Abstract
Diabetic neuropathy (DN) is the most common complication of diabetes and is characterized by distal-to-proximal loss of peripheral nerve axons. The idea of tissue-specific pathological alterations in energy metabolism in diabetic complications-prone tissues is emerging. Altered nerve metabolism in type 1 diabetes models is observed; however, therapeutic strategies based on these models offer limited efficacy to type 2 diabetic patients with DN. Therefore, understanding how peripheral nerves metabolically adapt to the unique type 2 diabetic environment is critical to develop disease-modifying treatments. In the current study, we utilized targeted LC/MS/MS to characterize the glycolytic and tricarboxylic acid (TCA) cycle metabolomes in sural nerve, sciatic nerve and dorsal root ganglia (DRG) from male type 2 diabetic mice (BKS.Cg-m+/+Leprdb; db/db) and controls (db/+). We report depletion of glycolytic intermediates in diabetic sural nerve and sciatic nerve (glucose-6-phosphate, fructose-6-phosphate, fructose-1,6-bisphosphate (sural nerve only), 3-phosphoglycerate, 2-phosphoglycerate, phosphoenolpyruvate, lactate), with no significant changes in DRG. Citrate and isocitrate TCA cycle intermediates were decreased in sural nerve, sciatic nerve and DRG from diabetic mice. Utilizing LC/ESI/MS/MS and HPLC methods, we also observed increased protein and lipid oxidation (nitrotyrosine; hydroxyoctadecadienoic acids, HODEs) in db/db tissue, with a proximal-to-distal increase in oxidative stress, with associated decreased aconitase enzyme activity. We propose a preliminary model, whereby the greater change in metabolomic profile, increase in oxidative stress, and decrease in TCA cycle enzyme activity may cause distal peripheral nerve to rely on truncated TCA cycle metabolism in the type 2 diabetes environment.
Keywords: diabetes, neuropathy, metabolomics, sural nerve, sciatic nerve, dorsal root ganglia
INTRODUCTION
According to statistics published by the Centers for Disease Control in 2011, approximately 8% of the US population has diabetes mellitus, with 1.9 million new cases diagnosed in 2010 (CDCP 2011). Although type 1 diabetes and type 2 diabetes are both characterized by impaired insulin signaling and hyperglycemia, the two disease forms are distinct. Type 2 diabetes, accounting for 95% of all cases (CDCP 2011), is a metabolic disease characterized by high pancreatic insulin production and insulin resistance in muscle, fat and liver. The remaining 5% of individuals have type 1 diabetes (CDCP 2011), characterized by autoimmune destruction of the pancreas and loss of insulin production.
Diabetic neuropathy (DN) is the most common complication of diabetes, and over time affects approximately 60% of all patients with the disease (Vincent and Feldman 2004). DN is characterized by progressive, length-dependent loss of peripheral nerve axons in a stocking and glove (distal-to-proximal) pattern (Said 2007), resulting in pain, decreased sensation, and eventually complete loss of sensation. In the United States, DN is the leading cause of diabetes-related hospital admissions and non-traumatic amputations (Edwards, et al. 2008; Feldman 2008). Despite the morbidity associated with DN, there is currently no treatment available to patients suffering from DN other than glycemic control, largely due to our incomplete understanding of disease mechanisms. In order to gain insight into DN pathogenesis and develop disease-modifying treatments, we have recently started to investigate the role of aberrant energy metabolism in the peripheral nervous system. We hypothesize that DN arises from tissue-specific metabolic reprogramming, resulting in alterations in fuel utilization which lead to dysfunction of the tissue.
Glucose is the major substrate for peripheral nerves, although they do not rely on insulin for its uptake (Greene and Winegrad 1979). In rodent models of type 1 diabetes, peripheral nerve glucose content is elevated (Kishi, et al. 1999; Obrosova, et al. 2005b; Thurston, et al. 1995) and oxidative stress is increased within the sciatic nerve and the dorsal root ganglia (DRG) containing the sensory neuron cell bodies (Vincent, et al. 2004). It is also established that all mechanisms known to be contributory to the onset and progression of DN are related in some way to oxidative stress (Vincent, et al. 2011). Based on findings primarily in cultured endothelial cells, oxidative stress was thought to be related to hyperglycemia-induced overproduction of superoxide (O2•−) by the mitochondrial electron transport chain (Brownlee 2001). Thus, it was proposed that increased glycolytic flux leads to increased hyperglycemia-derived electron donors from the tricarboxylic acid (TCA) cycle (FADH2 and NADH), which generates a high mitochondrial membrane potential, facilitating the reduction of O2 to O2•− (Brownlee 2001; Tomlinson and Gardiner 2008). Indeed, a study by Thurston and colleagues in the mid-1990s reported an increase in nerve glycolytic intermediates in long-term type 1 diabetes in rats (Thurston et al. 1995). Recent reports, however, suggest downregulation of glycolytic intermediates in complications-prone tissues in type 1 diabetes, including peripheral nerves (Gardiner, et al. 2007), retina (Ola, et al. 2006), and lens (Obrosova and Stevens 1999). Recent metabolomic studies in models of type 1 diabetes reported downregulation of key TCA cycle and mitochondrial proteins (Akude, et al. 2011) and enzyme activities (Chowdhury, et al. 2010) in DRG, with decreased mitochondrial O2•− production (Akude et al. 2011). Conversely, Schwann cells grown under hyperglycemic conditions demonstrated upregulation of TCA cycle and mitochondrial proteins (Zhang, et al. 2010). Critically, these data were produced in models of type 1 diabetes.
While the mechanisms underlying type 1 and type 2 diabetes are distinct, it is generally held that DN is due to hyperglycemic damage, regardless of the type of diabetes (Callaghan, et al. 2012). However, DN is more common in patients with type 2 diabetes (Young, et al. 1993), and DN in type 2 diabetic patients is less amenable to tight glycemic control, indicating differing underlying pathogenic mechanisms (Callaghan et al. 2012). Despite the significant progress in our understanding of altered peripheral nerve metabolism in models of type 1 diabetes, fundamental differences between type 1 and type 2 diabetes confer a critical need to understand how peripheral nerves adapt to the unique type 2 diabetic environment (hyperglycemia, hyperinsulinemia, hyperlipidemia). We hypothesize that measures of oxidative stress are increased in a proximal-to-distal gradient in peripheral nerves in type 2 diabetes, consistent with DN pathogenesis. This will be reflected in a decrease in the glycolytic and TCA cycle metabolomes and related enzymes moving proximally-to-distally from the DRG to the sciatic nerve and its terminal branches, including the sural nerve.
In the current study, we utilized liquid chromatography-tandem mass spectrometry (LC/MS/MS) and liquid chromatography-electrospray ionization-tandem mass spectrometry (LC/ESI/MS/MS) to explore glycolytic and TCA cycle metabolomic changes within sural nerve, sciatic nerve and DRG of the BKS.Cg-m+/+Leprdb (db/db) mouse model of type 2 diabetes. We present steady-state metabolomics data demonstrating decreased glycolytic intermediates in the sural and sciatic nerves, and a decrease in TCA cycle intermediates in DRG, sciatic nerve and sural nerve, complementing the published data in peripheral nerve in type 1 diabetes. We confirm that these changes occur concurrently with decreased aconitase enzyme activity as well as increased protein and lipid oxidation in sciatic nerve and DRG. We present the first evidence that oxidative stress is more pronounced distally in peripheral nerves from a mouse model of type 2 DN. Finally, we propose a preliminary model whereby these changes may cause sciatic nerve to rely on truncated TCA cycle metabolism in the type 2 diabetes environment.
