Abstract
This study aimed to investigate the effect of bone marrow- and adipose tissue-derived mesenchymal stem cell (BM-MSC and AD-MSC respectively) transplantation on left ventricular function and infarct area (IA) in the rat model of ischaemic heart failure. In anaesthetized Wistar rats, the left coronary artery (LCA) was occluded for 40 min with subsequent reperfusion for 7 days. Seven days following surgery, the animals with LCA occlusion/reperfusion were randomized into three groups: (i) Controls received intramyocardial injection of vehicle at three different locations within the peri-infarct zone, (ii) BM-MSC: cells were injected in the same way as in previous group (106), (iii) AD-MSC: using the same protocol as used in the BM-MSC group. In addition there was also a sham-treated group that had no injection. Two weeks following MSC transplantation, the hearts were isolated and perfused according to the Langendorff method followed by 30-min global ischaemia and 90-min reperfusion. After this IA was determined histologically. During Langendorff perfusion initial and postischaemic LV functions were the same in all groups although LV pressure at the 10th minute of reperfusion was higher in the AD-MSC group compared to controls. However, LV pressure during 30-min global ischaemia was significantly higher in BM-MSC as compared to controls and AD-MSC. The sham treated animals showed the same results as those seen with BM-MSC. Thus, BM-MSC transplantation, in contrast to transplantation of AD-MSC, resulted in better preservation of the LV ability to contract during ischaemia. Furthermore, IA was significantly smaller in BM-MSC group as compared to the controls and the AD-MSC groups. Thus this study has demonstrated that treatment with BM-MSC both ameliorates LV function and reduces histological scar size.
Keywords: adipose tissue-derived mesenchymal stem cells, bone marrow-derived mesenchymal stem cells, chronic heart failure, myocardial infarction
Although recent research has made significant strides in developing new treatment options for chronic heart failure (CHF), it remains a leading cause of mortality worldwide (Levi et al. 2002). In turn, myocardial infarction (MI) is one of the most common aetiologies of CHF associated with poor quality of life and high risk of life-threatening complications (Shiba et al. 2005).
It is generally known that cardiac myocytes have limited capacity to regenerate in adult mammalian heart (Smart et al. 2010). Thus, irreversible cardiac injury caused by ischaemia-reperfusion results in myocyte replacement by connective tissue eventually leading to scar formation. Transplantation of stem/progenitor cells of different origin, known as cell therapy, might offer a new approach to treatment of postinfarction CHF. Owing to their promising properties, mesenchymal stem cells (MSC) are considered as the most likely candidates for cardiac cell therapy. MSC are multipotent mesoderm-derived stem cells which are capable of adipogenic, osteogenic and chondrogenic differentiation and characterized by the expression of CD105, CD73, CD90 and lack of CD34 and CD45 expression (Dominici et al. 2006). In the recent years, both in vitro properties and in vivo applications of MSC have been extensively investigated (Baek et al. 2011; Tan et al. 2011; Schu et al. 2012). However, the optimal route of MSC administration, the amount of MSC required for the achievement of therapeutic effect, as well as the extent of cardiac function improvement after transplantation of MSC derived from different tissues are still debated (Orlic et al. 2001; Wang et al. 2011).
Although MSC are currently most commonly obtained from the bone marrow, they are present virtually in any adult tissue studied, including adipose tissue. Several in vitro studies demonstrated that adipose tissue-derived MSC (AD-MSC) are characterized by higher proliferation potential in comparison with bone marrow-derived MSC (BM-MSC) (Izadpanah et al. 2008; Peng et al. 2008; Dmitrieva et al. 2012). Moreover, it has been shown in the study of Zhang et al. (2008) that AD-MSC demonstrated greater potential for 5-azacytidine-induced differentiation into cardiac myocytes as compared to BM-MSC. Despite the apparent differences in the properties of MSC in vitro, little is known whether MSC other than BM-MSC have beneficial effects when administered in the peri-infarct area.
This study was aimed at the evaluation of the effect of BM-MSC and AD-MSC transplantation into the peri-infarct area on left ventricular function and myocardial scar size in the rat model of myocardial ischaemia-reperfusion.
Materials and methods
Male Wistar rats weighting 200-250 g and maintained on a 12:12 light-dark regimen were used throughout the experiments. The animals were fed regular chow, and water was available ad libitum.
Ethical Approval
The procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institute of Health (USA), and were approved by the Ethics committees at V.A. Almazov Federal Heart, Blood and Endocrinology Centre and I.P. Pavlov Federal Medical University, St. Petersburg, Russian Federation.
