Abstract
OBJECTIVES
The purpose of this study was to assess the prevalence and distribution of coronary artery calcium (CAC) across Framingham Risk Score (FRS) strata and therefore determine FRS levels at which asymptomatic, young to early middle-age individuals could potentially benefit from CAC screening.
BACKGROUND
High CAC burden is associated with increased risk of coronary events beyond the FRS. Expert panel recommendations for CAC screening are based on data obtained in middle-age and older individuals.
METHODS
We included 2,831 CARDIA (Coronary Artery Risk Development in Young Adults) study participants with an age range of 33 to 45 years. The number needed to screen ([NNS] number of people in each FRS stratum who need to be screened to detect 1 person with a CAC score above the specified cut point) was used to assess the yield of screening for CAC. CAC prevalence was compared across FRS strata using a chi-square test.
RESULTS
CAC scores >0 and ≥100 were present in 9.9% and 1.8% of participants, respectively. CAC prevalence and amount increased across higher FRS strata. A CAC score >0 was observed in 7.3%, 20.2%, 19.1%, and 44.8% of individuals with FRSs of 0 to 2.5%, 2.6% to 5%, 5.1% to 10%, and >10%, respectively (NNS = 14, 5, 5, and 2, respectively). A CAC score of ≥100 was observed in 1.3%, 2.4%, and 3.5% of those with FRSs of 0 to 2.5%, 2.6% to 5%, and 5.1% to 10%, respectively (NNS = 79, 41, and 29, respectively), but in 17.2% of those with an FRS >10% (NNS = 6). Similar trends were observed when findings were stratified by sex and race.
CONCLUSIONS
In this young to early middle-age cohort, we observed concordance between CAC prevalence/amount and FRS strata. Within this group, the yield of screening and possibility of identifying those with a high CAC burden (CAC score of ≥100) is low in those with an FRS of ≤10%, but considerable in those with an FRS >10%.
Keywords: coronary artery calcium, coronary heart disease, Framingham Risk Score, number needed to screen, risk factors
CAC is associated with an increased risk of coronary heart disease (CHD) events and provides incremental risk prediction beyond the Framingham Risk Score (FRS) (1). Coronary artery calcium (CAC) increases with age and is associated with traditional risk factor burden. In addition, higher CAC burden (CAC score ≥100) carries a greater risk of CHD events (1), and compared with traditional cardiovascular risk factors alone, CAC scoring improves risk classification for the prediction of CHD events (2).
To date, some expert panels have recommended testing for CAC in intermediate-risk individuals (FRS predicted 10-year risk 10% to 20%) (1,3) and state that although it could be reasonable to screen for CAC in low- to intermediate-risk (FRS 6% to 10%) individuals, it is reasonable to do so in those at intermediate risk (FRS 10% to 20%) (4). Others suggest that there is more harm than benefit resulting from CAC measurement in intermediate-risk individuals (5), and yet other panels suggest a benefit of widespread CAC screening in all asymptomatic men 45 to 75 years of age and asymptomatic women 55 to 75 years of age, except for those defined as very low risk based on the absence of any traditional cardiovascular risk factors (6). With the exception of a study by Taylor et al. (7), most of the studies cited by these consensus panels examining associations between CAC and CHD events included participants with mean ages older than 50 years, likely because of lower power to detect CHD events in the younger population. Thus, expert recommendations are even less clear about screening for CAC in the younger population even though this population is still at risk of the development of CAC and CHD events (7).
We previously observed, based on the distribution of CAC relative to FRS, in the Multi-Ethnic Study of Atherosclerosis cohort (mean age, 60.9 years), that there might be minimal benefit to screening for clinically significant levels of CAC in very low risk persons with an FRS of ≤5% (8). However, for young and early middle-age persons, an appropriate FRS threshold above which CAC screening might be useful is unclear. Although some studies examined the relationship between CAC distribution and FRS (9–15), none were performed in individuals younger than 50 years of age.
In the younger to early middle-age asymptomatic biracial cohort of the CARDIA (Coronary Artery Risk Development in Young Adults) study, we sought to ascertain the prevalence and distribution of CAC across Framingham risk categories, stratified by sex and race. These associations can then form the basis for determining the yield of CAC screening, and therefore the FRS ranges for which CAC scoring might be beneficial in risk assessment. Findings from this study may facilitate further risk stratification for young, asymptomatic individuals predicted to be at low or intermediate 10-year risk by age and traditional risk factors.
