Abstract
Aims:
This study aims to investigate the effects of intravitreal octreotide on the growth factors, which have significant roles in the pathogenesis of proliferative vitreoretinopathy (PVR).
Settings and Design:
An experimental trial.
Materials and Methods:
21 guinea pigs were randomly assigned to form 3 groups each including 7 animals. In group 1 (the control group), 0.2 ml saline solution was applied intravitreally in a location of 1.5 mm behind the limbus. In group 2 (the sham group), 0.07 IU dispase in 0.1 ml and 0.1 ml saline solution were applied via the same route. The guinea pigs in group 3 (the treatment group) were applied 0.07 IU dispase in 0.1 ml and 1 mg octreotide in 0.1 ml via the same route. Octreotide injection was applied twice during the period of 10 weeks of the experiment. At the end of the 10 weeks, eyes were enucleated and retinal homogenates were prepared. The platelet derivated growth factor (PDGF), insulin-like growth factor (IGF 1) and transforming growth factor (TGF ß) levels in homogenized retina tissue were measured by Enzyme Linked-Immuno-Sorbent Assay (ELISA) method.
Statistical Analysis Used:
Kruskal-Wallis variance analysis and Mann-Whitney U test.
Results:
In the treatment group, a significant decrease was observed in retinal PDGF levels (P < 0.01) while decreases in TGF ß and IGF 1 levels were not found to be significant (P > 0.05).
Conclusions:
Intravitreally applied octreotide at a dose of 1 mg has a highly strong effect on PDGF. This study suggests that intravitreal octreotide may suppress PVR development and that octreotide may merit investigation for PVR prophylaxis.
Keywords: Insulin-like growth factor 1, octreotide, platelet derivated growth factor, proliferative vitreoretinopathy, transforming growth factor ß
Proliferative vitreoretinopathy (PVR) is an abnormal tissue response, which is characterized by the proliferation of non-neoplastic cells on both surfaces of the retina, resulting in membrane formation and traction on the retina. This abnormal tissue response occurs in the substance of vitreous and retina.[1–6] Currently, PVR is one of the most important complications experienced in vitreoretinal surgery as well as one of the most important reasons for serious vision loss worldwide.[7]
Current therapy for the treatment of PVR is vitreoretinal surgery including the removal of scar tissue, peeling of the membranes on the retina and flattening the retina with various intraocular tamponades. As a result of the advances in the surgical techniques, anatomic success rate of 60% - 80% has been reported while the functional success rate remains around 30% - 40%.[8] The difficulties in surgical treatment of PVR, the necessity for more than one surgery in many cases and the fact that the resulting blindness rate is considerably high, have led the researchers to seek for an ideal PVR treatment. Although the preventive effects of various pharmacological agents in adjuvant treatment for PVR have been studied, up to now, none of these drugs have been put to routine use due to their relative ineffectiveness and toxicity.
Octreotide is a long-term effective analogue of somatostatin formed by neuro-endocrine tissues and the gastrointestinal system. Somatostatin is a natural inhibitor of the growth hormone and insulin-like growth factor 1 (IGF-1), and it has great inhibitory effects on some hormones including thyroid stimulant hormone (TSH), prolactin (PRL), glucagon and insulin. It causes decrease in tumor growth fraction via its direct anti-proliferative effect.[9–14] To our knowledge, although octreotide has not been used widely in the ophthalmology practice, it might be a promising drug, in particular for its anti-proliferative and anti-angiogenic effects. Octreotide has been reported to inhibit retinal pigment epithelium (RPE) cells, which have important roles in PVR development and endothelial cells of choriocapillary induced by growth factors, thus causing a decrease in retinal neovascularisation.[11,14–23] However, there has been no study carried out with octreotide in animal PVR models in the literature.
Up to date, although there have been some unexplained points in the pathogenesis of PVR; the role of growth factors is clear and precise. It has been demonstrated from the samples taken from vitreous and sub-retinal liquid that there are 5 significant growth factors - the platelet derivated growth factor (PDGF), IGF 1, transforming growth factor (TGF ß), fibroblast growth factor (FGF) and epidermal growth factor (EGF), which have active roles in the proliferation of RPE cells.[24–38]
Taking into account the above-mentioned features of octreotide and the fact that it has inhibitory effect on the growth factors, which play important roles in the PVR pathogenesis, it might be thought that octreotide will inhibit RPE cell proliferation, which is considered to be the key stage for PVR pathogenesis. Under the light of this information, we aimed to investigate the effects of intravitreally-applied octreotide on the growth factors, which have significant roles in the pathogenesis of PVR.
Materials and Methods
The study was carried out under the permission of the institutional ethics Committee. A total of 21 pigmented guinea pigs (mean weight: 500 grams) were used in the experiment. 1 eye each of 21 pigmented guinea pigs were used in the study. The animals were kept in Institutional Medicine Research Center under appropriate diet conditions and in special cages throughout the study.
