Abstract
Objective:
CT imaging may be an effective diagnostic method for assessing the extent and progression of pulmonary injury in patients with acute paraquat (PQ) poisoning.
Methods:
A retrospective review of 78 patients with acute PQ poisoning (survivor group, n=42; non-survivor group, n=36) was conducted to examine the lung segment involvement and CT image characteristics from baseline (first CT scan at a mean of 2.4 days after poisoning) to treatment time (second CT scan 3 days after the first). We examined the association between prognosis and pulmonary lesions indicated by characteristic effusion, fibrosis and consolidation in CT images.
Results:
Significant differences were apparent in CT images at baseline and after 3 days between the survivor and the non-survivor groups, with higher levels of pulmonary segment involvement, effusion, consolidation and fibrosis observed in the non-survivor group at baseline (p<0.05). The non-survivor group also showed rapid lesion progression. The receiver operating characteristic curve indicated that the best prognostic value of baseline CT scanning was achieved when performed 2–3 days following the initial exposure.
Conclusion:
Prognosis correlated with increasing lung segment involvement, extent of disease characteristics visualised using CT and speed of lesion progression from baseline. Prognostic evaluation using CT scanning can be used to effectively provide earlier treatment for patients at risk for severe complications associated with PQ toxicity, such as acidosis; leukocytosis; and renal, hepatic and pancreatic failures.
Advances in knowledge:
Chest CT scan can be used 2–3 days following acute PQ poisoning to determine prognosis.
Paraquat (PQ), or 1,1′-dimethyl-4,4′-bipyridium dichloride, is a highly toxic organic heterocyclic contact herbicide once extensively promoted for control of weeds and illegal plants in developing countries and rural areas of China [1]. The compound was shown to be dangerous to both workers applying the herbicide and individuals later exposed to contaminated areas. In developing countries, intentional and accidental poisoning with herbicides represents a significant public health threat, accounting for untold numbers of accidental deaths and up to one-third of all suicides worldwide [2]. Unlike many other herbicides, the mortality rate following acute PQ poisoning is very high; and emergency treatment is often ineffective [3].
Patients can be exposed to PQ by oral ingestion, percutaneous routes or inhalation. The prognosis is generally associated with the degree of exposure. Although many patients survive moderate inhalation and subcutaneous exposure, only 55.5% survive oral ingestion of a 25% PQ solution, roughly equivalent to the commercially available 24.6% solution [4]. Thus, only 7–8 ml of the commercial grade herbicide solution is necessary to achieve severe poisoning, with survival rates of <13% without treatment and of only 73% with immediate treatment [5]. Notably, dose-related mortality rates are significantly higher in suicide attempts (74%) than in accidental exposure (60%), independent of the exposure dosage [6].
For patients with severe PQ poisoning, death generally occurs rapidly owing to circulatory collapse. For surviving patients, the long-term clinical course often results in potentially fatal pulmonary damage and renal failure [7]. PQ primarily enters the body through the digestive tract, where it is mainly absorbed in the jejunum (nearly 17% absorption) and excreted by renal excretion. Because plasma concentrations of PQ peak within 2 h of exposure, treatment must be administered immediately [8]. Surviving patients may also develop severe side effects, such as tissue injury along the gastrointestinal tract, shock, coma, nosebleeds, seizures and stomach pains [1]. Because of its high toxicity and the relatively high rate of poisoning incidences, the commercial use of PQ has been hotly debated, and its use was restricted in some countries. Consequently, the incidence of PQ poisoning has been decreasing since the 1980s in areas using the herbicide, probably because of these measures [9].
Previous studies have also linked certain patient characteristics, including age, respiratory rate, haemoglobin and presence of organ failure, with prognosis following acute PQ poisoning [4]. Immediate treatment with anti-oxidants (such as vitamins C and E), glucocorticoids, cytotoxic agents and certain forms of newly developed antifibrotic agents can improve the prognosis [10]. Only recently have radiological findings, such as CT scanning, been employed to examine the pulmonary effects and progression of acute PQ poisoning, revealing the highly variant response of individual patients to PQ.