MATERIALS AND METHODS
Diabetic Mice
Male type 2 diabetic (BKS.Cg-m+/+Leprdb; db/db) and control (db/+) mice were purchased from Jackson Laboratories (Bar Harbor, Maine, USA). A mutation in the leptin receptor of the db/db mouse results in hyperphagia, severe obesity, hyperinsulinemia and hyperglycemia beginning at approximately 4 weeks of age (Jackson Labs, http://jaxmice.jax.org/jaxmicedb/html/model_66.shtml). At 24 weeks of age (20 weeks of diabetes) db/db mice exhibit insensitivity to mechanical and thermal stimuli, along with slowed nerve conduction velocities and reduced intra-epidermal nerve fiber (IENF) density (Cheng, et al. 2009; Sullivan, et al. 2007). Animals were maintained at the University of Michigan in a pathogen-free environment, and cared for following the University of Michigan Committee on the Care and Use of Animals guidelines. Mice were given continuous access to food (Purina 5001, Purina Mills LLC, St. Louis, MO, USA) and water.
Blood glucose (6 hr fasting) and body weight were measured every 4 weeks to document the onset and duration of diabetes. One drop of tail blood was analyzed using a standard glucometer (One Touch Profile, LIFESCAN, Inc., Milpitas, CA, USA). At the end of the experimental period, glycated hemoglobin (GHb) was measured using the Helena Laboratories Test Kit (Glyco-Tek Affinity Column Method).
Tissue Harvest
Mice were euthanized by sodium pentobarbital overdose at 24 weeks of age, followed by transcardial PBS perfusion to remove residual contaminating blood. This method was chosen over CO2/cervical dislocation (recommended by Stevens and colleagues (Stevens, et al. 2000)) as CO2/cervical dislocation is not compatible with transcardial perfusion, a step that was necessary to remove blood artifact and for collaborative use of the other tissues. The left sural and sciatic nerves and lumbar DRG were dissected, briefly rinsed in double-distilled water, frozen by immersion in liquid nitrogen and stored at −80°C until metabolomic analysis was conducted (8 db/+ and db/db DRG; 9 db/+ and db/db sural nerve and sciatic nerve). The right sciatic nerve and lumbar DRG were dissected and immediately submerged in ice-cold antioxidant buffer, rapidly frozen by immersion in liquid nitrogen, and stored at −80°C for quantification of end products of oxidative damage (6 db/+ and db/db DRG; 12 db/+ and db/db sciatic nerve). The right sciatic nerve from a separate cohort of mice was dissected, cut in thirds and the proximal and distal thirds prepared as above for quantification of oxidative damage (6 db/+ and 6 db/db). The left sciatic nerve and lumbar DRG were used for determination of aconitase enzyme activity as per manufacturer’s instructions (Cayman Chemical Company). Although the method of euthanasia confers a greater time from death to tissue harvest than CO2/cervical dislocation, the timings were standardized across all mice (sural and sciatic nerves 8 min, DRG 15 min), allowing comparative results within this study.
Targeted metabolomic analysis by LC/MS/MS
Frozen tissue samples were extracted with 150 µL of chilled 8:1:1 methanol:chloroform:water containing 13C-labeled glycolysis and TCA cycle standards. Samples were sonicated on ice for 20 seconds (20% duty cycle, 20% maximum power), held at 4°C for a further 5 minutes, and centrifuged at 15,000 × g for 5 minutes at 4°C. Supernatant was transferred to autosampler vials and directly analyzed by LC/MS.
Chromatographic separation of 18 targeted glycolytic and TCA intermediates was performed based on the methods of Lorenz et al. (Lorenz, et al. 2011). Briefly, hydrophilic interaction liquid chromatography (HILIC) was performed using a Phenomenex Luna NH2 column (3 µm, 150 mm × 1 mm i.d.) on an Agilent 1200 RRLC coupled to an Agilent 6410 triple quadrupole mass spectrometer. Mobile phase A was 5 mM ammonium acetate in water, adjusted to pH 9.9 with ammonium hydroxide. Mobile phase B was acetonitrile. The initial gradient was a 10-minute linear ramp from 20% to 100% B, followed by a 3-minute hold at 100% B. The mobile phase was returned to 20% B and held for 17 minutes for re-equilibration prior to the next run.
The instrument was operated in multiple reaction monitoring (MRM) mode using MS/MS transitions previously optimized by analysis of authentic standards. The following parameters were implemented: flow rate was 0.07 mL/min, column temperature 25°C, injection volume 20 µL, spray voltage 4.0 kV in negative ion mode, desolvation gas flow rate 10 L/min, desolvation gas temperature 325°C, and nebulizer pressure 40 psi.
The ratio of each metabolite peak area to that of the closest-matching 13C-labeled standard was calculated. Metabolite concentration was determined using calibration curves generated from known concentrations of authentic standards and equal concentrations of 13C-labeled compounds as were present in the samples. Concentrations were normalized to total protein content of the tissue sample, determined by the Bradford-Lowry method using the reagents and protocol supplied by Bio-Rad Laboratories (Hercules). Final values are expressed as pmol/µg tissue. Intermediates of the pathways not included in the LC/MS/MS output were due to lack of commercial availability of standard, insufficient concentration to reach detection limits, or degradation issues preventing accurate analysis by the current method.
Oxidative stress measures
DRG and sciatic nerve samples were analyzed for nitrated protein (3-nitrotyrosine) and oxidized lipids (hydroxyoctadecadienoic acids, HODEs) as previously described (Vivekanandan-Giri, et al. 2011; Wiggin, et al. 2008). Tissue was homogenized at 4°C in antioxidant buffer [100 µM diethylenetriaminepentaacetic acid, 50 µM butylated hydroxytoluene, 1% (v/v) ethanol, 10 mM 3-amino-1,2,4-triazole, and 50 mM sodium phosphate buffer (pH 7.4)] to prevent ex vivo oxidation, frozen, and thawed. Protein was precipitated with ice-cold trichloroacetic acid (10% v/v), collected by centrifugation, washed with 10% trichloroacetic acid, and delipidated twice with water/methanol/water-washed diethyl ether (1:3:7 v/v). Isotopically labeled internal standards were added and samples were hydrolyzed in 4 M methane sulfonic acid at 110°C for 24 hr under argon as described previously (Pennathur, et al. 2004). HODEs were quantified by reverse-phase C-18 HPLC analysis of triphenylphosphine-reduced lipid extracts after base hydrolysis. The protein content of tissue pellets was determined by a modified Lowry protein assay using BSA as a standard (Pennathur, et al. 2005). Amino acids were isolated from the acid hydrolysate using a solidphase C-18 column (Supelco) (Pennathur et al. 2004; Pennathur et al. 2005) and quantified by MRM using isotope dilution LC/ESI/MS/MS as previously described (Vivekanandan-Giri et al. 2011). Results were normalized to tyrosine content, the precursor of 3-nitrotyrosine.
Statistical Analysis
Data analysis was performed using GraphPad Prism 5.0 (GraphPad Software). Comparisons between groups were performed using either a one-way ANOVA with Tukey post test for multiple comparisons, or an unpaired t-test, as applicable. Assumptions about the Gaussian distribution of data and rules for transformation of non-normative data were made as previously described (Russell, et al. 1999). Significance was assigned when p<0.05.
