Abstract
A number of plant species were examined for the presence of pyruvate kinase (pyruvate-ATP phosphotransferase, EC 2.7.1.40), and of a phosphatase activity which hydrolyzes phosphoenolpyruvate. Of those examined, only cotton (Gossypium sp. L.) seeds were found to be sufficiently free of the phosphatase to permit a kinetic study of pyruvate kinase.
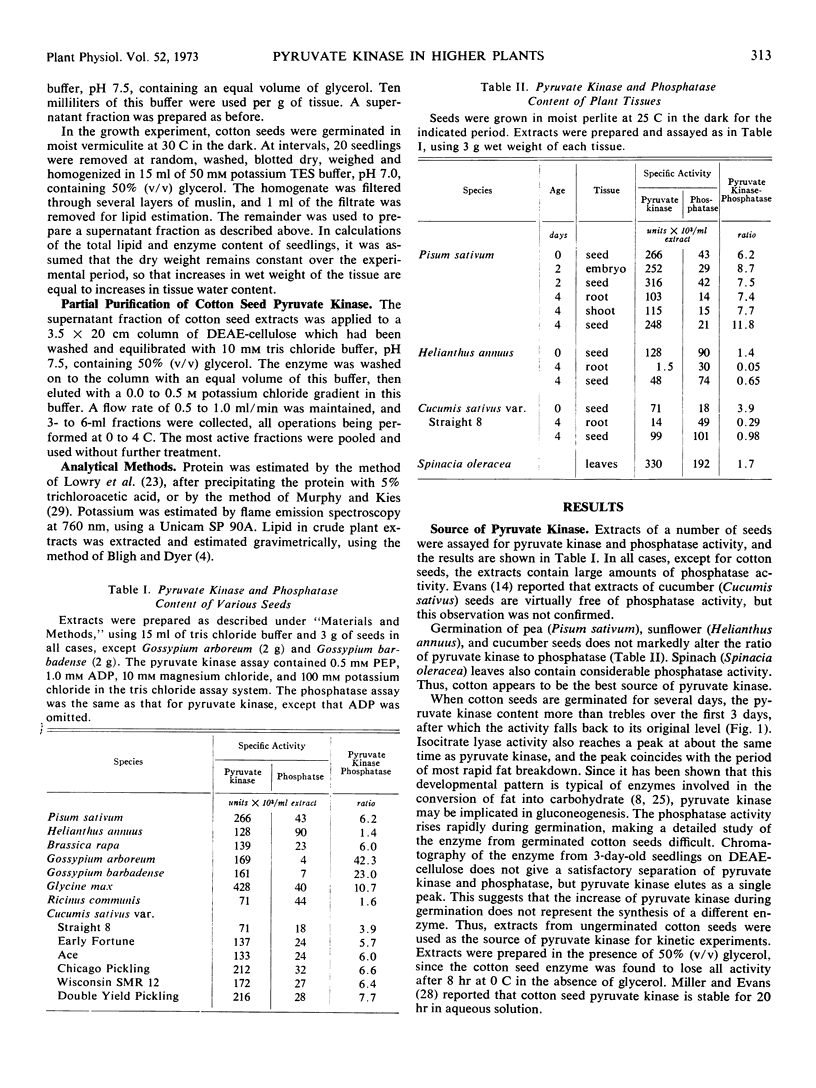
During germination of cotton seeds, pyruvate kinase activity rises for the first 3 days, after which it falls back to its original level. This developmental pattern is characteristic of enzymes involved in the conversion of fat into carbohydrate in fatstoring seeds. The phosphatase also rose rapidly during germination, which precluded the use of extracts from seedlings in the study of pyruvate kinase. No evidence was found for the presence of more than one pyruvate kinase in cotton seedlings.
