics has progressed greatly, the vast majority of targeting strategies have relied upon a single cellular surface receptor for the target. This strategy has been shown to work remarkably well for homogenous cell line populations. However, some target cells such as cancer do not comprise a homogenous population. As such, targeting a single cellular surface receptor may lead to a selected population that is resistant to further therapy. Thus, any molecular-based therapy should take this into account when designing a targeting strategy. Several groups have begun developing Ad-targeting strategies that utilize multiple ligands within the same virion. One of the first vectors reported was an Ad5 containing both an RGD motif and a polylysine ligand (Wu et al., 2002). This vector could thus target both cell surface integrins and heparin sulfate proteoglycans. Following that, Borovjagin et al. (2005) inserted an integrin-binding RGD motif into the C-terminus of a chimeric Ad fiber composed of the shaft domain of Ad5 and the knob domain of Ad3. This vector showed 55-fold increase in gene transduction of bladder cancer cell lines. Utilizing adapter-based retargeting, Grill et al. (2001) combined an adapter composed of an scFv against EGF receptor with genetically incorporated RGD motif in the HI loop of fiber knob. This virus was shown to be able to target both EGF receptor and cell surface integrins on primary glioma cells and spheroids.
Although these strategies provide insight into the efficacy of dual targeting over single, they are limited in their ability to insert multiple ligands within the same fiber. Using multiple fibers within the same Ad virion would bypass this restriction and provides a platform for the utilization of multiple complex targeting ligands. Pereboeva, Komarova, Mahasreshti, and Curiel (2004) first showed that this strategy was feasible by generating an Ad vector incorporating both the wild-type fiber and a knob-less fiber fibritin presenting a 6-His motif. Utilizing this mosaic vector, they showed both CAR and artificial 6-His receptor-specific gene transduction, though in high-CAR-expressing cells no additional gain was seen from the 6-His containing fiber fibritin. The combination of an Ad5/3 chimeric fiber with a fiber containing the reovirus σ-1 protein in the same Ad virion has also shown to provide an increase in infectivity enhancement of ovarian cancer cell lines and primary ovarian cancer tissue slices (Tsuruta et al., 2007). Murakami, Ugai, Wang, et al. (2010) additionally provided evidence that with the correct genetic construct, equal expression, and incorporation of the two distinct fibers can be accomplished. This vector, containing both Ad5 and Ad3 fiber, provided both CAR- and CD46-specific gene transduction. Of interest, they showed that this vector could target two distinct cells, PC-3 cells expressing CD46 and Cho-CAR cells expressing CAR, in a mixed culture experiment.
18. AD TARGETING: RECENT CLINICAL DEVELOPMENTS
Although Ad-based therapeutics have shown great promise in preclinical studies, in the clinical setting, therapeutic efficacy of Ad vectors has not followed. Although ample evidence concludes that lack of CAR expression upon cancer cells drastically limits Ad-based therapeutic efficacy, the vast majority of clinical trials utilizing Ad rely upon native CAR-based transduction. One hurdle limiting the translation of Ad-based targeting strategies has been the additional complexity of adapter/Ad conjugates. Since these strategies are two-component systems, they entail additional production complexity and scrutiny in regard to safety before being approved for clinical use. As such, the few clinical trials to date utilizing targeting have relied upon genetically inserted targeting ligands.
Recently, clinical studies involving Ad-based therapies utilizing genetically incorporated integrin-binding RGD motifs in the fiber knob have been reported. Kimball et al. recently finished a phase I clinical trial examining the therapeutic efficacy and maximum tolerated dose of a tropism-modified, infectivity enhanced conditionally replicative adenovirus (CRAd), Ad5-Δ24-RGD, in patients with malignant gynecologic diseases (Kimball et al., 2010; Page et al., 2007). Following treatment, of the 21 patients, 71% had stable disease, while 29% still showed disease progression after 1 month of follow-up. Of note, seven patients did show a decrease in CA-121 levels, with four of these being a greater than 20% decrease. Although no patients showed regression of disease in this study, toxicity associated with therapy was limited to grade 1/2 fever, fatigue, and abdominal pain. A similarly sized trial reported by Nokisalmi et al. (2010) analyzed an integrin-targeted CRAd, ICOVIR-7, in patients with a variety of solid tumors. Similarly, this trial saw only mild to moderate treatment-related side effects. Of note, 9 of 17 evaluable patients showed evidence of antitumor activity, with 1 patient showing partial response and 2 patients with minor responses. This strategy has also shown similar safety and therapeutic outcomes when combined with expression of granulocyte-macrophage colony-stimulating factor, an immune-stimulatory molecule (Pesonen et al., 2011). Progression of targeted Ad-based cancer therapies in the clinical setting has lead to new studies utilizing chimeric Ad-based vectors replacing the Ad5 knob domain with that of the Ad3 knob (Ad5/3) (Kim et al., 2011). Pesonen et al. (2010) showed similar safety in a trial of 18 patients with varying solid tumors. Of these patients, 61% showed evidence of antitumor activity.
In addition to these CRAd-based trials, Matthews et al. (2009) reported plans for and are currently finishing another phase I clinical trial involving Ad5.SSTR/TK.RGD. This Ad-based therapeutic utilizes a genetically incorporated RGD ligand to target the expression of a therapeutic suicide gene, herpes simplex virus thymidine kinase (TK), and an imaging motif, somatostatin receptor type 2 (SSTR), for viral tracking via nuclear imaging.
In all, translation of targeted Ad-based therapeutics to the clinical setting has shown a solid safety record similar to their untargeted counterparts. Although some levels of antitumor activity and therapeutic response have been noted, the response rate is still far below that required for therapeutic usage. This emphasizes the need for further targeting trials and the utilization of additional strategies involving Ad virion/host interactions such as liver detargeting and immune evasion.