METHODS
The CARDIA study is a multicenter, prospective cohort study designed to investigate the evolution of CHD risk in young adults. Details of the study design, as well as inclusion/exclusion criteria and baseline characteristics, were described previously (16). Briefly, the CARDIA study enrolled 5,115 black and white participants (55% women), ranging from 18 to 30 years of age, during the period 1985 to 1986 from 4 U.S. urban areas (Birmingham, Alabama; Oakland, California; Chicago, Illinois; and Minneapolis, Minnesota). The institutional review boards at all the study sites approved the study protocol, and written informed consent was obtained from all study participants. The study was designed to include approximately balanced numbers of participants by age, sex, race, and education level.
For the current study, we included men and women with measured coronary calcium at the year 15 (our study baseline) examination (n = 3,043), when the mean age was approximately 40 years. From this number, we excluded 170 participants with diabetes because they are considered high-risk under current National Cholesterol Education Program Adult Treatment Panel III guidelines (17), and we focused on evaluating the yield of screening in individuals at lower risk. Additionally, 41 participants were excluded due to missing FRS equation covariates.
Risk factor measurements
Data on cigarette smoking status, age, race, socioeconomic measures, diabetes history, and medication use were obtained by participant self-report (16). Current smoking was defined as at least 5 cigarettes per week almost every week for at least 3 months. Family history of myocardial infarction was obtained using a self-administered questionnaire. Blood pressure was measured 3 times with a random-zero device, and the average of the last 2 measurements was used. Body mass index was calculated by dividing weight in kilograms by the square of the height in meters. The CARDIA study physical activity history questionnaire was used to assess physical activity, which was coded as exercise units (18). Venous blood samples were obtained from participants after a 12-h fast. Plasma triglycerides and total and high-density lipoprotein cholesterol were determined using an enzymatic assay by Northwest Lipids Research Laboratory (Seattle, Washington). Low-density lipoprotein cholesterol was then derived using the Friedewald equation (19).
Agatston CAC measurement and scoring were previously described (20). The presence of CAC was defined as having a positive, nonzero Agatston score determined from the average of 2 scans. Because of the young age of the participants, each scan set with at least 1 nonzero score was reviewed and verified by an expert investigator who was blinded to the scan scores. There was reasonable agreement between scans (kappa = 0.79, with only 3.6% discordance). For this study, CAC scores were categorized as >0 or ≥100. The prevalence of advanced CAC (CAC score ≥300 or 400) was too low in this cohort because of the younger age of the participants. As such, we made use of a lower cut point (CAC score ≥100, previously shown to be associated with increased risk of CHD events) (1) in our definition of high CAC burden. Concurrent FRS 10-year risk of CHD was calculated and stratified as follows: 0 to 2.5%, 2.6% to 5%, 5.1% to 10%, and >10%. Further stratification of FRS categories for those with an FRS >10% would not have been meaningful due to the relative youth and therefore low-risk composition of our study cohort.
Statistical analysis
All analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, North Carolina). A 2-tailed p value <0.05 was considered statistically significant. The Framing-ham 10-year risk estimates for all participants were calculated using the risk prediction functions from the National Cholesterol Education Program Adult Treatment Panel III guidelines (17) based on an update from the Framingham methodology reported by Wilson et al. (21). The covariates included in the FRS calculation were age, total and high-density lipoprotein cholesterol levels, current smoking status, systolic blood pressure, and the use of antihypertensive medication. Baseline characteristics were compared according to FRS 10-year risk strata and by CAC categories using general linear models for continuous variables and cross-tabulations for categorical variables. A chi-square test was used to compare the prevalence of CAC categories across FRS 10-year risk strata for the participants included in this study, then after stratification by sex and race. All analyses performed for the current study (CARDIA year 15 examination; age range, 33 to 45 years) were also repeated in secondary analyses using data from the CARDIA year 20 examination (age range, 38 to 50 years). NNS was defined as the number of people who need to be screened to identify 1 individual with a CAC score above the pre-specified CAC cut point in each FRS category. It was calculated by dividing the total number of participants by the number of people with a CAC score >0 (or ≥100) in each FRS stratum. The CAC amount was represented by median CAC scores in FRS groups.
