Abstract
Femoral head avascular necrosis is a process leading to femoral head deformity and osteoarthritic changes in the hip joint. Alendronate slows down bone resorption and remodelling in rats, while core decompression hastens the healing processes. We evaluated the influence of daily alendronate treatment on the rat femoral head shape after surgical osteonecrosis with core decompression, compared with controls. No differences were found in shape factor and femoral head height/length ratios. It was concluded that alendronate treatment slows down the process of replacing osteonecrotic bone by new bone and prevents early immature new bone collapse resulting from early revascularization because of core decompression.
Keywords: alendronate, avascular necrosis, core decompression, femoral head, morphometry, rat model
Femoral head avascular necrosis (FAVN) is one of the processes leading to femoral head deformity which, if not treated, flattens later and results in hip joint osteoarthritis (Peled et al. 2006). There are many different aetiological factors that are implicated in the pathogensis of FAVN. It has been shown previously that increased osseous pressure on the end of the long bone is part of the pathogenesis of the osteonecrosis (Boss & Misselevich 2001). Various surgical procedures for FAVN and hip joint preservation have been described to prevent FAVN progression, including core decompression, drilling, vascularized and non-vascularized bone grafting, and osteotomies (Koo et al. 1995). Core decompression and drilling relieves intramedullary hypertension and venous reduction, especially in early Ficat stages. These procedures have resulted in success rates of up to 63.5% (Mont et al. 1996).
Surgical rat femoral head osteonecrosis is induced by stripping the femoral neck periosteum and detaching the ligamentum teres (Norman et al. 1998). Bone marrow haematopoietic necrosis is seen from the second day and bone necrosis from the fifth day (Levin et al. 1999). The inflammatory reaction around the joint and from the joint capsule invades the femoral head, promoting the healing process during the second week. This process involves macrophages and osteoclast activity, which removes the necrotic bone that is replaced by fibrous tissue and, eventually, by osteoblast deposit and intramembranous bone. During the remodelling process, the femoral head epiphysis and physis spherical shape is distorted and becomes flattened (Norman et al. 1998; Levin et al. 1999; Peskin et al. 2001). Rapid repair of the osteonecrotic femoral head with newly formed bone leads to flattening of the femoral head (Sabo et al. 2001; Boss et al. 2004; Peled et al. 2006, 2009).
Alendronate is a biphosphonate that retains the osteoclasts and, as a result, inhibits their activity, resulting in inhibition of the remodelling and healing processes of the necrotic femoral head bone. Alendronate absorption in rats is poor, and less than 1% of an oral dose is available systemically in rats, with food impairing its absorption further. Up to 60–70% of alendronate is taken up by the bone tissue, while up to 30–40% is excreted by the kidneys within 24 h (Lin et al. 1991). Alendronate gastrointestinal absorption is lower with food and higher when fasting. Bone uptake is lower in female rats and higher in young rats (Lin et al. 1992). Alendronate absorption is influenced by food that can enhance, delay or reduce its absorption. Using the parenteral route for administration, intravenous/subcutaneous/intramuscular, allows almost complete alendronate absorption with higher systemic levels and availability to the calcified tissue, specifically to bone tissue. Bone uptake is linear up to 5 mg/kg per day (Lin et al. 1994; Lin 1996). Alendronate daily dosage of 200 μg/kg has been widely used in rats, and the medication is redistributed to the bone with a half-life time of about 200 days (Lin et al. 1992, 1994; Lin 1996). Alendronate treatment does not affect normal bone histology (Reszka & Rodan 2004) or prevent side effects of core decompression. Alendronate treatment has become common for FAVN (Nishii et al. 2006; Agarwala et al. 2009).
We hypothesized that alendronate treatment might prevent femoral head distortion and that it might preserve head shape and its sphericity. To evaluate and to determine objectively the validity of our hypothesis, we created surgical rat femoral head osteonecrosis with core decompression. In this study, femoral head shape after FAVN decompression and daily treatment with alendronate for 6 weeks was compared with controls.
