Abstract
Objectives
This study evaluated the association of long- and short-term air pollutant exposures with flow-mediated dilation (FMD) and baseline arterial diameter (BAD) of the brachial artery using ultrasound in a large multicity cohort.
Background
Exposures to ambient air pollution, especially long-term exposure to particulate matter <2.5 μm in aerodynamic diameter (PM2.5), are linked with cardiovascular mortality. Short-term exposure to PM2.5 has been associated with decreased FMD and vasoconstriction, suggesting that adverse effects of PM2.5 may involve endothelial dysfunction. However, long-term effects of PM2.5 on endothelial dysfunction have not been investigated.
Methods
FMD and BAD were measured by brachial artery ultrasound at the initial examination of the Multi-Ethnic Study of Atherosclerosis. Long-term PM2.5 concentrations were estimated for the year 2000 at each participant’s residence (n = 3,040) using a spatio-temporal model informed by cohort-specific monitoring. Short-term PM2.5 concentrations were based on daily central-site monitoring in each of the 6 cities.
Results
An interquartile increase in long-term PM2.5 concentration (3 μg/m3) was associated with a 0.3% decrease in FMD (95% confidence interval [CI] of difference: −0.6 to −0.03; p = 0.03), adjusting for demographic characteristics, traditional risk factors, sonographers, and 1/BAD. Women, nonsmokers, younger participants, and those with hypertension seemed to show a greater association of PM2.5 with FMD. FMD was not significantly associated with short-term variation in PM2.5 (−0.1% per 12 μg/m3 daily increase [95% CI: −0.2 to 0.04] on the day before examination).
Conclusions
Long-term PM2.5 exposure was significantly associated with decreased endothelial function according to brachial ultrasound results. These findings may elucidate an important pathway linking air pollution and cardiovascular mortality.
Keywords: air pollution, atherosclerosis, cardiovascular mortality, endothelial function, flow-mediated dilation, traffic
Air pollution is a complex mixture of particulate matter, volatile organic compounds, and gaseous pollutants such as oxides of nitrogen. Epidemiological analyses have demonstrated an association between short- and long-term exposures to air pollution, especially particulate matter <2.5 μm in aerodynamic diameter (PM2.5) and increased cardiovascular morbidity and mortality (1,2). It is postulated that this adverse cardiovascular effect is related to systemic inflammation, oxidative stress, or autonomic nervous system imbalance effects in the artery wall (3). Hence, one of the intermediate steps by which PM2.5 exposure increases cardiovascular morbidity and mortality may be via functional changes in the endothelium–smooth muscle complex.
Previous studies have shown associations between short-term exposure to PM2.5 and nitric oxide (NO)-mediated endothelial dysfunction measured by using forearm plethysmography (4) or flow-mediated dilation (FMD) using brachial ultrasound (5,6). In a study of healthy volunteers situated for 2 h at different urban bus stops, a 30-μg/m3 increase in PM2.5 exposure corresponded to a 0.5% reduction in FMD (7). In contrast, other experimental studies have shown associations with vasoconstriction measured as a decrease in baseline arterial diameter (BAD) but not FMD (8,9). These studies provide different conclusions: PM2.5 exposure may primarily affect NO-mediated endothelial dysfunction or it may alter elaboration of vasoconstrictors.
Observations from short-term exposure studies provide only limited insight into effects of pollutants and mechanisms. Are the vascular responses transient or do lasting effects accrue after long-term exposure? We hypothesized that repeated short-term insults to the vasculature result in persistent endothelial dysfunction related to long-term exposures. To test this hypothesis, we investigated the relationship between PM2.5 exposures and changes in the brachial artery in the MESA (Multi-Ethnic Study of Atherosclerosis) cohort. Leveraging the detailed exposure assignment of the MESA Air Pollution (MESA Air) study, we examined whether long- and short-term exposure to PM2.5 is associated with decreased FMD and/or decreased BAD in this cohort.
Methods
Study design
MESA is a prospective study designed to investigate the prevalence and progression of subclinical atherosclerosis in participants aged 45 to 84 years who were free of clinical cardiovascular disease at the time of enrollment (10). The study includes 6,814 participants of white, African American, Hispanic, and Chinese descent from 6 US communities. The institutional review boards of each study site approved this study, and all participants gave written informed consent. Demographic characteristics, medical history, anthropometry, laboratory data, and brachial ultrasound measurements for the current analysis were taken at the first examination (July 2000 to August 2002). For this study, we included all participants with reliable brachial ultrasound measurements for whom complete covariate information and exposure estimates were available (N = 3,040).
