Abstract
Introduction
Breast cancer survivors experience diminished health-related quality of life (HRQOL). We report on the influence of tai chi chuan exercise (TCC) on HRQOL and explore associations between changes in HRQOL and biomarkers.
Methods
Breast cancer survivors (N=21) were randomly assigned to TCC or standard support therapy (SST) for 12 weeks (three times/week; 60 min/session). Interleukin-6, interleukin-8 (IL-8), insulin-like growth factor-1 (IGF-1), insulin-like growth factor-binding protein (IBFBP)-1, IGFBP-3, glucose, insulin, and cortisol were measured pre- and postintervention. Overall HRQOL and subdomains were assessed at preintervention (T1), midintervention (T2) and postintervention (T3) and biomarkers at T1 and T3.
Results
The TCC group improved in total HRQOL (T1–T2: CS=8.54, P=0.045), physical functioning (T1–T2:CS=1.89, P=0.030), physical role limitations (T1–T2 CS=1.55, P=0.023), social functioning (T1–T3:CS=1.50, P=0.020), and general mental health (T1–-T2:CS=2.67, P=0.014; T1–T3: CS=2.44, P=0.019). The SST improved in social functioning (T1–T2:CS=0.64, P=0.043) and vitality (T1–T2:CS=0.90, P=0.01). There were relationships between changes in IGF-1 and overall HRQOL (r=−0.56; P<0.05), physical role limitation (r=−0.68; P<0.05), and social functioning (r=−0.56; P<0.05). IGFBP-1 changes were associated with physical role limitation changes (r=0.60; P<0.05). IGFBP-3 changes were associated with physical functioning changes (r=0.46; P≤0.05). Cortisol changes were associated with changes in physical role limitations (r=0.74; P<0.05) and health perceptions (r=0.46; P<0.05). Glucose changes were associated with emotional role limitation changes (r=−0.70; P<0.001). IL-8 changes were associated with emotional role limitation changes (r=0.59; P<0.05).
Discussion/conclusions
TCC may improve HRQOL by regulating inflammatory responses and other biomarkers associated with side effects from cancer and its treatments. Implications for cancer survivors TCC may be an intervention capable of improving HRQOL in breast cancer survivors.
Keywords: Breast cancer, Inflammation, Exercise, Quality of life
Introduction
Breast cancer is the most common type of cancer, aside from skin cancer, in women in the USA [1]. Treatment for breast cancer often includes, alone or in combination, surgery, chemotherapy, radiation therapy, and hormone therapy. Although nearly 89% of women survive at least 5 years after diagnosis [1], treatment side effects can be debilitating and persistent, lasting months to years following diagnosis and treatment. It is important to consider not only clinical outcomes following cancer treatment, such as the likelihood of recurrence and survival, but also the impact of cancer and cancer treatment on function and well-being. Side effects from breast cancer and its treatments include cancer-related fatigue [2, 3], cognitive impairment [4, 5], impaired sleep quality [6, 7], depression [8–10], anxiety, and stress stemming from fear of recurrence [9, 10], bone loss [11, 12], reduced functional capacity [13], weight gain [14, 15], and reduced muscular strength and endurance [13], all of which ultimately affect quality of life (QOL). Fatigue and cognitive impairment, in particular, reduce QOL, with symptoms lasting beyond 10 years in a subset of breast cancer survivors [16, 17].
These side effects or late effects have been linked with dysregulated inflammatory and metabolic processes. For example, research has shown that insulin and insulin-like growth factors and binding proteins are linked with increased adiposity and weight gain with loss of lean muscle mass among breast cancer survivors [18–20]. Proinflammatory cytokines including interleukin-6 (IL-6) and interleukin-8 (IL-8) are produced at high levels in the tumor microenvironment [21, 22] and are also expressed at high rates during radiation and chemotherapy [22, 23]. They are implicated in the development of fatigue, cognitive impairment, and sleep disturbance in cancer survivors [24]. Additionally, chronic inflammation may be the result of dysregulated hypothalamic–pituitary–adrenal (HPA) axis, as recent studies have shown cancer patients and survivors have either flatted or aberrant cortisol rhythm [25] and fatigued breast cancer survivors in particular have lower morning serum cortisol [26]. Stress can result in the failure of the HPA to counterregulate the immune system which can cause a host of metabolic and inflammatory alterations [27]. Molecules involved in glucose metabolism have also been associated with cancer- and cancer treatment-related side effects. For example, insulin-like growth factor-1 (IGF-1), which stimulates mitosis [28], inhibits apoptosis [29], is associated with breast cancer risk [30, 31], and ultimately may play a role in reduced physical function and fatigue. Bone loss is common in breast cancer survivors during and following treatment [32, 33]. In breast cancer survivors participating in a tai chi chuan exercise (TCC) intervention, changes in bone markers are correlated with inflammatory cytokines and growth factors, such as insulin-like growth factor-binding protein (IGFBP) -1 and IL-6 [34].