Material and methods
Approval was obtained prior to the start of the study from The Committee for the Supervision of Animal Experiments of Technion-Israel Institute of Technology (#IL-040-05-2004). The blood circulation of the right femoral head of 18 female Sprague-Dawley rats, weighing 400–450 gm, was interrupted to create surgical femoral head osteonecrosis (Norman et al. 1998). The animals were housed in spacious cages to allow ample ambulation and were operated after a minimum of a week for acclimation to the cage. They had free access to water and regular laboratory chow at all times. The rats were anaesthetized with an intramuscular injection of ketamine (120 mg/kg) and xylazine (17 mg/kg) and placed on a heated operating table to prevent hypothermia. The skin was shaved, anti-sepsis was applied, the area was draped, and the cutis was proximally slit by a longitudinal incision over the greater trochanter. Then, the gluteus maximus muscle was split in the direction of its bundles, and the anterior two-thirds of the gluteus medius muscle were detached from the bone. The anterolateral insertion of the articular capsule was transected along the trochanteric ridge, the femoral head was dislocated, and the ligamentum teres was cut. With a number 11 blade, the periosteum at the base of the neck of the femoral head was incised together with the reflected capsular fibres by twice circumferentially sweeping the edge of the blade around the femoral neck, at a 1-mm interval, around the bone. Core decompression of the femoral head was carried out in a retrograde manner starting from the insertion point of the ligamentum teres through the head and the neck. The drilling was accomplished with a 21-gauge needle parallel to the neck axis. After drilling, the femoral head was relocated. The articular capsule and the gluteal muscles were sutured with vicryl 3/0, and the skin was closed with nylon 2/0 (Norman et al. 1998). Each group consisted of nine animals. Both groups were treated with daily subcutaneous injections: the treated group with alendronate and the control group with saline. A stock solution of alendronate was prepared by diluting tablets of ‘Fosalan’ (alendronate sodium) 70 mg (MSD Inc., Harlem, the Netherlands) at a concentration of 500 μg/ml of physiological saline and mixed in an electrical stirrer for 90 min. Postoperatively, the nine rats in the treated group received in the neck region daily subcutaneous injections of 200 μg of alendronate per 1 kg of body weight to which a saline solution was added, up to a total volume of 0.5 ml. The nine control rats were treated with 0.5 ml saline instead of the alendronate solution. All rats were sacrificed by CO2 inhalation on the 42nd postoperative day, both femoral heads were harvested, and the soft tissue was excised. The specimens were fixed in formalin for a week. Following decalcification in EDTA for 2 weeks, the femoral heads were halved at the residue of the ligamentum teres into anterior and posterior parts. Paraffin blocks were cut at 4 μm, and the sections were stained with haematoxylin and eosin. All the histological specimens were numbered randomly.
Histomorphometric analysis of the sections was performed using a computerized image analysis system. The system consisted of a trinocular Olympus CH-40 bright-field microscope (Olympus Corporation, Tokyo, Japan) fitted with a Zeiss Plan 1× objective. This ultra-low objective allows the structures in the preparation to be examined and measured on a single image. The image passes through a 2.5× projection lens to a WAT202D digital video camera, and the image is seen on a 19-inch video monitor. In parallel, a second similar monitor is used for data collection, management and spreadsheets. The PC is fitted with a dual video channel and a frame grabber. The software is from Soft Imaging System, analysis® version 3.0, Munster, Germany. The system is very accurately calibrated for the specific objective and images, and all the data were recorded in micrometres (μm) (Sabo et al. 2001).
As noted earlier, the femoral heads were halved at the residue of the ligamentum teres into anterior and posterior parts. The field of interest was chosen as the femoral head portion overlying an imaginary straight line at the insertion of the joint capsule to the cartilage–bone junction. Feret diameters were gauged at 10 degree intervals. The maximum and minimum Feret diameters, expressing the length and the height of the femoral head, were measured. These values were used to compare the height/length (H/L) ratio of the femoral heads. The shape factor (SF) of the femoral heads was determined to assess the femoral head profile. The SF was defined as 4πA/P2, where A is the femoral head area and P is the perimeter. Round objects have SF values close to 1, while linear objects have 0 values (Sabo et al. 2001).