Vascular outcomes
The brachial examination procedure, inclusion and exclusion criteria, and reproducibility measures are reported in detail elsewhere (11). Briefly, participants were examined in the supine position after at least a 6-h fast and 15 min of rest. A standard blood pressure cuff was positioned around the right arm, 2 inches below the antecubital fossa, and the brachial artery was imaged 5 to 9 cm above the antecubital fossa by using a linear-array multifrequency transducer operating at 9 MHz and the LOGIQ 700 ultrasound (General Electric Logic 700 Device General Electric Medical Systems, Waukesha, Wisconsin). After the baseline images were obtained continuously for 30 s to obtain the BAD, the cuff was inflated to 50 mm Hg for 5 min above the participant’s systolic blood pressure (SBP). After cuff deflation, the maximum arterial diameter (MAD) was measured. Image analysis was performed at the Wake Forest University Cardiology Image Processing Laboratory by using a previously validated semiautomated system that uses media-adventitial interfaces (12).
BAD (in millimeters) and FMD% were our main outcome measures and are based on recent guidelines (13). The number of sonographers acquiring images varied between the cities, ranging from 1 in St. Paul to 9 in Chicago. Each sonographer underwent central training, performed a supervised examination, and acquired at least 5 examinations acceptable to the core laboratory before certification. Intrareader reproducibility for BAD and FMD, evaluated by comparing an original and a blinded quality control reread of ultrasounds from 40 participants, was reported previously (11).
Exposure assessment. LONG-TERM PM2.5 EXPOSURE ASSESSMENT
Estimates of long-term average ambient PM2.5 concentrations were computed for each participant by using a hierarchical spatio-temporal model fit with an estimation procedure described elsewhere (14–16). Monitoring data came from the U.S. Environmental Protection Agency’s Air Quality System regulatory monitoring stations supplemented by several fixed-site monitoring stations in each MESA community and home-monitoring at 10% of MESA participant homes, in campaigns specifically deployed for the MESA Air cohort (17). The hierarchical model decomposed the space-time field of concentrations into 3 components: 1) spatially varying long-term averages; 2) spatially varying seasonal and long-term trends; and 3) spatially correlated but temporally independent residuals. The spatially varying long-term averages and seasonal trends were modeled by using land-use regression and spatial smoothing of residuals using universal kriging. A large suite of spatial covariates such as proximity to major roadways and local land-use were used as predictors in the universal kriging models, after dimension reduction by partial least-squares. The spatially correlated but temporally independent residuals were predicted by using ordinary kriging. These components were combined to predict concentrations at each subject’s home location for every 2-week period starting in January 1999. An average of exposures for the calendar year 2000 were used for this analysis. We used ArcGIS 9.1 software (Esri, Redlands, California); Dynamap/2000 street network and geocoding database (TeleAtlas, Boston, Massachusetts) were used to determine participants’ residential addresses.
SHORT-TERM PM2.5 EXPOSURE ASSESSMENT
We assessed each participant’s short-term exposure to PM2.5 concentrations on the day of the examination (day 0), 1 day prior, 2 days prior, and the average of days 0 to 2. Daily average PM2.5 concentrations were derived from an air quality system central-site monitoring station with complete daily average information available for the study city during the examination period (14).
COVARIATES
A number of covariates were considered in the multivariate analysis. These included age, gender, ethnicity, education, income, body surface area, smoking status, alcohol consumption, dietary fat intake, emotional distress derived from a standardized anxiety scale, and physical activity. We also adjusted for waist to hip ratio, blood glucose, SBP, diastolic blood pressure, high-density lipoprotein, total cholesterol, triglycerides, homocysteine, fibrinogen, and C-reactive protein. We also adjusted for specific medications, including anti-inflammatory agents, antihypertensives, lipid-lowering medications, and antioxidants (vitamin C).