The guinea pigs were randomly divided into 3 groups, each including 7 animals:
Group 1: Control group
Group 2: Sham group (in which PVR was developed)
Group 3: Treatment group (in which PVR was developed and octreotide was applied)
Anesthesia technique
A combination of intramuscular 50 mg/kg ketamine hydrochloride (Ketalar, Eczacıbaşı, Turkey) and 6 mg/kg xylazine hydrochloride (Rompun, Bayer, Turkey) was used for the anesthesia and analgesia. Before the operation, 1 drop of 0.5% proparacaine hydrochloride (Alcaine, Alcon, Turkey) was instilled on the animals' eyes.
Application of the experiment
After 10 mg of octreotide in the form of powder (Sandostatin LAR 10 mg flacon, Novartis Pharma AG, Basel, Switzerland) was solved by means of a special solvent containing 12.5 mg sodium carboxymethyl cellulose and 15 mg mannitole, 1 mg octreotide in 0.1 mL was prepared. 0.07 IU dispase in 0.1 mL was prepared by means of combining 25 IU of dispase in powder form (Boehringer Mannheim, Indianapolis, IN; Collaborative Biomedical Products, Bedfort, MA) and saline. In order to provide the prophylaxis of endophthalmitis, prior to all intravitreal applications, the surgical area was cleaned with 10% povidone iodine, and 5% povidone iodine was applied to the conjunctival sac; after waiting for 3 minutes, the conjunctival surface was washed with saline solution in all animals.
In the treatment group, a 27 G needle was inserted into the right eyes of the 7 guinea pigs in a location of 1.5 mm behind the limbus and 0.1 ml vitreous was aspired (vitreous tap). 0.07 U dispase and 1 mg octreotide were consequently injected without removing this needle from the globe. The vitreous tap was performed in order to minimize the 0.2 ml of volume effect.
A period of 10 weeks (70 days) was waited to provide an adequate time for dispase-induced PVR development.[39,40] Taking into account the fact that octreotide remains for 35 days in the vitreous; intravitreal octreotide injection was applied twice within 70 days. The first injection of octreotide was performed simultaneously with dispase while the second injection was applied 35 days after the first injection.[41,42]
In the sham group, a 27 G needle was inserted into the right eyes of the 7 guinea pigs via the same and 0.1 mL of vitreous tap was performed with the same method. Without removing the needle from the globe, 0.07 U dispase and 0.1 mL saline solution were injected intravitreally. Similarly, 10 weeks (70 days) were waited. On the 35th day, a second injection of 0.1 mL saline solution was repeated similar to the treatment group.
In order to maintain the similar mechanic effect formed in the eyes of the treatment and in the sham groups, 0.2 saline solution was intravitreally injected into the right eyes of the 7 subjects of the control group via the same method. On the 35th day, the same injection was repeated with 0.1 ml of saline solution.
At the end of the 10th week, the guinea pigs were applied a combination of intramuscular 50 mg/kg ketamine hydrochloride and 6 mg/kg xylazine hydrochloride, and enucleation was performed. The eyes, which were enucleated, were dissected into 2 in the direction of the sagittal surface passing through the optic nerve. The hematoxylen-eosine stained sections obtained from one half of the globe were analyzed.
After observing epiretinal membrane formation and retinal folds in the eyes of the animals of the sham and treatment groups by slit lamb examination, the eyes were enucleated on the 70th day.
Homogenization and biochemical assessment
The PDGF, IGF-1 and TGF-β levels in homogenized retina tissue were measured with Enzyme Linked- Immuno-Sorbent Assay (ELISA) method using Quantikine PDGF (R and D Systems, Inc., Minneapolis, USA) kit, Quantikine IGF-1 (R and D Systems, Inc., Minneapolis, USA) kit and Biosource TGF-β (Invitrogen Corporation, Carlsbad, USA) kit, respectively.
Statistical analysis
The means and standard deviations of the obtained data were calculated. Statistical analysis was performed with Statistical Package for the Social Sciences 13 (SPSS 13.0, Chicago, IL, USA). Kruskal-Wallis variance analysis was performed for multiple comparisons. Mann-Whitney U test was applied for between groups comparison. A P value <0.05 was accepted as significant.
Results
The maximum, minimum and mean values and the standard deviations of retinal PDGF, TGF-ß and IGF-1 levels in the groups are presented in Table 1. The comparisons of retinal PDGF, TGF-ß and IGF-1 levels in the groups are given in Figs. 1-3, respectively.
Table 1.
