Abstract
Background
Left bundle branch block (LBBB) is a marker of increased delay between septal and left ventricular (LV) lateral wall electrical activation, and is a predictor of which patients will benefit from cardiac resynchronization therapy (CRT). Recent analysis has suggested that one third of patients meeting conventional ECG criteria for LBBB are misdiagnosed and new strict LBBB criteria have been proposed. We tested the hypothesis that strict LBBB patients have greater LV mechanical dyssynchrony than patients meeting non-strict LBBB criteria while there is no difference between patients with non-strict LBBB and LV conduction delay with QRS duration 110–119 ms.
Methods
Sixty-four patients referred for primary prevention implantable cardioverter-defibrillators (ICD) underwent 12-lead ECG and cardiac magnetic resonance (CMR) myocardial tagging. The patients were classified as strict LBBB, non-strict LBBB or non-LBBB (nonspecific LV conduction delay with QRS duration 110–119 ms). The time delay between septal and lateral LV wall peak circumferential strain (septal-to-lateral wall delay) was measured by CMR.
Results
Patients with strict LBBB (n=31) had a greater septal-to-lateral wall delay, compared to patients with non-strict LBBB (n=19) (210±137 ms vs. 122±102 ms, p=0.045). There was no significant difference between non-strict LBBB and non-LBBB (n=14) septal-to-lateral wall delay (122±102 ms vs. 100±86 ms, p=0.51).
Conclusions
Strict-LBBB criteria identify patients with greater mechanical dyssynchrony compared to patients only meeting non-strict LBBB criteria, while there was no significant difference between non-strict LBBB and non-LBBB patients. The greater observed LV dyssynchrony may explain why strict-LBBB patients have better response to CRT.
Keywords: Cardiac Resynchronization Therapy, Left Bundle Branch Block, Tagged Cardiac Magnetic Resonance Imaging, Left Ventricular Dyssynchrony
Introduction
The goal of cardiac resynchronization therapy (CRT) using biventricular pacing is to improve global left ventricular (LV) function by synchronizing activation of the inter-ventricular septum with that of the LV lateral wall.1 Initial clinical trials for CRT enrolled patients with heart failure, QRS duration ≥ 120 ms and a left ventricular ejection fraction (LVEF) ≤ 35%,1 which comprise the current clinical criteria for CRT device implantation.2 However, the reason for the QRS prolongation was not considered. More recent clinical trials that also included patients with milder heart failure symptoms found that the clinical benefit was limited to patients with left bundle branch block (LBBB).3,4 There is a pathophysiological basis for this observation. In complete LBBB, there is an increased delay between electrical activation of the septum and LV lateral wall. However, in right bundle branch block (RBBB) and non-specific LV conduction delay [e.g. from left ventricular hypertrophy (LVH)], although QRS duration is prolonged, the LV endocardium is still activated synchronously by the rapidly conducting Purkinje system.5
The importance of LBBB in predicting response to CRT has led to renewed interest in accurately defining LBBB.5 A combination of endocardial mapping and simulation studies suggests that approximately one third of patients diagnosed with LBBB by conventional criteria do not have true complete LBBB.6,7,8 Consequently, strict LBBB criteria were proposed by Strauss et al. that require a QRS duration ≥ 130 ms in women or ≤ 140 ms in men, and also rS or QS morphology in lead V1 and mid QRS notching/slurring in at least two of the leads V1, V2, V5, V6, I or aVL.5 A recent study showed that only patients meeting strict LBBB criteria had a significant increase in LVEF in response to CRT and that patients meeting strict LBBB criteria had a higher event-free survival than those with non-strict LBBB (i.e. meeting conventional LBBB criteria, but not meeting the strict criteria).9 These findings support the premise that LBBB morphology is important for prediction of optimal CRT response.
Differences in the mechanical contraction patterns in patients with strict compared to non-strict LBBB have not previously been studied. Myocardial tissue tagging using cardiac magnetic resonance (CMR) is a method that can accurately quantify LV contraction and its temporal course.10 In this study, we tested the hypothesis that patients with strict LBBB criteria have greater septal-to-lateral wall mechanical dyssynchrony than patients meeting non-strict LBBB criteria. Furthermore, we also assessed our expectation that there is no difference between patients with non-strict LBBB and LV conduction delay with QRS duration 110–119 ms.