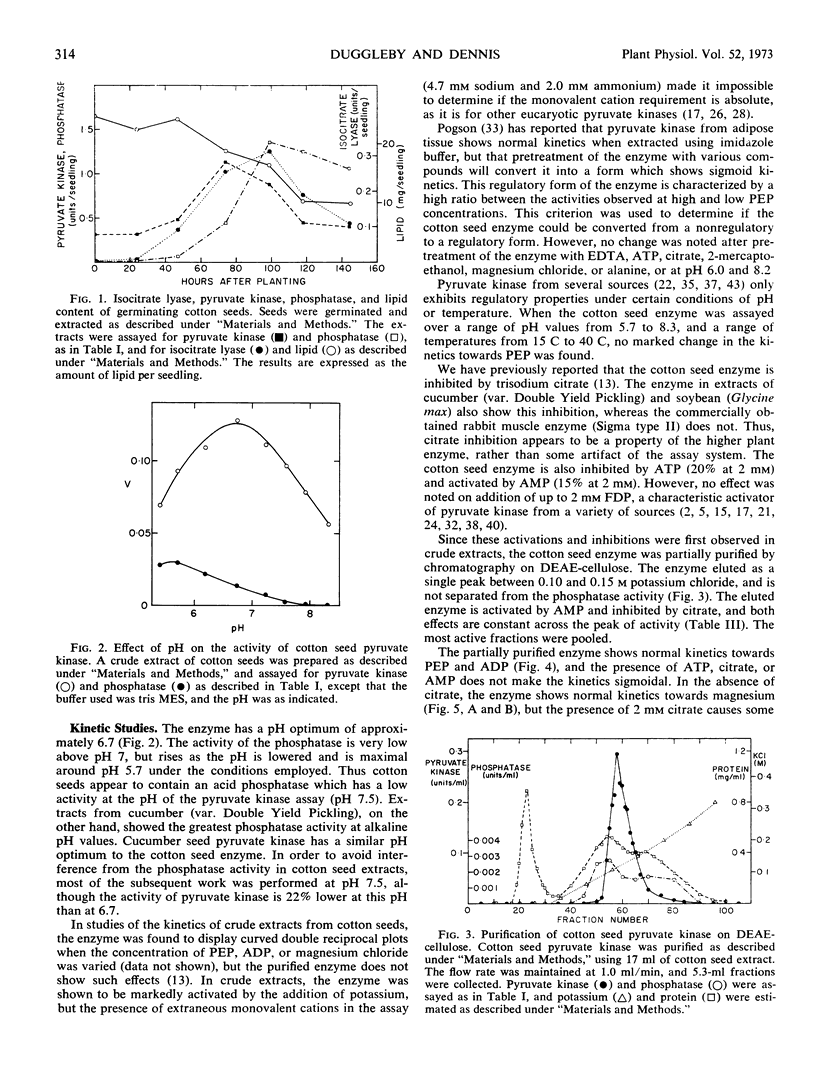
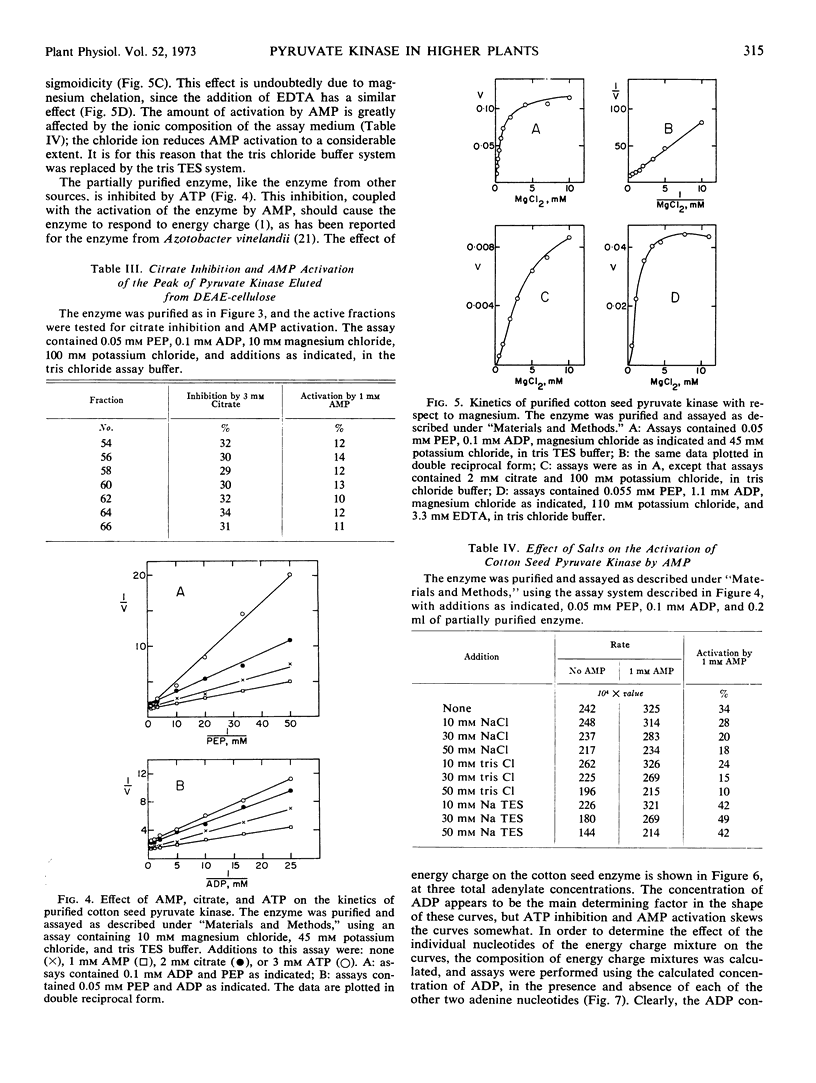
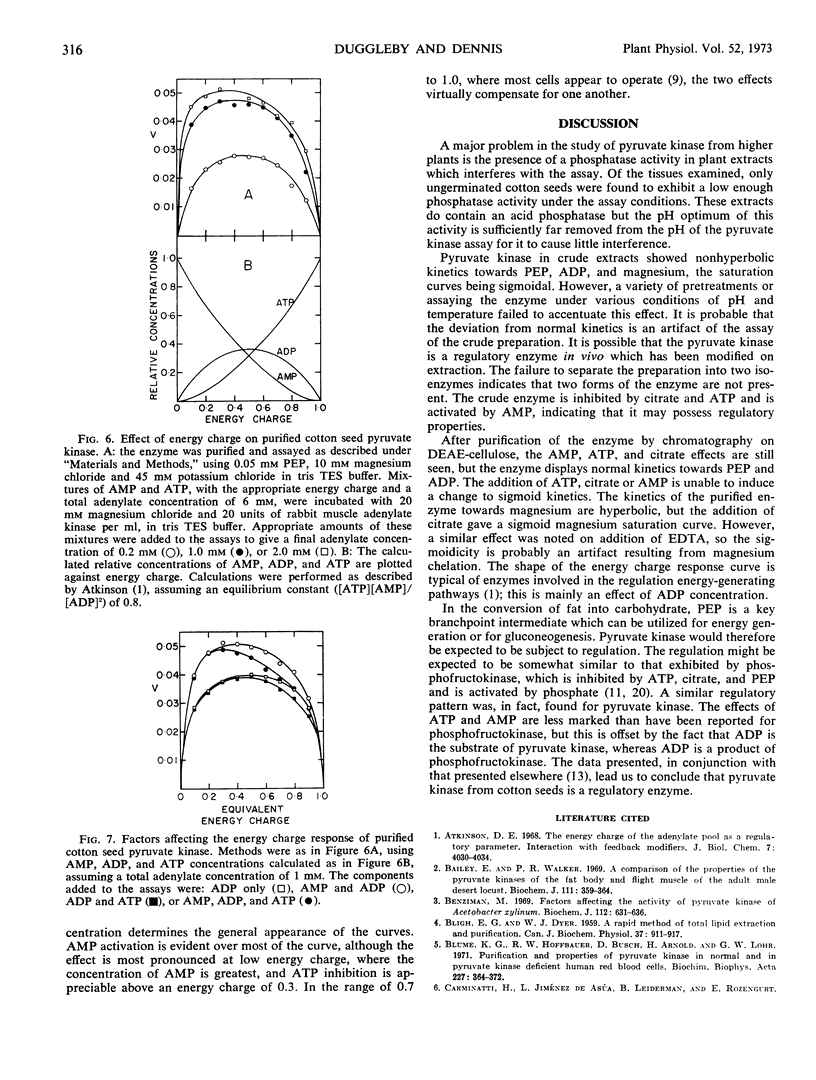
In crude extracts from ungerminated seeds, the enzyme shows slight deviations from normal kinetics with respect to phosphoenolpyruvate, magnesium, and to a lesser extent, ADP. After partial purification of the enzyme by ion exchange chromatography, the enzyme shows normal kinetics. The enzyme is activated by AMP, and inhibited by both ATP and citrate, in both crude and partially purified preparations. It is suggested that cotton seed pyruvate kinase is a regulatory enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bailey E., Walker P. R. A comparison of the properties of the pyruvate kinases of the fat body and flight muscle of the adult male desert locust. Biochem J. 1969 Feb;111(3):359–364. doi: 10.1042/bj1110359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benziman M. Factors afecting the activity of pyruvate kinase of Acetobacter xylinum. Biochem J. 1969 May;112(5):631–636. doi: 10.1042/bj1120631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume K. G., Hoffbauer R. W., Busch D., Arnold H., Löhr G. W. Purification and properties of pyruvate kinase in normal and in pyruvate kinase deficient human red blood cells. Biochim Biophys Acta. 1971 Feb 10;227(2):364–372. doi: 10.1016/0005-2744(71)90068-4. [DOI] [PubMed] [Google Scholar]
- Carminatti H., Jiménez de Asúa L., Recondo E., Passeron S., Rozengurt E. Some kinetic properties of liver pyruvate kinase (type L). J Biol Chem. 1968 Jun 10;243(11):3051–3056. [PubMed] [Google Scholar]
- Carpenter W. D., Beevers H. Distribution and Properties of Isocitritase in Plants. Plant Physiol. 1959 Jul;34(4):403–409. doi: 10.1104/pp.34.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish A. S., Johnson E. J. Regulation of pyruvate kinase from Thiobacillus neapolitanus. Arch Biochem Biophys. 1971 Feb;142(2):584–590. doi: 10.1016/0003-9861(71)90522-4. [DOI] [PubMed] [Google Scholar]