Statistical analysis
Using Stata version 10.1 (Stata Corp., College Station, Texas) and SAS version 9.1.3 (SAS Institute, Inc., Cary, North Carolina), summary statistics were calculated and analyses performed. We tested for differences between the full cohort and those with complete data by using a t test for continuous covariates and chi-square test for the categorical covariates. Linear regression modeling was performed to examine associations between PM2.5 estimates and the main vascular outcomes. Because the sonographers vary by study location and can influence these outcomes, the analyses were adjusted by using an indicator variable for sonographer. Each sonographer worked in only 1 study site; in effect, our analysis controls for study site as well.
The ratio outcome (FMD%) requires careful statistical consideration because the denominator (BAD) may itself be associated with the exposure. In our primary approach, we included 1/BAD as a covariate in regression models to obtain unbiased effect estimates and to increase precision (18). We also evaluated the FMD% outcome without adjusting for 1/BAD and have referred to it as “FMD% without adjustment for 1/BAD.” In a sensitivity analysis, we also used simple extent of dilation (“FMDmm”), calculated as MAD-BAD, as reported in the Framingham Heart Study (13,19). Results are reported per interquartile range (IQR) change in PM2.5.
The role of all the aforementioned covariates, including secondhand smoke exposures, family history of myocardial infarction, serum cotinine, and forced expiratory volume in 1 s, were examined. However, these were not included in the final model due to a large amount of missing data.
To control for temporal and meteorological confounding in short-term analyses, B-splines were used for city-specific trends in calendar time (12 df/year), temperature (6 df), and relative humidity (6 df) and included a city-specific day of the week indicator. In addition, long- and short-term exposures were evaluated jointly, including the temporal and meteorological confounders.
In another sensitivity analysis, the final model was not controlled for sonographer (or city) to determine between-city estimates. In separate city-specific analyses, the influence of within-city exposure contrasts was estimated in the final model controlled for sonographer. Differential susceptibility was investigated by stratifying for age categories, gender, ethnicity, diabetes mellitus status, hypertension categories (using the criteria of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, as derived from the blood pressure variables from examination 1) (20), antihypertensive use, obesity (obese if body mass index [BMI] ≥30 kg/m2), smoking status, and residential stability (people who lived in the same address for ≥5 versus <5 years’ duration). In addition, we tested for interaction for age, BMI, SBP, and diastolic blood pressure as continuous variables.
The effect of lipid-lowering medications and the antihypertensive medications classified as angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, beta-blockers, calcium channel blockers, diuretics, and vasodilators was investigated. Analysis was conducted by using stratification and by evaluating effect modification through interaction terms for the respective categories.
Results
Characteristics of the study population
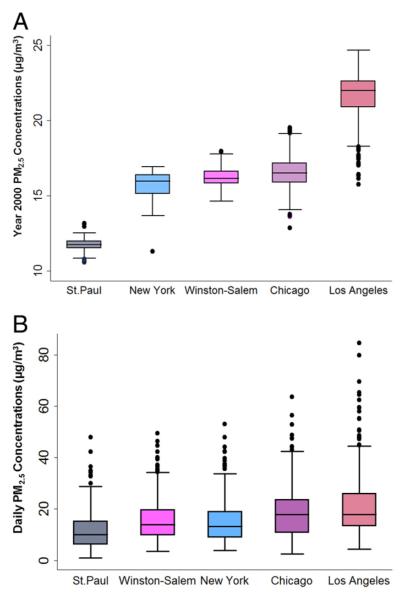
Of the 6,814 MESA participants, only a subset of images from the brachial ultrasound examinations was analyzed due to quality control and funding reasons and also only in 5 of the 6 MESA study sites. Similar to the main cohort, our study population of 3,040 subjects included 50% female patients and 18% with less than a high school education. The exclusion of 1 site resulted in a lower proportion of African Americans and higher proportion of Chinese Americans than in the overall MESA cohort. Fifteen percent of the study population used lipid-lowering drugs, and 34% were treated for hypertension (Table 1). Approximately 21% of the participants had moved residential location within 5 years before their brachial examination. Estimated long-term PM2.5 concentrations ranged from 10.6 to 24.7 μg/m3, with an IQR of 3 μg/m3. Short-term PM2.5 concentrations ranged from 1 to 74 μg/m3, with an IQR of 12 μg/m3 (Fig. 1). The mean BAD and FMD% in this cohort were 4.3 ± 0.8 mm and 4.4 ± 2.8%, respectively (Table 2).