Methods
Study population
This is a retrospective analysis of patients referred for a primary prevention implantable cardioverter defibrillator (ICD) who were enrolled between November, 2003 and February, 2012 at Johns Hopkins Hospital as part of a prospective cohort study, PROSE-ICD (Prospective Observational Study of Implantable Cardioverter Defibrillators). The inclusion and exclusion criteria have been described previously.11,12,13,14,15 Patient inclusion required 1) LVEF ≤35% measured by a clinically indicated non-CMR study (echocardiography, nuclear scintigraphy or ventriculography), 2) coronary angiography, 3) no other indications for ICD placement (e.g. syncope, sustained ventricular arrhythmias, or cardiac arrest), and 4) no contraindications to CMR (e.g. existing cardiac device). Both ischemic and nonischemic cardiomyopathy patients were included. The study was approved by the Johns Hopkins Hospital Institutional Review Board, the Duke Institutional Review Board and the FDA Research in Human Subjects Committee.
ECG analysis
Clinically indicated 12-lead ECGs were acquired with a GE-Marquette system (GE Healthcare, WI, USA) before ICD implantation for patients in the PROSE-ICD CMR study as previously described.13 ECGs were analyzed by two observers and classified in consensus according to the following criteria:
Strict LBBB (by Strauss et al.5) – QS or rS in V1, QRS duration ≥ 140 ms in men or ≥ 130 ms in women and mid-QRS notching/slurring in at least two of the leads I, aVL, V1, V2, V5 or V6. The mid QRS-notching/slurring was required to begin after the first 40 ms following the QRS onset but before 50% of the QRS duration.
Non-strict LBBB – QS or rS in V1 and QRS duration ≥ 120 ms, but not meeting the strict LBBB criteria.
Non-LBBB LV conduction delay – QS or rS in V1 and QRS duration 110–119 ms.
Of the 235 potential patients enrolled in the PROSE-ICD prospective cohort study, 129 patients did not meet the above ECG criteria (n=108 with QRS duration <110 ms, n=19 with RBBB, and n=2 with only paced ECGs available).
CMR protocol and analysis
The CMR protocol has been described previously.11,13,15 In summary, patients were imaged with a 1.5 T scanner (Signa CV/I, GE Healthcare Technologies, Milwaukee, Wisconsin or Avanto, Siemens Medical Systems, Erlangen, Germany). Using custom research software tool, CINEtool (GE Healthcare, WI, USA), delayed enhancement images were analyzed for total LV myocardial scar volume by previously reported methods11,14 and segmental LV scar involvement was visually assessed according to the American Heart Association 17-segment model.16 CMR myocardial tagging, performed before gadolinium administration, was used to determine circumferential myocardial strain. Typical image acquisition parameters for tagging included: vecto-electrocardiographic triggering, TR/TE 3.5–7.2ms/2.0–4.2ms; flip angle 12 degrees; slice thickness 8–10mm; field of view, 40cm; matrix, 256 *96–140; 4–9 phase-encoding views per segment; mean bandwidth 49MHz, temporal resolution 25–40ms; and tag spacing, 7mm. The tagged short axis slices were analyzed blinded to ECG classification with the harmonic phase method (HARP, Diagnosoft, Palo Alto, California, USA)17 to assess time-to-peak circumferential strain of the myocardium. The method has previously shown good intra- and inter-observer agreement.17 Peak strain was defined as the difference in length between tag intersects at systole compared to end diastolic circumferential length. Negative strain indicated shortening and contraction, while positive strain indicated lengthening and stretching.
The anterior RV insertion point was determined manually in the mid-ventricular short axis slice with the best image quality. The borders of the epicardium and endocardium were delineated on a late systolic image, creating a circular grid over the LV myocardium. The software then automatically tracked the grid onto the other time frames of the cardiac cycle in the same slice. Imperfect tracking was corrected manually. Next, the HARP software automatically calculated curves for circumferential strain for the LV divided into 6 segments per short-axis slice: two septal segments, two lateral segments, one anterior segment and one inferior segment (Figure 1). From the strain curves, the time from the ECG R-wave to peak circumferential strain was determined manually for each segment (Figure 2). Peak strain was defined as the first negative systolic peak in the strain curve. To be considered a peak it had to be followed by an upward slope of more than 3% of circumferential strain, and it had to continue positively for more than one time frame. This last criterion was not used if the peak was determined to be the last or second-to-last time frame analyzed.