- Dennis D. T., Coultate T. P. The regulatory properties of a plant phosphofructokinase during leaf development. Biochim Biophys Acta. 1967 Sep 12;146(1):129–137. doi: 10.1016/0005-2744(67)90079-4. [DOI] [PubMed] [Google Scholar]
- Duggleby R. G., Dennis D. T. The characterization and regulatory properties of pyruvate kinase from cotton seeds. Arch Biochem Biophys. 1973 Apr;155(2):270–277. doi: 10.1016/0003-9861(73)90115-x. [DOI] [PubMed] [Google Scholar]
- Evans H. J. Effect of Potassium & Other Univalent Cations on Activity of Pyruvate Kinase in Pisum sativum. Plant Physiol. 1963 Jul;38(4):397–402. doi: 10.1104/pp.38.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders L. E., Bamburg J. R., Sallach H. J. Pyruvate kinase isozymes in adult tissue and eggs of Rana pipiens. II. Physical and kinetic studies of purified skeletal and heart muscle pyruvate kinases. Biochim Biophys Acta. 1971 Sep 22;242(3):566–579. doi: 10.1016/0005-2744(71)90150-1. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M., Gancedo C., Sols A. Regulation of the concentration or activity of pyruvate kinase in yeasts and its relationship to gluconeogenesis. Biochem J. 1967 Feb;102(2):23C–25C. doi: 10.1042/bj1020023c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsley J. R., Suelter C. H. Yeast pyruvate kinase. II. Kinetic properties. J Biol Chem. 1969 Sep 25;244(18):4819–4822. [PubMed] [Google Scholar]
- Ibsen K. H., Schiller K. W., Haas T. A. Interconvertible kinetic and physical forms of human erythrocyte pyruvate kinase. J Biol Chem. 1971 Mar 10;246(5):1233–1240. [PubMed] [Google Scholar]