It stands to reason that these dysregulated metabolic and inflammatory processes may be the underlying mechanisms that explain different aspects of QOL that would be significantly influenced by these late effects. For example, health-related QOL (HRQOL), which encompasses health concepts such as physical functioning, physical role limitations, bodily pain, social functioning, general mental health which includes psychological distress and psychological well-being, emotional role limitations, vitality (energy/fatigue), and general health perceptions [35], would most likely be effected by dysregulated metabolic and inflammatory processes.
Figure 1 depicts the possible relationships between biomarkers and HRQOL. Cytokines such as IL-6 and IL-8, produced during treatment, may result in symptoms such as fatigue and anxiety, which, in turn, impact HRQOL. Cortisol dysregulation, due to HPA axis dysfunction, may result in a multitude of side effects including fatigue, which may impact HRQOL. Stress may result in HPA dysregulation and contribute to fatigue. Dysregulation of mediators of glucose metabolism, such as insulin, IGF-1, and glucose, may lead to weight gain, ultimately reducing HRQOL. Bone loss due to breast cancer treatment increases the fracture risk [36], which can impair HRQOL.
Fig. 1.
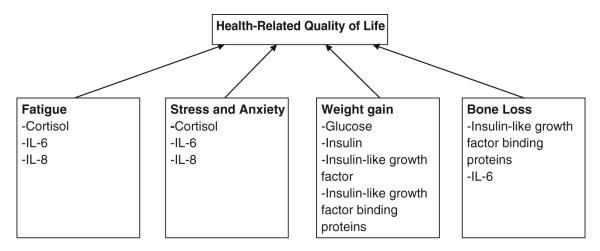
Relationship between health-related quality of life and biomarkers
Tai chi chuan has been found to be beneficial in those without a history of cancer for stress reduction, as assessed by salivary cortisol [37], reducing anxiety [38], and may boost the immune system [39]. Additionally, tai chi chuan can reduce blood pressure [38], favorably change the lipid profiles [38], and improve postural control [40, 41] and functional status [42]. Research also shows tai chi chuan may reduce systemic inflammation in older adults with high levels of IL-6 [43]. Tai chi chuan can reduce cardiovascular risk factors in those with dyslipidemia [44]. Previous research in breast cancer survivors has shown that tai chi chuan improves functional capacity [45], QOL [46], and self-esteem [47] and can regulate growth factors and binding proteins associated with weight gain and bone loss [34, 48].
No studies to date have shown whether these inflammatory markers, growth factors, or binding proteins are associated with HRQOL. Therefore we are conducting a secondary data analysis to determine how HRQOL and biomarkers change due to a 12-week tai chi chuan intervention and investigate the relationship between HRQOL and biomarkers.
Materials and methods
This is a phase II randomized controlled trial employing a traditional randomized controlled design. The results of this study have been previously reported [34, 45–47].
Study population
Breast cancer survivors were recruited by the Behavioral Health and Fitness Laboratory at the University of North Carolina Greensboro, Moses Cone Regional Cancer Center, and by the American Cancer Society through local mailings, flyers, and physician referrals. The principal investigator contacted those who expressed interest and provided a detailed explanation of the study and screened interested individuals for inclusion. In order to be eligible for participation, patients had to meet the following criteria: (1) female, (2) primary diagnosis of breast cancer stages 0–IIIb, (3) treatment completed more than 1 month prior but less than 30 months prior, (4) no drainage tubes or catheters, (5) not taking part in moderate or vigorous physical activity more than once a week, (6) physician’s permission for aerobic fitness testing and exercise, (7) physically able to participate in an exercise regimen, and (8) no clinical diagnosis of any mental disorder, as defined by the use of psychotropic drugs and self-report. The institutional review board at the University of North Carolina Greensboro approved the study [47].
Design and procedures
Participants were randomly assigned to either the TCC exercise group or a standard support therapy control (SST) group for the 12-week intervention. Randomization was achieved by the flip of a coin with group assignment concealed from participants until baseline assessments were completed. Baseline assessments included patient report questionnaires assessing HRQOL, clinical record information, demographics, as well as a fasting blood draw to assess biomarkers, including IL-6, IL-8, IGF-1, IGFBP-1, IGFBP-3, glucose, insulin, and cortisol. Baseline assessments were completed within 2 days prior to the start of the intervention. HRQOL was assessed again at 6 weeks (midintervention) and 12 weeks (postintervention). Biomarkers were assessed again at 12-weeks. During the 12- week intervention, participants in each study arm met with their respective groups for 60 min three times per week. The sessions took place in separate classrooms in the Behavioral Health and Fitness Laboratory at the University of North Carolina Greensboro at the same time of day for the duration of the trial. An American College of Sports Medicine certified health and fitness instructor with previous experience led the TCC sessions. Sessions consisted of a 10-min warm-up, 40 min of Yang-style tai chi chuan using the 15-move short form which are the first 15-moves of the traditional 104-move long form, and 10 min of guided breathing, imagery, and meditation. The psychosocial therapy control group was facilitated by a master’s trained counselor and supervised by a graduate exercise psychology student. Spiegel’s supportive–expressive group therapy model [49] was followed, and emphasis was placed on behavioral coping skills, group cohesion, and peer support. Participants in both groups were instructed to maintain their current level of physical activity outside of the TCC intervention. Self-report data were used to assess compliance with instructions.