The pulmonary system is one of the most vulnerable systems in patients being treated for acute PQ poisoning. To further examine the characteristic changes in CT imaging and the potential of CT findings as a prognostic indicator, the current study retrospectively examined pulmonary lesions, lung segment involvement and other characteristics in surviving and non-surviving patients with acute PQ poisoning using CT imaging. Better characterisation of CT characteristics for diagnosis and prognosis in these patients may contribute to earlier and more effective treatment.
MATERIALS AND METHODS
Patient selection
A retrospective study was conducted in 78 patients with acute PQ poisoning (33 males and 45 females) with a mean age of 27.1 years, treated between January 2007 and April 2009 at the Department of Radiology and the Emergency Department of the Affiliated Hospital of the Academy of Military Medical Sciences, Beijing, China. Patients were grouped as survivors (n=42) and non-survivors (n=36). All patients were followed up for >2 years. Survivors were defined as patients who survived and whose lung lesions disappeared or did not progress during the follow-up period. All patients were admitted to the emergency department and received baseline CT scans on average 2.4 days from admission, with additional scans conducted 3 days following the initial scan. This study was approved by the hospital’s ethics committee, and written informed consent was obtained from all included patients.
Patients were diagnosed with acute PQ poisoning based on initial symptom presentation or self-report of exposure, followed by confirmation with blood serum testing. Patients were included based on: (1) emergency admission within 24 h of poisoning, (2) confirmed diagnosis of acute PQ poisoning and (3) reported or suspected diagnosis of acute PQ poisoning consistent with observed symptoms at the time of admission (confirmed later by blood test). In addition, all included patients received appropriate treatment, including 2 h of haemoperfusion, hormone impact and anti-oxidative treatment. Patients were excluded if they (1) presented with or had a history of pulmonary disorders, (2) had been treated previously for acute PQ poisoning, (3) had received treatment with any medications prior to admission or (4) had symptoms consistent with ongoing lung infection. Only patients with complete data sets were included in the present study.
CT examination
Conventional chest CT scans were conducted using a Sensation 4 spiral CT scanner (Siemens Healthcare, Forchheim, Germany). Patients were asked to inhale, and scans were consistently performed at maximum thoracic volume. Gases were excluded and the scan range was set from the apex to the base of the lung, allowing a full view of the entire lungs. Standard lung algorithm settings (window width 1600 HU, window level −550 HU, slice thickness 10 mm and interlayer spacing 10 mm) were used. Two sequences of lung window and mediastinal window were obtained during examination of each patient.
Review of CT imaging data
Two independent physicians blindly reviewed the CT data for characteristic CT signs (effusion, consolidation and fibrosis) and disease extent (the number of involved lung segments). All data were based on the consensus of both physicians. If lesions of any amount or severity were observed in the lung segment, it was considered to be involved regardless of the CT signs or disease extent observed by other methods, because each lung segment has its own independent arterial and bronchial pathways and thus acts as a relatively independent functional unit.
Statistical analysis
All statistical analyses were conducted using SPSS® v. 8.0 (SPSS Inc., Chicago, IL). Comparisons between groups were performed using t-tests. The receiver operating characteristic (ROC) curve analysis was used to examine patient prognosis and the threshold of lung segments. p<0.05 was considered to be statistically significant.
RESULTS
Baseline disease extent and patient prognosis
Significantly fewer involved lung segments, or those presenting lesions, were observed in baseline CT images from the survivor group than in those from the non-survivor group (p<0.05; Table 1), representing a smaller baseline disease extent in surviving patients. Baseline CT scans showed abnormal lesions in 15 patients (36%), effusion in 14 (33%) patients and consolidation in 2 (5%) patients. A total of 27 (63%) patients exhibited no abnormal CT findings. Stable lesions were apparent in 9 (21%) patients, and two or three lesions were apparent in 8 (19%) patients. In addition, CT signs were significantly fewer in the survivor group than in the non-survivor group (p<0.05). In the survivor and non-survivor groups, abnormal CT signs of effusion, consolidation and fibrosis occurred in 14/42 (33%) vs 25/36 (69%), 2/42 (5%) vs 13/36 (36%) and 8/42 (19%) vs 8/36 (22%) of patients, respectively (Table 1).