- Jiménez de Asúa L., Rozengurt E., Carminatti H. Some kinetic properties of liver pyruvate kinase (type L). 3. Effect of monovalent cations on its allosteric behavior. J Biol Chem. 1970 Aug 10;245(15):3901–3905. [PubMed] [Google Scholar]
- Kelly G. J., Turner J. F. Cooperativity in pea-seed phosphofructokinase. Biochim Biophys Acta. 1971 Sep 22;242(3):559–565. doi: 10.1016/0005-2744(71)90149-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liao C. L., Atkinson D. E. Regulation at the phosphoenolpyruvate branchpoint in Azotobacter vinelandii: pyruvate kinase. J Bacteriol. 1971 Apr;106(1):37–44. doi: 10.1128/jb.106.1.37-44.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente P., Marco R., Sols A. Regulation of liver pyruvate kinase and the phosphoenolpyruvate crossroads. Eur J Biochem. 1970 Mar 1;13(1):45–54. doi: 10.1111/j.1432-1033.1970.tb00897.x. [DOI] [PubMed] [Google Scholar]
- MARCUS A., VELASCO J. Enzymes of the glyoxylate cycle in germinating peanuts and castor beans. J Biol Chem. 1960 Mar;235:563–567. [PubMed] [Google Scholar]
- Malcovati M., Kornberg H. L. Two types of pyruvate kinase in Escherichia coli K12. Biochim Biophys Acta. 1969 Apr 22;178(2):420–423. doi: 10.1016/0005-2744(69)90417-3. [DOI] [PubMed] [Google Scholar]
- Miller G., Evans H. J. The Influence of Salts on Pyruvate Kinase from Tissues of Higher Plants. Plant Physiol. 1957 Jul;32(4):346–354. doi: 10.1104/pp.32.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa T., Hochachka P. W. Catalytic and regulatory properties of pyruvate kinases in tissues of a marine bivalve. J Biol Chem. 1971 May 25;246(10):3196–3203. [PubMed] [Google Scholar]
- Ohmann E. Die Regulation der Pyruvat-Kinase in Euglena gracilis. Arch Mikrobiol. 1969;67(3):273–292. doi: 10.1007/BF00411262. [DOI] [PubMed] [Google Scholar]
- Passeron S., Terenzi H. Activation of pyruvate kinase of Mucor Rouxii by manganese ions. FEBS Lett. 1970 Feb 16;6(3):213–216. doi: 10.1016/0014-5793(70)80060-6. [DOI] [PubMed] [Google Scholar]