The maximum, minimum, mean values and standard deviations of retinal platelet derived growth factor, transforming growth factor-ß and insulin-like growth factor-1 levels in study groups
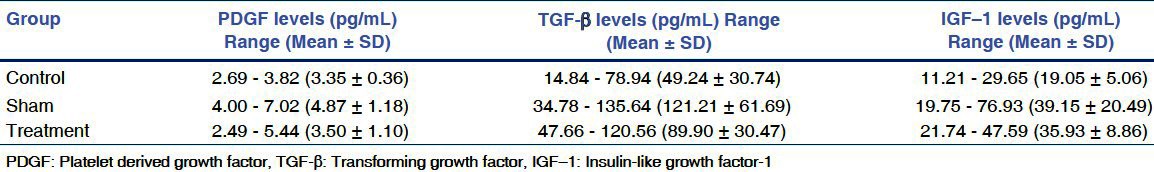
Figure 1.
A comparison of the levels of retinal PDGF among the groups. *P < 0.05 **P > 0.05
Figure 3.
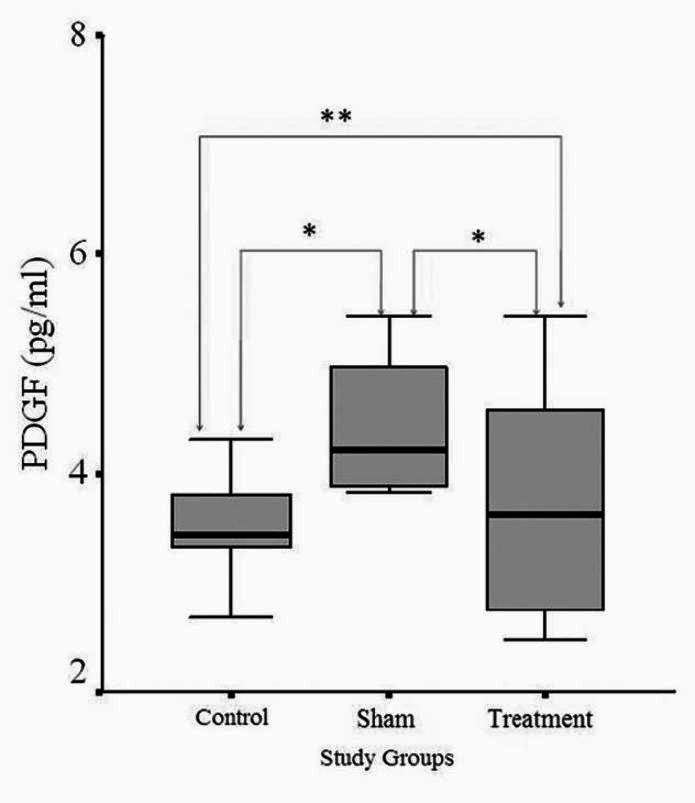
A comparison of the levels of retinal IGF-1 among the groups. *P < 0.05 **P > 0.05
Figure 2.
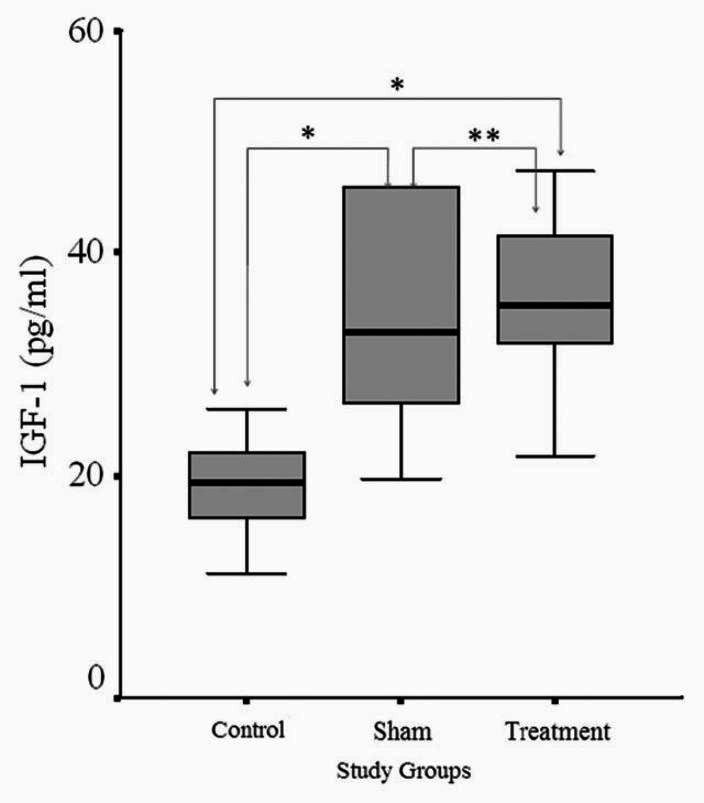
A comparison of the levels of retinal TGF-β among the groups. *P > 0.05
When compared with the control group, it was observed that mean PDGF level of the sham group was significantly higher than that of the control group (P < 0.05). A comparison of the mean PDGF levels of the sham and treatment groups revealed a significant decrease in PDGF level in the treatment group (P < 0.05). It was also found out that there is no significant difference between the PDGF levels of control and treatment groups (P = 0.937), and the mean PDGF level of the treatment group were close to that of control group.