19. CONCLUDING REMARKS
Ad-based vectors are a widely used therapeutic platform for gene delivery. They are especially prominent in the field of cancer gene therapy where shorter gene expression times are not an issue. However, biological hurdles stand between native Ad-based vectors and their full utilization as a therapeutically effective cancer treatment platform. Of these hurdles, effective gene transduction of cancer cells drastically limits potential of Ad-based vectors. Early clinical trials highlighted this issue by reporting Ad vectors safe but therapeutically nonefficacious. In this regard, studies have clearly illustrated the case for increased transduction of target cells leading to increased therapeutic efficacy. To this end, Ad-based vectors utilizing cancer-specific targeting should continue to be progressed and examined in stringent models of cancer with the goal of full therapeutic efficacy in the clinical setting.
ACKNOWLEDGMENT
We would like to acknowledge NIH Pancreatic Cancer SPORE Grant 2P50CA101955.
REFERENCES
- Alba R, Bradshaw AC, Coughlan L, Denby L, McDonald RA, Waddington SN, et al. Biodistribution and retargeting of FX-binding ablated adenovirus serotype 5 vectors. Blood. 2010;116:2656–2664. doi: 10.1182/blood-2009-12-260026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba R, Bradshaw AC, Parker AL, Bhella D, Waddington SN, Nicklin SA, et al. Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: Effect of mutagenesis on FX interactions and gene transfer. Blood. 2009;114:965–971. doi: 10.1182/blood-2009-03-208835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany R, Curiel DT. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Therapy. 2001;8:1347–1353. doi: 10.1038/sj.gt.3301515. [DOI] [PubMed] [Google Scholar]
- Araki K, Yamashita T, Reddy N, Wang H, Abuzeid WM, Khan K, et al. Molecular disruption of NBS1 with targeted gene delivery enhances chemosensitisation in head and neck cancer. British Journal of Cancer. 2010;103:1822–1830. doi: 10.1038/sj.bjc.6605980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnberg N, Edlund K, Kidd AH, Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor. Journal of Virology. 2000;74:42–48. [PMC free article] [PubMed] [Google Scholar]
- Bangari DS, Mittal SK. Porcine adenovirus serotype 3 internalization is independent of CAR and alphavbeta3 or alphavbeta5 integrin. Virology. 2005;332:157–166. doi: 10.1016/j.virol.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Bangari DS, Shukla S, Mittal SK. Comparative transduction efficiencies of human and nonhuman adenoviral vectors in human, murine, bovine, and porcine cells in culture. Biochemical and Biophysical Research Communications. 2005;327:960–966. doi: 10.1016/j.bbrc.2004.12.099. [DOI] [PubMed] [Google Scholar]
- Barker SD, Dmitriev IP, Nettelbeck DM, Liu B, Rivera AA, Alvarez RD, et al. Combined transcriptional and transductional targeting improves the specificity and efficacy of adenoviral gene delivery to ovarian carcinoma. Gene Therapy. 2003;10:1198–1204. doi: 10.1038/sj.gt.3301974. [DOI] [PubMed] [Google Scholar]
- Belousova N, Krendelchtchikova V, Curiel DT, Krasnykh V. Modulation of adenovirus vector tropism via incorporation of polypeptide ligands into the fiber protein. Journal of Virology. 2002;76:8621–8631. doi: 10.1128/JVI.76.17.8621-8631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belousova N, Mikheeva G, Gelovani J, Krasnykh V. Modification of adenovirus capsid with a designed protein ligand yields a gene vector targeted to a major molecular marker of cancer. Journal of Virology. 2008;82:630–637. doi: 10.1128/JVI.01896-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockmann M, Drosten M, Putzer BM. Discovery of targeting peptides for selective therapy of medullary thyroid carcinoma. The Journal of Gene Medicine. 2005;7:179–188. doi: 10.1002/jgm.648. [DOI] [PubMed] [Google Scholar]
- Borovjagin AV, Krendelchtchikov A, Ramesh N, Yu DC, Douglas JT, Curiel DT. Complex mosaicism is a novel approach to infectivity enhancement of adenovirus type 5-based vectors. Cancer Gene Therapy. 2005;12:475–486. doi: 10.1038/sj.cgt.7700806. [DOI] [PubMed] [Google Scholar]
- Breidenbach M, Rein DT, Everts M, Glasgow JN, Wang M, Passineau MJ, et al. Mesothelin-mediated targeting of adenoviral vectors for ovarian cancer gene therapy. Gene Therapy. 2005;12:187–193. doi: 10.1038/sj.gt.3302404. [DOI] [PubMed] [Google Scholar]
- Breidenbach M, Rein DT, Wang M, Nettelbeck DM, Hemminki A, Ulasov I, et al. Genetic replacement of the adenovirus shaft fiber reduces liver tropism in ovarian cancer gene therapy. Human Gene Therapy. 2004;15:509–518. doi: 10.1089/10430340460745829. [DOI] [PubMed] [Google Scholar]
- Burnett RM. The structure of the adenovirus capsid. II. The packing symmetry of hexon and its implications for viral architecture. Journal of Molecular Biology. 1985;185:125–143. doi: 10.1016/0022-2836(85)90187-1. [DOI] [PubMed] [Google Scholar]