RESULTS
Baseline characteristics
Our study sample consisted of a total of 2,832 black and white participants (mean age, 40.3 years [range, 33 to 45 years]; 53% women). With the exception of body mass index and some measures of socioeconomic status, there were significant differences in most of the traditional risk factors (including FRS) between those with a CAC score of 0 versus a CAC score >0 and a CAC score <100 versus a CAC score ≥100 (Table 1). Race and physical activity were significantly different for the CAC score of 0 versus >0 categories, but not CAC score <100 versus ≥100 categories. Ninety percent of individuals with a CAC score ≥100 and an FRS >10% smoked, so that cigarette smoking was the prevalent cardiovascular risk factor among this subset of our study population.
Table 1.
Baseline Characteristics Stratified by CAC Score (N = 2,832)
| CAC Score Categories
|
||||||
|---|---|---|---|---|---|---|
| Characteristics | 0 (n = 2,553) | >0 (n = 279) | p Value | <100 (n = 2,781) | >100 (n = 51) | p Value |
| Age, yrs | 40.1 ± 3.6 | 42 ± 3.1 | <0.01 | 40.2 ± 3.6 | 43.1 ± 2.6 | <0.01 |
|
| ||||||
| Female, % | 56 | 27.2 | <0.01 | 53.6 | 27.5 | <0.01 |
|
| ||||||
| Black race, % | 45.8 | 34.4 | <0.01 | 44.9 | 33.3 | 0.10 |
|
| ||||||
| SBP, mm Hg | 112.4 ± 14.1 | 118.5 ± 16.1 | <0.01 | 112.9 ± 14.3 | 120.8 ± 18.4 | <0.01 |
| DBP, mm Hg | 74.1 ± 11.1 | 78.1 ± 12.6 | <0.01 | 74.4 ± 11.2 | 81.4 ± 14.7 | <0.01 |
|
| ||||||
| Total cholesterol, mg/dl | 183.8 ± 34.3 | 198.4 ± 40.6 | <0.01 | 185.0 ± 34.9 | 199.6 ± 50.6 | <0.01 |
| HDL, mg/dl | 51.1 ± 14.3 | 46.1 ± 14.3 | <0.01 | 50.7 ± 14.3 | 45.9 ± 15.8 | 0.02 |
| LDL, mg/dl | 112.3 ± 30.6 | 127 ± 37.8 | <0.01 | 113.5 ± 31.4 | 125.4 ± 43.9 | 0.01 |
|
| ||||||
| Current smoking, % | 19.3 | 32.3 | <0.01 | 20.2 | 39.2 | <0.01 |
|
| ||||||
| Hypertension treatment, % | 6.0 | 11.1 | <0.01 | 6.4 | 11.8 | 0.12 |
| Lipid treatment, % | 1.7 | 4.7 | <0.01 | 1.8 | 11.8 | <0.01 |
|
| ||||||
| Family history of heart attack, % | 19.6 | 26.9 | <0.01 | 19.9 | 45.1 | <0.01 |
|
| ||||||
| FRS, % | 1.3 ± 2.5 | 3.5 ± 4.8 | <0.01 | 1.4 ± 2.7 | 5.2 ± 6.9 | <0.01 |
|
| ||||||
| BMI, kg/m2 | 28.3 ± 6.2 | 28.7 ± 5.9 | 0.28 | 28.3 ± 6.2 | 28.2 ± 6.3 | 0.88 |
|
| ||||||
| Physical activity (intensity score) | 352 ± 282.8 | 403.4 ± 310.8 | <0.01 | 357.0 ± 285.6 | 365.6 ± 314.3 | 0.83 |
|
| ||||||
| Education, % | 0.01 | 0.14 | ||||
| Less than high school | 0.3 | 0.7 | 0.3 | 2.0 | ||
| High school | 20.1 | 28 | 20.7 | 27.5 | ||
| College | 58 | 51.3 | 57.4 | 52.9 | ||
| Graduate school | 21.7 | 20.1 | 21.6 | 17.7 | ||
|
| ||||||
| Marital status: married, % | 54.3 | 50.9 | 0.29 | 54.0 | 52.9 | 0.89 |
|
| ||||||
| Income, % | 0.38 | 0.29 | ||||
| <$25,000 | 14 | 16.3 | 14.0 | 21.6 | ||
| $25,000–$50,000 | 24.2 | 27.1 | 24.6 | 15.7 | ||
| $50,000–$75,000 | 23.2 | 20.2 | 22.9 | 21.6 | ||
| >$75,000 | 38.7 | 36.5 | 38.4 | 41.2 | ||
|
| ||||||
| Health insurance, % | 87.8 | 85.7 | 0.30 | 87.7 | 86.3 | 0.77 |
Values are mean ± SD or %. Baseline refers to CARDIA year 15 examinations.