It was observed that there was an increase in the mean TGF-ß level of the sham group in comparison with the control group, yet this increase was not significant (P = 0.056). It was also seen that there was a decrease in the mean TGF-ß level in the treatment group in comparison with the sham group. However, this difference was also not significant (P = 0.273). As for the comparison of mean TGF-ß levels of control and treatment groups, no significant difference was observed between them, and it was noted that the mean level of the treatment group were close to that of control group (P = 0.329).
A comparison between the IGF-1 levels of the sham group and control group demonstrated that there was a significant increase in the sham group (P < 0.05). As for the sham group and treatment group, no significant difference was found between them (P = 0.731). Finally, a significant level of increase was found in the treatment group in comparison with the control group (P < 0.05).
Discussion
The most important stage in PVR development is the proliferative stage where the RPE cells and the myofibroblasts assumed to be formed with the transformation of the proliferation of these cells. Thus, for PVR prophylaxis, the emphasis has been widely put on anti-proliferative agents. As a matter of fact, many anti-proliferative drugs have been used experimentally or clinically in order to prevent PVR. Although they are available as potent drugs for the prevention of PVR, they have not been put in common use due to the critical retinal toxicity they have.[43–45]
In miscellaneous experimental studies, autologous and homologous fibroblasts and RPE cells, endothelial cells, chondrocytes, embrional cells, and photoreceptor cells stimulated by transgenic PDGF have been used in intravitreal injection for PVR induction. The PVR model performing by use of intravitreal dispase injection, described by Frenzel et al, is an easy and inexpensive method.[39] Dispase is a proteolytic enzyme derived from Bacillus polymyxa. It selectively breaks down type 4 collagen and fibronectin in the structure of the basement membrane. Basement membrane plays an important role in continuity of RPE cell layer. When RPE cells from disrupted RPE layer were dispersed into vitreal cavity, they can cause the formation of intravitreal contractile membranes, retinal detachment and consequently PVR development. In recent studies, it was shown that an effective dose of dispase is 0.05 - 0.07 U, and that average duration is 8 - 10 weeks for PVR development. In our study, dispase model was used for PVR induction because this method is easy and inexpensive method, and it does not need laboratory conditions compared to the others.[39,40]
Octreotide, whose efficacy was not previously studied in vivo PVR models, was used in this study. The fact that octreotide, which has been used in various fields recently, has lower side effects when compared to the strong anti-proliferative agents like 5-fluorouracil, mitomycin-C and daunomycin. Thus, this increases the importance of the drug.[43–46] Recent studies have shown that when octreotide was applied intravitreally in doses of 1 mg or lower, it does not lead to retinal toxiticity.[41,42] On the basis of this knowledge, in order to minimize present systemic side effects, intravitreal application was preferred in the present study.
As it is known, during PVR development, inflammatory blood cells and pro-inflammatory serum elements besides RPE cells also pass into vitreal cavity. The platelets which pass from the serum induce to release growth factors including PDGF, TGF-β and EGF. TGF-ß stimulates the synthesis of collagen and fibronectin in fibroblasts; in addition, they are chemo attractants for the monocytes. PDGF is a chemoattractant and mitogen for both RPE and glial cells.[8,47,48]
Octreotide inhibits DNA synthesis stimulated by IGF-1 and cell proliferation beside hormone excretion centrally in some tissues and at the same time IGF-1 excretion peripherally, and it regulates cell proliferation and secretion directly by affecting at least 3 different signal transmissions.[49,50] In a study on rats, it was observed that IGF-1 is effective in especially the proliferative phase of premature retinopathy, which is one of the vitreoretinal disorders characterized with proliferation.[37] Also, it was demonstrated that in the studies performed in vitro, IGF-1 increases the contraction stimulating effect of Muller cells together with PDGF.[36,38] In another in vitro study by Mukherjee et al.,[51] it was shown that IGF-1 have the stimulating effect for traction on RPE cells, and it was considered that IGF-1 may have a significant physiopathological role in fibro-contractile disorders like PVR. In the light of this issue, the IGF-1 levels and octreotide efficacy in retina were analyzed in animals, in which PVR was induced in this study. As expected, a significant increase was observed in IGF-1 levels in the sham group in comparison with the control group. This finding supports the potential role of IGF-1 in PVR. However, when the treatment and sham groups were compared, the expected decrease in IGF-1 level was not observed. The fact that the values of the treatment group were not close to those of the control group, we considered that intravitreally applied octreotide had no effect on IGF-1. The fact that octreotide could not maintain the expected inhibition on IGF-1 levels, the non-toxic 1 mg intravitreal dose is thought not to be sufficient for the inhibitory effect on IGF-1. Thus, further studies should be carried out in order to examine the effect of octreotide in higher doses on IGF-1 inhibition.