Table 1.
Patient demographics and data in the survivor and non-survivor groups
| Variables | Survival group (n=42) | Non-survivor group (n=36) | t-value | p-value |
| Age (years) | 26.4±8.7 | 27.9±13.1 | −0.60 | >0.05 |
| Baseline scan timea (days) | 2.5±0.6 | 2.3±1.0 | 1.31 | >0.05 |
| Number of lung segments involved | 2.42±0.7 | 7.0±0.3 | −5.22 | <0.05 |
| Abnormal CT signs | −2.36 | 0.0463 | ||
| Effusion | 14 (33%) | 25 (69%) | ||
| Consolidation | 2 (5%) | 13 (36%) | ||
| Fibrosis | 8 (19%) | 8 (22%) | ||
| No abnormal CT signsb | 27 (64%) | 10 (28%) | <0.05 | |
| Lesions | 15 (36%) | 26 (72%) | <0.05 | |
| Stable lesionsb | 9 (21%) | 0 (0%) | ||
| Two or three lesions | 8 (19%) | 16 (44%) | ||
| Blood PQ concentration (µg ml−1) | 0.767±1.66 | 6.383±4.98 | −3.59 | <0.05 |
| Urinary PQ concentration (µg ml−1) | 29.38±12.13 | 222.68±148.23 | −3.47 | <0.05 |
PQ, paraquat.
Time interval between admission and first CT scan.
Patients without abnormal signs were discharged before follow-up CT scans could be conducted 3 days after baseline.
CT signs and pulmonary injury in survivors
Among the 42 patients in the survivor group, CT scans revealed no clear abnormal signs at baseline in 27 (64%) patients and no lesions in 18 (43%) cases, resulting in them being discharged from the hospital. The remaining 9 (21%) patients exhibited lesions with tendencies to decrease or stabilise following treatments. Another 15 (36%) patients exhibited lesions in the baseline CT scans. CT revealed effusion in 14 (33%) patients, consolidation in 2 (5%) patients and fibrosis in 8 (19%) patients. Two or three pulmonary lesions were observed in 8 (19%) patients (Table 1). Pleural effusion was observed in 4 (9.5%) patients. After the treatment, CT scans revealed that the majority of lesions were decreased and stabilised (Figure 1).
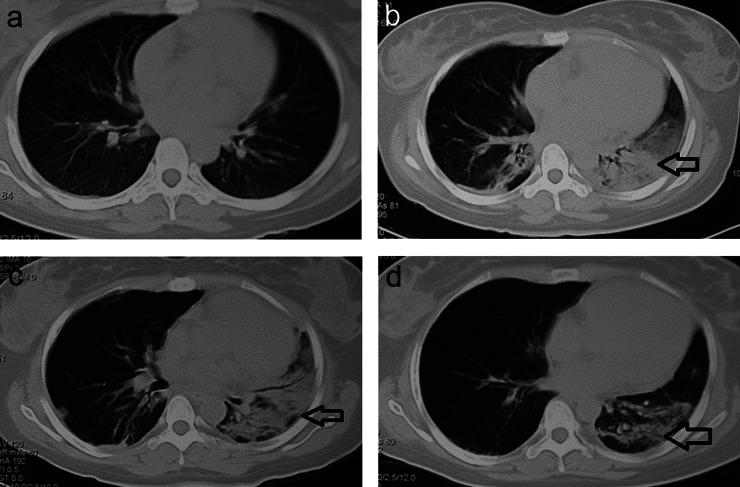
Figure 1.
CT scans in the survivor group. (a) A female (22 years) admitted 6 h after acute paraquat poisoning; baseline CT scan 3 days after admission showing scattered effusion in the lungs and (b) CT scan taken 3 days after baseline showing significant progression of lesions (arrow), as well as significant consolidation and fibrosis. (c) A partially resolved lesion in the lungs (arrow). (d) CT scan showing that the lesion had significantly resolved in the lungs (arrow). The patient was discharged when all lesions were stabilised.
