Abstract
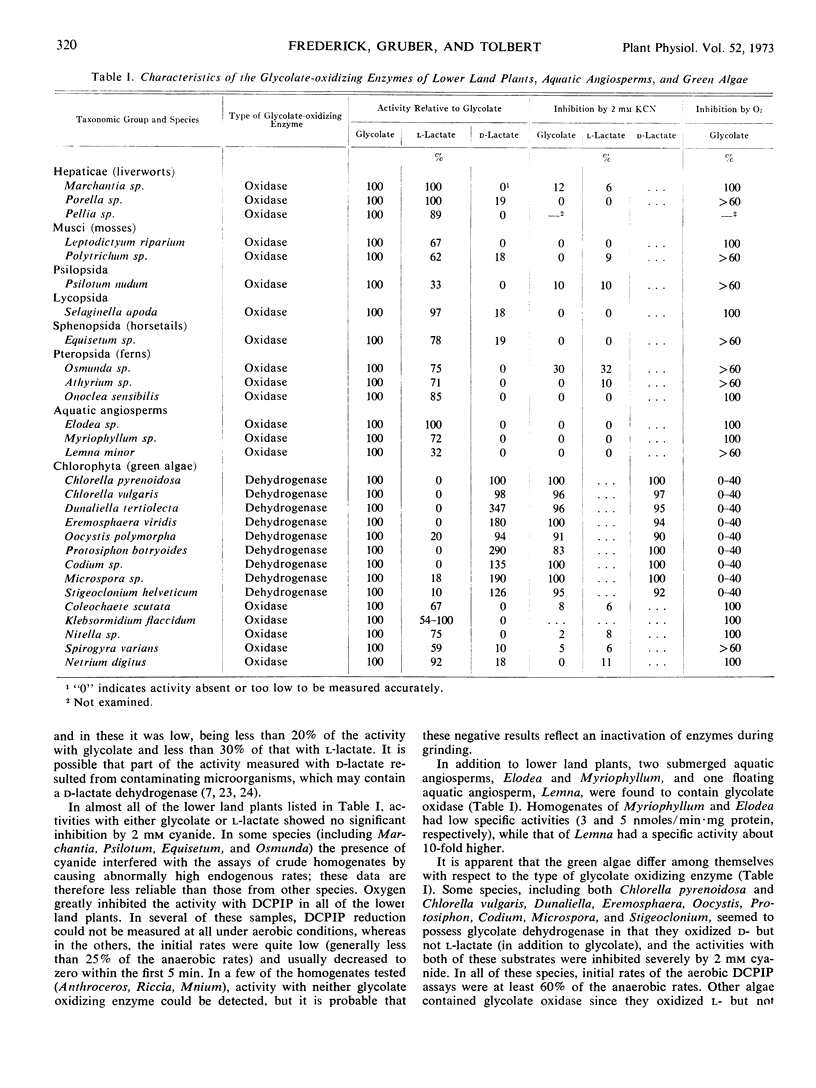
Homogenates of various lower land plants, aquatic angiosperms, and green algae were assayed for glycolate oxidase, a peroxisomal enzyme present in green leaves of higher plants, and for glycolate dehydrogenase, a functionally analogous enzyme characteristic of certain green algae. Green tissues of all lower land plants examined (including mosses, liverworts, ferns, and fern allies), as well as three freshwater aquatic angiosperms, contained an enzyme resembling glycolate oxidase, in that it oxidized l- but not d-lactate in addition to glycolate, and was insensitive to 2 mm cyanide. Many of the green algae (including Chlorella vulgaris, previously claimed to have glycolate oxidase) contained an enzyme resembling glycolate dehydrogenase, in that it oxidized d- but not l-lactate, and was inhibited by 2 mm cyanide. Other green algae had activity characteristic of glycolate oxidase and, accordingly, showed a substantial glycolate-dependent O2 uptake. It is pointed out that this distribution pattern of glycolate oxidase and glycolate dehydrogenase among the green plants may have phylogenetic significance.