BMI = body mass index; CAC = coronary artery calcium; DBP = diastolic blood pressure; FRS = Framingham Risk Score; HDL = high-density lipoprotein; LDL = low-density lipoprotein; SBP = systolic blood pressure.
Of 1,501 women in our study, 76 had a CAC score >0. Of these, 66 were premenopausal and 10 were post-menopausal (data not shown). Among pre-menopausal women, 4.9% had the presence of any CAC versus 6.2% of postmenopausal women (p = 0.45 for comparison of CAC prevalence between the 2 groups).
Distribution of CAC prevalence, amount, and NNS compared across FRS strata
Table 2 shows the distribution of CAC scores >0 and ≥100 across FRS strata. Overall, CAC scores >0 and ≥100 were present in 9.9% and 1.8% of participants, respectively. Among individuals with CAC, median CAC scores increased with higher FRS. As expected, the prevalence of CAC scores >0 and ≥100 increased across greater FRS strata (Fig. 1) (both p for trend <0.01). Consequently, the NNS (signifying the number of individuals who need to be screened to detect 1 person with a CAC score >0 [or ≥100]) decreased with a higher FRS. In each CAC category, the NNS was higher for lower than higher FRS strata (Table 2). For example, among those with a CAC score of ≥100, the NNS was 79 for participants with an FRS of 0 to 2.5% and 6 for those with an FRS >10%.
Table 2.
CAC Prevalence, Amount, and NNS Compared With FRS Categories (N = 2,832)
| FRS Categories
|
||||||
|---|---|---|---|---|---|---|
| CAC Score Group | 0%–2.5% (n = 2,372) | 2.6%–5% (n = 287) | 5.1%–10% (n = 115) | >10% (n = 58) | p Value | |
| Median CAC score (IQR)* | 17.9 (5.1–64.5) | 16.6 (5.6–52.2) | 18.9 (11.9–73.5) | 58.8 (20.6–215.7) | ||
|
| ||||||
| CAC score >0, % (n = 279) | 7.3 | 20.2 | 19.1 | 44.8 | <0.01 | |
| NNS | 13.7 | 5.0 | 5.2 | 2.2 | ||
|
| ||||||
| CAC score ≥100, % (n = 51) | 1.3 | 2.4 | 3.5 | 17.2 | <0.01 | |
| NNS | 79 | 41 | 28.8 | 5.8 | ||
Among those with a CAC score >0.
IQR = interquartile range, NNS = number needed to screen (number of people who need to be screened to identify 1 person with a CAC score above a pre-specified cut point in each Framingham risk score category); other abbreviations as in Table 1.
Figure 1. CAC Score Compared With FRS.
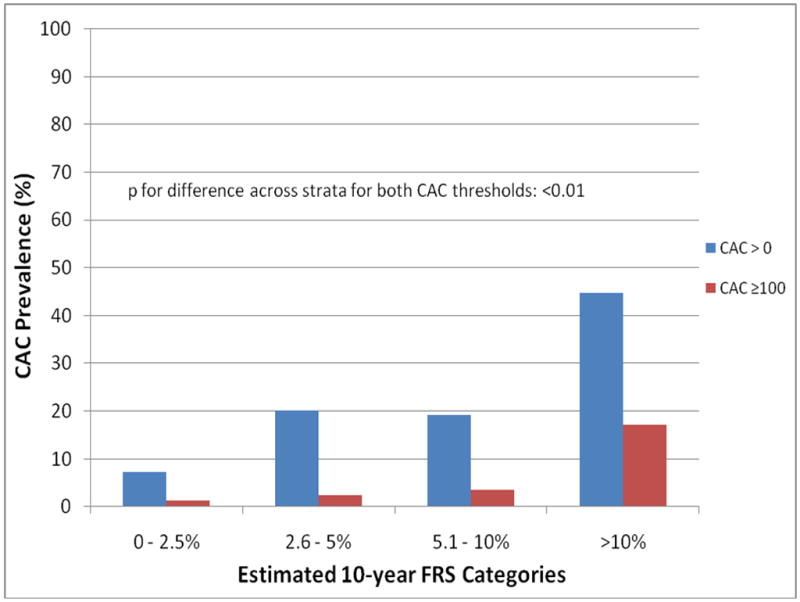
Prevalence of coronary artery calcium (CAC) scores >0 and ≥100 compared across 10-year Framingham Risk Score (FRS) strata in the CARDIA study. There was significant concordance between CAC prevalence/amount and FRS such that prevalence of CAC scores >0 and ≥100 were low in the lower FRS strata and increased with higher FRSs.