Surgical procedures
The animals were anaesthetized with chloral hydrate (Acros Organics, Fair Lawn, NJ, USA) given intraperitoneally at a dose of 450 mg/kg. The trachea was intubated after topical application of 2% lidocaine solution on the tongue, pharynx and larynx with subsequent mechanical ventilation (60 breaths per minute and tidal volume of 3 ml/100 g of body weight). Three-lead standard electrocardiogram (ECG) was registered during the experiments.
To secure access to the heart, left thoracotomy in the fourth intercostal space was performed. The pericardium was blunt dissected followed by the passage of atraumatic prolene suture (Ethicon, 6-0, Johnson & Johnson, New Brunswick, NJ, USA) beneath the left coronary artery (LCA). The LCA was occluded for 40 min with subsequent reperfusion. The occurrence of ischaemia was verified by visual inspection of the anterior surface of the left ventricle as well as by ECG criteria, such as ST segment elevation and development of ischaemic arrhythmias. After reperfusion the wound was closed in layers and this was followed by animal recovery.
MSC preparation and characterization
Bone marrow was extruded from tibia, humerus and femur bones by flushing the cavity of the bones with PBS. Bone marrow was then dissociated in cell culture media consisting of α-MEM (PanEco, St. Petersburg, Russia) supplemented with 10% FBS (HyClone, Waltham, MA, USA), L-glutamine and antibiotics and cultured in T75 flasks in CO2-incubator in standard conditions (step designated Passage 0; P0). Fragments of adipose tissue were washed extensively using HBSS, dissociated in 5 mg/ml collagenase III solution (Worthington Biochemical Corp., Lakewood, NJ, USA) and cultured as described previously. For both MSC populations (BM-MSC and AD-MSC), non-adherent cells were removed after 48 h, and the medium was replaced completely. Subsequently, the medium was changed once every 2–3 days. A small number of cells developed colonies by day 5–7. Passages were performed for subconfluent cultures enzymatically, using trypsin-EDTA solution.
Cellular growth characteristics of BM-MSC and AD-MSC were assessed by evaluation of the cumulative population doublings (cumPD) and the population doubling time (PDT), based on the cell counts at passages 0–3 (P0-P3). Population doubling time was calculated as follows: PDT = (T2-T1)/[(LgN2 – LgN1) × 3.32], where (T2-T1) is culturing (days); N2, number of cells harvested; and N1, number of cells seeded. Cumulative population doublings were calculated as the following: cumPD = (LgN2-LgN1)/Lg2, where N2 and N1 are as above. At all passages, a proportion of colony-forming units in culture (CFU-F) was calculated according to Slaoui et al. (1984), using serial dilutions of cells in the wells of 96-well plates (0.4–50 cells per well).
Rat MSC are generally characterized by the following phenotype: CD45−/CD44low/CD106low/CD90 + . In our study, surface marker analysis was performed for BM-MSC and AD-MSC using Guava HT easyCyte 8 Flow Cytometry System (Millipore, USA). Cells were stained with antibodies against CD45, CD44, CD106 and CD90 and isotype control (IgG) (Millipore), at saturating concentrations. Data were analysed using guavasoft 2.2.3 program (Millipore).
Plasticity of BM-MSC and AD-MSC was assessed quantitatively at passage 3 (P3) via induced differentiation into default osteogenic and adipogenic lineages. Osteogenic differentiation of MSC was induced by supplementing cell culture media with 0.1% Dexamethasone, 0.1% ascorbic acid and 1% β-glycerophosphate (Sigma, St. Louis, MO, USA); after 21 days, differentiated cells were visualized with Alizarin R (Sigma). Adipogenic differentiation of MSC was induced by supplementing cell culture media with 0.1% Dexamethasone, 0.1% Insulin and 0.5% isobutylmethylxanthine; after 14 days, differentiated cells were visualized with Oil Red (Sigma). A proportion of differentiated cells was then calculated using AxioVision software (Carl Zeiss, Germany).
Replicative senescence of MSC was assessed quantitatively at passages 0–3 via a β-galactosidase expression assay (Sigma). Briefly, cells were stained with X-gal overnight, and following washing steps were further stained with DAPI (Sigma). Visualization was performed with a fluorescent AxioVert 40 CFL microscope (Carl Zeiss, Germany); the proportion of stained cells was calculated.