RESULTS
Metabolomic intermediates are decreased in sural nerve, sciatic nerve and DRG tissue in db/db mice
Diabetes was confirmed in db/db mice by significant elevations in body weight, blood glucose, and GHb compared with their age-matched db/+ controls (p<0.001) (Table 1). To begin to characterize the changes in glycolytic and TCA cycle intermediates within peripheral nervous system tissue of the db/db mouse, we performed targeted LC/MS/MS metabolomic analysis on sural nerve, sciatic nerve and DRG extracts (Table 2). At 24 weeks of age (20 weeks of diabetes) there was a significant decrease in four of the five measured glycolytic intermediates within both diabetic sural nerve and sciatic nerve compared with db/+ controls (glucose-6-phosphate/fructose-6-phosphate (G6P/F6P), 62%, 45%; 3-phosphoglycerate/2-phosphoglycerate (3PG/2PG), 75%, 63%; phosphoenolpyruvate (PEP), 75%, 75%; lactate, 46%, 56%; respectively), with additional diabetic decrease in fructose-1,6-bisphosphate content of sural nerve (63%) (Table 2). In contrast, diabetes had no effect on measured glycolytic intermediates in DRG (Table 2). Among the TCA cycle metabolites, citrate and isocitrate were significantly lower in all three tissues from db/db mice compared with db/+ controls, with a greater decrease seen in sural nerve and sciatic nerve (48%, 50% sural nerve; 55%, 50% sciatic nerve; 34%, 50% DRG). Sural nerve, sciatic nerve and DRG content of the remaining measured TCA cycle intermediates were not significantly altered with diabetes (Table 2). These observations indicate that type 2 diabetes affects the TCA cycle metabolome of sural nerve, sciatic nerve and DRG, as well as the glycolytic metabolome of the sural nerve and sciatic nerve, and suggest that the effect is greater in the peripheral nerve than the DRG.
Table 1.
Body weight, fasting blood glucose, and GHb for db/+ and db/db mice at 24 weeks of age
| Genotype | Weight (g) | Blood glucose (mg/dL) |
GHb (%) |
|---|---|---|---|
| 24wk db/+ (9) | 32.0 ± 1.0 | 203 ± 9 | 6.6 ± 0.2 |
| 24wk db/db (9) | 52.6 ± 4.8*** | 672 ± 67*** | 13.7 ± 0.4*** |
Data are mean ± SEM.
p< 0.001 vs db/+. 9 db/+ and db/db.
Table 2.
Quantified LC/MS/MS metabolomic data from db/+ and db/db mouse sural nerve, sciatic nerve, and DRG
| Metabolite | 24wk db/+ sural nerve |
24wk db/db sural nerve |
24wk db/+ sciatic nerve |
24wk db/db sciatic nerve |
24wk db/+ DRG | 24wk db/db DRG |
|---|---|---|---|---|---|---|
| G6P/F6P | 16.3 ± 4.9 | 6.2 ± 3.1* | 10.9 ± 1.9 | 6.0 ± 1.1* | 5.5 ± 1.0 | 6.8 ± 1.2 |
| FBP | 3.8 ± 0.6 | 1.4 ± 0.3** | 9.7 ± 1.6 | 6.2 ± 1.3 | 7.1 ± 0.9 | 6.3 ± 0.9 |
| 3PG/2PG | 0.4 ± 0.1 | 0.1 ± 0.03* | 2.2 ± 0.4 | 0.8 ± 0.2** | 0.1 ± 0.03 | 0.1 ± 0.02 |
| PEP | 0.4 ± 0.1 | 0.1 ± 0.02** | 0.4 ± 0.1 | 0.1 ± 0.02** | 0.1 ± 0.03 | 0.1 ± 0.03 |
| LAC | 136.8 ± 19.0 | 73.3 ± 19.7* | 220.7 ± 45.8 | 96.1 ± 14.4* | 149.8 ± 14.7 | 162.4 ± 14.1 |
| CIT | 8.3 ± 1.0 | 4.3 ± 0.6** | 49.7 ± 6.9 | 22.2 ± 4.4** | 8.5 ± 0.8 | 5.6 ± 0.4** |
| ICIT | 0.2 ± 0.03 | 0.1 ± 0.03* | 0.6 ± 0.1 | 0.3 ± 0.1* | 0.2 ± 0.02 | 0.1 ± 0.01* |
| SUC | 1.4 ± 0.05 | 1.1 ± 0.2 | 5.9 ± 0.9 | 4.7 ± 0.8 | 0.6 ± 0.04 | 0.9 ± 0.2 |
| FUM | 4.4 ± 0.4 | 3.6 ± 0.8 | 6.0 ± 1.1 | 3.6 ± 0.6 | 4.9 ± 0.6 | 3.6 ± 0.1 |
| MAL | 8.0 ± 0.9 | 6.2 ± 1.0 | 16.9 ± 2.5 | 11.5 ± 2.0 | 10.9 ± 0.9 | 10.6 ± 0.7 |
Data are mean pmol/µg protein for each group ± SEM.
p <0.05;
p< 0.01 vs db/+. 8 db/+ and db/db DRG; 9 db/+ and db/db sciatic nerve and sural nerve; all data from male mice.
p <0.05;
p< 0.01 vs. db/+. G6P/F6P, glucose-6-phosphate/fructose-6-phosphate; FBP, fructose-1,6-bisphosphate; 3PG/2PG, 3-phosphoglycerate/2-phosphoglycerate; PEP, phosphoenolpyruvate; LAC, lactate; CIT, citrate; ICIT, isocitrate; SUC, succinate; FUM, fumarate; MAL, malate.
Protein oxidation and lipid peroxidation markers are elevated in sciatic nerve and DRG tissue in db/db mice
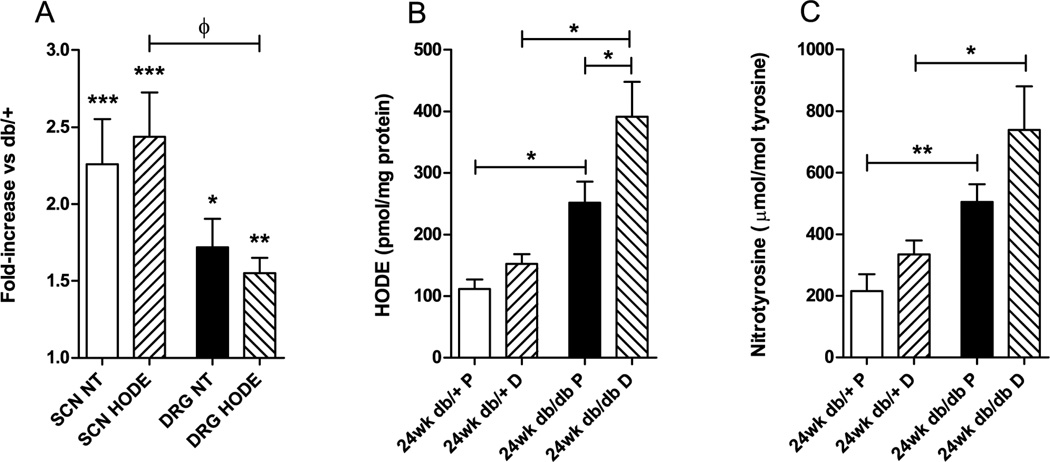
Oxidative stress is a major mechanism of hyperglycemia-induced DN in humans and rodents, particularly through the oxidation of proteins and lipids (Vincent and Feldman 2004; Vincent, et al. 2009b; Vincent et al. 2004). Hydrogen peroxide (H2O2) and O2•− react with nitrite, derived from NO, to produce peroxynitrite in peripheral nerves in experimental diabetes (Obrosova, et al. 2005a). 3-nitrotyrosine accumulates when peroxynitrite oxidizes tyrosine (Corbett, et al. 1992; Du, et al. 2002; van der Veen and Roberts 1999). To confirm whether protein oxidation was elevated in the db/db peripheral nervous system in vivo, we used isotope dilution LC/ESI/MS/MS to quantify levels of protein-bound 3-nitrotyrosine in sciatic nerve and DRG tissue from the db/+ and db/db mice (Fig. 1A). Nitrotyrosine in tissues of the peripheral nervous system was significantly increased by diabetes (sciatic nerve nitrotyrosine 2.3-fold increase, p<0.001; DRG nitrotyrosine 1.7-fold increase, p<0.05). To determine whether lipid peroxidation was also elevated, we used HPLC to quantify HODEs in lipid extracts from the sciatic nerve and DRG of db/+ and db/db mice (Fig. 1A). The sciatic nerve and DRG samples from the db/db mice contained significantly more HODEs than those from the db/+ mice (sciatic nerve HODEs 2.4-fold increase, p<0.001; DRG HODEs 1.6-fold increase, p<0.01). This increase in oxidized lipids was greater in sciatic nerve than DRG (p<0.05).