Figure 1. Cardiac magnetic resonance tagging and LV segmentation.
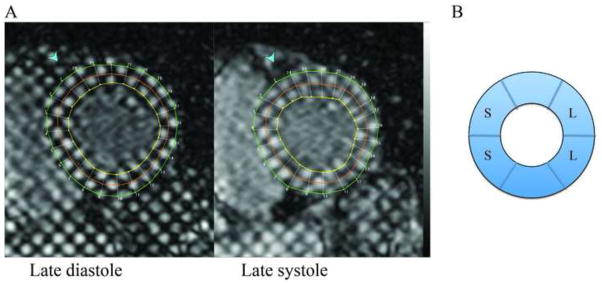
A: shows 2 tagged cardiac magnetic resonance images with a short axis view of the left ventricle, one late diastolic and one late systolic image, with the grid from the HARP analysis software over the left ventricular myocardium. B: shows the segmentation of the mid-ventricular short-axis slice used for strain analysis. Segments labeled with “S” denote the septal segments and segments labeled “L” denote the lateral wall segments.
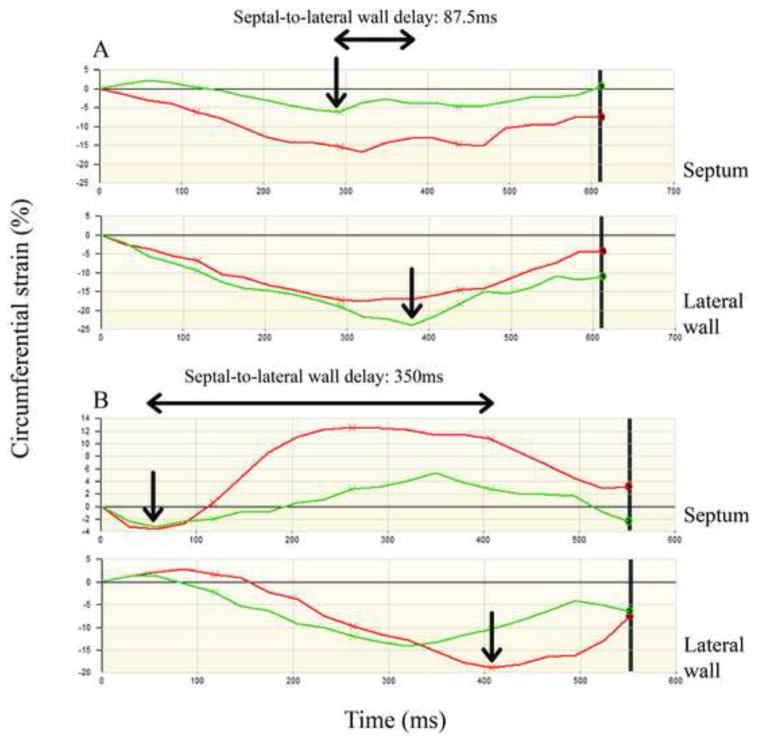
Figure 2. Cardiac magnetic resonance tagging strain curves.
Strain curves for 2 patients (patient A and patient B). For each patient, the strain curves for 2 septal segments and 2 lateral wall segments are presented in red and green. In each patient, the peak circumferential strain in the septal segment with the earliest peak and the peak circumferential strain in the lateral wall segment with the latest peak are denoted with arrows. Peak circumferential strain is defined as the first negative peak followed by an upward slope of more than 3% of the circumferential strain that continued positively for more than one time frame. Patient A has a septal-to-lateral wall delay of 87.5 ms and patient B has a septal-to-lateral wall delay of 350 ms. Note also that the differences in septal contraction pattern between the two patients. Patient A has a normal and relatively undisturbed septal contraction. However, for patient B, the septal peak strain – peak of contraction – was early with an amplitude of −4% (marked with an arrow for the red strain curve) followed by positive strain (stretching) in the septum.