- Pogson C. I. Adipose-tissue pyruvate kinase. Properties and interconversion of two active forms. Biochem J. 1968 Nov;110(1):67–77. doi: 10.1042/bj1100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson C. I. Two interconvertible forms of pyruvate kinase in adipose tissue. Biochem Biophys Res Commun. 1968 Feb 15;30(3):297–302. doi: 10.1016/0006-291x(68)90450-6. [DOI] [PubMed] [Google Scholar]
- Somero G. N. Pyruvate kinase variants of the Alaskan king-crab. Evidence for a temperature-dependent interconversion between two forms having distinct and adaptive kinetic properties. Biochem J. 1969 Sep;114(2):237–241. doi: 10.1042/bj1140237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. R., Moore D. Factors affecting the level and activity of pyruvate kinase from Coprinus lagopus sensu Buller. J Gen Microbiol. 1971 Jun;66(3):361–370. doi: 10.1099/00221287-66-3-361. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Goto Y., Akazawa T. Pyruvate Kinase Activity of Wheat Plants Grown under Potassium Deficient Conditions. Plant Physiol. 1968 May;43(5):730–734. doi: 10.1104/pp.43.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen F. W., Bernlohr R. W. Pyruvate kinase of the spore-forming bacterium, Bacillus licheniformis. II. Kinetic properties. J Biol Chem. 1971 Mar 25;246(6):1746–1755. [PubMed] [Google Scholar]
- Vijayvargiya R., Schwark W. S., Singhal R. L. Metabolic control mechanisms in mammalian systems. XI. Pyruvate kinase modulation in the rat prostate and seminal vesicles. Can J Biochem. 1970 Nov;48(11):1268–1277. doi: 10.1139/o70-196. [DOI] [PubMed] [Google Scholar]
- Wieker H. J., Hess B. Allosteric interactions of yeast pyruvate kinase as a function of pH. Biochemistry. 1971 Mar 30;10(7):1243–1248. doi: 10.1021/bi00783a022. [DOI] [PubMed] [Google Scholar]