Figure 1. Increased oxidative stress in diabetic sciatic nerve and DRG.
A. Fold changes in oxidative stress measures from whole sciatic nerve and DRG were calculated from the ratio of db/db to db/+. The nitrated protein content and HODEs were increased in whole sciatic nerve and DRG from diabetic mice compared with those from db/+ controls (* p<0.05; ** p<0.01; *** p<0.001 vs. db/+). The diabetic fold increase in oxidized lipids was greater in sciatic nerve than DRG (ф p<0.05). Data expressed as mean fold-change ±SEM; 6 db/+ and db/db DRG; 12 db/+ and db/db sciatic nerve. There were no changes in oxidative stress measures between proximal and distal segments of db/+ control sciatic nerves (B, C). B. Oxidized lipids (HODEs) were elevated in proximal and distal db/db sciatic nerve, respectively, compared with those of db/+ mice. Diabetic sciatic nerve HODEs were greater distally than proximally. C. The nitrotyrosine-to-tyrosine ratio was elevated in proximal and distal db/db sciatic nerve, respectively, compared with those of db/+ mice. Data expressed as mean ±SEM; 6 db/+ and db/db sciatic nerve. * p<0.05; ** p<0.01. SCN, sciatic nerve; DRG, dorsal root ganglia; P, proximal; D, distal; NT, 3-nitrotyrosine. All data from male mice.
The data presented in Fig. 1A are those from whole sciatic nerve. Due to the greater observed effect of diabetes on the metabolome of sural nerve and sciatic nerve than DRG (Table 2), and the peripheral presentation of signs of DN in rodents (Sullivan et al. 2007) and in humans (Edwards et al. 2008), we explored whether oxidative stress is greater distally in the sciatic nerve by repeating the analyses for 3-nitrotyrosine and HODEs on proximal and distal segments of the sciatic nerve from db/+ and db/db mice (Fig. 1B, 1C). There were no differences in oxidative stress measures between proximal and distal segments of db/+ control nerves. Oxidized lipids (HODEs) were 2.3-fold and 2.6-fold greater in proximal and distal db/db sciatic nerve, respectively, compared with those of db/+ mice (p<0.05). Furthermore, diabetic sciatic nerve HODEs were 1.6-fold greater distally than proximally (p<0.05) (proximal db/+ 112 ±15, proximal db/db 252 ±35, distal db/+ 152 ±16, distal db/db 391 ±56 pmol/mg protein) (Fig. 1B). Nitrated protein was 2.3-fold and 2.2-fold greater in proximal and distal db/db sciatic nerve, respectively, compared with those of db/+ mice (proximal db/+ 216 ±54, proximal db/db 505 ±57 µmol/mol tyrosine, p<0.01) (distal db/+ 335 ±45, distal db/db 739 ±142 µmol/mol tyrosine, p<0.05). Nitrated protein content was not significantly altered proximally-to-distally in diabetic sciatic nerve (Fig. 1C).
Together, these observations indicate that type 2 diabetes increases both protein and lipid oxidation in db/db mouse sciatic nerve and DRG. The diabetic increase in lipid peroxidation is greater in sciatic nerve than DRG, and the extent of this oxidation is greater distally in sciatic nerve.
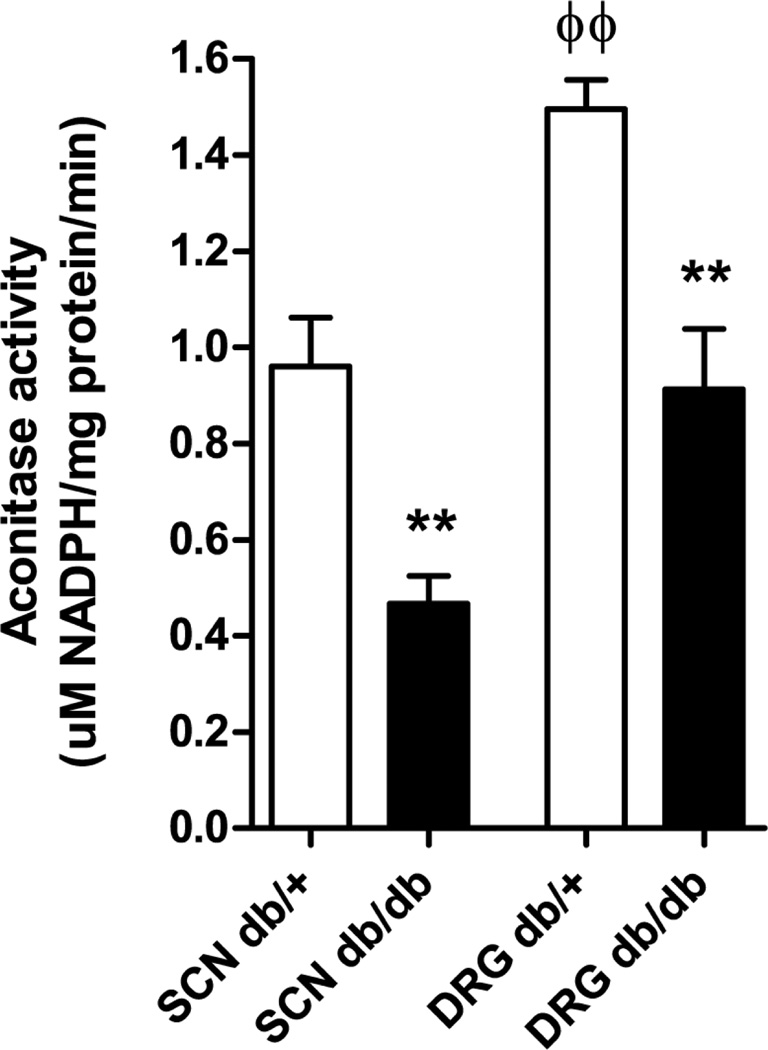
Aconitase enzyme activity is decreased in sciatic nerve and DRG tissue in db/db mice
Aconitase catalyzes the conversion of citrate to isocitrate in the TCA cycle and is the most sensitive TCA cycle enzyme to reactive oxygen species (ROS)-inhibition (Tretter and Adam-Vizi 2000). Work by Tretter and colleagues established that when aconitase is inhibited, a segment of the TCA cycle between α-ketoglutarate (α-KG) and oxaloacetate still functions via anaplerosis of glutamate in to the TCA cycle (Tretter and Adam-Vizi 2000). To begin to understand the lack of change in TCA cycle intermediates other than citrate and isocitrate, we measured aconitase enzyme activity in sciatic nerve and DRG from the db/+ and db/db mice (Fig. 2). Aconitase activity was 1.6-fold greater in DRG than sciatic nerve in control (db/+) mice (DRG 1.50 ±0.06; sciatic nerve 0.96 ±0.10 uM NADPH/mg protein/min, p<0.01), with this tissue difference maintained in the db/db mice (DRG 0.91 ±0.12; sciatic nerve 0.46 ±0.06 uM NADPH/mg protein/min p<0.05). Type 2 diabetes was associated with a decrease in aconitase activity, with a greater effect in sciatic nerve than DRG (DRG 0.39-fold decrease, p<0.01; sciatic nerve 0.51-fold decrease, p<0.01).
Figure 2. Decrease in aconitase enzyme activity in diabetic sciatic nerve and DRG.
Aconitase activity was 1.6-fold greater in DRG than sciatic nerve in control (db/+) mice (ϕϕ p<0.01), with this tissue difference maintained in the db/db mice (ф p<0.05). Type 2 diabetes was associated with a decrease in aconitase activity in both tissues (** p<0.01 compared with db/+). Data expressed as mean ±SEM; 6 db/+ and db/db. SCN, sciatic nerve; DRG, dorsal root ganglia. All data from male mice.