Septal-to-lateral wall delay was defined as the time difference between peak circumferential strain in the septal segment with the earliest peak, and peak circumferential strain in the lateral wall segment with the latest peak (Figure 2). Inclusion of tagging data was based on the ability to monitor the LV throughout the cardiac cycle long enough to observe the peak strain deflection in at least 1 of the 2 lateral wall segments, as well as 1 of the 2 septal segments. Of the 106 patients meeting ECG inclusion criteria, 33 patients did not have CMR-tagging images available and 9 patients did not meet the above tagging data requirements (n=13 with strict LBBB, n=16 with non-strict LBBB and n=13 with non-LBBB LV-conduction delay).
Statistical analysis
Data are presented as mean ± standard deviation (SD) or number (%). In comparing characteristics of the strict versus non-strict and non-strict verus non-LBBB groups, we used the Chi-square test for categorical variables and the Wilcoxon Rank Sum test for continuous ones. For the entire population and in the subgroups, the association between dyssychrony and QRS duration was examined using Spearman correlation. All statistical analyses were performed using STATA (version 11.1, College Station, TX, USA). P-values <0.05 were considered statistically significant.
Results
The baseline characteristics of the 64 patients meeting the ECG criteria with tagging data are presented in Table 1. Compared to non-strict LBBB patients (n=19), strict-LBBB patients (n=31) were more likely to have nonischemic cardiomyopathy, smaller total scar mass, and a lower prevalence of lateral wall scar. There were no differences in LVEF or LV volumes between the groups. In comparison to the non-strict LBBB patients, non-LBBB patients (n=14) were younger and had less severe NYHA heart failure class. As expected, patients with non-LBBB had the shortest QRS duration and those with strict LBBB had the longest QRS duration.
Table 1.
Characteristics of the study population.
| Strict LBBB (n=31) | Non-strict LBBB (n=19) | Non-LBBB (n=14) | P-value (Strict vs. Non-strict) | P-value (Non-strict vs. Non) | |
|---|---|---|---|---|---|
| Age, y (SD) | 58 ± 11 | 63 ± 15 | 52 ± 11 | 0.07 | 0.012 |
| Female, n (%) | 10 (32) | 4 (21) | 1 (7) | 0.39 | 0.27 |
| Ischemic etiology, n (%) | 8 (26) | 12 (63) | 8 (57) | 0.003 | 0.73 |
| NYHA, n (%) | |||||
| Class I | 2 (6) | 4 (21) | 7 (50) | 0.12 | 0.005 |
| Class II | 12 (39) | 3 (16) | 6 (43) | ||
| Class III | 17 (55) | 12 (63) | 1 (7) | ||
| LVEF (%) | 25.2 ± 8.4 | 23.8 ± 8.2 | 27.9 ± 7.5 | 0.76 | 0.23 |
| LVEDV (ml) | 289.0 ± 109.3 | 287.0 ± 128.4 | 247.5 ± 74.1 | 0.76 | 0.51 |
| LVESV (ml) | 221.3 ± 105.0 | 221.3± 111.3 | 182.0 ± 62.9 | 0.98 | 0.49 |
| Scar size (% LV) | 4.2 ± 6.6 | 11.7 ± 10.0 | 14.9 ± 14.5 | 0.003 | 0.80 |
| Septal Scar, n (%) | 11 (35) | 11 (58) | 9 (64) | 0.12 | 0.71 |
| Lateral Scar, n (%) | 7 (23) | 11 (58) | 7 (50) | 0.012 | 0.65 |
| QRS duration (ms) | 163 ± 21 | 129 ± 16 | 114 ± 3 | <0.0001 | <0.0001 |
| Patients without detectable contraction in 1 of 2 septal segments, n (%) | 15 (48) | 3 (16) | 2 (14) | 0.020 | 0.90 |
LVEF=left ventricular ejection fraction, LVEDV=left ventricular end diastolic volume, LVESV= left ventricular end systolic volume
Cardiac Magnetic Resonance Tagging
Analysis of myocardial tagging revealed a contraction pattern characterized by lower strain amplitude in the septum than in the lateral wall; 26 patients (48%) had <5% negative (i.e. shortening) strain in the septum. Furthermore, 20 of the 64 patients (40%) exhibited positive strain (i.e. dyskinesis) in 1 of the 2 septal segments (see Table 1). See Figure 2 for examples of septal strain curves. In comparison, lateral wall contraction was more robust, and all 64 patients had detectable circumferential lateral wall strain deflections in both segments (Figure 2).