Experimental protocol
Seven days after myocardial ischaemia-reperfusion, the animals were anaesthetized again as described previously, and the heart was re-exposed (for procedures, see above) to ensure intramyocardial MSC transplantation. The animals were randomly allocated into one of three groups:
Controls (n = 20): the phosphate buffer was administered in the peri-infarct area. Three injections at distinct anatomical sites were performed, each at a volume of 30 μl.
BM-MSC (n = 17) – a total of 106 bone marrow-derived MSC diluted in 100 μl of PBS were administered in the peri-infarct area at three different sites.
AD-MSC (n = 14) – adipose tissue-derived MSC were administered in the peri-infarct area in the same way as in the previous group.
Sham-operated animals (n = 15) were subjected to all surgical manipulations except myocardial ischaemia-reperfusion and received the phosphate buffer injection in the peri-infarct area. After intramyocardial administration of either MSC or PBS, the chest wound was closed, and the animals were recovered.
Isolated heart perfusion
Two weeks after MSC transplantation, the left ventricular (LV) function as well as the response of the injured myocardium to global ischaemia-reperfusion were studied in the hearts isolated according to the Langendorff method. Briefly, the thorax was opened via the transdiaphragmal bilateral thoracotomy, the heart was rapidly excised and placed into ice-cold Krebs–Henseleit buffer with heparin containing (in mM): glucose 11, NaCl 118, KCl 4,7, CaCl2 3,0, MgSO4 1,2, KH2PO4 1,2, NaHCO3 25. Immediately after heart arrest, it was mounted on the Langendorff apparatus and retrogradely perfused with oxygenated Krebs–Henseleit buffer kept at 37°C through the aorta under constant pressure of 85 mm Hg. Global ischaemia was induced by cessation of perfusate flow to the heart for 30 min followed by 90-min reperfusion. The following functional parameters were registered throughout the experiments: end-diastolic (LVEDP), developed LV pressures (LVDP) and coronary flow (CF). Mean LV pressure was also monitored during 30-min global ischaemia for assessment of the magnitude and time to onset of ischaemic LV contracture. Haemodynamic parameters were digitized and registered with PhysExp software (Cardioprotect Ltd., St-Petersburg, Russia).
Histological analysis
After the end of 90-min reperfusion, the hearts were fixed in 10% buffered formaldehyde solution. Subsequently, the hearts were embedded in paraffin, cut into five transverse slices from apex to base and stained with haematoxylin and eosin. The slides were analysed under light microscope (Axiostar plus, Carl Zeiss, Germany) by the pathologist blinded to the treatment used in the groups, and the infarct area (IA) was calculated as a percentage of infarct scar area relative to the outer circumference of the LV free wall. The values of IA obtained in each of the five levels from apex to base were averaged to obtain the mean value of IA.
Statistical analysis
All data were expressed as mean ± standard error of mean. statistica software (StatSoft, Tulsa, OK, USA) was used for all statistical analyses. Inter-group differences in functional data and infarct area were determined using anova with repeated measures and nonparametric Mann–Whitney test respectively. P < 0.05 was considered statistically significant.
Results
MSC cultures
Primary cultures of rat BM-MSC and AD-MSC were established and characterized as described in Materials and methods section. Cells had a characteristic MSC morphology (Figure 1a,b) and demonstrated high rate of proliferation (Figure 2a-c). FACS analysis had confirmed a characteristic immunophenotype of both BM-MSC and AD-MSC populations (CD45−/CD44low/CD106low/CD90+) (Figure 3). Cell plasticity was evaluated at passage 3, when MSC were induced to differentiate into default osteogenic and adipogenic lineages: following differentiation protocols, a number of MSC-derived cells stained positive for Alizarin R (26% cells) and Oil Red (19% cells) respectively (Figure 1c,d). Finally, a replicative senescence of MSC was assessed quantitatively, and a resulting growth curve had an anticipated shape (Figure 2d).
Figure 1.
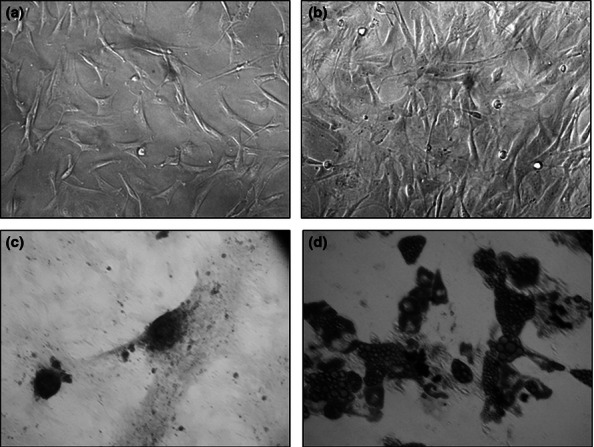
Morphology of rat (a) BM-MSC and (b) AD-MSC; MSC-derived cells differentiated into osteogenic ((c); Alizarin R staining) and adipogenic ((d); Oil Red staining) lineages.