- Campos SK, Parrott MB, Barry MA. Avidin-based targeting and purification of a protein IX-modified, metabolically biotinylated adenoviral vector. Molecular Therapy. 2004;9:942–954. doi: 10.1016/j.ymthe.2004.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette JE, Graat HC, Schagen FH, Mastenbroek DC, Rots MG, Haisma HJ, et al. A conditionally replicating adenovirus with strict selectivity in killing cells expressing epidermal growth factor receptor. Virology. 2007;361:56–67. doi: 10.1016/j.virol.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Chen CY, May SM, Barry MA. Targeting adenoviruses with factor x-single-chain antibody fusion proteins. Human Gene Therapy. 2010;21:739–749. doi: 10.1089/hum.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124:119–131. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Davison AJ, Benko M, Harrach B. Genetic content and evolution of adenoviruses. The Journal of General Virology. 2003;84:2895–2908. doi: 10.1099/vir.0.19497-0. [DOI] [PubMed] [Google Scholar]
- Davison E, Diaz RM, Hart IR, Santis G, Marshall JF. Integrin alpha5beta1-mediated adenovirus infection is enhanced by the integrin-activating antibody TS2/16. Journal of Virology. 1997;71:6204–6207. doi: 10.1128/jvi.71.8.6204-6207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrij J, Uil TG, van den Hengel SK, Cramer SJ, Koppers-Lalic D, Verweij MC, et al. Adenovirus targeting to HLA-A1/MAGE-A1-positive tumor cells by fusing a single-chain T-cell receptor with minor capsid protein IX. Gene Therapy. 2008;15:978–989. doi: 10.1038/gt.2008.26. [DOI] [PubMed] [Google Scholar]
- Diaconu I, Denby L, Pesonen S, Cerullo V, Bauerschmitz GJ, Guse K, et al. Serotype chimeric and fiber-mutated adenovirus Ad5/19p-HIT for targeting renal cancer and untargeting the liver. Human Gene Therapy. 2009;20:611–620. doi: 10.1089/hum.2008.108. [DOI] [PubMed] [Google Scholar]
- Dmitriev IP, Kashentseva EA, Curiel DT. Engineering of adenovirus vectors containing heterologous peptide sequences in the C terminus of capsid protein IX. Journal of Virology. 2002;76:6893–6899. doi: 10.1128/JVI.76.14.6893-6899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev I, Kashentseva E, Rogers BE, Krasnykh V, Curiel DT. Ectodomain of coxsackievirus and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor-positive cells. Journal of Virology. 2000;74:6875–6884. doi: 10.1128/jvi.74.15.6875-6884.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. Journal of Virology. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas JT, Rogers BE, Rosenfeld ME, Michael SI, Feng M, Curiel DT. Targeted gene delivery by tropism-modified adenoviral vectors. Nature Biotechnology. 1996;14:1574–1578. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus C, Al-Jaibaji A, Operschall E, Schoffel A, Peter I, Greber UF, et al. Functional and selective targeting of adenovirus to high-affinity Fcgamma receptor I-positive cells by using a bispecific hybrid adapter. Journal of Virology. 2001;75:480–489. doi: 10.1128/JVI.75.1.480-489.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts M, Kim-Park SA, Preuss MA, Passineau MJ, Glasgow JN, Pereboev AV, et al. Selective induction of tumor-associated antigens in murine pulmonary vasculature using double-targeted adenoviral vectors. Gene Therapy. 2005;12:1042–1048. doi: 10.1038/sj.gt.3302491. [DOI] [PubMed] [Google Scholar]
- Fabry CM, Rosa-Calatrava M, Conway JF, Zubieta C, Cusack S, Ruigrok RW, et al. A quasi-atomic model of human adenovirus type 5 capsid. The EMBO Journal. 2005;24:1645–1654. doi: 10.1038/sj.emboj.7600653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechner H, Haack A, Wang H, Wang X, Eizema K, Pauschinger M, et al. Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Therapy. 1999;6:1520–1535. doi: 10.1038/sj.gt.3301030. [DOI] [PubMed] [Google Scholar]
- Figures MR, Wobb J, Araki K, Liu T, Xu L, Zhu H, et al. Head and neck squamous cell carcinoma targeted chemosensitization. Otolaryngology and Head and Neck Surgery. 2009;141:177–183. doi: 10.1016/j.otohns.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nature Medicine. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- Glasgow JN, Kremer EJ, Hemminki A, Siegal GP, Douglas JT, Curiel DT. An adenovirus vector with a chimeric fiber derived from canine adenovirus type 2 displays novel tropism. Virology. 2004;324:103–116. doi: 10.1016/j.virol.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Glasgow JN, Mikheeva G, Krasnykh V, Curiel DT. A strategy for adenovirus vector targeting with a secreted single chain antibody. PLoS One. 2009;4:e8355. doi: 10.1371/journal.pone.0008355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman CK, Rogers BE, Douglas JT, Sosnowski BA, Ying W, Siegal GP, et al. Targeted gene delivery to Kaposi’s sarcoma cells via the fibroblast growth factor receptor. Cancer Research. 1997;57:1447–1451. [PubMed] [Google Scholar]
- Grellier E, Lecolle K, Rogee S, Couturier C, D’Halluin JC, Hong SS, et al. A fiber-modified adenoviral vector interacts with immunoevasion molecules of the B7 family at the surface of murine leukemia cells derived from dormant tumors. Molecular Cancer. 2011;10:105. doi: 10.1186/1476-4598-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill J, Van Beusechem VW, Van Der Valk P, Dirven CM, Leonhart A, Pherai DS, et al. Combined targeting of adenoviruses to integrins and epidermal growth factor receptors increases gene transfer into primary glioma cells and spheroids. Clinical Cancer Research. 2001;7:641–650. [PubMed] [Google Scholar]