Table 1.
Demographic and Health Characteristics of the MESA Participants During Examination 1 (2000–2002)
| Characteristic | All Participants (N = 6,814) |
Participants With Complete Data (n = 3,040) |
p Value |
|---|---|---|---|
| Age (yrs) | 62.2 ± 10.2 | 61.2 ± 9.9 | <0.001 |
| Systolic blood pressure (mm Hg) | 126.6 ± 21.5 | 125.5 ± 19.7 | <0.001 |
| Diastolic blood pressure (mm Hg) | 72.0 ± 10.0 | 72.2 ± 10.3 | 0.45 |
| Body mass index (kg/m2) | 28.3 ± 5.5 | 27.7 ± 5.1 | 0.01 |
| Body surface area (m2) | 1.85 ± 2.3 | 1.86 ± 2.3 | <0.001 |
| Blood glucose (mg/dl)* | 97.5 ± 30.2 | 96.2 ± 28.1 | 0.05 |
| High-density lipoprotein (mg/dl)* | 50.9 ± 14.8 | 50.6 ± 14.6 | 0.3 |
| Total cholesterol (mg/dl)* | 194.2 ± 35.7 | 194.4 ± 34.9 | 0.6 |
| C-reactive protein (mg/l)* | 3.8 ± 5.9 | 3.4 ± 5.4 | 0.002 |
| Physical activity (MET min/week) | 807.3 ± 793.3 | 821.3 ± 842.3 | 0.4 |
| Less than high school education | 1,225 (18.0) | 559 (18.4) | 0.4 |
| Current alcohol users | 3,749 (55.4) | 1,681 (55.3) | 0.001 |
| Gender | |||
| Men | 3,601 (52.9) | 1,545 (50.8) | 0.06 |
| Women | 3,213 (47.2) | 1,495 (49.2) | |
| Ethnicity | |||
| White | 2,622 (38.5) | 1,023 (33.9) | <0.001 |
| Chinese American | 803 (11.8) | 605 (19.8) | |
| African American | 1,893 (27.8) | 641 (21.1) | |
| Hispanic | 1,496 (21.9) | 765 (25.1) | |
| Smoking status* | |||
| Never | 3,418 (50.3) | 1,654 (54.4) | 0.001 |
| Former | 2,487 (36.6) | 1,033 (33.9) | |
| Current | 887 (13.1) | 355 (11.6) | |
| Diabetes mellitus status (ADA 2003 criteria)* | |||
| Normal | 5,087 (74.9) | 2,340 (76.9) | |
| Impaired fasting glucose | 844 (12.4) | 369 (12.1) | 0.1 |
| Nontreated DM | 179 (2.6) | 71 (2.3) | |
| Treated DM | 680 (10.0) | 260 (8.5) | |
| Drug use | |||
| Antihypertensive medications* | 2,536 (37.2) | 1,042 (34.3) | 0.01 |
| Lipid-lowering drugs* | 1,100 (16.1) | 465 (15.3) | 0.3 |
Values are mean ± SD or n (%), and p values were obtained from a t test or chi-square test for difference between all Multi-Ethnic Study of Atherosclerosis (MESA) participants and those with complete data.
Indicates the covariates for which the total number of available participants was less due to missing information.
ADA = American Diabetes Association; DM = diabetes mellitus; MET = metabolic equivalent.
Figure 1. Distribution of the PM2.5 Concentrations.
(A) Long-term particulate matter <2.5 μm in aerodynamic diameter (PM2.5) concentrations estimated for the year 2000 annual average. (B) Short-term PM2.5 concentrations for 2 days before the brachial examination. Similar distribution pattern was seen for all other averaging time points.
Table 2.