Figure 2.
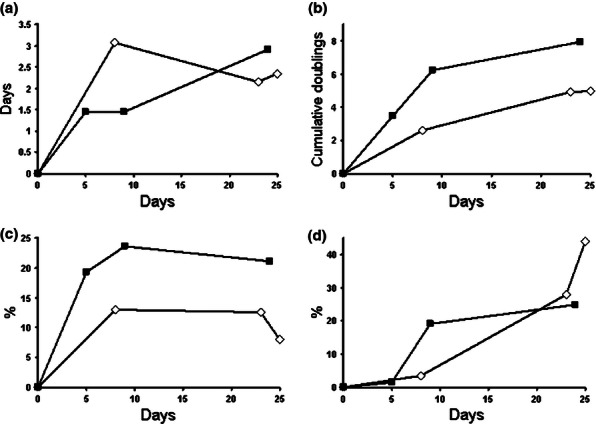
Proliferation (a–c) and replicative senescence (d) of rat BM-MSC (open diamonds, ◊) and AD-MSC (filled rectangles, ▪). Population doubling time (PDT) (a); cumulative population doublings (cumPD) (b); colony-forming units in culture (CFU-F) (c); and β-galactosidase-positive cells (d) at passages 1–3 (P1-P3) are shown.
Figure 3.
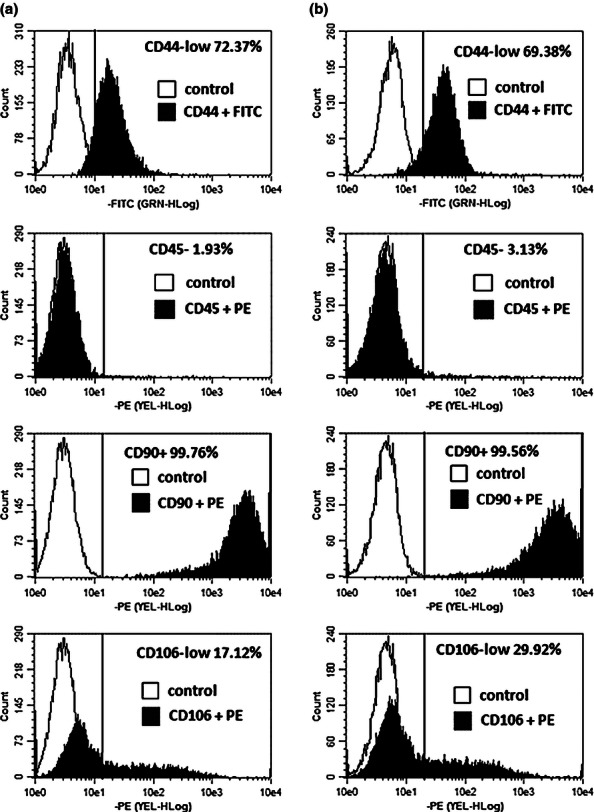
FACS analysis of rat BM-MSC (a) and AD-MSC (b) immunophenotype.
Mortality rates
At the end of the study, results from 53 of 66 rats (80.3%) could be obtained. The mortality rates therefore were 5 of 20, 3 of 17, 3 of 14 and 0 of 15 in Controls, BM-MSC, AD-MSC and Sham-operated animals respectively.
Left ventricular function
The data on pre- and postischaemic values of LVEDP, LVDP and CF are shown on the Figure 4a-c respectively. Both initial and postischaemic LV function was not different among all groups although LV pressure at the 10th minute of reperfusion tended to be higher in the AD-MSC group in comparison with controls (129 ± 15 and 115 ± 30 mm Hg, respectively, P = 0.08). However, LV pressure during 30-min global ischaemia was significantly higher in sham-treated animals and BM-MSC as compared to controls and AD-MSC (Figure 4d). Thus, at the 20th minute of global ischaemia, LV pressure was 79 ± 17, 82 ± 15, 57 ± 23 and 53 ± 22 mm Hg in BM-MSC (P < 0.05 vs. AD-MSC and controls), sham-operated rats (P < 0.05 vs. AD-MSC and controls), AD-MSC and controls respectively. It follows, therefore, that BM-MSC transplantation, in contrast to transplantation of AD-MSC, resulted in better preservation of the LV ability to contract under ischaemic conditions.