- Gu DL, Gonzalez AM, Printz MA, Doukas J, Ying W, D’Andrea M, et al. Fibroblast growth factor 2 retargeted adenovirus has redirected cellular tropism: Evidence for reduced toxicity and enhanced antitumor activity in mice. Cancer Research. 1999;59:2608–2614. [PubMed] [Google Scholar]
- Guardado-Calvo P, Munoz EM, Llamas-Saiz AL, Fox GC, Kahn R, Curiel DT, et al. Crystallographic structure of porcine adenovirus type 4 fiber head and galectin domains. Journal of Virology. 2010;84:10558–10568. doi: 10.1128/JVI.00997-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haisma HJ, Grill J, Curiel DT, Hoogeland S, van Beusechem VW, Pinedo HM, et al. Targeting of adenoviral vectors through a bispecific single-chain antibody. Cancer Gene Therapy. 2000;7:901–904. doi: 10.1038/sj.cgt.7700198. [DOI] [PubMed] [Google Scholar]
- Haisma HJ, Kamps GK, Bouma A, Geel TM, Rots MG, Kariath A, et al. Selective targeting of adenovirus to alphavbeta3 integrins, VEGFR2 and Tie2 endothelial receptors by angio-adenobodies. International Journal of Pharmaceutics. 2010;391:155–161. doi: 10.1016/j.ijpharm.2010.02.032. [DOI] [PubMed] [Google Scholar]
- Haisma HJ, Pinedo HM, Rijswijk A, der Meulen-Muileman I, Sosnowski BA, Ying W, et al. Tumor-specific gene transfer via an adenoviral vector targeted to the pan-carcinoma antigen EpCAM. Gene Therapy. 1999;6:1469–1474. doi: 10.1038/sj.gt.3300969. [DOI] [PubMed] [Google Scholar]
- Hakkarainen T, Hemminki A, Pereboev AV, Barker SD, Asiedu CK, Strong TV, et al. CD40 is expressed on ovarian cancer cells and can be utilized for targeting adenoviruses. Clinical Cancer Research. 2003;9:619–624. [PubMed] [Google Scholar]
- Harvey TJ, Burdon D, Steele L, Ingram N, Hall GD, Selby PJ, et al. Retargeted adenoviral cancer gene therapy for tumour cells overexpressing epidermal growth factor receptor or urokinase-type plasminogen activator receptor. Gene Therapy. 2010;17:1000–1010. doi: 10.1038/gt.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heideman DA, van Beusechem VW, Offerhaus GJ, Wickham TJ, Roelvink PW, Craanen ME, et al. Selective gene transfer into primary human gastric tumors using epithelial cell adhesion molecule-targeted adenoviral vectors with ablated native tropism. Human Gene Therapy. 2002;13:1677–1685. doi: 10.1089/104303402760293529. [DOI] [PubMed] [Google Scholar]
- Hemminki A, Belousova N, Zinn KR, Liu B, Wang M, Chaudhuri TR, et al. An adenovirus with enhanced infectivity mediates molecular chemotherapy of ovarian cancer cells and allows imaging of gene expression. Molecular Therapy. 2001;4:223–231. doi: 10.1006/mthe.2001.0446. [DOI] [PubMed] [Google Scholar]
- Henning P, Andersson KM, Frykholm K, Ali A, Magnusson MK, Nygren PA, et al. Tumor cell targeted gene delivery by adenovirus 5 vectors carrying knobless fibers with antibody-binding domains. Gene Therapy. 2005;12:211–224. doi: 10.1038/sj.gt.3302408. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Meyer B, Wildner O. Improved glioblastoma treatment with Ad5/35 fiber chimeric conditionally replicating adenoviruses. The Journal of Gene Medicine. 2007;9:764–778. doi: 10.1002/jgm.1076. [DOI] [PubMed] [Google Scholar]
- Huch M, Abate-Daga D, Roig JM, Gonzalez JR, Fabregat J, Sosnowski B, et al. Targeting the CYP2B 1/cyclophosphamide suicide system to fibroblast growth factor receptors results in a potent antitumoral response in pancreatic cancer models. Human Gene Therapy. 2006;17:1187–1200. doi: 10.1089/hum.2006.17.1187. [DOI] [PubMed] [Google Scholar]
- Izumi M, Kawakami Y, Glasgow JN, Belousova N, Everts M, Kim-Park S, et al. In vivo analysis of a genetically modified adenoviral vector targeted to human CD40 using a novel transient transgenic model. The Journal of Gene Medicine. 2005;7:1517–1525. doi: 10.1002/jgm.806. [DOI] [PubMed] [Google Scholar]
- Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, et al. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashentseva EA, Seki T, Curiel DT, Dmitriev IP. Adenovirus targeting to c-erbB-2 oncoprotein by single-chain antibody fused to trimeric form of adenovirus receptor ectodomain. Cancer Research. 2002;62:609–616. [PubMed] [Google Scholar]
- Kelly FJ, Miller CR, Buchsbaum DJ, Gomez-Navarro J, Barnes MN, Alvarez RD, et al. Selectivity of TAG-72-targeted adenovirus gene transfer to primary ovarian carcinoma cells versus autologous mesothelial cells in vitro. Clinical Cancer Research. 2000;6:4323–4333. [PubMed] [Google Scholar]