Figure 3 shows the comparison of septal-to-lateral wall delay in strict LBBB versus non-strict LBBB groups. The patients with strict LBBB had significantly greater septal-to-lateral wall delay than patients with non-strict LBBB (210 ± 137 ms vs. 122 ± 102 ms, p=0.045). The results for comparison of non-strict LBBB versus non-LBBB are also presented, and there was no difference between the groups (122 ± 102 ms vs. 100 ± 86 ms, p=0.51).
Figure 3. Septal-to-lateral wall delay differences between groups.
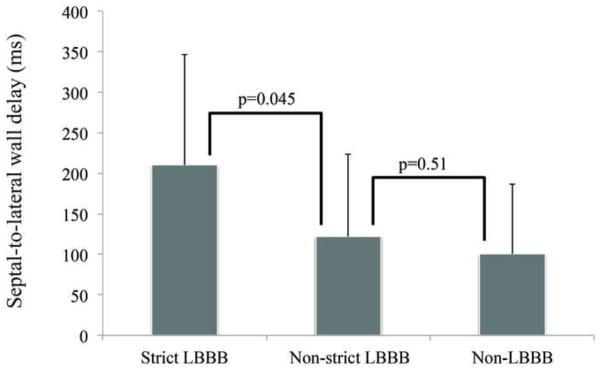
A difference was found for strict LBBB patients compared to non-strict LBBB patients (210 ± 137 ms vs. 122 ± 102 ms, p=0.045). The difference in the mean values was 88 ms, indicating an almost doubled septal-to-lateral wall delay in strict LBBB patients. However, no difference was found for non-strict LBBB patients compared to non-LBBB patients (122 ± 102 ms vs. 100 ± 86 ms, p=0.51). Error bars denote the standard deviation.
We also assessed whether there was a direct association between absolute QRS duration and septal-to-lateral wall delay. For the whole population, there was a modest relationship (r=0.35, p=0.005), however, no significant relationship was found within any of the three study groups (strict LBBB: r=0.14, p=0.44, non-strict LBBB: r=0.21, p=0.39, non-LBBB: r=0.29, p=0.32).
Discussion
In this study, we found that strict LBBB ECG criteria identify patients with greater LV mechanical dyssynchrony compared to patients meeting non-strict LBBB criteria, while non-strict LBBB and non-LBBB patients do not differ. This suggests that patients who only meet non-strict LBBB criteria may not have a true complete LBBB. Interestingly, patients who had non-strict LBBB criteria more commonly had ischemic cardiomyopathy and a greater amount of total LV scar compared to strict LBBB patients. This further highlights the differences between strict LBBB and non-strict LBBB patients and could explain differential responses to CRT that have been reported previously.
Cardiac Resynchronization Therapy and Left Bundle Branch Block
Prior studies have demonstrated that LBBB is a critical predictor of which patients will benefit from CRT.18 In the Multicenter Automated Defibrillator Implantation Trial – Cardiac Resynchronization (MADIT-CRT), patients with LBBB had a 53% reduction in heart failure events or death while those without LBBB did not benefit.3 Furthermore, in patients with nonspecific LV conduction delay, there was a trend toward CRT causing an increase in heart failure events.3 This highlights the importance of differentiating complete LBBB from nonspecific LV conduction delay. Interestingly, Mascioli et al. assessed the ability of the same strict LBBB criteria used in the present study to predict response to CRT in comparison to patients meeting non-strict LBBB criteria. They showed that patients meeting non-strict LBBB criteria had a four-fold higher rate of heart failure hospitalizations or death compared to strict LBBB patients, which was independent of QRS duration. This suggests that patients with strict LBBB respond better to CRT and in fact, these patients were the most likely to show evidence of improved LVEF after CRT.9 The relationship between absolute QRS duration and mechanical dyssynchrony has been studied previously by Bleeker, et al., and they found no relationship.19 In the current study, we found a weak relationship between QRS duration and septal-to-lateral wall delay for the whole population with an R-value of 0.35. However, within each subgroup, no relationship was found. This indicates that QRS duration alone does not appear to be a major predictor of mechanical dyssynchrony.