Measures
Demographics and related medical information
Medical history and demographic information were obtained by questionnaires and included breast cancer stage, type of surgical treatment, and use of adjuvant treatment (chemotherapy, radiotherapy, hormone therapy, or none), age, height, weight, race, employment history, household income, educational background, and partnered status.
Health-related quality of life
Health-related quality of life was assessed using the MOS SF-36 which was developed for use in clinical research [35]. The MOS SF-36 is a patient report instrument used to determine medical care outcomes related to perceived health and biopsychosocial functioning. The multiscale MOS SF-36 measures eight health concepts: physical functioning, physical role limitations, pain, social functioning, general mental health, emotional role limitations, vitality, and general health perceptions [35]. Physical functioning was assessed with 10 items, and scores range from “limited a lot in performing all physical activities including bathing or dressing” to “performs all types of physical activities including the most vigorous without limitations due to health” [35]. Physical role limitations was assessed using four items, and scores ranged from “problems with work or other daily activities as a result of physical health” to “no problems with work or other daily activities as a result of physical health, past 4 weeks” [35]. Pain frequency and severity were assessed with two items, and scores ranged from “very severe and extremely limiting pain” to “no pain or limitations due to pain, past 4 weeks” [35]. Social functioning was assessed with two items, and scores ranged from “extreme and frequent interference with normal social activities due to physical and emotional problems” to “performs normal social activities without interference due to physical or emotional problems, past 4 weeks” [35]. General mental health was assessed with five items, and scores ranged from “feelings of nervousness and depression all of the time” to “feels peaceful, happy, and calm all of the time, past 4 weeks” [35]. Emotional role limitations were assessed with three items, and scores ranged from “problems with work or other daily activities as a result of emotional problems” to “no problems with work or other daily activities as a result of emotional problems, past 4 weeks” [35]. Vitality was assessed with four items and was scored from “feels tired and worn out all of the time” to “feels full of pep and energy all of the time, past 4 weeks” [35]. Lastly, general health perception was scored using five items, and scores ranged from “believes personal health is poor and likely to get worse” to “believes personal health is excellent” [35].
Biomarkers
Blood samples were collected during a fasted state pre- and postintervention and centrifuged to allow serum collection. Serum samples were aliquoted and stored at −80°C. Commercially available enzyme-linked immunosorbent assays and radioimmunoassays from Becton Dickinson, Franklin Lakes, New Jersey, and Diagnostic Systems Laboratories, Inc., Webster, Texas, were used to determine the serum concentrations of IL-6, IL-8, IGF-1, IGFBP-1, IGFBP-3, glucose, insulin, and cortisol.
Statistical analyses
Data analyses were conducted using SPSS PASW version 18 software. All statistical tests were performed at a two-tailed 5% level of significance. Patient data were analyzed on an “intent-to-treat” basis. Assumptions underlying all analyses were checked, no outliers or influential data were found, and therefore analyses included all evaluable patients. Missing data were minimal, and no imputations were necessary for analyses. Descriptive statistics, frequency distributions, means, mean change scores, and standard deviations for measures were assessed in the two study arms. Independent samples t tests were used to compare baseline characteristics of the two groups. Repeated measures ANOVAs were used to assess change over time in HRQOL and in biomarkers. Pearson correlations were used to assess associations change scores, from pre- to postintervention, for health-related quality of life and changes scores, from pre- to postintervention, for biomarkers.
Results
Participants
Seventy-five women expressed interest in the study and were contacted by the principal investigator who determined eligibility. Of the 75 women who expressed interest, 35 met eligibility requirements and were randomized to the TCC or SST group. Twenty one of the participants completed the study. Six participants in the TCC and four in the SST group dropped out of the study for reasons including severe side effects from the treatment, time constraints due to work or family obligations, and dissatisfaction with the group to which they were assigned. Participants randomly assigned to the SST group were offered 4 weeks of TCC following the 12-week intervention. There were no significant differences between groups at baseline in demographic or clinical (treatment) variables. The demographic variables have previously been presented [34, 45–47]. Participants were, on average, 53 years old, 147 lb, and had a body mass index of 24.93 kg/m2. Participants were diagnosed with nonmetastatic breast cancer, and all underwent surgical treatment, followed by chemotherapy in 84%, radiation in 61%, and hormonal therapy in 56%. See Table 1 for baseline participant characteristics.
Table 1.