Distribution of Brachial Artery Outcomes and Sonographers Among the MESA Participants Included in the Final Study Model (N = 3,040)
| Outcome | Chicago (n = 681) |
Los Angeles (n = 800) |
New York (n = 584) |
St. Paul (n = 473) |
Winston-Salem (n = 502) |
Total (N = 3,040) |
|---|---|---|---|---|---|---|
| Baseline arterial diameter (mm) | 4.2 ± 0.8 | 4.4 ± 0.8 | 4.4 ± 0.8 | 4.3 ± 0.9 | 4.3 ± 0.9 | 4.3 ± 0.8 |
| Flow-mediated dilation (mm) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Flow-mediated dilation (%) | 4.8 ± 2.9 | 4.2 ± 2.5 | 4.2 ± 2.6 | 5.0 ± 3.2 | 4.0 ± 2.9 | 4.4 ± 2.8 |
| No. of sonographers | 9 | 5 | 7 | 1 | 2 | 24 |
Abbreviation as in Table 1.
Long-term exposure to PM2.5 and its association with reduced NO-mediated endothelial function
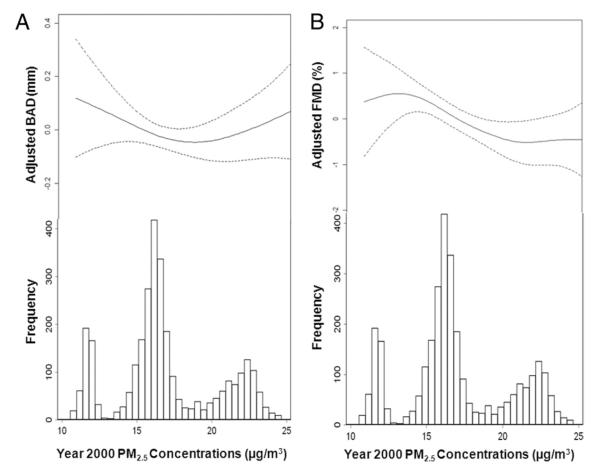
We found a significant inverse association between long-term PM2.5 concentrations and FMD% but not with BAD. For every 3-μg/m3 increase in the annual average of PM2.5, FMD% decreased by 0.3% (95% CI: −0.6 to −0.03; p = 0.03) independent of cardiovascular risk factors (Table 3, Fig. 2). A weaker but highly statistically significant relationship was observed between long-term PM2.5 concentrations and FMD% (−0.1% [95% CI: −0.2 to −0.04]; p = 0.005) without adjustment for sonographer or city (Online Table 1). Exclusion of sonographers who performed 1 to 2 examinations did not affect these estimates. Restricting analysis to those with a stable residential address for ≥5 years did not affect the association (Online Table 2). In sensitivity analyses using the FMD% without adjustment for 1/BAD (−0.3% [95% CI: −0.5 to 0.01]; p = 0.06) or the simple FMDmm (−0.01 mm [95% CI: −0.02 to 0.001]; p = 0.07), this negative association persisted.
Table 3.
Associations Between Brachial Artery Outcomes and the Long-Term Exposures to PM2.5
| Final Model | BAD (mm) |
FMD (%) |
||
|---|---|---|---|---|
| Final Model | β-Coefficient (95% CI) | p Value | β-Coefficient (95% CI) | p Value |
| Total population (n = 3,040) | −0.002 (−0.06 to 0.06) | 1.0 | −0.3 (−0.6 to −0.03) | 0.03 |
| Chicago (n = 681) | −0.1 (−0.3 to 0.002) | 0.05 | −0.6 (−1.1 to 0.01) | 0.05 |
| Los Angeles (n = 800) | 0.1 (−0.02 to 0.2) | 0.13 | −0.2 (−0.6 to 0.2) | 0.3 |
| New York (n = 584) | −0.2 (−0.4 to −0.02) | 0.03 | −0.3 (−1.1 to 0.4) | 0.4 |
| St. Paul (n = 473) | 0.02 (−0.4 to 0.5) | 0.9 | 0.2 (−1.9 to 2.4) | 0.8 |
| Winston-Salem (n = 502) | 0.2 (−0.1 to 0.5) | 0.2 | −0.1 (−1.5 to 1.3) | 0.9 |
All associations reported for baseline arterial diameter (BAD) as millimeters and for flow-mediated dilation (FMD) as percentage per interquartile range of 3 μg/m3 in particulate matter <2.5 μm in aerodynamic diameter (PM2.5) for the long-term exposure. Effect estimates (β-coefficients) and 95% confidence intervals (CIs) are shown for these associations derived from the multiple regression modeling. Final model includes age, gender, body surface area, sonographer, income, education, smoking, alcohol use, dietary fat intake, emotional distress, physical activity, waist to hip ratio, systolic and diastolic blood pressure, total cholesterol, high-density lipoprotein, C-reactive protein, fibrinogen, homocysteine, fasting blood glucose, anti-inflammatory agents, antihypertensive agents, lipid-lowering drugs, and ascorbate (also 1/BAD for FMD). p Values are based on the final model of the multiple regression analysis controlled for all the these covariates for the total population and the city-specific analysis. Interaction testing did not reveal any significant effect modification for the city-specific analysis.