Conventional vs. Strict Left Bundle Branch Block Criteria
Conventional criteria for LBBB were developed 70 to 100 years ago.5 In 1956, work by Grant and Dodge indicated that more than one third of the patients diagnosed with LBBB by conventional criteria were likely misdiagnosed because there was no change in the direction of electrical activation in the beginning of the QRS with the onset of the supposed LBBB.20 In the normal heart, the first 30–40 ms of the QRS complex is dominated by left to right activation of the interventricular septum.8 However, a heart with LBBB only has activation from right to left and Grant and Dodge noted that the direction of electrical forces changes in the first 30–40 ms of the QRS complex after onset of LBBB. In addition, they noted that QRS duration was commonly prolonged by 70–80 ms in true LBBB, not 40 ms as had been proposed by canine studies.20 More recently, two endocardial mapping studies have demonstrated that one third of patients with LBBB by conventional ECG criteria do not have endocardial activation consistent with LBBB.6,7 Specifically, Aurrichio et al. found that in two thirds of patients there was a >40 ms delay from the start of endocardial activation in the RV to endocardial activation in the LV, while in one third of patients the delay was <20 ms. There were no patients with a 20 to 40 ms delay.7 Computer simulations of left bundle branch block8 confirmed that the minimum amount of time to activate the septum from right to left is 40 ms, indicating that the third of patients in the Aurrichio et al. study with <20 ms delay likely did not have a complete LBBB. Taken together, these findings support the assumption that one third of the patients do not have a true LBBB but rather a non-specific LV conduction delay. In the present study, patients with strict LBBB had longer QRS duration (163 ± 21 ms) than patients meeting non-strict LBBB criteria (129 ± 16).
Prior simulations also highlighted the importance of having mid-QRS notching/slurring that begins after the first 40 ms, which coincides with the time when activation breaks through to the endocardium of the LV in LBBB.8 Of note, in 1985 the World Health Organization presented criteria for LBBB21,22 which recommend the presence of “broad and notched or slurred” R in I and V5 or V6. However, there was no specification as to when the notching should occur. Hence, the strict LBBB criteria used in the current study, which includes notching criteria, may provide higher specificity for true LBBB.
Myocardial strain in Left Bundle Brach Block patients
Myocardial tagging with CMR provides highly reproducible results that are largely user independent.10 The strain patterns in healthy subjects have been studied in detail in other studies and have demonstrated that normal mechanical activation starts in the septum and proceeds to the lateral wall.17 A CMR animal study where the hearts were paced from different locations showed that when pacing from the right atrium, the mechanical activation spread fast through the LV but when pacing from the right ventricle it started in the septum and spread slowly through the LV,17 causing the same type of activation pattern found in hearts with LBBB.
In this study, we found markedly abnormal septal contraction, with many patients, especially those with strict LBBB, having only positive strain (i.e. dyskinesia) in one or both of the septal segments. In the cases with negative strain (i.e. shortening) in the septum it was often of very low magnitude, almost akinetic, and the peak of strain often occurred very early following the QRS R-wave and was followed by positive strain signifying stretching. This early peak of contraction in the septum followed by stretching has been shown in a previous study of patients with LBBB.23 Another study investigated the early contraction in the septum in patients with LBBB and concluded that it indeed was true contraction, but stretching of the septum from other forces prematurely halted the negative strain and therefore the very early peak occurred.24
There are possible explanations for the early peak followed by stretching of the septum and one could speculate that in the cases with a true LBBB, the right ventricle stretches the septum by pulling it toward the RV since it is activated before the LV.21 In addition, the right ventricle also empties earlier, which results in higher relative pressure in the LV than in the right ventricle that could push the septum towards the right ventricle. Another explanation could be that because of the dyssynchronous activation and contraction of the LV, there may be differences in local loading conditions over the cardiac cycle.25 Hence, in the early part of systole, there is early septal activation leading to unopposed septal contraction but this septal contraction is not sufficiently strong to eject blood out from the LV. Instead, the force from the septal contraction results in stretching of the lateral wall. When the lateral wall is finally activated, the force of its contraction overrides the force in the septum and prematurely terminates its contraction. This competitive action tends to prohibit the attainment of the “true peak” of the septal strain, and creates an earlier “false peak” of contraction in the septum. This phenomenon will require further investigation in a study where the synchrony of LV wall contraction of completely healthy subjects is compared to that of patients meeting strict LBBB criteria.