In various studies, it was demonstrated that TGF- ß levels were high in the vitreous of the eyes with PVR and that these elevations were in accordance with the degree of intraocular fibrosis in various diseases such as PVR. Therefore, TGF- ß has a critical role in disorders, which appear with fibrosis.[26–30,46,52,53] In some studies, it was reported that octreotide decreases wound healing response similar to corticosteroids and mitomycin.[54,55] In this the present study, it was observed that there was an increase in the TGF- ß levels of the animals in which PVR was formed; however, this increase was not found to be significant in comparison with the control group. The fact that TGF- ß levels increase in accordance with fibrosis degree, has been shown in previous studies, leads one to think that the experimental PVR model applied in this study may have led to a lower level of fibrosis when compared to previous ones. Although no significant differences were observed in the comparisons between the treatment, control and sham groups, numerically the treatment group values were found to be highly close to control group, and the sham group values were found to be higher than these 2 groups. This shows that the intravitreally applied octreotide in treatment group has inhibiting effect over TGF- ß levels, yet this effectiveness is limited.
The fact that somatostatin inhibits growth factor effects by maintaining activation of tyrosine phosphatase enzyme, which establishes inactivation of growth factors, makes one think that it will also have an inhibiting effect over PDGF, which is a growth factor.[12,13] PDGF is a significant mitogen, chemo attractant and mediator, playing a role in cellular contraction, therefore, it has a key role in PVR pathogenesis.[30–35] In this study, when compared to the control group, a significant increase was observed in the PDGF levels of animals in which PVR was formed. This is a supportive finding on the potential role of PDGF in PVR pathogenesis. In an in vitro study by Amann et al., it was demonstrated that octreotide significantly decreases RPE proliferation stimulated by PDGF.[56] However, there has been no in vivo study on the inhibiting effect of octreotide on PDGF. In this study, a significant decrease was observed in PDGF values of the treatment group in comparison with sham group. The fact that there was no significant difference between the treatment group levels and control group levels, leads one to consider that intravitreally applied octreotide at this dose has an inhibitory effect on PDGF levels.
In conclusion, it was found that intravitreally applied octreotide at a dose of 1 mg has no effect over IGF 1, yet has a partial effect on TGF-ß and a highly strong effect on PDGF. In this study, it was concluded that octreotide may inhibit PVR effectively. Further studies are needed for the usage of intravitreal octreotide in the PVR prophylaxis.
Acknowledgements
Involved in conduct of study (B.T., O.E.); collection of data, typing and editing of manuscript and preparation, review, or approval of the manuscript (B.T., O.E., U.C., K.A.). All authors read and approved the final manuscript.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Glaser BM, Lemor M. Pathobiology of Proliferative Vitreoretinopathy. In: Ryan SJ, editor. Retina. 2nd ed. St Lous: CV Mosby; 1994. pp. 2249–63. [Google Scholar]
- 2.Miller B, Miller H, Patterson R, Ryan SJ. Effect of the vitreous on retinal wound-healing. Graefes Arch Clin Exp Ophthalmol. 1986;224:576–9. doi: 10.1007/BF02154747. [DOI] [PubMed] [Google Scholar]
- 3.Peyman GA, Shulman JA. East Norwalk: Appleton and Lange; 1995. Vitreous Substitutes. [Google Scholar]
- 4.Scott JD. Pathogenesis of prolifeative vitreoretinopathy with analisis of events leading to recurrent retinal detachment. In: Heimann K, Wiedemann P, editors. Proliferative Vitreoretinopathy. Heilderberg: Kaden; 1989. pp. 150–3. [Google Scholar]