To evaluate the specimens objectively and to prevent biased results, all the slides were numbered randomly and mixed. The examiner who received the slides knew that they were mixed. After completing the measurements, the slide results were uncovered. For each rat SF and H/L, the operated right side femoral head was compared with the non-operated left side femoral head, and the alendronate-treated group was compared with the control group (Sabo et al. 2001).
Statistical evaluation was performed by a qualified statistician. Mean, standard deviation (SD) and range were calculated. The Wilcoxon signed-rank test was used to compare between the right and the left femoral head of each animal. Mann–Whitney U-test was used to compare between treated and control groups. P values up to 0.05 were considered significant.
Results
Four rats were lost during the investigated period, one from the alendronate-treated group that died of wound complications and infection, leaving eight treated rats, and three from the control group, one of which died on the second postoperative day due to wound complications and two of which died of the same causes after a week, leaving six rats in the control group. Among the alendronate-treated group (Figure 1), one femoral head was so severely damaged during the harvesting that it was not possible to do the measuring needed for its evaluation, leaving seven rats in the alendronate-treated group. The histomorphometry of the femoral heads was evaluated with computer assistance and consisted of the SF and the H/L ratio. During the treatment period, the rats were weighed daily before the alendronate injection to determine the alendronate injectable dose. We did not recognize weight differences between the subgroups, and the animal's weight was minimally, if at all, changed during the study period.
Figure 1.
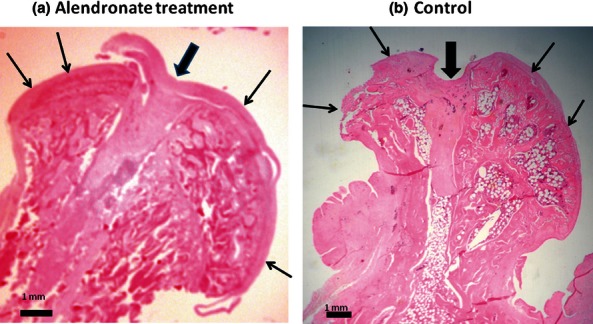
Rat femoral heads 42 days after surgical avascular osteonecrosis of the head and core decompression at the mid-coronal section at the point of the ligamentum teres insertion and after H&E staining. The thin arrows are directed at the femoral head articular surface. The wide arrows are directed at the point of the ligamentum teres insertion and the core decompression tract, which was made using a 21-gauge needle. (a) The right-sided head of the treated rat shows preservation of the epiphyseal height as well as its sphericity. (b) The left-sided head of the control shows that the epiphyseal height is lost along with its roundness and sphericity.
There were no SF or H/L ratio differences in the alendronate-treated group when comparing the operated right side head to the non-operated head (P > 0.05), meaning that there were no differences concerning sphericity among the treated animals. In the control group (Figure 1), there were significant differences when comparing SF (P = 0.03) and H/L (P = 0.03) of the right and left femoral heads. These differences meant that the shape of the operated femoral head was distorted and had lost its sphericity, which was preserved on the non-operated side.
Differences were found between the SF and H/L ratios when comparing the right femoral heads of the alendronate-treated group to the control group when using the Mann–Whitney U-test (P = 0.03 for SF and P = 0.01 for H/L), meaning that there were differences in sphericities of the operated femoral heads.
Discussion
This study describes the effects and the influences of daily subcutaneous alendronate injections on rat femoral head shape after surgical avascular osteonecrosis-associated core decompression. The dead bone marrow and bone, as a result of the ischaemic event, initiate reparative processes. Lesions larger than 15% of the femoral head have difficulties in healing and might collapse (Mont et al. 1998). Collapse occurs when the necrotic bone affects the weight-bearing area or when the subchondral bone under this area is flattened and tends to collapse (Ohzono et al. 1991; Sabo et al. 2001). Femoral head core decompression or an intraosseous conduit decompresses the head and expedites the reparative and remodelling processes, which stimulate ingrowth of sinusoidal blood vessels compared with those without core decompression (Norman et al. 2004). The presence of the canal created along the femoral head accelerates the healing processes by means of opening pathways for vascular ingrowth from the viable neck towards the head (Norman et al. 2004). This process hastens the replacement of the necrotic bone by the newly weak bone, which might have difficulties in withstanding the weight-bearing loads (Peled et al. 2009).