When data were stratified by sex, the general pattern of distribution of CAC scores >0 and ≥100 across FRS strata remained the same, with a higher prevalence of CAC scores >0 and ≥100 across FRS strata (Table 3). The prevalence of CAC scores >0 and ≥100 was higher in men than women. Likewise, the overall median CAC scores (among those with CAC) were higher in men.
Table 3.
CAC Prevalence and Amount Compared With FRS Categories, Stratified by Sex (N = 2,832)
| FRS Categories
|
|||||
|---|---|---|---|---|---|
| CAC Score Group | 0%–2.5% (n = 2,372) | 2.6%–5% (n = 287) | 5.1%–10% (n = 115) | >10% (n = 58) | p Value |
| Males (n = 1,327) | n = 927 | n = 241 | n = 106 | n = 53 | |
|
| |||||
| Median CAC score (IQR)* | 20.6 (5.6–64.6) | 16.6 (5.6–41.8) | 18.9 (10.8–76.5) | 60.6 (20.6–215.7) | |
| CAC score >0, % (n = 203) | 11.9 | 20.8 | 18.9 | 43.4 | <0.01 |
| CAC score ≥100, % (n = 37) | 1.9 | 2.5 | 3.8 | 17.0 | |
|
| |||||
| Females (n = 1,505) | n = 1,445 | n = 46 | n = 9 | n = 5 | |
|
| |||||
| Median CAC score (IQR)* | 15.0 (4.2–61.7) | 19.1 (4.9–57.9) | 30.6 (15.9–45.4) | 33.0 (16.4–571.1) | |
| CAC score >0, % (n = 76) | 4.4 | 17.4 | 22.2 | 60.0 | <0.01 |
| CAC score ≥100, % (n = 14) | 0.8 | 2.2 | 0.0 | 20.0 | |
Among those with a CAC score >0.
Abbreviations as in Table 2.
Further stratification by race revealed that although the overall prevalence of CAC was greater in white compared with black participants, the overall median CAC scores were higher in black than in white participants (Table 4). As with the overall distribution in Table 2, the prevalence of CAC scores > 0 and ≥100 increased with higher FRSs.
Table 4.
CAC Prevalence and Amount Compared With FRS Categories, Stratified by Race (N = 2,832)
| FRS Categories
|
|||||
|---|---|---|---|---|---|
| CAC Score Group | 0%–2.5% (n = 2,372) | 2.6%–5% (n = 287) | 5.1%–10% (n = 115) | >10% (n = 58) | p Value |
| White (n = 1,566) | n = 1,307 | n = 159 | n = 64 | n = 36 | |
|
| |||||
| Median CAC score (IQR)* | 16.5 (5.1–63.3) | 22.4 (6.6–53.5) | 16.8 (9.8–31.8) | 24.1 (10.0–161.2) | |
| CAC score >0, % (n = 183) | 9.3 | 19.5 | 20.3 | 47.2 | <0.01 |
| CAC score ≥100, % (n = 34) | 1.7 | 3.1 | 1.6 | 16.7 | |
|
| |||||
| Black (n = 1,266) | n = 1,065 | n = 128 | n = 51 | n = 22 | |
|
| |||||
| Median CAC score (IQR)* | 34.6 (4.7–64.6) | 13.2 (3.7–30.0) | 45.4 (12.5–108.2) | 76.9 (36.3–215.7) | |
| CAC score >0, % (n = 96) | 4.8 | 21.1 | 17.7 | 40.9 | <0.01 |
| CAC score ≥100, % (n = 17) | 0.8 | 1.6 | 5.9 | 18.2 | |
Among those with a CAC score >0.
Abbreviations as in Table 2.
The observed prevalence and NNS patterns were similar when CARDIA year 20 examination data were analyzed in the same fashion.