CT signs and pulmonary injury in non-survivors
Of the 36 patients in the non-survivor group, CT scans revealed no clear abnormal signs at baseline in 10 (28%) patients. A total of 26 (72%) patients had lesions on baseline CT images, and 4 (11%) patients exhibited lesions on the treatment CT images taken 3 days later. Notably, rapid progression of these lesions was observed in the non-survivor group between baseline and treatment CT scans. Consolidation, fibrosis and effusion were present in all patients, continuing to rapidly emerge and multiply until death occurred (Figure 2). The remaining 6 (17%) patients died sooner than 3 days following initial treatment, and no 3-day CT scans could be performed.
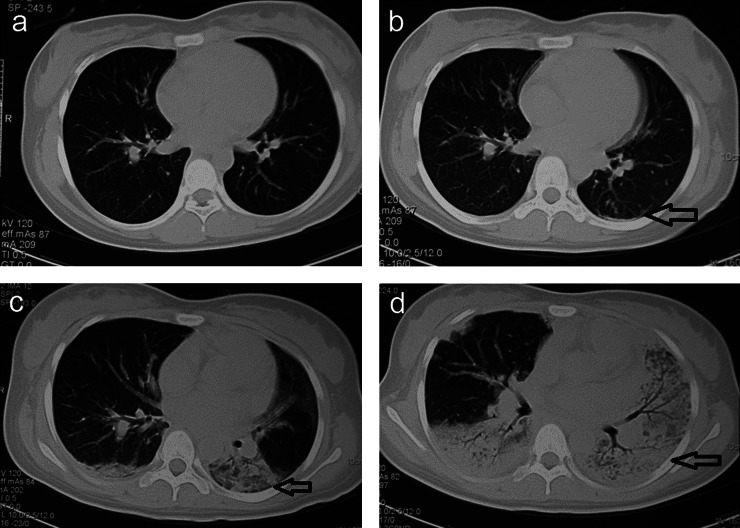
Figure 2.
CT scans in the non-survivor group. (a) A female (16 years) admitted 10 h after acute paraquat poisoning; baseline CT scan 1 day after admission showing no clear abnormal signs and (b) scattered effusion and fibrosis (arrow) after 3 days. (c) Lesions progressed and consolidation rapidly appeared (arrow). (d) Further lesion progression was observed, showing consolidation in most of the lungs (arrow) and elevated bronchial gas volumes. The patient died from respiratory failure 15 days after admission.
Pleural effusion was observed in 5 (13.9%) patients at baseline. After treatment, 26 (72%) patients exhibited lesions in CT images. CT images revealed effusion in 25 (69%) patients, consolidation in 13 (36%) patients and fibrosis in 8 (22%) patients. Two or three pulmonary lesions were observed in 16 (44%) cases. Lesions progressed rapidly between baseline and treatment CT scans, with consolidation and fibrosis rapidly emerging (Figure 2). These CT signs ultimately highlighted the characteristics leading to respiratory failure and death. Patients died from respiratory or renal failure.
CT scanning as a tool for prognosis evaluation of patients
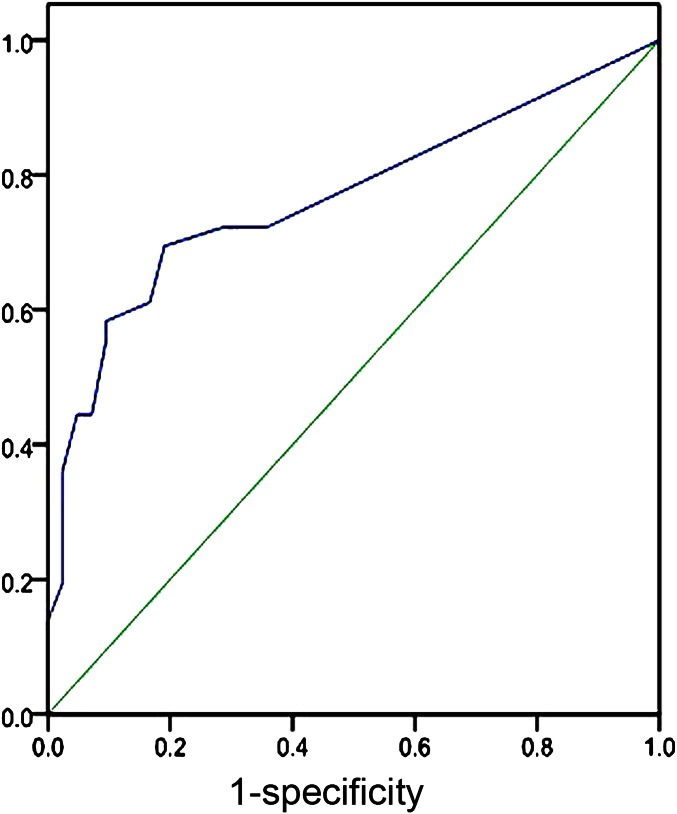
An ROC curve analysis was performed to assess the prognosis of patients according to the number of injured lung segments in the baseline CT examination (Figure 3), resulting in an area under the curve of 0.767 (95% confidence interval 0.656–0.878). Analysis revealed that using two lung segments as the threshold allowed discrimination between the non-survivor and the survivor groups (p<0.05). The sensitivity and specificity of CT scanning to predict prognosis in these patients were 72.2% and 28.6%, respectively, based on the number of injured lung segments in the baseline CT examination. Although this sensitivity and specificity were not very high for patients’ prognostic evaluation, mortality was shown to be associated with increased disease extent in patients with more than two involved lung segments. This initial statistical analysis is of clinical significance, indicating the prognostic value of baseline CT scanning at 2–3 days following initial admission and treatments.
Figure 3.
Receiver operating characteristic curve. The area under the curve was 0.767 (95% confidence interval 0.656–0.878), and the calculated threshold for the involved lung segments of the paraquat-poisoned patients was 2.0 (death occurred above this value). The sensitivity and specificity of the number of involved lung segments in the initial CT scan were used to indicate the patient’s prognosis.