- Kim KH, Ryan MJ, Estep JE, Miniard BM, Rudge TL, Peggins JO, et al. A new generation of serotype chimeric infectivity-enhanced conditionally replicative adenovirals: The safety profile of ad5/3-Delta24 in advance of a phase I clinical trial in ovarian cancer patients. Human Gene Therapy. 2011;22:821–828. doi: 10.1089/hum.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Smith T, Idamakanti N, Mulgrew K, Kaloss M, Kylefjord H, et al. Targeting adenoviral vectors by using the extracellular domain of the coxsackie-adenovirus receptor: Improved potency via trimerization. Journal of Virology. 2002;76:1892–1903. doi: 10.1128/JVI.76.4.1892-1903.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball KJ, Preuss MA, Barnes MN, Wang M, Siegal GP, Wan W, et al. A phase I study of a tropism-modified conditionally replicative adenovirus for recurrent malignant gynecologic diseases. Clinical Cancer Research. 2010;16:5277–5287. doi: 10.1158/1078-0432.CCR-10-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korokhov N, de Gruijl TD, Aldrich WA, Triozzi PL, Banerjee PT, Gillies SD, et al. High efficiency transduction of dendritic cells by adenoviral vectors targeted to DC-SIGN. Cancer Biology & Therapy. 2005;4:289–294. doi: 10.4161/cbt.4.3.1499. [DOI] [PubMed] [Google Scholar]
- Kraaij R, van Rijswijk AL, Oomen MH, Haisma HJ, Bangma CH. Prostate specific membrane antigen (PSMA) is a tissue-specific target for adenoviral transduction of prostate cancer in vitro. The Prostate. 2005;62:253–259. doi: 10.1002/pros.20150. [DOI] [PubMed] [Google Scholar]
- Krasnykh V, Belousova N, Korokhov N, Mikheeva G, Curiel DT. Genetic targeting of an adenovirus vector via replacement of the fiber protein with the phage T4 fibritin. Journal of Virology. 2001;75:4176–4183. doi: 10.1128/JVI.75.9.4176-4183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnykh V, Dmitriev I, Mikheeva G, Miller CR, Belousova N, Curiel DT. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. Journal of Virology. 1998;72:1844–1852. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavilla-Alonso S, Bauerschmitz G, Abo-Ramadan U, Halavaara J, Escutenaire S, Diaconu I, et al. Adenoviruses with an alphavbeta integrin targeting moiety in the fiber shaft or the HI-loop increase tumor specificity without compromising antitumor efficacy in magnetic resonance imaging of colorectal cancer metastases. Journal of Translational Medicine. 2010;8:80. doi: 10.1186/1479-5876-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E, Brown SL, Stupack DG, Puente XS, Cheresh DA, Nemerow GR. Integrin alpha(v)beta1 is an adenovirus coreceptor. Journal of Virology. 2001;75:5405–5409. doi: 10.1128/JVI.75.11.5405-5409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E, Brown SL, Von Seggern DJ, Brown GB, Nemerow GR. Signaling antibodies complexed with adenovirus circumvent CAR and integrin interactions and improve gene delivery. Gene Therapy. 2000;7:1593–1599. doi: 10.1038/sj.gt.3301271. [DOI] [PubMed] [Google Scholar]
- Li HJ, Everts M, Yamamoto M, Curiel DT, Herschman HR. Combined transductional untargeting/retargeting and transcriptional restriction enhances adenovirus gene targeting and therapy for hepatic colorectal cancer tumors. Cancer Research. 2009;69:554–564. doi: 10.1158/0008-5472.CAN-08-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Guang W, Abuzeid WM, Roy S, Gao GP, Sauk JJ, et al. Novel adenoviral gene delivery system targeted against head and neck cancer. The Laryngoscope. 2008;118:650–658. doi: 10.1097/MLG.0b013e3181613aba. [DOI] [PubMed] [Google Scholar]
- Li E, Stupack D, Bokoch GM, Nemerow GR. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. Journal of Virology. 1998;72:8806–8812. doi: 10.1128/jvi.72.11.8806-8812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E, Stupack D, Klemke R, Cheresh DA, Nemerow GR. Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-OH kinase. Journal of Virology. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson MK, Hong SS, Boulanger P, Lindholm L. Genetic retargeting of adenovirus: Novel strategy employing “deknobbing” of the fiber. Journal of Virology. 2001;75:7280–7289. doi: 10.1128/JVI.75.16.7280-7289.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson MK, Kraaij R, Leadley RM, De Ridder CM, van Weerden WM, Van Schie KA, et al. A transductionally retargeted adenoviral vector for virotherapy of Her2/neu-expressing prostate cancer. Human Gene Therapy. 2011;23:70–82. doi: 10.1089/hum.2011.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh MP, Campos SK, Baker ML, Chen CY, Chiu W, Barry MA. Cryoelectron microscopy of protein IX-modified adenoviruses suggests a new position for the C terminus of protein IX. Journal of Virology. 2006;80:11881–11886. doi: 10.1128/JVI.01471-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Noker PE, Tian B, Grimes SD, Fulton R, Schweikart K, et al. Identifying the safety profile of Ad5.SSTR/TK.RGD, a novel infectivity-enhanced bicistronic adenovirus, in anticipation of a phase I clinical trial in patients with recurrent ovarian cancer. Clinical Cancer Research. 2009;15:4131–4137. doi: 10.1158/1078-0432.CCR-08-3354. [DOI] [PubMed] [Google Scholar]
- Meier O, Boucke K, Hammer SV, Keller S, Stidwill RP, Hemmi S, et al. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. The Journal of Cell Biology. 2002;158:1119–1131. doi: 10.1083/jcb.200112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CR, Buchsbaum DJ, Reynolds PN, Douglas JT, Gillespie GY, Mayo MS, et al. Differential susceptibility of primary and established human glioma cells to adenovirus infection: Targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Research. 1998;58:5738–5748. [PubMed] [Google Scholar]