DISCUSSION
We report the prevalence of CAC scores >0 and ≥100 relative to FRS strata in a cohort of young to early middle-age black and white men and women without diabetes. There was significant concordance between CAC prevalence/amount and FRS such that the prevalence of CAC scores >0 and ≥100 and median CAC scores were low in the lower FRS strata and increased with higher FRSs. Correspondingly, the NNS to detect CAC scores >0 or ≥100 was lower with higher FRSs. Findings were similar when stratified by sex and race.
Potential implications
The FRS is a useful tool for predicting coronary events, but fails to identify a significant number of individuals who will have events (22,23). Clinical trial data showing reduced event rates due to CAC screening are lacking. Nevertheless, recent data showed that compared with no CAC testing, randomization to CAC screening was associated with improved coronary artery disease risk factor control without increased downstream medical testing (24). In addition, CAC predicts CHD events independent of the FRS (1,23) and appropriately reclassifies low-risk persons who will have events into the high-risk category (2). In addition, CAC is useful for guiding and monitoring effects of therapy and for motivating patients in lifestyle and/or drug therapy for cardiovascular risk factor modification (4). As such, CAC testing is a topic of discussion for different consensus panels.
In defining FRS thresholds for CAC screening, expert panels have generally focused on individuals 50 years of age and older and differ in their recommendations for what constitutes a reasonable FRS threshold at which to screen for CAC (1,3–6). In the current study, we attempted to determine FRS thresholds at which screening for the presence of CAC, and especially a high CAC burden, might be useful in young to early middle-age individuals based on distribution of CAC by FRS strata. Compared with the presence of any CAC, a high CAC burden (CAC score ≥100) has been associated with greater risk (>2-fold and as high as a 7-fold increase in multivariable-adjusted relative risk) for CHD events (1,6,25,26). We therefore focus our discussion for the current study on determining possible FRS screening thresholds for CAC scores ≥100.
We used the NNS as a tool to aid our prevalence data in determining potential thresholds for CAC screening across FRS strata. The NNS is an extension of the concept of the number needed to treat and is typically defined as the number of people who need to be screened to prevent 1 death or 1 adverse event (27). As in a previous study by our group (8), we defined the NNS as the number of people who need to be screened to detect 1 person with CAC above a specified cut point in each FRS stratum. The prevalence and NNS data from our previous study (of multiethnic men and women 45 to 84 years of age) suggested a low yield of screening for clinically significant levels of CAC in individuals with an FRS ≤5%.
In the current study, the prevalence of CAC scores ≥100 was low (<5%) among all FRS predicted strata <10%, and considerably higher (~17%) in those with an FRS >10%. Correspondingly, the NNS was much higher (NNS>28) in participants with an FRS of 0 to 2.5%, 2.6% to 5%, and 5.1% to 10% compared with those with an FRS >10% (NNS = 6). Furthermore, the relative difference in NNS for CAC scores ≥100 was reasonably high (5-fold) for FRSs >10% versus 5.1% to 10%. This relative difference for adjacent FRS strata was much less (1.4-fold) for FRSs of 5.1% to 10% compared with 2.6% to 5% and ~2-fold for FRSs of 2.6% to 5% versus 0 to 2.5%. Putting our findings in context, it should be noted that in the MASS (Multicentre Aneurysm Screening Study) (28), which used abdominal ultrasound to evaluate the benefit of screening for abdominal aortic aneurysms, the NNS to prevent 1 death secondary to abdominal aortic aneurysm was 20.4 among those screened.
The prevalence and NNS data from our study suggest a low yield of screening for CAC scores ≥100 in those young individuals identified as being at lower 10-year risk of CHD events (FRSs ≤10%). Thus, in this population, an FRS of 10% might represent a logical threshold for CAC screening in younger adults. This is in agreement with some consensus guidelines (1,3) that suggest that persons at intermediate 10-year risk of CHD events (FRSs of 10% to 20%) are more likely to benefit from screening for CAC to aid further risk factor interventions, especially in situations in which there is uncertainty regarding the use of drug therapy. According to the guidelines, those with an FRS >20% are considered to be at high risk of CHD events and should be appropriately managed with drug therapy and lifestyle modifications (17). Also in support of expert panels (1,6), our study suggests that decisions regarding CAC measurement should be made in the context of traditional cardiovascular risk factors rather than in isolation. As such, data from our study support the avoidance of radiation exposure, discovery of incidental findings requiring follow-up computed tomography scans, as well as time, money, and effort spent on CAC measurement for clinical guidance in young, low-risk patients with an FRS <10%.