- 5.Weller M, Wiedemann P, Heimann K. Proliferative Vitreoretinopathy. Is it anything more than wound healing at the wrong place? Int Ophthalmol. 1990;14:105–17. doi: 10.1007/BF00154210. [DOI] [PubMed] [Google Scholar]
- 6.Wiedemann P, Weller M. The Pathophysiology of proliferative vitreoretinopathy. Acta Ophtalmol Suppl. 1988;189:3–15. [PubMed] [Google Scholar]
- 7.Charteris DG, Sethi CS, Lewis GP, Fisher SK. Proliferative vitreoretinopathy-developments in adjunctive treatment and retinal pathology. Eye (Lond) 2002;16:369–74. doi: 10.1038/sj.eye.6700194. [DOI] [PubMed] [Google Scholar]
- 8.Charteris DG. Proliferative vitreoretinopathy: Pathobiology, surgical management and adjunctive treatment. Br J Ophthalmol. 1995;79:953–60. doi: 10.1136/bjo.79.10.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezzat S, Mehmed S. Are Patients with acromegaly at increased risk for neoplasia? J Clin Endocrinol Metab. 1991;72:245–9. doi: 10.1210/jcem-72-2-245. [DOI] [PubMed] [Google Scholar]
- 10.Spraul CW, Kawen CK, Kampmeier JK, Lang GK, Lang GE. Effect of thalidomide, octreotide, and prednisolone on the migration and proliferation of RPE cells in vitro. Curr Eye Res. 1999;19:483–90. doi: 10.1076/ceyr.19.6.483.5281. [DOI] [PubMed] [Google Scholar]
- 11.Baldysiak-Figiel A, Lang GK, Kampmeier J, Lang GE. Octreotide prevents growth factor induced proliferation of bovine retinal endothelial cells under hypoxia. Journal of Endocrinology. 2004;180:417–24. doi: 10.1677/joe.0.1800417. [DOI] [PubMed] [Google Scholar]
- 12.Cool DE, Andreassen PR, Tonks NK, Krebs EG, Fischer EH, Morgolis RL. Cytokinetic failure and asynchronous nuclear division in BHK cells overexpressing a truncated protein-tyrosine-phosphates. Proc Natl Acad Sci. 1992;89:5422–6. doi: 10.1073/pnas.89.12.5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant MB, Wargovich TJ, Ellis EA, Caballero BS, Mansour M, Pepine CJ. Localization of insulin-like growth factor-I and inhibition of coronary smooth muscle cell growth by somatostatin analogues in human coronary smooth muscle cells. Circulation. 1994;89:1511–7. doi: 10.1161/01.cir.89.4.1511. [DOI] [PubMed] [Google Scholar]
- 14.Luo Q, Peyman GA, Conway MD, Woltering EA. Effect of somatostatin analog (octreotide acetate) on the growth of retinal pigment epithelial cells in culture. Curr Eye Res. 1996;15:909–13. doi: 10.3109/02713689609017634. [DOI] [PubMed] [Google Scholar]
- 15.Mallet B, Vialettes B, Haroche S, Escoffier P, Gastaut P. Stabilization of severe proliferative diabetic retinopathy by long-term treatment with SMS 201-995. Diabet and Metabolism. 1992;18:438–44. [PubMed] [Google Scholar]
- 16.Casani G, Catalani E, Dal Monte M, Bagnoli P. Functional aspects of the somatostatinergic system in the retina and the potential therapeutic role of somatostatin in retinal disease. Histol Histopathol. 2005;20:615–32. doi: 10.14670/HH-20.615. [DOI] [PubMed] [Google Scholar]
- 17.Qu Y, Zhang S, Xu X, Wang H, Li J, Zhou F, et al. Octreotide inhibits choroidal neovascularization in rats. Ophthalmic Res. 2009;42:36–42. doi: 10.1159/000219683. [DOI] [PubMed] [Google Scholar]
- 18.Spraul CW, Baldysiak-Figiel A, Lang GK, Lang GE. Octreotide inhibits growth factor-induced bovine choriocapillary endothelial cells in vitro. Graefes Arch Clin Exp. 2002;240:227–31. doi: 10.1007/s00417-002-0441-7. [DOI] [PubMed] [Google Scholar]
- 19.Meng RH, Yang L, Sun L, Zhang WY. The experimental study of octreotide suppressing retinal neovascularization. Zhonghua Yan Ke Za Zhi. 2005;41:423–7. [PubMed] [Google Scholar]
- 20.Weill CL. Somatostatin prevents natural motoneuron cell death in embriyonic chick spinal cord. Dev Neurosci. 1991;13:377–81. doi: 10.1159/000112188. [DOI] [PubMed] [Google Scholar]
- 21.Celiker U, Ilhan N, Ozercan I, Demir T, Celiker H. Octreotide reduces ischaemia-reperfusion injury in the retina. Acta Ophthalmol Scand. 2002;80:395–400. doi: 10.1034/j.1600-0420.2002.800409.x. [DOI] [PubMed] [Google Scholar]