Figure 2. Dose-Response Associations Between Brachial Outcomes and Modeled Long-Term Pollutant Concentrations After Controlling for Covariates.
The values for (A) baseline arterial diameter (BAD) and (B) flow-mediated dilation (FMD) represent partial residuals from a final model controlled for age, gender, ethnicity, body surface area, sonographer, income, education, smoking, alcohol use, dietary fat intake, emotional distress, physical activity, waist to hip ratio, systolic blood pressure, diastolic blood pressure, total cholesterol, high-density lipoprotein, C-reactive protein, fibrinogen, homocysteine, fasting blood glucose, anti-inflammatory agents, antihypertensive agents, lipid-lowering drugs, and vitamin C. FMD% includes adjustment for 1/BAD. Data are plotted as penalized thin-plate regression splines with smoothness parameter selected by generalized cross-validation for BAD and FMD%.
Short-term PM2.5 concentrations were associated with a small but not statistically significant reduction in FMD% (−0.1% [95% CI: −0.2 to 0.04]; p = 0.4) and BAD (−0.01 mm [95% CI: −0.05 to 0.01]; p = 0.4) for the day 1 before examination when adjusted for risk factors, seasonality, and meteorology (Table 4). Associations with long-term PM2.5 exposure did not significantly change when short-term exposure estimates or temperature or season were included in the model (Online Table 3).
Table 4.
Association Between Short-Term PM2.5 Concentrations and the Brachial Artery Outcomes
| BAD (mm) |
FMD (%) |
|||
|---|---|---|---|---|
| Outcome | β-Coefficient (95% CI) | p Value | β-Coefficient (95% CI) | p Value |
| Day 0 (n = 2,695) | −0.02 (−0.06 to 0.01) | 0.1 | −0.06 (−0.2 to 0.08) | 0.4 |
| Day 1 (n = 2,715) | −0.01 (−0.05 to 0.01) | 0.4 | −0.10 (−0.2 to 0.04) | 0.2 |
| Day 2 (n = 2,709) | −0.001 (−0.04 to 0.04) | 0.9 | −0.08 (−0.2 to 0.05) | 0.2 |
| Average of 3 days (n = 2,550) | −0.02 (−0.06 to 0.02) | 0.3 | −0.1 (−0.3 to 0.05) | 0.3 |
All associations reported for BAD as millimeters and for FMD as percentage for a daily interquartile range increase of 12 μg/m3 of PM2.5 concentrations derived from central-site monitors on days 0, 1, and 2, and the average of these 3 days, before the brachial ultrasound examination. Effect estimates (β-coefficients) and 95% CIs are shown for these associations derived from the multiple regression modeling. Final model includes age, gender, body surface area, sonographer, income, education, smoking, alcohol use, dietary fat intake, emotional distress, physical activity, waist to hip ratio, systolic and diastolic blood pressure, total cholesterol, high-density lipoprotein, C-reactive protein, fibrinogen, homocysteine, fasting blood glucose, anti-inflammatory agents, antihypertensive agents, lipid-lowering drugs, and ascorbate (also 1/BAD for FMD%). In addition, we also included interaction for each city and day of the week as well as B-splines of temporal variables temperature (4 df/year), seasonality (12 df/year), and humidity (6 df/year) for these short-term exposures.
Abbreviations as in Table 3.
City-specific differences for vascular outcomes and PM2.5 association
Models stratified according to city demonstrated that the relationship between long-term PM2.5 concentration and reduced FMD% was consistently negative across the 5 communities, except for St. Paul, which had the lowest mean PM2.5 concentrations. Chicago participants showed the largest decrease in FMD% per IQR increase in PM2.5 (Table 3). Similar effect estimates were noted when sonographer was not included as a covariate.