DISCUSSION
Recent metabolomic studies in models of type 1 diabetes reported downregulation of key TCA cycle and mitochondrial proteins (Akude et al. 2011) and enzyme activities (Chowdhury et al. 2010) in DRG, and upregulation of TCA cycle and mitochondrial proteins in Schwann cells (Zhang et al. 2010). Differences in metabolic profiles, prevalence of DN, and efficacy of glycemic control on DN in type 1 and type 2 diabetes suggest that therapeutic strategies based on models of type 1 diabetes may offer limited efficacy to the type 2 diabetic patient with DN (Callaghan et al. 2012). There is therefore a critical need to understand peripheral nerve-specific metabolomic changes in type 2 diabetes, and their role in nerve injury, to develop disease-modifying treatments. To this end, we evaluated changes in the glycolytic and TCA cycle metabolomes of key components of the peripheral nervous system in the db/db mouse model of type 2 diabetes. We performed targeted LC/MS/MS metabolomic analysis on sural nerve, sciatic nerve and DRG from db/db mice. To confirm that ROS is elevated in the db/db peripheral nervous system, we used both LC/ESI/MS/MS and HPLC to measure nitrated protein and peroxidated lipid content, respectively. We observed decreased glycolytic and TCA cycle intermediates in the db/db tissue, with concurrent increases in protein and lipid oxidation. Additionally, our data suggest a greater metabolic impact of diabetes distally, a proximal- distal increase in the severity of oxidative stress, and a proximal-distal decrease in aconitase enzyme activity in the db/db nerve.
Oxidative stress is strongly implicated in DN pathogenesis, particularly through the oxidation of proteins and lipids (Vincent and Feldman 2004; Vincent et al. 2009b; Vincent et al. 2004). To confirm whether protein and lipid oxidation are elevated in the db/db peripheral nervous system in vivo, we quantified levels of protein-bound 3-nitrotyrosine in tissue extracts, and HODEs in lipid extracts, respectively, from the sciatic nerve and DRG of db/+ and db/db mice. Nitrotyrosine and HODEs were significantly elevated in the db/db peripheral nerve and DRG (Fig. 1), consistent with previous work showing that diabetes increases neuronal oxidized protein (Obrosova et al. 2005a; Pennathur et al. 2005; Vincent, et al. 2005; Vincent et al. 2004; Wiggin et al. 2008) and lipids (Vincent et al. 2005; Vincent et al. 2004; Wiggin et al. 2008). Elevated oxidized proteins and oxidized lipids in distal diabetic sciatic nerve compared with distal control sciatic nerve indicate greater oxidative stress at peripheral nerve extremities in diabetes (Fig. 1B, 1C), paralleling the dying-back axonopathy observed in animal models (Sullivan et al. 2007) and human patients (Edwards et al. 2008). The diabetic elevation in lipid peroxidation (HODEs) was greater in the sciatic nerve than DRG and increased moving distally along the db/db sciatic nerve (Fig. 1). There are at least two possible explanations for these findings. First, the increased HODEs as a measure of oxidative stress could simply represent the greater lipid content in sciatic nerve compared to DRG (data not shown), or alternatively, the increase is a true reflection of impaired axonal function with secondary impairment of axon/Schwann cell interactions leading to Schwann cell injury. To our knowledge, these are novel data that oxidative stress increases proximally-to-distally in the neuropathic diabetic peripheral nerve. DN predominantly occurs in a distal symmetrical pattern, with skin denervation (reduced IENF density) (Said 2007; Sullivan et al. 2007) increasing with diabetes duration (Shun, et al. 2004), and proximal-to-distal graded loss of myelinated fiber density observed in diabetic patients (Sullivan, et al. 2003). The current data suggest there may be a link between this proximal-to-distal graded loss of myelinated fiber density and a graded increase in oxidative stress. In addition to its role in nitration of proteins (Corbett et al. 1992; Du et al. 2002; Obrosova et al. 2005a; van der Veen and Roberts 1999), peroxynitrite formation is required for myelin-lipid peroxidation in in vitro myelin suspensions (van der Veen and Roberts 1999); the greater fold-increases observed in sciatic nerve than DRG lipid peroxidation measures (Fig. 1A) may reflect oxidation of myelin in the peripheral nerve.
Aconitase catalyzes the conversion of citrate to isocitrate in the TCA cycle and is the most sensitive TCA cycle enzyme to ROS-inhibition (Tretter and Adam-Vizi 2000). To begin to understand the specificity of the change in citrate and isocitrate TCA cycle intermediates, we measured aconitase enzyme activity in sciatic nerve and DRG from the db/+ and db/db mice. The diabetes-related depletion of aconitase activity (Fig 2) is consistent with our previous report in cultured DRG neurons (Vincent et al. 2005). The greater diabetic impairment observed in sciatic nerve than DRG is likely related to greater oxidative stress-mediated aconitase inhibition (Gardner and Fridovich 1992; Gardner, et al. 1995; Tretter and Adam-Vizi 2000) in the sciatic nerve. This is contradictory to our previous in vitro observation that DRG neurons are more susceptible to oxidative stress than non-myelinating Schwann cells (Vincent, et al. 2009c). It is important to note that our 2009 study investigated the effects of mild, acute oxidative stress induced by high glucose and H2O2 in cells derived from immature rat embryos and pups. We did not assess the effects of myelin-lipid peroxidation, or high free fatty acids and elevated ox-LDL, both of which are associated with type 2 diabetes and linked to oxidative stress-mediated injury in these cells (Suzuki, et al. 2011; Vincent, et al. 2009a).
Lactate production via the reversible lactate dehydrogenase-catalyzed reaction from pyruvate, the last metabolic intermediate of glycolysis, is intimately linked to the NAD+/NADH cellular redox status. Further flux experiments are required, but the pattern of glycolytic and TCA cycle intermediates in the sural nerve and sciatic nerve in diabetic mice compared with controls suggests the observed significant depletion of sural nerve and sciatic nerve lactate (Table 2) is related to inhibition of glycolysis. These data are in contrast with findings of elevated lactate in sciatic nerve from type 1 diabetic streptozotocin (STZ)-treated rats (Obrosova, et al. 1999; Stevens et al. 2000). Our finding that lactate levels were unchanged in db/db DRG is in agreement with reports in STZ-diabetic lens (Obrosova, et al. 1998; Obrosova and Stevens 1999). Work by Stevens and colleagues highlighted concerns over anesthetic-induced elevations in lactate levels in control peripheral nerves when compared with the CO2/cervical dislocation method of euthanasia (Stevens et al. 2000). Absolute values stated in Table 2 may therefore be an underestimate of true tissue lactate content, but relative comparisions between db/+ and db/db mice may be made. Interestingly, the observed depletions in db/db sural and sciatic nerves are in contrast with elevations reported in STZ rats euthanized via the CO2/cervical dislocation method (Obrosova et al. 1999; Stevens et al. 2000). The significant decrease in lactate content we observe argues against the potential for an anesthesia-driven lactate elevation to introduce artifact into the data. In addition, it should be noted that the above studies by Obrosova and Stevens were performed in a model of type 1 diabetes in rats and over shorter diabetes duration (3–6 weeks).
Our finding that glucose-6-phosphate (G6P) and fructose-6-phosphate (F6P) were decreased in sciatic nerve, with additional decrease in fructose-1,6-bisphosphate (FBP) in sural nerve, in db/db mice at 24 weeks of age (Table 2) is in contrast to findings in STZ-diabetic lens (Obrosova et al. 1998; Obrosova and Stevens 1999). As G6P and F6P are derived from the same pool in the current MS approach, we cannot determine the contribution from each intermediate to the observed measurements. Whether there is a true decrease in both F6P and upstream G6P, or a decrease in F6P due to redirection of G6P into the polyol (or other) pathways remains unclear.