Baseline participant characteristics
| Variable | TCC (n= 9) | SST (n= 10) |
|---|---|---|
| Age (years ± SEM) | 54.33±3.55 | 52.70±2.11 |
| Race, white (N, %) | 9 (100) | 10 (100) |
| Married (N, %) | 6 (67) | 4 (40) |
| High school education | 9 (100) | 9 (90) |
| Treatment type (N, %) | ||
| Surgery | ||
| Mastectomy | 4 (44) | 4 (40) |
| Lumpectomy | 5 (56) | 6 (60) |
| Chemotherapy (N) | 6 | 3 |
| Radiation therapy (N) | 8 | 9 |
| Weight (lb ± SEM) | 146.67±10.91 | 146.67±6.84 |
| Body mass index (kg/m2) | 24.89±1.93 | 24.97±1.39 |
| Body fat percentage | 38.62±1.98 | 41.58±1.68 |
| Distance walked in 6 min (m) | 610.00±24.35 | 611.65±25.02 |
Intervention and adherence
The 11 TCC participants who completed all study requirements had an adherence rate of 72% to the exercise intervention, completing 100% of TCC activities when in attendance. SST attendance was slightly lower, with 10 participants completing all study requirements and attending 67% of sessions. All participants (n =11) in the TCC intervention group reported adhering to the request to maintain their current level of physical activity outside of the TCC intervention, while 80% (n=8) of women in the psychosocial therapy control group complied and 20% (n= 2) reported beginning or increasing an aerobic walking program. Two participants from the TCC group and one participant from the SST group did not provide blood samples for biomarker analysis, therefore analyses are conducted on nine TCC and 10 SST participants.
Health-related quality of life
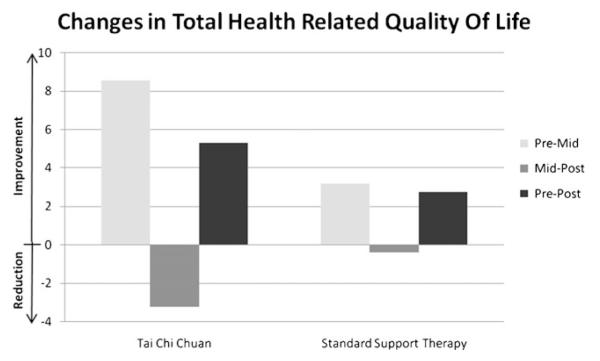
Baseline, midintervention (6 weeks), and postintervention (12 weeks) means±SEM for all HRQOL domains are presented in Table 2. Total HRQOL significantly improved in the TCC group from pre- to midintervention (P=0.045) but not from pre- to postintervention or mid- to postintervention. There were no significant changes over time in the SST group (see Fig. 2). Physical functioning significantly improved in the TCC group from pre- to midintervention (P=0.030), and there was a statistical trend for improvements in physical functioning from pre- to postintervention (P=0.062). There were no significant changes over time in the SST group, but there was a slight reduction in physical functioning from pre- to postintervention. Physical role limitations significantly improved in the TCC group from pre- to midintervention (P=0.023) with a statistical trend towards improvements from mid- to postintervention (P=0.094). There were no significant changes over time in the SST group. There was no significant improvement in pain across time in either group. There was a trend towards a significant improvement in social functioning in the TCC group from pre- to midintervention (P=0.078) and a significant improvement from pre- to postintervention (P=0.020). There was a significant improvement in social functioning in the SST group from pre- to midintervention (P=0.043).
Table 2.
Health-related quality of life and biomarkers
| Measurement | TCC (n=9) |
SST (n=10) |
Difference in change, p value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre ± SEM | Mid ± SEM | Post ± SEM | Pre–post change | Pre–post p value |
Pre ± SEM | Mid ± SEM | Post ± SEM | Pre–post change | Pre–post p value |
||
| Health-related QOL | |||||||||||
| Total HRQOL | 99.63±6.03 | 108.17±8.32 | 104.94±6.60 | 7.15±4.18 | 0.17 | 106.20±4.45 | 109.36±13.35 | 108.96±6.06 | 2.75±2.95 | 0.43 | 0.43 |
| Physical functioning | 25.00±1.88 | 26.89±1.31 | 26.89±1.37 | 1.89±0.87 | 0.06 | 26.70±1.07 | 26.70±3.02 | 26.50±1.31 | −0.20 ±0.51 | 0.91 | 0.05 |
| Physical role limitations | 1.89±0.56 | 3.44±0.73 | 2.44±0.50 | 0.56±0.53 | 0.33 | 2.00±0.58 | 2.60±1.58 | 2.80±0.51 | 0.80±0.49 | 0.11 | 0.74 |
| Pain | 9.33±0.44 | 9.44±1.13 | 9.11±0.45 | −0.22 ±0.43 | 0.81 | 9.10±0.38 | 9.00±1.05 | 9.10±0.55 | 0.00±0.45 | 0.96 | 0.64 |