- 22.Celiker U, Ilhan N. Nitric oxide and octreotide in retinal ischemia-reperfusion injury. Doc Ophthalmol. 2002;105:327–38. doi: 10.1023/a:1021243126512. [DOI] [PubMed] [Google Scholar]
- 23.Krassas GE, Dumas A, Pontikides N, Kaltsas T. Somatostatin receptor scintigraphy and octreotide treatment in patients with thyroid eye disease. Clin Endocrinol Oxf. 1995;42:571–80. doi: 10.1111/j.1365-2265.1995.tb02682.x. [DOI] [PubMed] [Google Scholar]
- 24.Schultz G, Khaw PT, Oxford K, MaCauley S, Van Setten G, Chegini N. Growth factors and ocular wound healing. Eye (Lond) 1994;8:184–7. doi: 10.1038/eye.1994.43. [DOI] [PubMed] [Google Scholar]
- 25.Baudouin C, Fredj-Reygrobellet D, Brignole F, Negre F, Lapalus P, Gastaud P. Growth factors in vitreous and subretinal fluid cells from patients with proliferative vitreoretinopathy. Ophthalmic Res. 1993;25:52–9. doi: 10.1159/000267221. [DOI] [PubMed] [Google Scholar]
- 26.Pfeffer BA, Flanders KC, Guerin CJ, Danielpour D, Anderson DH. Transforming growth factor beta 2 is the predominant isoform in the neural retina, retinal pigment epithelium-choroid and vitreous of the monkey eye. Exp Eye Res. 1994;59:323–3. doi: 10.1006/exer.1994.1114. [DOI] [PubMed] [Google Scholar]
- 27.Bornstein P. Thrombospondins as matricelllular modulators of cell function. J Clin Invest. 2001;107:929–34. doi: 10.1172/JCI12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinton DR, He S, Jin ML, Barron E, Ryan SJ. Novel growth factors involved in the pathogenesis of proliferative vitreoretinopathy. Eye (Lond) 2002;16:422–8. doi: 10.1038/sj.eye.6700190. [DOI] [PubMed] [Google Scholar]
- 29.Jester JV, Huang J, Petroll WM, Cavanagh HD. TGF beta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGF beta, PDGF and integrin signaling. Exp Eye Res. 2002;75:645–57. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- 30.Hinton DR, He S, Graf K, Yang D, Hsueh WA, Ryan SJ, et al. Mitogen activated protein kinase activation mediates PDGF directed migration of RPE cells. Exp Cell Res. 1998;239:11–5. doi: 10.1006/excr.1997.3873. [DOI] [PubMed] [Google Scholar]
- 31.Limb GA, Hollifield RD, Webster L, Charteris DG, Chignell AH. Soluble TNF receptors in vitreoretinal proliferative disease. Invest Opthalmol Vis Sci. 2001;42:1586–91. [PubMed] [Google Scholar]
- 32.Campochiaro PA, Sugg R, Grotendorst G, Hjelmeland LM. Retinal pigment epithelial cells produce PDGF- like proteins and secrete them into their media. Exp Eye Res. 1989;49:217–27. doi: 10.1016/0014-4835(89)90092-4. [DOI] [PubMed] [Google Scholar]
- 33.Andrews A, Balciunaite E, Leong FL, Tallquist M, Soriano P, Refojo M, et al. Platelet-derived growth factor plays a key role in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1999;40:2683–9. [PubMed] [Google Scholar]
- 34.Ikuno Y, Leong FL, Kazlauskas A. Attenuation of experimental proliferative vitreoretinopathy by inhibiting the platelet-derived growth factor receptor. Invest Opthalmol Vis Sci. 2000;41:3107–16. [PubMed] [Google Scholar]
- 35.Seo MS, Okamoto N, Vinores MA, Vinores SA, Hackett SF, Yamada H, et al. Photoreceptor-specific expression of platelet-derived growth factor-B results in traction retinal detachment. Am J Pathol. 2000;157:995–1005. doi: 10.1016/S0002-9440(10)64612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardwick C, Feist R, Morris R, White M, Angus R, Guidry C. Tractional force generation by porcine muller cells stimulation by growth factors in human vitreous. Invest Ophthalmol Vis Sci. 1997;38:2053–63. [PubMed] [Google Scholar]
- 37.Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med. 1999;5:1390–5. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- 38.Guidry C, Mamballikalathil I, Mann C. Tractional force generation by porcine muller cells development and differential stimulation by growth factors. Invest Ophthalmol Vis Sci. 1997;38:456–68. [PubMed] [Google Scholar]
- 39.Frenzel E, Neely KA, Walsh AW, Cameron JD. A new model of proliferativevitreoretinopathy. Invest Ophthalmol Vis Sci. 1998;39:2157–64. [PubMed] [Google Scholar]