Hexokinase saturation and maximal glycolytic flux is one proposed mechanism underlying the accumulation of nerve glucose and its direction into the polyol pathway in diabetes (Tomlinson and Gardiner 2008). Gardiner and colleagues observed diminished hexokinase activity in DRG from STZ-diabetic rats (Gardiner et al. 2007). Studies on excised rat retinas (Ola et al. 2006) concluded that glycolysis, and glucose metabolism downstream of hexokinase, are not elevated by hyperglycemia or type 1 diabetes, but that intermediates of alternative glucose metabolism, such as those of the polyol pathway, are increased. This is in agreement with conclusions of studies by the Greene group in STZ-diabetic rat sciatic nerve (Obrosova et al. 1999; Stevens et al. 2000). Thus, it is possible that glucose is being preferentially directed into the polyol pathway in the db/db sural nerve and sciatic nerve, away from energy-producing glycolysis.
Decreased steady-state concentrations of metabolites in the lower segment of glycolysis (3-phosphoglycerate/2-phosphoglycerate, 3PG/2PG; phosphoenolpyruvate, PEP) in db/db sural and sciatic nerves compared with controls agrees with previously published reports in the STZ-diabetic lens (Obrosova et al. 1998; Obrosova and Stevens 1999) (levels of these intermediates were similar between control and diabetic DRG in the current study) which concluded that sites of regulation are at glyceraldehyde 3-phosphate dehydrogenase (GAPDH) reaction and downstream (e.g. enolase, pyruvate kinase). This conclusion is supported in the current model by our recent report of downregulation of the gene encoding enolase (Eno2) in db/db sciatic nerve at 24 weeks of age (Pande, et al. 2011), the fact that GAPDH is vulnerable to oxidative damage (Du, et al. 2000), and greater markers of lipid and protein oxidative damage in distal sciatic nerve compared with DRG (Fig. 1). In the more distal sural nerve, data suggest additional upstream sites of glycolysis inhibition (e.g. hexokinase, phosphoglucose isomerase, phosphofructokinase), with a greater diabetic decrease in metabolic intermediates in sural nerve compared with sciatic nerve (Table 2). In addition, slow axonal transport, responsible for the transport of glycolytic enzymes, is impaired in db/db diabetes at 20–24 weeks of age (Vitadello, et al. 1983; Vitadello, et al. 1985). Decreased peripheral enzyme availability coupled with increased distal oxidative stress may be related to the greater distal metabolomic changes observed in the current study.
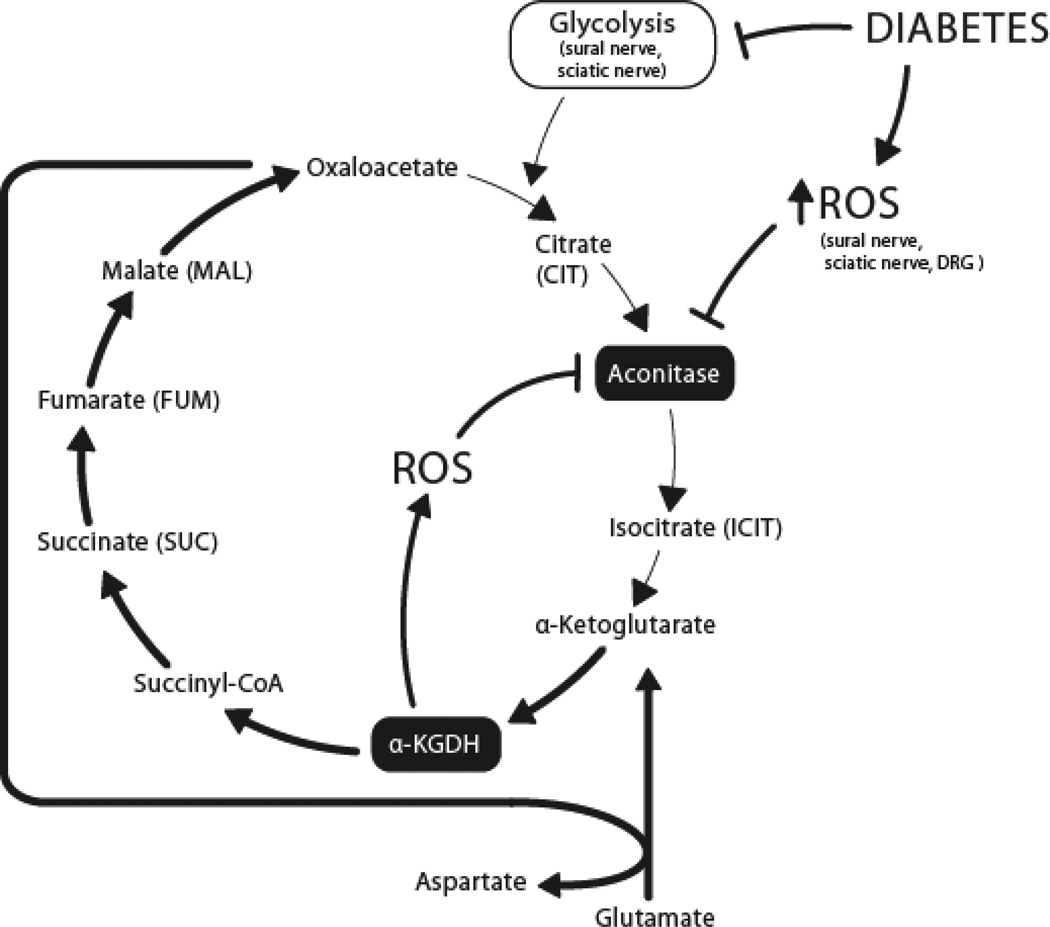
Our observed decreases in citrate and isocitrate (Table 2) are consistent with work by Fernyhough and colleagues reporting downregulation of key TCA cycle proteins, including citrate synthase, in DRG from 22-week STZ-diabetic rats (Akude et al. 2011). One question arising from the TCA cycle metabolomic data is the lack of change in additional intermediates other than citrate and isocitrate. Aconitase, catalyzing the citrate to isocitrate reaction in the TCA cycle (Fig. 3), is inhibited by ROS, including O2•− (Gardner and Fridovich 1992; Gardner et al. 1995) and H2O2 (Tretter and Adam-Vizi 2005). When aconitase is fully inhibited by H2O2 in nerve terminals, α-ketoglutarate dehydrogenase (α-KGDH; catalyzing the α-KG to succinyl-CoA reaction) remains functional and a segment of the TCA cycle (α-KG to oxaloacetate) is maintained by glutamate, which is converted to α-KG via transamination (see (Tretter and Adam-Vizi 2005) for comprehensive explanation). ROS-mediated inhibition of aconitase (Fig. 2) may activate this truncated TCA in diabetic sural nerve, sciatic nerve and DRG (Fig. 3), explaining the maintenance of levels of succinate, fumarate, and malate in the face of decreased citrate and isocitrate observed in the present study. This truncated segment of the TCA cycle may function in the absence of glucose (Erecinska, et al. 1996; Yudkoff, et al. 1994). In this respect, a state of oxidative stress when aconitase is completely inhibited but α-KGDH remains active is similar to a glucose-free state. Additionally, α-KGDH is itself a source of ROS production (see (Gibson, et al. 2010) for review of α-KGDH in neurodegeneration), the level of which increases when α-KG is utilized as a fuel source over glucose in isolated brain synaptosomes (Tretter and Adam-Vizi 2004). The greater decrease in citrate and isocitrate observed in db/db sural and sciatic nerves (compared with DRG) may be due to reduced anaplerosis of intermediates from upstream glycolysis to the TCA cycle. This may be interpreted as a glucose-deficient state, activating the α-KG-utilizing truncated TCA cycle pathway, further contributing to ROS production, and potentially contributing to the greater degree of oxidative stress observed in diabetic sciatic nerve than DRG (Fig. 1A). Contribution to these pathways from an altered diabetic lipid metabolome should be considered, but has not been explored in the current study.