DISCUSSION
Acute PQ poisoning is associated with high mortality rates, even with immediate treatment, in part owing to long-term pulmonary effects. To better diagnose and evaluate patients' prognoses, CT scanning can be used to monitor the progression of the condition, with rapid worsening in CT signs indicating a high mortality risk. CT signs, including the number of lesions, effusion, consolidation and fibrosis, are readily apparent on CT images. Furthermore, progression in these signs over the days immediately following initial treatments is a strong indicator of prognosis.
PQ produces toxic peroxide ions that result in cell membrane injury and cell death, finally inducing permanent organ damage or failure if not immediately treated (normally within 4 h) [11]. Serum levels of PQ have been widely used to assess the accumulation of dangerous lung uptake, which normally peaks 4–5 h following exposure, although some studies reported that maximum lung concentration occurs as late as 15 h after exposure [7]. Plasma PQ levels have long been considered the most valuable prognostic indicator, but they have been shown to be highly variable between individuals, necessitating further examination by CT scanning and other imaging methods [12,13]. Because the serum response time varies, investigation by tissue biopsy may yield more accurate results [14]. CT images of the lungs have recently been used to produce the most accurate and effective individual prognosis and treatment plans [15]. CT scans and other radiographic images reveal changes during the first week after ingestion, characterised by diffuse consolidation, pneumomediastinum with or without pneumothorax and cardiomegaly coupled with widening of areas in the superior mediastinum. By the end of the first week, cystic and linear shadows vaguely appear and preponderant parenchymal abnormality appears after 2–4 weeks. At 4 weeks, focal honeycombing is the primary characteristic that is visible on high-resolution CT (HRCT) scans of the lung, and localised fibrosis containing small cysts persist up to 9 months after PQ exposure [15]. These findings were consistent with the present CT results shortly after PQ poison exposure, although further study will be required to observe long-term results.
Using CT scanning, clinicians can obtain a better prognosis and use appropriate treatments for acute PQ poisoning, aiming to remove PQ from the blood, including 2–5 days of treatment with haemoperfusion (clearance 116±32 ml min−1), haemodialysis (clearance 90±54 ml min−1) and haemodiafiltration for 2–5 days [7]. A recent study also reported successful treatment of acute PQ poisoning using 50 mg kg−1 glutathione therapy administered intravenously over a 12-h period [16]. Other alternative treatments were reported by Noh [17], who described the successful treatment of PQ-poisoned patients with combined high-dose cyclophosphamide and steroid therapies. Similarly, Eddleston et al [18] reported the use of antineutrophil therapy to prevent lung fibrosis, with mixed outcomes.