Figure 4.
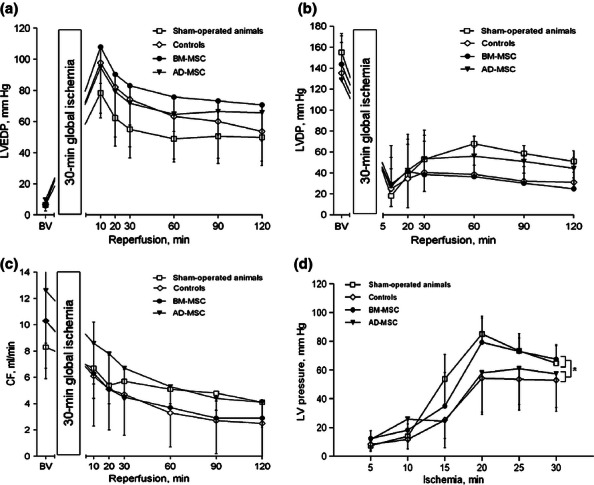
Functional parameters of the isolated Langendorff-perfused hearts subjected to 30-min global ischaemia plus 120-min reperfusion. The hearts were harvested from sham-treated animals or rats subjected to intramyocardial administration of either BM-MSC or AD-MSC after 30-min ischaemia followed by 7 days of reperfusion. The animals subjected to intramyocardial administration of PBS were controls. (a) left ventricular end-diastolic pressure, (b) left ventricular developed pressure, (c) coronary flow, (d) mean LV pressure during 30-min global ischaemia. There were no differences in both pre- and postischaemic values of LV pressures and coronary flow between groups. Mean pressure in the LV was significantly lower in AD-MSC and controls as compared to both BM-MSC and sham-operated group. * - P < 0.005 for controls and AD-MSC vs. BM-MSC and sham-operated groups.
Infarct area
Infarct area was significantly smaller in the BM-MSC group in comparison with controls (22 ± 5 vs. 37 ± 3%, respectively, P < 0.05, Figure 5). Besides, the infarct area was not different between the AD-MSC and controls (32 ± 6 vs. 37 ± 3%, respectively, P > 0.05, Figure 5).
Figure 5.
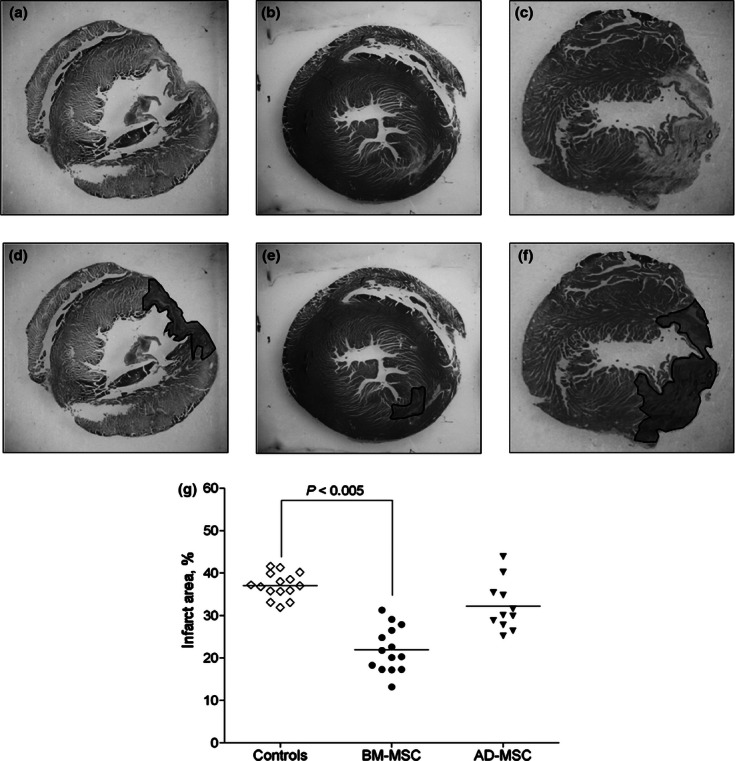
Representative haematoxylin/eosin-stained cross-sections of the heart from control (a), AD-MSC- and BM-MSC-treated rats ((b) and (c) respectively). (d–f) the infarct area in the respective slides is highlighted. (g) quantitative values of infarct area defined as a percentage infarct scar area relative to the outer circumference of the LV free wall in the above groups. The data are expressed as dot plots with median values. Infarct area was significantly smaller in the BM-MSC group as compared to both controls and AD-MSC group.