| Social functioning | 7.44±0.47 | 8.14±0.34 | 8.63±0.63 | 1.50±0.50 | 0.02 | 8.56±0.47 | 9.20±1.03 | 9.00±0.58 | 0.44±0.37 | 0.13 | 0.10 |
| General mental health | 22.33±1.17 | 25.00±2.78 | 24.78±0.68 | 2.44±0.84 | 0.02 | 24.70±0.76 | 24.00±2.58 | 24.80±1.01 | 0.10±0.53 | 0.43 | 0.03 |
| Emotional role limitations | 2.00±0.44 | 2.67±0.50 | 2.25±0.41 | 0.25±0.31 | 0.33 | 2.20±0.36 | 2.50±0.85 | 2.40±0.40 | 0.20±0.44 | 0.76 | 0.93 |
| Vitality | 13.22±1.63 | 15.67±3.00 | 15.22±1.79 | 2.00±1.33 | 0.11 | 14.80±1.37 | 15.70±4.08 | 15.20±1.50 | 0.40±0.69 | 0.32 | 0.29 |
| General health perceptions | 18.38±1.40 | 19.38±4.26 | 18.60±1.59 | 0.22±0.64 | 0.65 | 18.62±1.19 | 19.67±3.55 | 19.30±0.92 | 0.68±0.79 | 0.39 | 0.66 |
| Biomarkers | |||||||||||
| IL-6 (pg/mL) | 2.63±1.32 | 4.63±2.32 | 2.00±1.84 | 0.31 | 2.44±0.56 | 2.42±0.55 | −0.01±0.48 | 0.98 | 0.28 | ||
| IL-8 (pg/mL) | 9.37±1.80 | 9.69±2.05 | 0.32±1.25 | 0.80 | 11.06±2.57 | 7.24±1.93 | −3.82±2.52 | 0.17 | 0.17 | ||
| IGF-1 (ng/mL) | 156.81±19.58 | 129.49±14.61 | 27.32±15.02 | 0.11 | 111.76±26.15 | 95.12±18.55 | −16.64±21.03 | 0.45 | 0.69 | ||
| IGFBP-1 (ng/mL) | 72.64±8.55 | 76.40±14.25 | 3.76±9.10 | 0.69 | 92.22±12.34 | 101.34±15.82 | 9.12±11.53 | 0.45 | 0.72 | ||
| IGFBP-3 (ng/mL) | 39.22±2.09 | 40.11±2.43 | 0.89±1.04 | 0.42 | 40.81±4.28 | 40.11±4.79 | −0.70±1.19 | 0.57 | 0.33 | ||
| Glucose (mg/dL) | 80.00±4.83 | 85.78±4.39 | 5.78±2.86 | 0.08 | 85.50±4.95 | 89.00±11.41 | 3.50±7.10 | 0.63 | 0.78 | ||
| Insulin (μIU/mL) | 15.34±1.79 | 16.75±2.66 | 1.34±2.35 | 0.56 | 15.83±2.88 | 30.85±9.44 | 15.03±7.47 | 0.08 | 0.10 | ||
| Cortisol (μg/dL) | 21.52±2.91 | 23.50±3.77 | 1.98±2.30 | 0.42 | 26.04±2.08 | 28.69±2.52 | 2.65±2.76 | 0.36 | 0.86 | ||
SEM standard error of the mean
Fig. 2.
Mean change in health-related quality of life assessed using the multiple outcomes study short form-36
General mental health significantly improved in the TCC group from pre- to midintervention (P=0.014) and from pre- to postintervention (P=0.019). There were no significant changes over time in the SST group. Emotional role limitations did not improve in either group. There was a statistical trend toward improvement in vitality in the TCC group from pre- to midintervention (P=0.061) and a significant improvement in the SST group from pre- to midintervention (P=0.010). General health perceptions did not improve over time in either group.
Biomarkers
Baseline and postintervention (12 weeks) means±SEM for all biomarkers are presented in Table 2. There were no significant changes over time in either group in IL-6, IL-8, IGF-1, IGFBP-1, IGFPB-3, and cortisol. There was a statistical trend for an increase in glucose among the TCC participants but not among the SST participants. There was a statistical trend toward an increase in insulin among the SST participants (P=0.08), but not among the TCC participants (P=0.56).
Relationship between HRQOL and biomarkers
Analyses demonstrated an inverse relationship between changes, from pre- to postintervention, in IGF-1 and overall HRQOL (r=−0.56; P=0.02), role limitations due to physical health problems (r=−0.68; P=0.001), social functioning (r=−0.56; P=0.02), and a statistical trend for vitality (r=−0.44; P=0.06). Changes in IGFBP-1 from pre- to postintervention were directly correlated with changes in role limitations due to physical health problems (r=0.60; P=0.007). Changes in IGFBP-3 from pre- to postintervention were directly correlated with changes in physical functioning (r=0.46; P=0.05). Changes in glucose from pre- to postintervention were inversely associated with role limitations due to emotional problems (r=−0.70; P=0.001). Changes in cortisol from pre- to postintervention were directly associated with changes role limitations due to physical health problems (r=0.74; P<0.001) and health perceptions (r=0.46; P=0.05). Changes in IL-8 from pre- to postintervention were directly correlated with changes in role limitations due to emotional problems (r=0.59; P=0.01).