- 40.Cantó Soler MV, Gallo JE, Dodds RA, Suburo AM. A mouse model of proliferative vitreoretinopathy induced by dispase. Exp Eye Res. 2002;75:491–504. doi: 10.1006/exer.2002.2031. [DOI] [PubMed] [Google Scholar]
- 41.Liang C, Peyman GA, Conway MD, Woltering EA. Retinal toxicity of intravitreous octreotide in the rabbit. Can J Ophthalmol. 1997;32:229–32. [PubMed] [Google Scholar]
- 42.Robertson JE, Westra I, Woltering EA, Winthrop KL, Barrie R, O'Dorisio TM, et al. Intravitreal injection of octreotide acetate. J Ocul Pharmacol Ther. 1997;13:171–7. doi: 10.1089/jop.1997.13.171. [DOI] [PubMed] [Google Scholar]
- 43.Stern WH, Guerin CJ, Erickson PA, Levvis GP, Anderson DH, Fisher SK. Ocular toxicity of fluorouracil after vitrectomy. Am J Ophthalmol. 1983;96:43–51. doi: 10.1016/0002-9394(83)90453-1. [DOI] [PubMed] [Google Scholar]
- 44.Steinhorst UH, Hatchell DL, Chen EP, Machemer R. Ocular toxicity of daunomycin: Effects of subdivided doses on the rabbit retina after vitreous gas compression. Graefe's Arch Clin Exp Ophthalmol. 1993;231:591–4. doi: 10.1007/BF00936524. [DOI] [PubMed] [Google Scholar]
- 45.Imai K, Loevvenstein A, Koroma B, Grebe R, de Juan EJ. Herbimycin A in the treatment of experimental proliferative vitreoretinopathy: Toxicity and efficacy studies. Graefes Arch Clin Exp Ophthalmol. 2000;238:440–7. doi: 10.1007/s004170050376. [DOI] [PubMed] [Google Scholar]
- 46.Günal AI, Duman S, Sen S, Unsal A, Terzioğlu E, Akçiçek F, et al. By reducing TGF beta 1, octreotide lessens the peritoneal derangements induced by a high glucose solution. J Nephrol. 2001;14:184–9. [PubMed] [Google Scholar]
- 47.Ryan SJ. The pathophysiology of proliferative vitreoretinopathy in its management. Am J Ophthalmol. 1985;100:188–93. doi: 10.1016/s0002-9394(14)75004-4. [DOI] [PubMed] [Google Scholar]
- 48.Pastor JC, de la Rua ER, Martin F. Proliferative vitreoretinopathy: Risk factors and pathobiology. Prog Ret Eye Res. 2002;21:127–44. doi: 10.1016/s1350-9462(01)00023-4. [DOI] [PubMed] [Google Scholar]
- 49.Chen F, O'Dorisio MS, Hermann G, Hayes J, Malarkey WB, O'Dorisio TM. Mechanisms of action of long-acting analogs of somatostatin. Regul Pept. 1993;44:285–95. doi: 10.1016/0167-0115(93)90138-x. [DOI] [PubMed] [Google Scholar]
- 50.Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ. Octreotide. N Engl J Med. 1996;334:246–54. doi: 10.1056/NEJM199601253340408. [DOI] [PubMed] [Google Scholar]
- 51.Mukherjee S, Guidry C. The Insulin-Like Growth Factor System Modulates Retinal Pigment Epithelial Cell Tractional Force Generation. Invest Ophthalmol Vis Sci. 2007;48:1892–9. doi: 10.1167/iovs.06-1095. [DOI] [PubMed] [Google Scholar]
- 52.Günal AI, Iºik A, Celiker H, Eren O, Celebi H, Günal SY, et al. Short term reduction of ventricular mass in primary hypertrophic cardiomyopathy by octreotide injections. Heart. 1996;76:418–21. doi: 10.1136/hrt.76.5.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cunliffe IA, Rees RC, Rennie , IG The effect of TGF-beta 1 and TGF-beta 2 on the proliferation of human Tenon's capsule fibroblasts in tissue culture. Acta Ophthalmol Scand. 1996;74:31–5. doi: 10.1111/j.1600-0420.1996.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 54.Cordeiro MF, Gay JA, Khaw PT. Human anti-transforming growth factor-beta2 antibody: A new glaucoma anti-scarring agent. Invest Ophthalmol Vis Sci. 1999;40:2225–34. [PubMed] [Google Scholar]
- 55.Demir T, Turgut B, Celiker U, Ozercan I, Ulas F, Akyol N. Effects of octreotide acetate and amniotic membrane on wound healing in experimental glaucoma surgery. Doc Ophthalmol. 2003;107:87–92. doi: 10.1023/a:1026257227194. [DOI] [PubMed] [Google Scholar]
- 56.Amann J, Kaven C, Spraul CW, Lang GK, Lang GE. Effect of octreotide combined with growth factors on proliferation of RPE cells in vitro. Ophthalmologe. 2000;97:737–41. doi: 10.1007/s003470070020. [DOI] [PubMed] [Google Scholar]