Activities of catalase, a marker enzyme for peroxisomes, were also determined and were generally lower in the algae than in the land plants or aquatic angiosperms. Among the algae, however, there were no consistent correlations between levels of catalase and the type of enzyme which oxidized glycolate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brody M., White J. E. Environmental factors controlling enzymatic activity in microbodies and mitochondria of Euglena gracilis. FEBS Lett. 1972 Jun 15;23(2):149–152. doi: 10.1016/0014-5793(72)80327-2. [DOI] [PubMed] [Google Scholar]
- Bruin W. J., Nelson E. B., Tolbert N. E. Glycolate pathway in green algae. Plant Physiol. 1970 Sep;46(3):386–391. doi: 10.1104/pp.46.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt J. S. The CO2 compensation point, Hill acitivity and photorespiration. Biochem Biophys Res Commun. 1969 Jun 6;35(5):748–753. doi: 10.1016/0006-291x(69)90469-0. [DOI] [PubMed] [Google Scholar]
- CREMONA T. THE LACTIC DEHYDROGENASES OF YEAST. IV. D-ALPHA-HYDROXY ACID DEHYDROGENASE. J Biol Chem. 1964 May;239:1457–1465. [PubMed] [Google Scholar]
- Codd G. A., Lord J. M., Merrett M. J. The glycollate oxidising enzyme of algae. FEBS Lett. 1969 Dec 30;5(5):341–342. doi: 10.1016/0014-5793(69)80352-2. [DOI] [PubMed] [Google Scholar]
- Codd G. A., Schmid G. H., Kowallik W. Enzymic evidence for peroxisomes in a mutant of Chlorella vulgaris. Arch Mikrobiol. 1972;81(3):264–272. doi: 10.1007/BF00412245. [DOI] [PubMed] [Google Scholar]
- Downton W. J., Tregunna E. B. Photorespiration and Glycolate Metabolism: A Re-examination and Correlation of Some Previous Studies. Plant Physiol. 1968 Jun;43(6):923–929. doi: 10.1104/pp.43.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick S. E., Newcomb E. H. Cytochemical localization of catalase in leaf microbodies (peroxisomes). J Cell Biol. 1969 Nov;43(2):343–353. doi: 10.1083/jcb.43.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. Zur Lokalisation von Enzymen der Microbodies in Polytomella caeca. Arch Mikrobiol. 1971;80(3):205–218. [PubMed] [Google Scholar]
- Graves L. B., Jr, Hanzely L., Trelease R. N. The occurrence and fine structural characterization of microbodies in Euglena gracilis. Protoplasma. 1971;72(2):141–152. doi: 10.1007/BF01279047. [DOI] [PubMed] [Google Scholar]
- Graves L. B., Jr, Trelease R. N., Grill A., Becker W. M. Localization of glyoxylate cycle enzymes in glyoxysomes in Euglena. J Protozool. 1972 Aug;19(3):527–532. doi: 10.1111/j.1550-7408.1972.tb03521.x. [DOI] [PubMed] [Google Scholar]
- Graves L. B., Trelease R. N., Becker W. M. Particulate nature of glycolate dehydrogenase in euglena: possible localization in microbodies. Biochem Biophys Res Commun. 1971 Jul 16;44(2):280–286. doi: 10.1016/0006-291x(71)90596-1. [DOI] [PubMed] [Google Scholar]
- HUGHES E. O., GORHAM P. R., ZEHNDER A. Toxicity of a unialgal culture of Microcystis aeruginosa. Can J Microbiol. 1958 Jun;4(3):225–236. doi: 10.1139/m58-024. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Kemp M. B. D- and L-lactate dehydrogenases of Pseudomonas aeruginosa. Biochem J. 1972 Nov;130(1):307–309. doi: 10.1042/bj1300307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lord J. M., Merrett M. J. The intracellular localization of glycollate oxidoreductase in Euglena gracilis. Biochem J. 1971 Sep;124(2):275–281. doi: 10.1042/bj1240275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Merrett M. J. The pathway of glycollate utilization in Chlorella pyrenoidosa. Biochem J. 1970 May;117(5):929–937. doi: 10.1042/bj1170929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. Glycolate dehydrogenase in green algae. Arch Biochem Biophys. 1970 Nov;141(1):102–110. doi: 10.1016/0003-9861(70)90112-8. [DOI] [PubMed] [Google Scholar]
- Pulich W. M., Ward C. H. Physiology and Ultrastructure of an Oxygen-resistant Chlorella Mutant under Heterotrophic Conditions. Plant Physiol. 1973 Feb;51(2):337–344. doi: 10.1104/pp.51.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart K. D., Floyd G. L., Mattox K. R., Davis M. E. Cytochemical demonstration of a single peroxisome in a filamentous green alga. J Cell Biol. 1972 Aug;54(2):431–434. doi: 10.1083/jcb.54.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]