Discussion
The purpose of this secondary data analysis was to assess the effect of a TCC intervention on HRQOL and markers of inflammation in breast cancer survivors who had completed standard breast cancer treatment. This preliminary investigation presents promising results regarding the efficacy of tai chi for the improvement of HRQOL in breast cancer survivors. Participants in the TCC group improved throughout the course of the 12-week intervention in numerous components of HRQOL, including total HRQOL, physical functioning, physical role limitations, social functioning, and general mental health. The only significant improvements in the SST group were in social functioning and vitality. The lack of more improvements in HRQOL in the SST group is somewhat surprising since participants in the SST group attended the supportive-expressive group therapy sessions three times per week for 12 weeks. While we do not know why, we can speculate that the women in our study were not experiencing enough distress per our inclusion criteria, with recent studies showing that supportive-expressive therapy was less effective for women with primary breast cancer [50].
Tai chi, despite being of low to moderate aerobic intensity, still resulted in improvements in physiological components of HRQOL, in particular physical functioning and role limitations due to physical health problems. Additionally, we began with a relatively “psychologically healthy” group of breast cancer survivors based on our criteria which excluded women with a self-reported clinical diagnosis of any mental disorder, as defined by the use of psychotropic medications and self-reported history. Despite this, we found improvements in social functioning, general mental health, and overall HRQOL. This leads us to hypothesize that using a more broad inclusion criteria may result in greater improvements in HRQOL.
We also found some interesting relationships between components of HRQOL and inflammatory markers. The mechanism by which cancer and its treatments result in reduced HRQOL may include altered inflammatory and metabolic profiles. Exercise interventions have proven effective for reducing low-grade inflammation and may attenuate treatments side effects through the regulation of inflammatory cytokines. We found an inverse relationship between changes in IGF-1 and changes in overall health-related quality of life, physical role limitations, and social functioning. Changes in IGFBP-1 and IGFBP-3, binding proteins for IGF-1, were correlated with changes in physical role limitations and physical functioning, respectively. Changes in cortisol were directly associated with changes in physical role limitations. Cortisol dysregulation occurs in fatigued breast cancer survivors [25, 26], so this association between cortisol change and physical role limitation may be explained by the underlying role on fatigue. Lastly, changes in emotional role limitations were inversely related to changes in glucose and directly related to changes in IL-8.
This hypothesis-generating study supports further investigation into the relationships between HRQOL, cytokines, and metabolic processes during and following exercise interventions for cancer survivors. However, there are limits to this study. First, the small sample size limited our ability to elucidate changes from pre- to postintervention. Additionally, the use of breast cancer survivors alone limits the generalizability of these results to a more broad population of cancer survivors. This work needs to be replicated in future large, randomized controlled trials of various types of cancer survivors at various points throughout the cancer continuum to identify relationships between inflammatory mediators, metabolic processes, and QOL in cancer survivors. Lastly, due to the voluntary nature of this study, participants who choose to participate may have been more motivated than those who did not.
Despite these limitations, these promising results lead us to believe that tai chi chuan, an enjoyable and well-accepted exercise intervention with proven mental and physical benefits, is safe and effective for breast cancer survivors. It is important to find exercise interventions that are beneficial while also being enjoyable to encourage long-term participation. Tai chi shows promise as an intervention that offers physiological and psychological benefits while being well received.
Acknowledgments
The authors thank the Sally Schindel Cone Foundation (KMM), NCI K07CA120025 (KMM), and NCI R25CA10618 (GRM) for the financial support.
Footnotes
Conflict of interest The authors have no conflicts of interest to disclose.
Contributor Information
Lisa K. Sprod, James P. Wilmot Cancer Center, University of Rochester, Rochester, NY, USA Department of Radiation Oncology, University of Rochester, Rochester, NY, USA.
Michelle C. Janelsins, James P. Wilmot Cancer Center, University of Rochester, Rochester, NY, USA Department of Radiation Oncology, University of Rochester, Rochester, NY, USA.
Oxana G. Palesh, School of Medicine, Stanford University, Palo Alto, CA, USA
Jennifer K. Carroll, James P. Wilmot Cancer Center, University of Rochester, Rochester, NY, USA Department of Radiation Oncology, University of Rochester, Rochester, NY, USA; Department of Medicine, University of Rochester, Rochester, NY, USA; Department of Family Medicine, University of Rochester, Rochester, NY, USA.
Charles E. Heckler, James P. Wilmot Cancer Center, University of Rochester, Rochester, NY, USA
Luke J. Peppone, James P. Wilmot Cancer Center, University of Rochester, Rochester, NY, USA Department of Radiation Oncology, University of Rochester, Rochester, NY, USA.