Limitations
A limitation of this study is the temporal resolution of CMR tagging, which ranged from 25–40 frames per second (25–40 ms between frames). This may have adversely influenced the detection of peak strain and thus contributed to the exclusion of 9 patients. The temporal resolution also made it impossible to detect differences in the onset of contraction between different ECG groups. However, it was possible to detect the time-to-peak contraction, which is of longer duration. Future studies should compare findings to other measures of mechanical dyssynchrony, such as echocardiography color tissue Doppler imaging, which has a temporal resolution >130 frames per second.25 Between the strict and non-strict LBBB groups, there were differences in scar size and ischemic etiology, two factors that can affect mechanical contraction. However, there was more ischemic etiology and scar in the non-strict LBBB group, which had less septal-to-lateral wall dyssynchrony than the strict LBBB patients.
Conclusions
Patients with strict LBBB ECG criteria have greater LV dyssynchrony, defined as prolonged time between septal and lateral wall contraction, compared to patients meeting non-strict LBBB criteria (i.e. conventional LBBB criteria but not meeting the strict LBBB criteria). Patients with non-strict LBBB have more ischemic cardiomyopathy and lateral wall scar and their septal-to-lateral wall delay is not different from patients with nonspecific LV conduction delay and QRS duration 110–119 ms. The strict LBBB ECG criteria thus identify patients with greater mechanical intraventricular dyssynchrony. Further investigation should confirm prior findings that the strict LBBB criteria can better predict response to CRT and clinical outcomes.
Acknowledgments
Use of the custom research software, CINEtool, was obtained through a research agreement between Dr. Wu and GE Healthcare, WI, USA.
Financial Support
The study was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (HL103812 to KCW, HL91062 to GFT, and HL61912 to RGW), the DW Reynolds Foundation and the FDA Critical Path Initiative.
Footnotes
Disclaimer
The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bristow M, Feldman A, Saxon L. Heart failure management using implantable devices for ventricular resynchronization: Comparison of medical therapy, pacing, and defibrillation in chronic heart failure (COMPANION) trial. J Card Fail. 2000;6(3):276–285. doi: 10.1054/jcaf.2000.9501. [DOI] [PubMed] [Google Scholar]
- 2.Epstein AE, DiMarco JP, Ellenbogen Ka, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline. Circulation. 2008;117(21):e350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 3.Zareba W, Klein H, Cygankiewicz I, et al. Effectiveness of Cardiac Resynchronization Therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) Circulation. 2011;123(10):1061–72. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 4.Tang AS, Wells GA, Talajic M, et al. Cardiac-Resynchronization Therapy for Mild-to-Moderate Heart Failure. N Engl J Med. 2012;363(25):2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 5.Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol. 2011;107(6):927–34. doi: 10.1016/j.amjcard.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Vassallo JA, Cassidy DM, Marchlinski FE, et al. Endocardial activation of left bundle branch block. Circulation. 1984;69(5):914–923. doi: 10.1161/01.cir.69.5.914. [DOI] [PubMed] [Google Scholar]
- 7.Auricchio A, Fantoni C, Regoli F, et al. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation. 2004;109(9):1133–9. doi: 10.1161/01.CIR.0000118502.91105.F6. [DOI] [PubMed] [Google Scholar]