To investigate the influence of alendronate treatment on bone metabolism, Nishii et al. (2006) compared the influence of alendronate treatment in 22 patients with osteonecrosis of the femoral head for a year. Twenty hips in 14 patients were treated on a daily basis with alendronate and compared with 13 hips in eight patients in a control group. All patients had measurements of biochemical markers of bone turnover at entry into the study, and the measurements were repeated for the patients in the alendronate group at 3, 6 and 12 months. The alendronate group showed a greater decrease in biochemical markers of bone resorption, a lower frequency of collapse of the femoral head and reported less hip pain than the control group.
Femoral head sphericity as presented by the SF (Table 1), as well as the H/L ratio (Table 2), was preserved among the treated group. There were no differences when comparing the operated right side femoral head to the left side. In the control group, the one that did not receive alendronate, femoral head sphericity was lost, and the head was deformed as represented by the SF and H/L ratio, when comparing the operated right side femoral head to the left, and these changes were significant (P < 0.05).
Shape factor (SF) of the osteonecrotic femoral head of both groups, comparing operated right side to native left side
| Treated group N = 7 | Control group N = 6 | ||
|---|---|---|---|
| Right femoral head | Mean ± SD | 0.62 ± 0.04 | 0.55 ± 0.05 |
| Range | 0.54–0.67 | 0.33–0.62 | |
| Left femoral head | Mean ± SD | 0.63 ± 0.02 | 0.63 ± 0.04 |
| Range | 0.37–0.67 | 0.57–0.69 | |
| P value (right/left) | >0.05 | 0.03 | |
Shape factor, 4πA/P2, where A is the femoral head area and P is the perimeter.
P value was calculated by comparing the right and left femoral heads using Wilcoxon signed-rank test.
N, number of rats; Mean ± SD, mean ± standard deviation; range, minimum–maximum.
Height/Length (H/L) ratio of the osteonecrotic femoral head of both groups, comparing operated right side to native left side
| Treated group N = 7 | Control group N = 6 | ||
|---|---|---|---|
| Right femoral head | Mean ± SD | 0.51 ± 0.06 | 0.44 ± 0.06 |
| Range | 0.37–0.56 | 0.31–0.53 | |
| Left femoral head | Mean ± SD | 0.52 ± 0.07 | 0.53 ± 0.06 |
| Range | 0.46–0.62 | 0.45–0.64 | |
| P value (right/left) | >0.05 | 0.03 | |
Height/Length ratio, compare the height/length (H/L) ratio of the femoral heads.
P value was calculated by comparing the right and left femoral heads using Wilcoxon signed-rank test.
N, number of rats; Mean ± SD, mean ± standard deviation; range, minimum–maximum.
Significant changes were also found when comparing the alendronate-treated group and the control group concerning the operated right side femoral head SF and H/L ratio, with P values of 0.03 and 0.01 respectively. Core decompression of the surgically osteonecrotic rat femoral head was associated with severely damaged and deformed sphericity shape when compared with other treatment modalities, such as non-weight-bearing loads and enoxaparin (Norman et al. 2004).
To assess the long-term follow-up after core decompression of the FAVN, Marker et al. (2008) reviewed the literature dealing with core decompression and tried to differentiate between follow-up up to 15 years and follow-up longer than 15 years. In their review, they summarized and analysed 1268 hips in the first group and 1337 in the second. Additional surgery was needed in 30% in the first group and 41% in the second group. Core decompression for FAVN was found to be a safe and effective early treatment procedure for patients with small–medium-sized lesions.
Lai et al. (2005) reported about 40 patients with femoral head AVN with a necrotic area greater than 30%. Half the patients were treated with alendronate once a week for 25 weeks and compared with untreated control patients. After a minimum of 2 years of follow-up, two of 29 femoral heads in the alendronate group collapsed, one of which underwent total hip replacement, while 19 of 25 femoral heads in the control group collapsed, 16 of which needed total hip replacement.