Other findings
Consistent with other studies, stratification by sex and race revealed the prevalence and amount of CAC to be higher in men than in women and CAC prevalence to be higher in whites compared with blacks (29–33). Contrary to expectations, however, median CAC scores were higher in black than in white participants, likely due to the skewed distribution of data as a result of fewer participants in the higher FRS categories. Because of the young age of the cohort, we did not stratify our data by age. Not surprisingly in this young cohort, cigarette smoking was the most predominant risk factor among those with CAC scores of ≥100 and FRSs >10%. This represents individuals already at higher risk of CHD/cardiovascular disease events based on FRS for whom smoking cessation should be emphasized as a modifiable risk factor, especially if CAC screening revealed significant CAC burden.
Study limitations
The very low number of CHD events in this young cohort to date precluded validation of our suggested FRS cut points for CAC screening using event data. Furthermore, due to the relative youth of our cohort, we had few participants with high CAC burden. As such, we used a lower cut point for high CAC burden (CAC score ≥100) and could not examine NNS and FRS distributions relative to advanced CAC burden (CAC scores ≥300 or 400). Our study, however, is likely representative of the distribution of CAC burden among U.S. young adults. For the same reason, there were fewer participants with a CAC score of ≥100 in each FRS category when stratified by sex and race. Finally, we did not separate the intermediate FRS (10% to 20%) from the high FRS (>20%) risk groups because, of 58 individuals with an FRS >10% in this young cohort, only 6 persons had an FRS >20% (3 of whom had the presence of any CAC).
CONCLUSIONS
In this young to early middle-age nondiabetic, asymptomatic cohort, there was concordance between CAC prevalence/amount and FRS strata. Our study suggests that in this group of relatively young individuals, the yield of screening for high CAC burden (CAC score ≥100) among low-risk persons with an FRS of ≤10% is low. However, CAC testing might be considered in younger persons with an FRS of ≥10%.
Acknowledgments
Work was supported (or partially supported) by contracts from University of Alabama at Birmingham, Coordinating Center, N01-HC-95095; University of Alabama at Birmingham, Field Center, N01-HC-48047; University of Minnesota, Field Center and Diet Reading Center (Year 20 Exam), N01-HC-48048; Northwestern University, Field Center, N01-HC-48049; Kaiser Foundation Research Institute, N01-HC-48050; University of California, Irvine, Echocardiography Reading Center (Year 5 & 10), N01-HC-45134; Harbor-UCLA Research Education Institute, Computed Tomography Reading Center (Year 15 Exam), N01-HC-05187; Wake Forest University (Year 20 Exam), N01-HC-45205; New England Medical Center (Year 20 Exam), N01-HC-45204 from the National Heart, Lung, and Blood Institute. All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CAC
coronary artery calcium
- CHD
coronary heart disease
- FRS
Framingham Risk Score
- NNS
number needed to screen
References
- 1.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378– 402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–6. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 4.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 5.U S. Preventive Services Task Force. Using nontraditional risk factors in coronary heart disease risk assessment: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:474–82. doi: 10.7326/0003-4819-151-7-200910060-00008. [DOI] [PubMed] [Google Scholar]
- 6.Naghavi M, Falk E, Hecht HS, et al. From vulnerable plaque to vulnerable patient–Part III: Executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol. 2006;98:2H–15H. doi: 10.1016/j.amjcard.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46:807–14. doi: 10.1016/j.jacc.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 8.Okwuosa TM, Greenland P, Ning H, et al. Distribution of coronary artery calcium scores by Framingham 10-year risk strata in the MESA (Multi-Ethnic Study of Atherosclerosis): potential implications for coronary risk assessment. J Am Coll Cardiol. 2011;57:1838–45. doi: 10.1016/j.jacc.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nucifora G, Schuijf JD, van Werkhoven JM, et al. Prevalence of coronary artery disease across the Framingham risk categories: coronary artery calcium scoring and MSCT coronary angiography. J Nucl Cardiol. 2009;16:368–75. doi: 10.1007/s12350-009-9059-z. [DOI] [PubMed] [Google Scholar]