- 8.Strauss DG, Selvester RH. The QRS complex--a biomarker that “images” the heart: QRS scores to quantify myocardial scar in the presence of normal and abnormal ventricular conduction. J Electrocardiol. 2009;42(1):85–96. doi: 10.1016/j.jelectrocard.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Mascioli G, Padeletti L, Sassone B, et al. Electrocardiographic Criteria of True Left Bundle Branch Block: A Simple Sign to Predict a Better Clinical and Instrumental Response to CRT. Pacing Clin Electrophysiol. 2012;35:927–34. doi: 10.1111/j.1540-8159.2012.03427.x. [DOI] [PubMed] [Google Scholar]
- 10.Lardo AC, Abraham TP, Kass Da. Magnetic resonance imaging assessment of ventricular dyssynchrony: current and emerging concepts. J Am Coll Cardiol. 2005;46(12):2223–8. doi: 10.1016/j.jacc.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt A, Azevedo CF, Cheng A, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115(15):2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51(25):2414–21. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strauss DG, Selvester RH, Lima JaC, et al. ECG quantification of myocardial scar in cardiomyopathy patients with or without conduction defects: correlation with cardiac magnetic resonance and arrhythmogenesis. Circ Arrhythm Electrophysiol. 2008;1(5):327–36. doi: 10.1161/CIRCEP.108.798660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu KC, Gerstenblith G, Guallar E, et al. Combined Cardiac MRI and C-Reactive Protein Levels Identify a Cohort at Low Risk for Defibrillator Firings and Death. Circ Cardiovasc Imaging. 2012:178–186. doi: 10.1161/CIRCIMAGING.111.968024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandes VR, Wu KC, Rosen BD, Tomaselli G, Marba E. Enhanced Infarct Border Zone Inducibility of Monomorphic Ventricular Tachycardia in Patients with Ischemic Cardiomyopathy. Radiology. 2007;245(3) doi: 10.1148/radiol.2452061615. [DOI] [PubMed] [Google Scholar]
- 16.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart. Circulation. 2002:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 17.Castillo E, Osman N, Rosen B, et al. Quantitative Assessment of Regional Myocardial Function with MR-Tagging in a Multi-Center Study: Interobserver and Intraobserver Agreement of Fast Strain Analysis with Harmonic Phase (HARP) MRI. J Cardiovasc Magn Reson. 2005;7(5):783–791. doi: 10.1080/10976640500295417. [DOI] [PubMed] [Google Scholar]
- 18.Sipahi I, Chou JC, Hyden M, et al. Effect of QRS morphology on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials. Am Heart J. 2012;163(2):260–7.e3. doi: 10.1016/j.ahj.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bleeker GB, Schalij MJ, Molhoek SG, et al. Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol. 2004;15(5):544–9. doi: 10.1046/j.1540-8167.2004.03604.x. [DOI] [PubMed] [Google Scholar]
- 20.Grant R, Dodge H. Mechanisms of QRS Complex Prolongation. Am J Med. 1956;20:834–852. doi: 10.1016/0002-9343(56)90204-2. [DOI] [PubMed] [Google Scholar]
- 21.Willems JL, Robles de Medina EO, Bernard R, et al. Criteria for intraventricular conduction disturbances and pre-excitation. J Am Coll Cardiol. 1985;5(6):1261–1275. doi: 10.1016/s0735-1097(85)80335-1. [DOI] [PubMed] [Google Scholar]
- 22.Surawicz B, Childers R, Deal BJ, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee. J Am Coll Cardiol. 2009;53(11):976–81. doi: 10.1016/j.jacc.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Lecoq G, Leclercq C, Leray E, et al. Clinical and electrocardiographic predictors of a positive response to cardiac resynchronization therapy in advanced heart failure. Eur Heart J. 2005;26(11):1094–100. doi: 10.1093/eurheartj/ehi146. [DOI] [PubMed] [Google Scholar]
- 24.Gjesdal O, Remme EW, Opdahl A, et al. Mechanisms of abnormal systolic motion of the interventricular septum during left bundle-branch block. Circ Cardiovasc Imagin. 2011;4(3):264–73. doi: 10.1161/CIRCIMAGING.110.961417. [DOI] [PubMed] [Google Scholar]
- 25.Risum N, Jons C, Olsen NT, et al. Simple regional strain pattern analysis to predict response to cardiac resynchronization therapy: rationale, initial results, and advantages. Am Heart J. 2012;163(4):697–704. doi: 10.1016/j.ahj.2012.01.025. [DOI] [PubMed] [Google Scholar]