Figure 3. Proposed relationship between diabetes, ROS, and the TCA cycle.
ROS-mediated inhibition of aconitase may truncate the TCA cycle in diabetic sural nerve, sciatic nerve and DRG (bold arrows). This would result in the observed maintenance of succinate, fumarate, and malate levels in the face of decreased citrate and isocitrate. Reduced glycolytic intermediates feeding into the TCA cycle in sural nerve and sciatic nerve may contribute to the observed decreases in citrate and isocitrate and be interpreted as a glucose-deficient state. Activation of the α-KG-utilizing truncated TCA cycle pathway in the sural and sciatic nerves, would further contribute to ROS production, and to the greater oxidative stress observed in diabetic sciatic nerve than DRG. α-KGDH, alpha-ketoglutarate dehydrogenase; DRG, dorsal root ganglia; TCA, tricarboxylic acid; ROS, reactive oxygen species.
Collectively, our data and those of the Fernyhough, LaNoue, and Obrosova groups support the emerging idea of tissue-specific alterations in energy metabolism in diabetic complications-prone tissues such as the peripheral nerve (Akude et al. 2011; Chowdhury et al. 2010; Gardiner et al. 2007), retina (Ola et al. 2006), and lens (Obrosova et al. 1998; Obrosova and Stevens 1999). For the first time, we expand this knowledge to neuropathy in a type 2 model of diabetes, additionally demonstrating different metabolomic profiles within specific tissues of the peripheral nervous system. The greater metabolomic changes in sural nerve and sciatic nerve than DRG may reflect increased oxidative stress and subsequent inhibition of key metabolic enzymes, including aconitase. However, our static measurements of metabolites are not direct indicators of flux through a pathway: these data are the beginnings of a full characterization of bioenergetic alterations in diabetic peripheral nerve, and are necessary to develop effective disease-modifying treatments for DN.
ACKNOWLEDGEMENTS
We thank Drs. Anne M. Heacock, Stacey A. Sakowski and Kelli A. Sullivan for critical review of the manuscript, and Mrs. Judith Bentley for assistance with preparation of the manuscript.
FUNDING
This work was supported by the National Institutes of Health [NIH 1 DP3 DK094292, NIH 1 R24 DK082841-01, NIH 1 UO1 DK076160, and NIH 1RC 1NS068182 (to E.L.F.)], The A. Alfred Taubman Medical Research Institute and The Program for Neurology Research and Discovery. This work utilized Molecular Phenotyping Core Services of the Michigan Nutrition and Obesity Research Center, supported by the National Institutes of Health [DK089503 (University of Michigan)].
Abbreviations
- 3PG/2PG
3-phosphoglycerate/2-phosphoglycerate
- AR
aldose reductase
- CIT
citrate
- DN
diabetic peripheral neuropathy
- DRG
dorsal root ganglia
- G6P/F6P
glucose-6-phosphate/fructose-6-phosphate
- FBP
fructose-1,6-bisphosphate
- FUM
fumarate
- GHb
glycated hemoglobin
- GLUT
glucose transporter
- HODEs
hydroxyoctadecadienoic acids
- ICIT
isocitrate
- α-KG
alpha-ketoglutarate
- α-KGDH
alpha-ketoglutarate dehydrogenase
- LAC
lactate
- MAL
malate
- PEP
phosphoenolpyruvate
- ROS
reactive oxygen species
- STZ
streptozotocin
- SUC
succinate
- TCA
tricarboxylic acid.
Footnotes
DECLARATION OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
REFERENCES
- Akude E, Zherebitskaya E, Chowdhury SK, Smith DR, Dobrowsky RT, Fernyhough P. Diminished superoxide generation is associated with respiratory chain dysfunction and changes in the mitochondrial proteome of sensory neurons from diabetic rats. Diabetes. 2011;60:288–297. doi: 10.2337/db10-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Callaghan BC, Hur J, Feldman EL. Diabetic Neuropathy: One disease or two? Current Opinion in Neurology. 2012 doi: 10.1097/WCO.0b013e328357a797. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDCP. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- Cheng HT, Dauch JR, Hayes JM, Hong Y, Feldman EL. Nerve growth factor mediates mechanical allodynia in a mouse model of type 2 diabetes. J Neuropathol Exp Neurol. 2009;68:1229–1243. doi: 10.1097/NEN.0b013e3181bef710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SK, Zherebitskaya E, Smith DR, Akude E, Chattopadhyay S, Jolivalt CG, Calcutt NA, Fernyhough P. Mitochondrial respiratory chain dysfunction in dorsal root ganglia of streptozotocin-induced diabetic rats and its correction by insulin treatment. Diabetes. 2010;59:1082–1091. doi: 10.2337/db09-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett JA, Tilton RG, Chang K, Hasan KS, Ido Y, Wang JL, Sweetland MA, Lancaster JR, Jr, Williamson JR, McDaniel ML. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992;41:552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Smith MA, Miller CM, Kern TS. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J Neurochem. 2002;80:771–779. doi: 10.1046/j.0022-3042.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: Mechanisms to management. Pharmacol Ther. 2008;120:1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Nelson D, Deas J, Silver IA. Limitation of glycolysis by hexokinase in rat brain synaptosomes during intense ion pumping. Brain Res. 1996;726:153–159. doi: 10.1016/0006-8993(96)00324-1. [DOI] [PubMed] [Google Scholar]
- Feldman EL. Diabetic neuropathy. Curr Drug Targets. 2008;9:1–2. doi: 10.2174/138945008783431709. [DOI] [PubMed] [Google Scholar]
- Gardiner NJ, Wang Z, Luke C, Gott A, Price SA, Fernyhough P. Expression of hexokinase isoforms in the dorsal root ganglion of the adult rat and effect of experimental diabetes. Brain Res. 2007;1175:143–154. doi: 10.1016/j.brainres.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Gardner PR, Fridovich I. Inactivation-reactivation of aconitase in Escherichia coli. A sensitive measure of superoxide radical. J Biol Chem. 1992;267:8757–8763. [PubMed] [Google Scholar]
- Gardner PR, Raineri I, Epstein LB, White CW. Superoxide radical and iron modulate aconitase activity in mammalian cells. J Biol Chem. 1995;270:13399–13405. doi: 10.1074/jbc.270.22.13399. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Starkov A, Blass JP, Ratan RR, Beal MF. Cause and consequence: mitochondrial dysfunction initiates and propagates neuronal dysfunction, neuronal death and behavioral abnormalities in age-associated neurodegenerative diseases. Biochim Biophys Acta. 2010;1802:122–134. doi: 10.1016/j.bbadis.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DA, Winegrad AI. In vitro studies of the substrates for energy production and the effects of insulin on glucose utilization in the neural components of peripheral nerve. Diabetes. 1979;28:878–887. doi: 10.2337/diab.28.10.878. [DOI] [PubMed] [Google Scholar]
- Kishi Y, Schmelzer JD, Yao JK, Zollman PJ, Nickander KK, Tritschler HJ, Low PA. Alpha-lipoic acid: effect on glucose uptake, sorbitol pathway, and energy metabolism in experimental diabetic neuropathy. Diabetes. 1999;48:2045–2051. doi: 10.2337/diabetes.48.10.2045. [DOI] [PubMed] [Google Scholar]