Supriya G. Mohile, James P. Wilmot Cancer Center, University of Rochester, Rochester, NY, USA Department of Medicine, University of Rochester, Rochester, NY, USA.
Gary R. Morrow, James P. Wilmot Cancer Center, University of Rochester, Rochester, NY, USA Department of Radiation Oncology, University of Rochester, Rochester, NY, USA; Department of Psychiatry, University of Rochester, Rochester, NY, USA.
Karen M. Mustian, James P. Wilmot Cancer Center, University of Rochester, Rochester, NY, USA Department of Radiation Oncology, University of Rochester, Rochester, NY, USA; Department of Community and Preventive Medicine, University of Rochester, Rochester, NY, USA.
References
- 1.American Cancer Society Cancer facts and figures. 2009.
- 2.Sadler IJ, Jacobsen PB. Progress in understanding fatigue associated with breast cancer treatment. Cancer Invest. 2001;19(7):723–31. doi: 10.1081/cnv-100106147. [DOI] [PubMed] [Google Scholar]
- 3.Andrykowski MA, et al. Use of a case definition approach to identify cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. J Clin Oncol. 2005;23(27):6613–22. doi: 10.1200/JCO.2005.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermelink K, et al. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer. 2007;109(9):1905–13. doi: 10.1002/cncr.22610. [DOI] [PubMed] [Google Scholar]
- 5.Vardy J, Tannock I. Cognitive function after chemotherapy in adults with solid tumours. Crit Rev Oncol Hematol. 2007;63(3):183–202. doi: 10.1016/j.critrevonc.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Banthia R, et al. Fatigued breast cancer survivors: the role of sleep quality, depressed mood, stage and age. Psychol Health. 2009;24(8):965–80. doi: 10.1080/08870440802110831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo H-H, et al. Quality of sleep and related factors during chemotherapy in patients with stage I/II breast cancer. J Formos Med Assoc. 2006;105(1):64–9. doi: 10.1016/S0929-6646(09)60110-8. [DOI] [PubMed] [Google Scholar]
- 8.Fann JR, et al. Major depression after breast cancer: a review of epidemiology and treatment. Gen Hosp Psychiatry. 2008;30(2):112–26. doi: 10.1016/j.genhosppsych.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Lueboonthavatchai P. Prevalence and psychosocial factors of anxiety and depression in breast cancer patients. J Med Assoc Thai. 2007;90(10):2164–74. [PubMed] [Google Scholar]
- 10.Burgess C, et al. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330(7493):702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maxwell C, Viale PH. Cancer treatment-induced bone loss in patients with breast or prostate cancer. Oncol Nurs Forum. 2005;32(3):589–603. doi: 10.1188/05.onf.589-603. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, et al. Osteoporosis and rate of bone loss among postmenopausal survivors of breast cancer. Cancer. 2005;104(7):1520–30. doi: 10.1002/cncr.21335. [DOI] [PubMed] [Google Scholar]
- 13.Newton RU, Galvao DA. Exercise in prevention and management of cancer. Curr Treat Options Oncol. 2008;9(2-3):135–46. doi: 10.1007/s11864-008-0065-1. [DOI] [PubMed] [Google Scholar]
- 14.Makari-Judson G, Judson CH, Mertens WC. Longitudinal patterns of weight gain after breast cancer diagnosis: observations beyond the first year. Breast J. 2007;13(3):258–65. doi: 10.1111/j.1524-4741.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 15.McInnes JA, Knobf MT. Weight gain and quality of life in women treated with adjuvant chemotherapy for early-stage breast cancer. Oncol Nurs Forum. 2001;28(4):675–84. [PubMed] [Google Scholar]
- 16.Ahles TA, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20(2):485–93. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 17.Bower JE, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106(4):751–8. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 18.McTiernan A, et al. Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol. 2003;21(10):1961–6. doi: 10.1200/JCO.2003.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irwin ML, et al. Relationship of obesity and physical activity with C-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2881–8. doi: 10.1158/1055-9965.EPI-05-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2(11):862–71. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 21.Oppenheim JJ, et al. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617–48. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 22.Walter M, et al. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28(30):2745–55. doi: 10.1038/onc.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The cytokine handbook. 1st ed Academic Press; San Diego, CA: 2003. [Google Scholar]
- 24.Seruga B, et al. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8(11):887–99. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 25.Bower JE, et al. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30(1):92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Bower JE, et al. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64(4):604–11. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Ronson A. Psychological stress in oncology: the role of glucocorticoids. Bull Cancer. 2006;93(7):699–708. [PubMed] [Google Scholar]