Discussion
In the present study intramyocardial transplantation of BM-MSC 7 days following myocardial ischaemia-reperfusion resulted in both significant decrease in the LV scar area and better preservation of myocardial response to global ischaemia as judged from the magnitude of LV contracture. In contrast, administration of AD-MSC in the peri-infarct zone did not result in cardiac protection.
The effects of intramyocardial MSC transplantation have been investigated before in the rat models of reversible (Jain et al. 2001) and permanent (Imanishi et al. 2008) myocardial ischaemia. Although the permanent LCA ligation model has been most frequently used in these reports, we have selected the model of cardiac ischaemia-reperfusion in this study. This model seems to be more relevant to the clinical scenario of patients with acute coronary syndrome who underwent coronary revascularization via primary percutaneous intervention or thrombolysis.
The question might arise as to the route of MSC administration used in the present study as optimal. Several ways of MSC transplantation for cell therapy of MI were examined previously, including intracoronary (Hou et al. 2005), intravenous (Nagaya et al. 2004) and intramyocardial administration either into peri-infarct area (Li et al. 2010) or directly into the infarct zone (Imanishi et al. 2008). In the present study, the intramyocardial route of MSC administration was chosen on the basis of the data showing greater effectiveness of this particular approach owing to local accumulation of the cells in the target area as well as better safety (Freyman et al. 2006). Moreover, intramyocardial administration of MSC has become more available in clinical practice over the last decade because of the application of such interventional techniques as the three-dimensional NOGA electromechanical mapping system which allows precise transendocardial administration of MSC (Chazaud et al. 2003; Banovic et al. 2011).
There have been some controversy about the optimal dose of MSC for intramyocardial administration and the exact time interval for their transplantation in the post-MI period. In the present study, both types of MSC were administered into the myocardium at a total dose of 106 cells in 1 ml of phosphate buffer. This dosage is currently most commonly used in the animal models of myocardial ischaemia (e.g. Poncelet et al. 2010). The MSC were administered in the myocardium 7 days after ischaemia-reperfusion, which is in agreement with the data of Jiang et al. (2008). This time point seems to be the best suited for the preservation of both MSC viability and their functional activity because of two major factors. First, the acute inflammatory response which might interfere with MSC activity is almost completely resolved. Second, the fibrous tissue is still quite immature at this stage of AMI evolution, which may contribute to the limitation of scar tissue formation be means of MSC growth and differentiation.
Therefore, the documented positive effect of BM-MSC transplantation might be accounted for by two factors, that is, optimal choice of MSC dose and route of administration as well as the administration time point characterized by minimal inflammation and presence of still immature scar tissue.
Despite the promising in vitro data and relative abundance of AD-MSC in the visceral fat tissue, the intra-cardiac AD-MSC transplantation failed to protect the heart from ischaemia-reperfusion injury in this study. This fact might be explained by the apparent genotypic and phenotypic characteristics of this type of MSC. The clear prediction of the effectiveness of treatment with certain population of MSC is hampered by the as yet unknown mechanism(s) of MSC-mediated protective effect. To date, there are at least two main hypotheses explaining the mechanism of MSC-mediated amelioration of LV function after MI. According to the first hypothesis, MSC transplantation contributes to improvement of LV function via enhanced production of cytokines that might control cellular microenvironment (Jiang et al. 2008), regulate cell metabolism (Feygin et al. 2007) and stimulate proliferation and differentiation of cardiac resident progenitor cells (Sassoli et al. 2011; Wang et al. 2012). The second hypothesis implies that MSC might directly differentiate into both cardiac myocytes and endothelial cells (Jiang et al. 2008; Numasawa et al. 2011). Without clear understanding of the relative contribution of the above mechanisms to the cardioprotective effect of MSC, we might only consider the key phenotypic characteristics of MSC as the determinants of their protective effect. There is a close similarity between AD-MSC and BM-MSC because both cell populations stem from embryonic mesoderm. In particular, both cell types fulfil the general criteria for MSC proposed by Dominici et al. (2006), that is, adherence to plastic when maintained in culture, expression of CD105, CD73, CD90, lack of expression of CD34, CD45, and the ability to differentiate in vitro into osteoblasts, adipocytes and chondroblasts. However, certain differences in the phenotypic properties of AD-MSC and BM-MSC were shown previously. For example, such markers as CD44 and CD49d are not found on BM-MSC but are typically present on AD-MSC. Further, CD106 is characteristic for BM-MSC but not for AD-MSC (Zhu et al. 2012). As to the proliferative capacity of MSC, there is some evidence that AD-MSC have greater proliferative potential than BM-MSC (Izadpanah et al. 2008; Peng et al. 2008; Dmitrieva et al. 2012). Moreover, the cardiomyocyte-specific differentiation of AD-MSC has been demonstrated in in vitro studies (Zhu et al. 2008; Yang et al. 2012). Despite these encouraging results with AD-MSC in vitro, their intra-cardiac transplantation failed to protect the LV from postinfarction remodelling in the present study. Thus, more detailed information on the paracrine factors produced by the two types of MSC is required to elucidate not only the functional differences between distinct MSC populations but also the basic mechanisms of cardiac protection afforded by MSC transplantation.