- Miura Y, Yoshida K, Nishimoto T, Hatanaka K, Ohnami S, Asaka M, et al. Direct selection of targeted adenovirus vectors by random peptide display on the fiber knob. Gene Therapy. 2007;14:1448–1460. doi: 10.1038/sj.gt.3303007. [DOI] [PubMed] [Google Scholar]
- Murakami M, Ugai H, Belousova N, Pereboev A, Dent P, Fisher PB, et al. Chimeric adenoviral vectors incorporating a fiber of human adenovirus 3 efficiently mediate gene transfer into prostate cancer cells. The Prostate. 2010;70:362–376. doi: 10.1002/pros.21070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Ugai H, Wang M, Belousova N, Dent P, Fisher PB, et al. An adenoviral vector expressing human adenovirus 5 and 3 fiber proteins for targeting heterogeneous cell populations. Virology. 2010;407:196–205. doi: 10.1016/j.virol.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Murugesan SR, Akiyama M, Einfeld DA, Wickham TJ, King CR. Experimental treatment of ovarian cancers by adenovirus vectors combining receptor targeting and selective expression of tumor necrosis factor. International Journal of Oncology. 2007;31:813–822. [PubMed] [Google Scholar]
- Myhre S, Henning P, Friedman M, Stahl S, Lindholm L, Magnusson MK. Re-targeted adenovirus vectors with dual specificity; binding specificities conferred by two different Affibody molecules in the fiber. Gene Therapy. 2009;16:252–261. doi: 10.1038/gt.2008.160. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Both GW, Banizs B, Tsuruta Y, Yamamoto S, Kawakami Y, et al. An adenovirus serotype 5 vector with fibers derived from ovine atadenovirus demonstrates CAR-independent tropism and unique biodistribution in mice. Virology. 2006;350:103–115. doi: 10.1016/j.virol.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Nettelbeck DM, Miller DW, Jerome V, Zuzarte M, Watkins SJ, Hawkins RE, et al. Targeting of adenovirus to endothelial cells by a bispecific single-chain diabody directed against the adenovirus fiber knob domain and human endoglin (CD105) Molecular Therapy. 2001;3:882–891. doi: 10.1006/mthe.2001.0342. [DOI] [PubMed] [Google Scholar]
- Nettelbeck DM, Rivera AA, Kupsch J, Dieckmann D, Douglas JT, Kontermann RE, et al. Retargeting of adenoviral infection to melanoma: Combining genetic ablation of native tropism with a recombinant bispecific single-chain diabody (scDb) adapter that binds to fiber knob and HMWMAA. International Journal of Cancer. 2004;108:136–145. doi: 10.1002/ijc.11563. [DOI] [PubMed] [Google Scholar]
- Nicklin SA, Dishart KL, Buening H, Reynolds PN, Hallek M, Nemerow GR, et al. Transductional and transcriptional targeting of cancer cells using genetically engineered viral vectors. Cancer Letters. 2003;201:165–173. doi: 10.1016/j.canlet.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Nishimoto T, Yoshida K, Miura Y, Kobayashi A, Hara H, Ohnami S, et al. Oncolytic virus therapy for pancreatic cancer using the adenovirus library displaying random peptides on the fiber knob. Gene Therapy. 2009;16:669–680. doi: 10.1038/gt.2009.1. [DOI] [PubMed] [Google Scholar]
- Nokisalmi P, Pesonen S, Escutenaire S, Sarkioja M, Raki M, Cerullo V, et al. Oncolytic adenovirus ICOVIR-7 in patients with advanced and refractory solid tumors. Clinical Cancer Research. 2010;16:3035–3043. doi: 10.1158/1078-0432.CCR-09-3167. [DOI] [PubMed] [Google Scholar]
- Okada Y, Okada N, Mizuguchi H, Hayakawa T, Nakagawa S, Mayumi T. Transcriptional targeting of RGD fiber-mutant adenovirus vectors can improve the safety of suicide gene therapy for murine melanoma. Cancer Gene Therapy. 2005;12:608–616. doi: 10.1038/sj.cgt.7700824. [DOI] [PubMed] [Google Scholar]
- Okegawa T, Pong RC, Li Y, Bergelson JM, Sagalowsky AI, Hsieh JT. The mechanism of the growth-inhibitory effect of coxsackie and adenovirus receptor (CAR) on human bladder cancer: A functional analysis of car protein structure. Cancer Research. 2001;61:6592–6600. [PubMed] [Google Scholar]
- Page JG, Tian B, Schweikart K, Tomaszewski J, Harris R, Broadt T, et al. Identifying the safety profile of a novel infectivity-enhanced conditionally replicative adenovirus, Ad5-delta24-RGD, in anticipation of a phase I trial for recurrent ovarian cancer. American Journal of Obstetrics and Gynecology. 2007;196:389.e9–389.e10. doi: 10.1016/j.ajog.2006.12.016. discussion 389 e9–389.e10. [DOI] [PubMed] [Google Scholar]
- Papanikolopoulou K, Forge V, Goeltz P, Mitraki A. Formation of highly stable chimeric trimers by fusion of an adenovirus fiber shaft fragment with the foldon domain of bacteriophage t4 fibritin. The Journal of Biological Chemistry. 2004;279:8991–8998. doi: 10.1074/jbc.M311791200. [DOI] [PubMed] [Google Scholar]
- Parrott MB, Adams KE, Mercier GT, Mok H, Campos SK, Barry MA. Metabolically biotinylated adenovirus for cell targeting, ligand screening, and vector purification. Molecular Therapy. 2003;8:688–700. doi: 10.1016/s1525-0016(03)00213-2. [DOI] [PubMed] [Google Scholar]