Agarwala et al. (2009) reported clinico-radiological follow-up up to 8 years after oral alendronate treatment for FAVN for 3 years. Alendronate improved the clinical function and reduced the collapse rate and the need for total hip replacement, especially in the early stages.
Kang et al. (2012) treated 93 patients with FAVN with multiple drilling core decompression and who received calcium and vitamin D3 additionally. Forty-seven patients (67 hips) were combined with alendronate treatment of 70 mg per week for 24 weeks. Of these patients, 39 (55 hips) completed a minimum of 48 months of follow-up. In the control group, there were 46 patients (60 hips). Of these, 40 patients (52 hips) completed follow-up. Nine hip joints from the alendronate group required total hip replacement, compared with 15 among the control group. In addition, among those who did not need joint replacement, the alendronate-treated group showed better results with a higher Harris Hip Score and a smaller incidence of late bone collapse.
According to our findings, we assume that the association of alendronate treatment after core decompression of the rat femoral head had a crucial effect and influence on the preservation of sphericity and in preventing head collapse. By adding alendronate, and perhaps other bisphosphonates, there may be an option for increasing the success rate for FAVN.
Conclusion
Alendronate treatment for FAVN in rats slows down the replacement process of the osteonecrotic bone by new bone and prevents early immature new bone collapse as a result of early revascularization because of the core decompression. The association of alendronate treatment and core decompression for FAVN is expected to increase clinical and radiological success rates and might preserve femoral head sphericity and the hip joint.
Acknowledgments
The authors thank Prof. Raymond Coleman for his assistance concerning the technical details, Mrs. Ronit Leiba for statistical analysis and Mrs. Myrna Perlmutter for her help in the preparation of the manuscript.
Conflict of interest
The authors declare that there are no conflict of interests.
References
- Agarwala S, Shah S, Joshi VR. The use of alendronate in the treatment of avascular necrosis of the femoral head: follow-up to eight years. J. Bone Joint Surg. Br. 2009;91:1013–1018. doi: 10.1302/0301-620X.91B8.21518. [DOI] [PubMed] [Google Scholar]
- Boss JH, Misselevich I. Review: experimental avascular osteonecrosis. Curr. Orthop. 2001;15:57–67. [Google Scholar]
- Boss JH, Misselevich I, Bejar J, Norman D, Zinman C, Reis DN. Experimentally gained insight-based proposal apropos the treatment of osteonecrosis of the femoral head. Med. Hypotheses. 2004;62:958–965. doi: 10.1016/j.mehy.2003.12.036. [DOI] [PubMed] [Google Scholar]
- Kang P, Pei F, Shen B, Zhou Z, Yang J. Are the results of multiple drilling and alendronate for osteonecrosis of the femoral head better than those of multiple drilling? A pilot study. Joint Bone Spine. 2012;79:67–72. doi: 10.1016/j.jbspin.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Koo KH, Kim R, Ko GH, Song HR, Leong ST, Cho SH. Preventing collapse in early osteonecrosis of the femoral head. J. Bone Joint Surg. Br. 1995;77:870–874. [PubMed] [Google Scholar]
- Lai KA, Shen WJ, Yang CY, Shao CJ, Hsu JT, Lin RM. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J. Bone Joint Surg. Am. 2005;87:2155–2159. doi: 10.2106/JBJS.D.02959. [DOI] [PubMed] [Google Scholar]
- Levin D, Norman D, Zinman C, Misselevich I, Reis NR, Boss JH. Osteoarthritis-like disorder in rats with vascular deprivation-induced necrosis of the femoral head. Pathol. Res. Pract. 1999;195:637–642. doi: 10.1016/S0344-0338(99)80129-0. [DOI] [PubMed] [Google Scholar]
- Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18:75–85. doi: 10.1016/8756-3282(95)00445-9. [DOI] [PubMed] [Google Scholar]
- Lin JH, Duggan DE, Chen IW, Ellsworth RL. Physiological disposition of alendronate, a potent anti-osteolytic bisphosphonate, in laboratory animals. Drug Metab. Dispos. 1991;19:926–932. [PubMed] [Google Scholar]
- Lin JH, Chen IW, Duggan DE. Effects of dose, sex, and age on the disposition of alendronate, a potent antiosteolytic bisphosphonate, in rats. Drug Metab. Dispos. 1992;20:608–613. [PubMed] [Google Scholar]
- Lin JH, Chen IW, deLuna FA. On the absorption of alendronate in rats. J. Pharm. Sci. 1994;83:1741–1746. doi: 10.1002/jps.2600831218. [DOI] [PubMed] [Google Scholar]
- Marker RD, Seyler MT, Ulrich DS, Srivastava S, Mont AM. Do modern techniques improve core decompression outcomes for hip osteonecrosis? Clin. Orthop. Relat. Res. 2008;466:1093–1103. doi: 10.1007/s11999-008-0184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mont MA, Carbone JJ, Fairbank AC. Core decompression vs. non-operative management for avascular necrosis of the femoral head. Clin. Orthop. Relat. Res. 1996;324:169–178. doi: 10.1097/00003086-199603000-00020. [DOI] [PubMed] [Google Scholar]
- Mont MA, Jones LC, Einhorn TA, Hungerford DS, Reddi AH. Osteonecrosis of the femoral head: potential treatment with growth and differentiations. Clin. Orthop. 1998;355:S314–S335. [PubMed] [Google Scholar]
- Nishii T, Sugano N, Miki H, Hashimoto J, Yoshikawa H. Does alendronate prevent collapse in osteonecrosis of the femoral head? Clin. Orthop. Relat. Res. 2006;443:273–279. doi: 10.1097/01.blo.0000194078.32776.31. [DOI] [PubMed] [Google Scholar]
- Norman D, Reis D, Zinman C, Misselevich I, Boss JH. Vascular deprivation-induced necrosis of the femoral head of the rat. An experimental model of avascular necrosis in the skeletally immature individual or Legg-Perthes disease. Int. J. Exp. Path. 1998;79:173–181. doi: 10.1046/j.1365-2613.1998.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman D, Misselevich I, Peled E, Salman S, Boss JH, Zinman C. Intraosseous conduit-induced enhancement of ingrowth of blood vessels into the necrotic femoral head of rats. J. Orthop. Traumatol. 2004;5:26–33. [Google Scholar]
- Ohzono K, Saito M, Takaoka K, et al. Natural history of nontraumatic avascular necrosis of the femoral head. J. Bone Joint Surg. Br. 1991;73:68–72. doi: 10.1302/0301-620X.73B1.1991778. [DOI] [PubMed] [Google Scholar]
- Peled E, Boss JH, Norman D, Bejar J, Ben-Noon H, Zinman C. Effects of alendronate medication on the fate of the necrotic femoral head of rats with or without core decompression. J. Orthop. Traumatol. 2006;7:88–92. [Google Scholar]
- Peled E, Bejar J, Zinman C, et al. The osteonecrotic femoral head shape treated by alendronate: computer-assisted analysis. Indian J. Orthop. 2009;43:22–26. doi: 10.4103/0019-5413.44630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskin B, Shupak A, Misselevich I, et al. Transphyseal osseous bridges in experimental osteonecrosis of the femoral head of the rat. Histologic study of the bony bridges connecting the epiphyseal with the metaphyseal bony trabeculae through gaps in the physeal cartilage. J. Pediatr. Orthop. 2001;10B:214–218. [PubMed] [Google Scholar]
- Reszka AA, Rodan GA. Nitrogen-containing bisphosphonate mechanism of action. Mini Rev. Med. Chem. 2004;4:711–719. [PubMed] [Google Scholar]
- Sabo E, Peskin B, Misselevich I, et al. Computer-assisted image analysis of the rat postosteonecrotic remodeled femoral head. Exp. Mol. Pathol. 2001;71:256–264. doi: 10.1006/exmp.2001.2394. [DOI] [PubMed] [Google Scholar]