The present study has several limitations. First, infarct area was determined using haematoxylin and eosin staining only. The quantification of interstitial collagen deposition with Masson's trichrome staining would be more informative for assessment of LV remodelling. Second, the mechanism(s) of the protective effect of MSC transplantation on postinfarction myocardial remodelling were not addressed. It should be noted, however, that a definite experimental proof of either hypothesis explaining the mechanism of MSC-mediated improvement of LV function after MI is still hampered by serious methodological limitations, including the verification of MSC differentiation into cardiac myocytes in vivo. Sex-mismatched transplantation of MSC can provide a useful tool in the understanding of the exact mechanisms of beneficial MSC effects in the setting of MI. Future studies will address these important issues.
In conclusion, this study demonstrated that postischaemic intramyocardial administration of BM-MSC both ameliorated LV function and reduced histological scar size. In contrast to BM-MSC, AD-MSC transplantation did not affect postinfarction LV remodelling.
Fundind source
The study is supported by the Ministry of Healthcare of the Russian Federation (State Contract K-32-NIR/111-3) within the framework of the Russia/Belarus Union State "Stem Cells" programme.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Baek SJ, Kang SK, Ra JC. In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp. Mol. Med. 2011;43:596–603. doi: 10.3858/emm.2011.43.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banovic M, Ostojic MC, Bartunek J, Nedeljkovic M, Beleslin B, Terzic A. Brachial approach to NOGA-guided procedures: electromechanical mapping and transendocardial stem-cell injections. Tex. Heart Inst. J. 2011;38:179–182. [PMC free article] [PubMed] [Google Scholar]
- Chazaud B, Hittinger L, Sonnet C, et al. Endoventricular porcine autologous myoblast transplantation can be successfully achieved with minor mechanical cell damage. Cardiovasc. Res. 2003;58:444–450. doi: 10.1016/s0008-6363(02)00834-9. [DOI] [PubMed] [Google Scholar]
- Dmitrieva RI, Minullina IR, Bilibina AA, Tarasova OV, Anisimov SV, Zaritskey AY. Bone marrow- and subcutaneous adipose tissue-derived mesenchymal stem cells: differences and similarities. Cell Cycle. 2012;11:377–383. doi: 10.4161/cc.11.2.18858. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotentmesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Feygin J, Mansoor A, Eckman P, Swingen C, Zhang J. Functional and bioenergetic modulations in the infarct border zone following autologous mesenchymal stem cell transplantation. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1772–H1780. doi: 10.1152/ajpheart.00242.2007. [DOI] [PubMed] [Google Scholar]
- Freyman T, Polin G, Osman H, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur. Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- Hou D, Youssef EA, Brinton TJ, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:150–156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- Imanishi Y, Saito A, Komoda H, et al. Allogenic mesenchymal stem cell transplantation has a therapeutic effect in acute myocardial infarction in rats. J. Mol. Cell. Cardiol. 2008;44:662–671. doi: 10.1016/j.yjmcc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Izadpanah R, Kaushal D, Kriedt C, et al. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68:4229–4238. doi: 10.1158/0008-5472.CAN-07-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, DerSimonian H, Brenner DA, et al. Performance after myocardial infarction cell therapy attenuates deleterious ventricular remodeling and improves cardiac performance after myocardial infarction. Circulation. 2001;103:1920–1927. doi: 10.1161/01.cir.103.14.1920. [DOI] [PubMed] [Google Scholar]