- Pereboeva L, Komarova S, Mahasreshti PJ, Curiel DT. Fiber-mosaic adenovirus as a novel approach to design genetically modified adenoviral vectors. Virus Research. 2004;105:35–46. doi: 10.1016/j.virusres.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Pereboeva L, Komarova S, Roth J, Ponnazhagan S, Curiel DT. Targeting EGFR with metabolically biotinylated fiber-mosaic adenovirus. Gene Therapy. 2007;14:627–637. doi: 10.1038/sj.gt.3302916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen S, Diaconu I, Cerullo V, Escutenaire S, Raki M, Kangasniemi L, et al. Integrin targeted oncolytic adenoviruses Ad5-D24-RGD and Ad5-RGD-D24-GMCSF for treatment of patients with advanced chemotherapy refractory solid tumors. International Journal of Cancer. 2011;130:1937–1947. doi: 10.1002/ijc.26216. [DOI] [PubMed] [Google Scholar]
- Pesonen S, Nokisalmi P, Escutenaire S, Sarkioja M, Raki M, Cerullo V, et al. Prolonged systemic circulation of chimeric oncolytic adenovirus Ad5/3-Cox2L-D24 in patients with metastatic and refractory solid tumors. Gene Therapy. 2010;17:892–904. doi: 10.1038/gt.2010.17. [DOI] [PubMed] [Google Scholar]
- Philipson L, Pettersson RF. The coxsackie-adenovirus receptor—A new receptor in the immunoglobulin family involved in cell adhesion. Current Topics in Microbiology and Immunology. 2004;273:87–111. doi: 10.1007/978-3-662-05599-1_3. [DOI] [PubMed] [Google Scholar]
- Piao Y, Jiang H, Alemany R, Krasnykh V, Marini FC, Xu J, et al. Oncolytic adenovirus retargeted to Delta-EGFR induces selective antiglioma activity. Cancer Gene Therapy. 2009;16:256–265. doi: 10.1038/cgt.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin KL, Lanthier RM, Smith AC, Christou C, Risco Quiroz M, Powell KL, et al. Retargeting of adenovirus vectors through genetic fusion of a single-chain or single-domain antibody to capsid protein IX. Journal of Virology. 2010;84:10074–10086. doi: 10.1128/JVI.02665-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Printz MA, Gonzalez AM, Cunningham M, Gu DL, Ong M, Pierce GF, et al. Fibroblast growth factor 2-retargeted adenoviral vectors exhibit a modified biolocalization pattern and display reduced toxicity relative to native adenoviral vectors. Human Gene Therapy. 2000;11:191–204. doi: 10.1089/10430340050016265. [DOI] [PubMed] [Google Scholar]
- Reddy VS, Natchiar SK, Stewart PL, Nemerow GR. Crystal structure of human adenovirus at 3.5 Å resolution. Science. 2010;329:1071–1075. doi: 10.1126/science.1187292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein DT, Breidenbach M, Wu H, Han T, Haviv YS, Wang M, et al. Gene transfer to cervical cancer with fiber-modified adenoviruses. International Journal of Cancer. 2004;111:698–704. doi: 10.1002/ijc.20295. [DOI] [PubMed] [Google Scholar]
- Rein DT, Volkmer A, Beyer IM, Curiel DT, Janni W, Dragoi A, et al. Treatment of chemotherapy resistant ovarian cancer with a MDR1 targeted oncolytic adenovirus. Gynecologic Oncology. 2011;123:138–146. doi: 10.1016/j.ygyno.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Renaut L, Colin M, Leite JP, Benko M, D’Halluin JC. Abolition of hCAR-dependent cell tropism using fiber knobs of Atadenovirus serotypes. Virology. 2004;321:189–204. doi: 10.1016/j.virol.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Reynolds PN, Zinn KR, Gavrilyuk VD, Balyasnikova IV, Rogers BE, Buchsbaum DJ, et al. A targetable, injectable adenoviral vector for selective gene delivery to pulmonary endothelium in vivo. Molecular Therapy. 2000;2:562–578. doi: 10.1006/mthe.2000.0205. [DOI] [PubMed] [Google Scholar]
- Rocconi RP, Zhu ZB, Stoff-Khalili M, Rivera AA, Lu B, Wang M, et al. Treatment of ovarian cancer with a novel dual targeted conditionally replicative adenovirus (CRAd) Gynecologic Oncology. 2007;105:113–121. doi: 10.1016/j.ygyno.2006.10.057. [DOI] [PubMed] [Google Scholar]
- Saban SD, Silvestry M, Nemerow GR, Stewart PL. Visualization of alpha-helices in a 6-angstrom resolution cryoelectron microscopy structure of adenovirus allows refinement of capsid protein assignments. Journal of Virology. 2006;80:12049–12059. doi: 10.1128/JVI.01652-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Martin C, Glasgow JN, Borovjagin A, Beatty MS, Kashentseva EA, Curiel DT, et al. Localization of the N-terminus of minor coat protein IIIa in the adenovirus capsid. Journal of Molecular Biology. 2008;383:923–934. doi: 10.1016/j.jmb.2008.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Eipel C, Furst K, Sommer N, Pahnke J, Putzer BM. Evaluation of systemic targeting of RET oncogene-based MTC with tumor-selective peptide-tagged Ad vectors in clinical mouse models. Gene Therapy. 2011;18:418–423. doi: 10.1038/gt.2010.165. [DOI] [PubMed] [Google Scholar]
- Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. Journal of Virology. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short JJ, Pereboev AV, Kawakami Y, Vasu C, Holterman MJ, Curiel DT. Adenovirus serotype 3 utilizes CD80 (B7.1) and CD86 (B7.2) as cellular attachment receptors. Virology. 2004;322:349–359. doi: 10.1016/j.virol.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Short JJ, Rivera AA, Wu H, Walter MR, Yamamoto M, Mathis JM, et al. Substitution of adenovirus serotype 3 hexon onto a serotype 5 oncolytic adenovirus reduces factor X binding, decreases liver tropism, and improves antitumor efficacy. Molecular Cancer Therapeutics. 2010;9:2536–2544. doi: 10.1158/1535-7163.MCT-10-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Mei YF. Transduction and oncolytic profile of a potent replication-competent adenovirus 11p vector (RCAd11pGFP) in colon carcinoma cells. PLoS One. 2011;6:e17532. doi: 10.1371/journal.pone.0017532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T, Idamakanti N, Kylefjord H, Rollence M, King L, Kaloss M, et al. In vivo hepatic adenoviral gene delivery occurs independently of the coxsackievirus-adenovirus receptor. Molecular Therapy. 2002;5:770–779. doi: 10.1006/mthe.2002.0613. [DOI] [PubMed] [Google Scholar]