- 10.Desai MY, Nasir K, Braunstein JB, et al. Underlying risk factors incrementally add to the standard risk estimate in detecting subclinical atherosclerosis in low- and intermediate-risk middle-aged asymptomatic individuals. Am Heart J. 2004;148:871–7. doi: 10.1016/j.ahj.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 11.Achenbach S, Nomayo A, Couturier G, et al. Relation between coronary calcium and 10-year risk scores in primary prevention patients. Am J Cardiol. 2003;92:1471–5. doi: 10.1016/j.amjcard.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann U, Massaro JM, Fox CS, Manders E, O’Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study) Am J Cardiol. 2008;102:1136–41. 1141.e1. doi: 10.1016/j.amjcard.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung J, Lim SJ, Choe Y, et al. Comparison of the coronary calcium score with the estimated coronary risk. Coron Artery Dis. 2008;19:475–9. doi: 10.1097/MCA.0b013e3283078f9f. [DOI] [PubMed] [Google Scholar]
- 14.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals [published correction appears in JAMA 2004;291:563] JAMA. 2004;291:210–5. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 15.Pletcher MJ, Tice JA, Pignone M, McCulloch C, Callister TQ, Browner WS. What does my patient’s coronary artery calcium score mean? Combining information from the coronary artery calcium score with information from conventional risk factors to estimate coronary heart disease risk. BMC Med. 2004;2:31. doi: 10.1186/1741-7015-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 17.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz KH, Jacobs DR, Jr, Leon AS, Schreiner PJ, Sternfeld B. Physical activity and body weight: associations over ten years in the CARDIA study. Coronary Artery Risk Development in Young Adults. Int J Obes Relat Metab Disord. 2000;24:1475– 87. doi: 10.1038/sj.ijo.0801415. [DOI] [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.Loria CM, Liu K, Lewis CE, et al. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA study. J Am Coll Cardiol. 2007;49:2013–20. doi: 10.1016/j.jacc.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 22.Brindle P, Beswick A, Fahey T, Ebrahim S. Accuracy and impact of risk assessment in the primary prevention of cardiovascular disease: a systematic review. Heart. 2006;92:1752–9. doi: 10.1136/hrt.2006.087932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy TP, Dhangana R, Pencina MJ, Zafar AM, D’Agostino RB. Performance of current guidelines for coronary heart disease prevention: optimal use of the Framingham-based risk assessment. Atherosclerosis. 2011;216:452–7. doi: 10.1016/j.atherosclerosis.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011;57:1622–32. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vliegenthart R, Oudkerk M, Song B, van der Kuip DA, Hofman A, Witteman JC. Coronary calcification detected by electron-beam computed tomography and myocardial infarction. The Rotterdam Coronary Calcification Study. Eur Heart J. 2002;23:1596–603. doi: 10.1053/euhj.2002.3240. [DOI] [PubMed] [Google Scholar]
- 26.Lakoski SG, Greenland P, Wong ND, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2007;167:2437–42. doi: 10.1001/archinte.167.22.2437. [DOI] [PubMed] [Google Scholar]
- 27.Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ. 1998;317:307–12. doi: 10.1136/bmj.317.7154.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashton HA, Buxton MJ, Day NE, et al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360:1531–9. doi: 10.1016/s0140-6736(02)11522-4. [DOI] [PubMed] [Google Scholar]
- 29.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 30.Doherty TM, Tang W, Detrano RC. Racial differences in the significance of coronary calcium in asymptomatic black and white subjects with coronary risk factors. J Am Coll Cardiol. 1999;34:787–94. doi: 10.1016/s0735-1097(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 31.Lee TC, O’Malley PG, Feuerstein I, Taylor AJ. The prevalence and severity of coronary artery calcification on coronary artery computed tomography in black and white subjects. J Am Coll Cardiol. 2003;41:39–44. doi: 10.1016/s0735-1097(02)02618-9. [DOI] [PubMed] [Google Scholar]
- 32.Newman AB, Naydeck BL, Whittle J, Sutton-Tyrrell K, Edmundowicz D, Kuller LH. Racial differences in coronary artery calcification in older adults. Arterioscler Thromb Vasc Biol. 2002;22:424–30. doi: 10.1161/hq0302.105357. [DOI] [PubMed] [Google Scholar]
- 33.Tang W, Detrano RC, Brezden OS, et al. Racial differences in coronary calcium prevalence among high-risk adults. Am J Cardiol. 1995;75:1088–91. doi: 10.1016/s0002-9149(99)80735-8. [DOI] [PubMed] [Google Scholar]