- 28.Pollak M. Insulin, insulin-like growth factors and neoplasia. Best Pract Res Clin Endocrinol Metab. 2008;22(4):625–38. doi: 10.1016/j.beem.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Samani AA, et al. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28(1):20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 30.Schernhammer ES, et al. Circulating levels of insulin-like growth factors, their binding proteins, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14(3):699–704. doi: 10.1158/1055-9965.EPI-04-0561. [DOI] [PubMed] [Google Scholar]
- 31.Allen NE, et al. A prospective study of serum insulin-like growth factor-I (IGF-I), IGF-II, IGF-binding protein-3 and breast cancer risk. Br J Cancer. 2005;92(7):1283–7. doi: 10.1038/sj.bjc.6602471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greep NC, et al. The effects of adjuvant chemotherapy on bone density in postmenopausal women with early breast cancer. Am J Med. 2003;114(8):653–9. doi: 10.1016/s0002-9343(03)00127-x. [DOI] [PubMed] [Google Scholar]
- 33.Lonning PE. Bone safety of aromatase inhibitors versus tamoxifen. Int J Gynecol Cancer. 2006;16(Suppl 2):518–20. doi: 10.1111/j.1525-1438.2006.00685.x. [DOI] [PubMed] [Google Scholar]
- 34.Peppone LJ, et al. Effects of a structured weight-bearing exercise program on bone metabolism among breast cancer survivors: a feasibility trial. Clin Breast Cancer. 2010;10(3):224–9. doi: 10.3816/CBC.2010.n.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 36.Chen Z, et al. Fracture risk among breast cancer survivors: results from the Women’s Health Initiative Observational Study. Arch Intern Med. 2005;165(5):552–8. doi: 10.1001/archinte.165.5.552. [DOI] [PubMed] [Google Scholar]
- 37.Jin P. Efficacy of Tai Chi, brisk walking, meditation, and reading in reducing mental and emotional stress. J Psychosom Res. 1992;36(4):361–70. doi: 10.1016/0022-3999(92)90072-a. [DOI] [PubMed] [Google Scholar]
- 38.Tsai J-C, et al. The beneficial effects of Tai Chi Chuan on blood pressure and lipid profile and anxiety status in a randomized controlled trial. J Altern Complement Med. 2003;9(5):747–54. doi: 10.1089/107555303322524599. [DOI] [PubMed] [Google Scholar]
- 39.Irwin MR, Olmstead R, Oxman MN. Augmenting immune responses to varicella zoster virus in older adults: a randomized, controlled trial of Tai Chi. J Am Geriatr Soc. 2007;55(4):511–7. doi: 10.1111/j.1532-5415.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- 40.Tse SK, Bailey DM. T’ai chi and postural control in the well elderly. Am J Occup Ther. 1992;46(4):295–300. doi: 10.5014/ajot.46.4.295. [DOI] [PubMed] [Google Scholar]
- 41.Wolf SL, et al. The effect of Tai Chi Quan and computerized balance training on postural stability in older subjects. Atlanta FICSIT Group. Frailty and Injuries: Cooperative Studies on Intervention Techniques. Phys Ther. 1997;77(4):371–81. doi: 10.1093/ptj/77.4.371. discussion 382-4. [DOI] [PubMed] [Google Scholar]
- 42.Li F, et al. An evaluation of the effects of Tai Chi exercise on physical function among older persons: a randomized controlled trial. Ann Behav Med. 2001;23(2):139–46. doi: 10.1207/S15324796ABM2302_9. [DOI] [PubMed] [Google Scholar]
- 43.Irwin MR, Olmstead R. Mitigating cellular inflammation in older adults: a randomized controlled trial of Tai Chi Chih. Am J Geriatr Psychiatry. 2011 doi: 10.1097/JGP.0b013e3182330fd3. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lan C, et al. Effect of T’ai chi chuan training on cardiovascular risk factors in dyslipidemic patients. J Altern Complement Med. 2008;14(7):813–9. doi: 10.1089/acm.2008.0143. [DOI] [PubMed] [Google Scholar]
- 45.Mustian KM, Katula JA, Zhao H. A pilot study to assess the influence of tai chi chuan on functional capacity among breast cancer survivors. J Support Oncol. 2006;4(3):139–45. [PubMed] [Google Scholar]
- 46.Mustian KM, Palesh OG, Flecksteiner SA. Tai Chi Chuan for breast cancer survivors. Med Sport Sci. 2008;52:209–17. doi: 10.1159/000134301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mustian KM, et al. Tai Chi Chuan, health-related quality of life and self-esteem: a randomized trial with breast cancer survivors. Support Care Cancer. 2004;12(12):871–6. doi: 10.1007/s00520-004-0682-6. [DOI] [PubMed] [Google Scholar]
- 48.Janelsins MC, et al. Effects of Tai Chi Chuan on insulin and cytokine levels in a randomized controlled pilot study on breast cancer survivors. Clin Breast Cancer. 2011;11(3):161–70. doi: 10.1016/j.clbc.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spiegel D. Essentials of psychotherapeutic intervention for cancer patients. Support Care Cancer. 1995;3(4):252–6. [PubMed] [Google Scholar]
- 50.Classen CC, et al. Supportive-expressive group therapy for primary breast cancer patients: a randomized prospective multicenter trial. Psychooncology. 2008;17(5):438–47. doi: 10.1002/pon.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]