- Smith TA, Idamakanti N, Rollence ML, Marshall-Neff J, Kim J, Mulgrew K, et al. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Human Gene Therapy. 2003;14:777–787. doi: 10.1089/104303403765255165. [DOI] [PubMed] [Google Scholar]
- Stoff-Khalili MA, Rivera AA, Stoff A, Michael Mathis J, Rocconi RP, Matthews QL, et al. Combining high selectivity of replication via CXCR4 promoter with fiber chimerism for effective adenoviral oncolysis in breast cancer. International Journal of Cancer. 2007;120:935–941. doi: 10.1002/ijc.22338. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kato K, Nakamura K, Nakano R, Kubota K, Hamada H. Neural cell adhesion molecule 2 as a target molecule for prostate and breast cancer gene therapy. Cancer Science. 2011;102:808–814. doi: 10.1111/j.1349-7006.2011.01855.x. [DOI] [PubMed] [Google Scholar]
- Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM, et al. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Molecular Therapy. 2001;3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- Tillman BW, de Gruijl TD, Luykx-de Bakker SA, Scheper RJ, Pinedo HM, Curiel TJ, et al. Maturation of dendritic cells accompanies high-efficiency gene transfer by a CD40-targeted adenoviral vector. Journal of Immunology. 1999;162:6378–6383. [PubMed] [Google Scholar]
- Tsuruta Y, Pereboeva L, Glasgow JN, Luongo CL, Komarova S, Kawakami Y, et al. Reovirus sigma1 fiber incorporated into adenovirus serotype 5 enhances infectivity via a CAR-independent pathway. Biochemical and Biophysical Research Communications. 2005;335:205–214. doi: 10.1016/j.bbrc.2005.07.054. [DOI] [PubMed] [Google Scholar]
- Tsuruta Y, Pereboeva L, Glasgow JN, Rein DT, Kawakami Y, Alvarez RD, et al. A mosaic fiber adenovirus serotype 5 vector containing reovirus sigma 1 and adenovirus serotype 3 knob fibers increases transduction in an ovarian cancer ex vivo system via a coxsackie and adenovirus receptor-independent pathway. Clinical Cancer Research. 2007;13:2777–2783. doi: 10.1158/1078-0432.CCR-06-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellinga J, de Vrij J, Myhre S, Uil T, Martineau P, Lindholm L, et al. Efficient incorporation of a functional hyper-stable single-chain antibody fragment protein-IX fusion in the adenovirus capsid. Gene Therapy. 2007;14:664–670. doi: 10.1038/sj.gt.3302908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellinga J, Rabelink MJ, Cramer SJ, van den Wollenberg DJ, Van der Meulen H, Leppard KN, et al. Spacers increase the accessibility of peptide ligands linked to the carboxyl terminus of adenovirus minor capsid protein IX. Journal of Virology. 2004;78:3470–3479. doi: 10.1128/JVI.78.7.3470-3479.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellinga J, Van der Heijdt S, Hoeben RC. The adenovirus capsid: Major progress in minor proteins. The Journal of General Virology. 2005;86:1581–1588. doi: 10.1099/vir.0.80877-0. [DOI] [PubMed] [Google Scholar]
- Vigne E, Dedieu JF, Brie A, Gillardeaux A, Briot D, Benihoud K, et al. Genetic manipulations of adenovirus type 5 fiber resulting in liver tropism attenuation. Gene Therapy. 2003;10:153–162. doi: 10.1038/sj.gt.3301845. [DOI] [PubMed] [Google Scholar]
- Vigne E, Mahfouz I, Dedieu JF, Brie A, Perricaudet M, Yeh P. RGD inclusion in the hexon monomer provides adenovirus type 5-based vectors with a fiber knob-independent pathway for infection. Journal of Virology. 1999;73:5156–5161. doi: 10.1128/jvi.73.6.5156-5161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Tzeng E, Shears LL, 2nd, Roelvink PW, Li Y, Lee GM, et al. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. Journal of Virology. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M, Perrotte P, Onishi E, Harper ME, Dinney C, Pagliaro L, et al. Biodistribution of an adenoviral vector carrying the luciferase reporter gene following intravesical or intravenous administration to a mouse. Cancer Gene Therapy. 1999;6:367–372. doi: 10.1038/sj.cgt.7700090. [DOI] [PubMed] [Google Scholar]
- Wu H, Han T, Belousova N, Krasnykh V, Kashentseva E, Dmitriev I, et al. Identification of sites in adenovirus hexon for foreign peptide incorporation. Journal of Virology. 2005;79:3382–3390. doi: 10.1128/JVI.79.6.3382-3390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Seki T, Dmitriev I, Uil T, Kashentseva E, Han T, et al. Double modification of adenovirus fiber with RGD and polylysine motifs improves coxsackievirus-adenovirus receptor-independent gene transfer efficiency. Human Gene Therapy. 2002;13:1647–1653. doi: 10.1089/10430340260201734. [DOI] [PubMed] [Google Scholar]
- Zubieta C, Schoehn G, Chroboczek J, Cusack S. The structure of the human adenovirus 2 penton. Molecular Cell. 2005;17:121–135. doi: 10.1016/j.molcel.2004.11.041. [DOI] [PubMed] [Google Scholar]