- Jiang CY, Gui C, He AN, et al. Optimal time for mesenchymal stem cell transplantation in rats with myocardial infarction. J. Zhejiang Univ. Sci. B. 2008;9:630–637. doi: 10.1631/jzus.B0820004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi F, Lucchini F, Negri E, La Vecchia C. Trends in mortality from cardiovascular and cerebrovascular diseases in Europe and other areas of the world. Heart. 2002;88:119–124. doi: 10.1136/heart.88.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Turdi S, Thomas DP, Zhou T, Ren J. Intra-myocardial delivery of mesenchymal stem cells ameliorates left ventricular and cardiomyocyte contractile dysfunction following myocardial infarction. Toxicol. Lett. 2010;195:119–126. doi: 10.1016/j.toxlet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya N, Fujii T, Iwase T, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am. J. Physiol. Heart Circ. Physiol. 2004;287:2670–2676. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- Numasawa Y, Kimura T, Miyoshi S, et al. Treatment of human mesenchymal stem cells with angiotensin receptor blocker improved efficiency of cardiomyogenic transdifferentiation and improved cardiac function via angiogenesis. Stem Cells. 2011;29:1405–1414. doi: 10.1002/stem.691. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Peng L, Jia Z, Yin X, et al. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev. 2008;17:761–773. doi: 10.1089/scd.2007.0217. [DOI] [PubMed] [Google Scholar]
- Poncelet AJ, Hiel AL, Vercruysse J, Hermans D, Zech F, Gianello P. Intracardiac allogeneic mesenchymal stem cell transplantation elicits neo-angiogenesis in a fully immunocompetent ischaemic swine model. Eur. J. Cardiothorac. Surg. 2010;38:781–787. doi: 10.1016/j.ejcts.2010.03.035. [DOI] [PubMed] [Google Scholar]
- Sassoli C, Pini A, Mazzanti B, et al. Mesenchymal stromal cells affect cardiomyocyte growth through juxtacrine Notch-1/Jagged-1 signaling and paracrine mechanisms: clues for cardiac regeneration. J. Mol. Cell. Cardiol. 2011;51:399–408. doi: 10.1016/j.yjmcc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Schu S, Nosov M, O'Flynn L, et al. Immunogenicity of allogeneic mesenchymal stem cells. J. Cell Mol. Med. 2012;16:2094–2103. doi: 10.1111/j.1582-4934.2011.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba N, Watanabe J, Shinozaki T, et al. Poor prognosis of Japanese patients with chronic heart failure following myocardial infarction comparison with nonischemic cardiomyopathy. Circ. J. 2005;69:143–149. doi: 10.1253/circj.69.143. [DOI] [PubMed] [Google Scholar]
- Slaoui M, Leo O, Marvel J, Moser M, Hiernaux J, Urbain J. Idiotypic analysis of potential and available repertoires in the arsonate system. J. Exp. Med. 1984;160:1–11. doi: 10.1084/jem.160.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Risebro CA, Clark JE, et al. Thymosin beta4 facilitates epicardial neovascularization of the injured adult heart. Ann. N. Y. Acad. Sci. 2010;1194:97–104. doi: 10.1111/j.1749-6632.2010.05478.x. [DOI] [PubMed] [Google Scholar]
- Tan Z, Su ZY, Wu RR, et al. Immunomodulative effects of mesenchymal stem cells derived from human embryonic stem cells in vivo and in vitro. J. Zhejiang Univ. Sci. B. 2011;12:18–27. doi: 10.1631/jzus.B1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hu X, Xie X, He A, Liu X, Wang JA. Effects of mesenchymal stem cells on matrix metalloproteinase synthesis in cardiac fibroblasts. Exp. Biol. Med. (Maywood) 2011;236:1197–1204. doi: 10.1258/ebm.2011.010317. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang G, Hou Y, et al. Transplantation of microencapsulated Schwann cells and mesenchymal stem cells augment angiogenesis and improve heart function. Mol. Cell. Biochem. 2012;366:139–147. doi: 10.1007/s11010-012-1291-1. [DOI] [PubMed] [Google Scholar]
- Yang J, Song T, Wu P, et al. Differentiation potential of human mesenchymal stem cells derived from adipose tissue and bone marrow to sinus node-like cells. Mol. Med. Report. 2012;5:108–113. doi: 10.3892/mmr.2011.611. [DOI] [PubMed] [Google Scholar]
- Zhang DZ, Gai LY, Liu HW. Differences between adipose-derived stem cells and mesenchymal stem cells in differentiation into cardiomyocytes. Sheng Li Xue Bao. 2008;60:341–347. [PubMed] [Google Scholar]
- Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem. Funct. 2008;26:664–675. doi: 10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]
- Zhu X, Du J, Liu G. The comparison of multilineage differentiation of bone marrow and adipose-derived mesenchymal stem cells. Clin. Lab. 2012;58:897–903. [PubMed] [Google Scholar]