- Lorenz MA, Burant CF, Kennedy RT. Reducing time and increasing sensitivity in sample preparation for adherent Mammalian cell metabolomics. Anal Chem. 2011;83:3406–3414. doi: 10.1021/ac103313x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrosova I, Cao X, Greene DA, Stevens MJ. Diabetes-induced changes in lens antioxidant status, glucose utilization and energy metabolism: effect of DL-alpha-lipoic acid. Diabetologia. 1998;41:1442–1450. doi: 10.1007/s001250051090. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Fathallah L, Lang HJ, Greene DA. Evaluation of a sorbitol dehydrogenase inhibitor on diabetic peripheral nerve metabolism: a prevention study. Diabetologia. 1999;42:1187–1194. doi: 10.1007/s001250051290. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Mabley JG, Zsengeller Z, Charniauskaya T, Abatan OI, Groves JT, Szabo C. Role for nitrosative stress in diabetic neuropathy: evidence from studies with a peroxynitrite decomposition catalyst. FASEB J. 2005a;19:401–403. doi: 10.1096/fj.04-1913fje. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Pacher P, Szabo C, Zsengeller Z, Hirooka H, Stevens MJ, Yorek MA. Aldose reductase inhibition counteracts oxidative-nitrosative stress and poly(ADP-ribose) polymerase activation in tissue sites for diabetes complications. Diabetes. 2005b;54:234–242. doi: 10.2337/diabetes.54.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrosova IG, Stevens MJ. Effect of dietary taurine supplementation on GSH and NAD(P)-redox status, lipid peroxidation, and energy metabolism in diabetic precataractous lens. Invest Ophthalmol Vis Sci. 1999;40:680–688. [PubMed] [Google Scholar]
- Ola MS, Berkich DA, Xu Y, King MT, Gardner TW, Simpson I, LaNoue KF. Analysis of glucose metabolism in diabetic rat retinas. Am J Physiol Endocrinol Metab. 2006;290:E1057–E1067. doi: 10.1152/ajpendo.00323.2005. [DOI] [PubMed] [Google Scholar]
- Pande M, Hur J, Hong Y, Backus C, Hayes JM, Oh SS, Kretzler M, Feldman EL. Transcriptional Profiling of Diabetic Neuropathy in the BKS db/db Mouse: A Model of Type 2 Diabetes. Diabetes. 2011;60:1981–1989. doi: 10.2337/db10-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennathur S, Bergt C, Shao B, Byun J, Kassim SY, Singh P, Green PS, McDonald TO, Brunzell J, Chait A, et al. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. Journal of Biological Chemistry. 2004;279:42977–42983. doi: 10.1074/jbc.M406762200. [DOI] [PubMed] [Google Scholar]
- Pennathur S, Ido Y, Heller JI, Byun J, Danda R, Pergola P, Williamson JR, Heinecke JW. Reactive carbonyls and polyunsaturated fatty acids produce a hydroxyl radical-like species: a potential pathway for oxidative damage of retinal proteins in diabetes. J Biol Chem. 2005;280:22706–22714. doi: 10.1074/jbc.M500839200. [DOI] [PubMed] [Google Scholar]
- Russell JW, Sullivan KA, Windebank AJ, Herrmann DN, Feldman EL. Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiol Dis. 1999;6:347–363. doi: 10.1006/nbdi.1999.0254. [DOI] [PubMed] [Google Scholar]
- Said G. Diabetic neuropathy--a review. Nat Clin Pract Neurol. 2007;3:331–340. doi: 10.1038/ncpneuro0504. [DOI] [PubMed] [Google Scholar]
- Shun CT, Chang YC, Wu HP, Hsieh SC, Lin WM, Lin YH, Tai TY, Hsieh ST. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain. 2004;127:1593–1605. doi: 10.1093/brain/awh180. [DOI] [PubMed] [Google Scholar]
- Stevens MJ, Obrosova I, Cao X, Van Huysen C, Greene DA. Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes. 2000;49:1006–1015. doi: 10.2337/diabetes.49.6.1006. [DOI] [PubMed] [Google Scholar]
- Sullivan KA, Brown MS, Harmon L, Greene DA. Digital electron microscopic examination of human sural nerve biopsies. Journal of the Peripheral Nervous System. 2003;8:260–270. doi: 10.1111/j.1085-9489.2003.03030.x. [DOI] [PubMed] [Google Scholar]
- Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI, Brosius F, 3rd, Feldman EL. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28:276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Akahane K, Nakamura J, Naruse K, Kamiya H, Himeno T, Nakamura N, Shibata T, Kondo M, Nagasaki H, et al. Palmitate induces apoptosis in Schwann cells via both ceramide-dependent and independent pathways. Neuroscience. 2011;176:188–198. doi: 10.1016/j.neuroscience.2010.11.035. [DOI] [PubMed] [Google Scholar]
- Thurston JH, McDougal DB, Jr, Hauhart RE, Schulz DW. Effects of acute, subacute, and chronic diabetes on carbohydrate and energy metabolism in rat sciatic nerve. Relation to mechanisms of peripheral neuropathy. Diabetes. 1995;44:190–195. doi: 10.2337/diab.44.2.190. [DOI] [PubMed] [Google Scholar]
- Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9:36–45. doi: 10.1038/nrn2294. [DOI] [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: A key role of [alpha]-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J Neurosci. 2000;20:8972–8979. doi: 10.1523/JNEUROSCI.20-24-08972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V. Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J Neurosci. 2004;24:7771–7778. doi: 10.1523/JNEUROSCI.1842-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V. Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philos Trans R Soc Lond B Biol Sci. 2005;360:2335–2345. doi: 10.1098/rstb.2005.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen RC, Roberts LJ. Contrasting roles for nitric oxide and peroxynitrite in the peroxidation of myelin lipids. J Neuroimmunol. 1999;95:1–7. doi: 10.1016/s0165-5728(98)00239-2. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat rev neurol. 2011;7:573–583. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Feldman EL. New insights into the mechanisms of diabetic neuropathy. Rev Endocr Metab Disord. 2004;5:227–236. doi: 10.1023/B:REMD.0000032411.11422.e0. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009a;58:2376–2385. doi: 10.2337/db09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009b;14:257–267. doi: 10.1111/j.1529-8027.2009.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, Kato K, McLean LL, Soules ME, Feldman EL. Sensory neurons and schwann cells respond to oxidative stress by increasing antioxidant defense mechanisms. Antioxid Redox Signal. 2009c;11:425–438. doi: 10.1089/ars.2008.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, McLean LL, Backus C, Feldman EL. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J. 2005;19:638–640. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocrine Reviews. 2004;25:612–628. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- Vitadello M, Couraud JY, Hassig R, Gorio A, Di GL. Axonal transport of acetylcholinesterase in the diabetic mutant mouse. Experimental Neurology. 1983;82:143–147. doi: 10.1016/0014-4886(83)90249-2. [DOI] [PubMed] [Google Scholar]
- Vitadello M, Filliatreau G, Dupont JL, Hassig R, Gorio A, Di Giamberardino L. Altered axonal transport of cytoskeletal proteins in the mutant diabetic mouse. J Neurochem. 1985;45:860–868. doi: 10.1111/j.1471-4159.1985.tb04073.x. [DOI] [PubMed] [Google Scholar]
- Vivekanandan-Giri A, Byun J, Pennathur S. Quantitative analysis of amino Acid oxidation markers by tandem mass spectrometry. Methods Enzymol. 2011;491:73–89. doi: 10.1016/B978-0-12-385928-0.00005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggin TD, Kretzler M, Pennathur S, Sullivan KA, Brosius FC, Feldman EL. Rosiglitazone treatment reduces diabetic neuropathy in streptozotocin-treated DBA/2J mice. Endocrinology. 2008;149:4928–4937. doi: 10.1210/en.2008-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36:150–154. doi: 10.1007/BF00400697. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Nelson D, Daikhin Y, Erecinska M. Tricarboxylic acid cycle in rat brain synaptosomes. Fluxes and interactions with aspartate aminotransferase and malate/aspartate shuttle. J Biol Chem. 1994;269:27414–27420. [PubMed] [Google Scholar]
- Zhang L, Yu C, Vasquez FE, Galeva N, Onyango I, Swerdlow RH, Dobrowsky RT. Hyperglycemia alters the schwann cell mitochondrial proteome and decreases coupled respiration in the absence of superoxide production. J Proteome Res. 2010;9:458–471. doi: 10.1021/pr900818g. [DOI] [PMC free article] [PubMed] [Google Scholar]