Lung damage from acute PQ poisoning is caused by increasing PQ concentrations in lung tissues, resulting in a free radical build-up that triggers inflammatory responses leading to lung fibrosis [19]. Thus, lung damage and respiratory failure are common causes of death. However, mortality may be reduced by accurately assessing the extent of disease progression, which varies highly from one individual to another. In fact, case studies by Lee et al [4] suggest that lung damage may not be irreversible if treated early. Thus, identification of lesions in the lungs and their severity may be crucial to improve patients’ outcomes, especially since the number of lung lesions has been associated with greater mortality rates in these patients [20]. Notably, the current study confirms the association between the number of lesions and mortality, although further studies will be required to identify whether earlier treatment or alternative treatment modalities, such as anti-oxidant or immunosuppressive treatments, could be used to improve the outcome.
CT examination of PQ-poisoned patients at 7 days has been conventionally used to assess fully developed PQ poisoning in the lungs, and HRCT has been previously shown to have a prognostic predictive value through the use of CT signs called ground-glass opacities [19]. However, the current study indicates that prognosis may be obtained much earlier, at only 2–3 days, using a variety of CT signs. Notably, lesions complicated by less consolidation and fibrosis were significantly more likely to be resolved and stabilised, or even to disappear entirely, following treatment. In non-surviving patients, lesions quickly progressed, as indicated by the proliferation of visible CT signs. Rapid consolidation, fibrosis and pleural effusion were strong indicators of poor prognosis. However, for patients with no apparent lesions on baseline CT scans, survival rates were higher, independent of the PQ exposure severity. These findings are consistent with the previously reported use of chest HRCT scanning in PQ-poisoned patients to reveal obvious pulmonary inflammation, pleural effusion and fibrous lesions several days after ingestion of PQ and to examine fibrous lesions, exudation and clearance of lung auscultation [21]. Similar techniques have also been using positron emission tomography/CT to assess the uptake of PQ in foetal tissues in utero [22].
Although CT scanning was shown to be valuable in assessing several signs associated with higher mortality, the use of only one method may limit our results. In addition, the present study included a relatively small number of patients from a single hospital and thus does not account for ethnic or geographic variation, which may play a role in an individual’s natural anti-oxidant response to PQ poisoning, affecting maximum concentration and survival times. Finally, CT has low sensibility and specificity to detect early renal damage, and, because of very high radiation, dynamic-enhanced CT was not used in our patients, thus preventing us from assessing renal damage.
Lesions on initial CT scans indicated a poorer prognosis for patients with acute PQ poisoning, with rapid progression visible on analysis of CT signs in later scans indicative of high mortality rates. In the current study, lesions were significantly more common in the non-survivor group, and rates of effusion, consolidation and fibrosis were also elevated compared with the survivor group. Early emergence of these signs was also indicative of poorer prognosis. CT findings can serve as important indicators of prognosis in acute PQ poisoning. Furthermore, consecutive CT scans may be used to observe the evolution of lesions during pulmonary injury, providing important information for improved clinical treatment.
REFERENCES
- 1.Robbe WCI, Meggs WJ. Insecticides, herbicides, rodenticides. New York, NY: McGraw-Hill; 2004 [Google Scholar]
- 2.Bertolote JM, Fleischmann A, Eddleston M, Gunnell D. Deaths from pesticide poisoning: a global response. Br J Psychiatry 2006;189:201–3 doi: 10.1192/bjp.bp.105.020834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabzghabaee AM, Eizadi-Mood N, Montazeri K, Yaraghi A, Golabi M. Fatality in paraquat poisoning. Singapore Med J 2010;51:496–500 [PubMed] [Google Scholar]
- 4.Lee EY, Hwang KY, Yang JO, Hong SY. Predictors of survival after acute paraquat poisoning. Toxicol Ind Health 2002;18:201–6 [DOI] [PubMed] [Google Scholar]
- 5.Cavalli RD, Fletcher K. An effective treatment for paraquat poisoning. New York, NY: Academic Press; 1977 [Google Scholar]
- 6.Van der Stuyft P. Paraquat poisoning. Lancet 1999;353:322–3 [DOI] [PubMed] [Google Scholar]
- 7.Yoon SC. Clinical outcome of paraquat poisoning. Korean J Intern Med 2009;24:93–4 doi: 10.3904/kjim.2009.24.2.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houzé P, Baud FJ, Mouy R, Bismuth C, Bourdon R, Scherrmann JM. Toxicokinetics of paraquat in humans. Hum Exp Toxicol 1990;9:5–12 [DOI] [PubMed] [Google Scholar]
- 9.Casey P, Vale JA. Deaths from pesticide poisoning in England and Wales: 1945-1989. Hum Exp Toxicol 1994;13:95–101 [DOI] [PubMed] [Google Scholar]
- 10.Seifirad S, Keshavarz A, Taslimi S, Aran S, Abbasi H, Ghaffari A. Effect of pirfenidone on pulmonary fibrosis due to paraquat poisoning in rats. Clin Toxicol (Phila) 2012;50:754–8 doi: 10.3109/15563650.2012.718783 [DOI] [PubMed] [Google Scholar]
- 11.Hoet PH, Nemery B. Polyamines in the lung: polyamine uptake and polyamine-linked pathological or toxicological conditions. Am J Physiol Lung Cell Mol Physiol 2000;278:L417–33 [DOI] [PubMed] [Google Scholar]
- 12.Kim YT, Jou SS, Lee HS, Gil HW, Yang JO, Lee EY, et al. The area of ground glass opacities of the lungs as a predictive factor in acute paraquat intoxication. J Korean Med Sci 2009;24:636–40 doi: 10.3346/jkms.2009.24.4.636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeBlanc JJ, Davidson RJ, Hoffman PS. Compensatory functions of two alkyl hydroperoxide reductases in the oxidative defense system of Legionella pneumophila. J Bacteriol 2006;188:6235–44 doi: 10.1128/JB.00635-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, Remião F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol 2008;38:13–71 doi: 10.1080/10408440701669959 [DOI] [PubMed] [Google Scholar]
- 15.Im JG, Lee KS, Han MC, Kim SJ, Kim IO. Paraquat poisoning: findings on chest radiography and CT in 42 patients. AJR Am J Roentgenol 1991;157:697–701 doi: 10.2214/ajr.157.4.1892020 [DOI] [PubMed] [Google Scholar]
- 16.Hong SY. New approaches of treatment in paraquat poisoning. Toxicol Lett 2010;196S:S31 [Google Scholar]
- 17.Noh SH. Four cases of 24.5% paraquat poisoning, successfully treated with combination therapy of hemoperfusion, high dose of cyclophosphamide, and steroid pulse therapy. Inje Med J 2002;23:821–6 [Google Scholar]
- 18.Eddleston MM, Wilks MF, Buckley NA. Prospects for treatment of paraquat-induced lung fibrosis with immunosuppressive drugs and the need for better prediction of outcome: a systematic review. QJM 2003;96:809–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SH, Lee KS, Ahn JM, Kim SH, Hong SY. Paraquat poisoning of the lung: thin-section CT findings. Radiology 1995;195:271–4 [DOI] [PubMed] [Google Scholar]
- 20.Gil HW, Kang MS, Yang JO, Lee EY, Hong SY. Association between plasma paraquat level and outcome of paraquat poisoning in 375 paraquat poisoning patients. Clin Toxicol 2008;46:515–18 doi: 10.1080/15563650701549403 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Wu W, Lu Y, Wang J, Shang A, Yao F, et al. Successful treatment of patients with paraquat intoxication: three case reports and review of the literature. J Zhejiang Univ Sci B 2012;13:413–18 doi: 10.1631/jzus.B1200008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartlett RM, Murali D, Nickles RJ, Barnhart TE, Holden JE, DeJesus OT. Assessment of fetal brain uptake of paraquat in utero using in vivo PET/CT imaging. Toxicol Sci 2011;122:551–6 doi: 10.1093/toxsci/kfr104 [DOI] [PMC free article] [PubMed] [Google Scholar]