Assessing effect modification of association between long-term PM2.5 exposure and NO-mediated endothelial dysfunction
A significant interaction was found between age and the association between long-term PM2.5 and the FMD% (p < 0.001) but not for BMI or blood pressure. Younger age was associated with a larger magnitude of effect of PM2.5 on reduced endothelial function (FMD% −0.5% [95% CI: −0.8 to −0.2%]; p = 0.001). Stratified analyses suggest that women, never smokers, participants with stages 1 and 2 hypertension, and those not taking antihypertensive medications tend to have a slightly greater negative association of PM2.5 with FMD% (Online Table 3). Although no category of medication usage demonstrated statistically significant effect modification, the association between long-term PM2.5 and FMD% seemed to be ameliorated by ACE inhibitor use (n = 163); there was a 0.3% increase (95% CI: −1.04 to 1.6) in FMD% per 3-μg/m3 annual increase in PM2.5 (Online Table 4).
Discussion
In this large, multisite, multiethnic cohort, a significant association was observed between long-term residential PM2.5 concentrations and NO-mediated endothelial dysfunction as assessed by FMD, independent of major cardiovascular risk factors. Although previous studies (5,6,21) have assessed the relation between short-term exposure to PM2.5 and endothelial function, this is the first investigation of the relation between long-term PM2.5 exposure and endothelial function. Our study provides unique insights into the mechanisms of long-term PM2.5 exposure and increased cardiovascular mortality.
Increasing age, SBP, BMI, and smoking are inversely related to FMD (19). The magnitude of the long-term effect of an IQR (difference between the 25th and 75th percentile) increase in PM2.5 on FMD% is comparable to the effect of 5 years’ increase in age, or of active tobacco smoking, in this population. A 3-μg/m3 increase in PM2.5 concentration occurs as a contrast in residential exposure between less polluted and more polluted areas in most major U.S. metropolitan areas. Taken together, these data suggest that PM2.5 exerts a clinically relevant degree of effect on endothelial dysfunction.
Endothelial dysfunction measured by using FMD is a precursor for atherogenesis (22) and is associated with hypertension (23), passive smoking (24), and cardiovascular events (11,25). Although the role of FMD as a predictor is still controversial, it is consistently associated with future cardiovascular events (26). In a nested case-cohort study using MESA data, abnormal FMD was predictive of incident cardiovascular events independent of other major cardiovascular risk factors (11). Furthermore, a recent meta-analysis using pooled data from 14 studies (including 5,447 participants) found that a 1% decrease in FMD% was associated with an 8% increase in cardiovascular mortality independent of risk factors (26). In our study, a 10 μg/m3 annual increase in PM2.5 concentration was associated with a 1% decrease in FMD% (regardless of the adjustment for 1/BAD). Although we report our outcomes for an IQR increase in the PM2.5 contrast, the equivalent of a 10-μg/m3 exposure contrast has previously been associated with a 9% increase in cardiopulmonary mortality in the American Cancer Society Cancer Prevention Study II (27) and a 24% increase in cardiovascular events in the Women’s Health Initiative Observational Study (28).
Our finding regarding short-term exposures, although not statistically significant, is still notable given the prior association with endothelial dysfunction in both experimental (8,9) and observational (5,21) studies. Our results of larger associations with long-term compared with short-term associations (one-tenth of the effect estimate for short-term compared with long-term) parallel the larger magnitude of effect on cardiovascular mortality noted in previous population-based studies (27–29). For example, a 10-μg/m3 increase in short-term exposure to particulate matter is typically associated with a 0.1% to 0.5% mortality increase in the large multicity studies (30) compared with 10% or greater mortality increases in long-term exposure cohort studies (1,27,28). The lack of more robust findings for short-term analysis may be due to limited statistical power or the simplified approach to short-term exposure estimation compared with long-term exposures.
Several mechanisms have been proposed to explain the potential cardiovascular risk associated with pollutants, involving oxidative stress, inflammation, and autonomic imbalance, each of which can affect the endothelium directly or indirectly. A recent study found an association between arterial stiffness and annual average concentrations of nitrogen dioxide and sulfur dioxide but not PM2.5 (31). Reduced production and efficacy of NO in the vasculature is a hallmark of endothelial dysfunction. Previous studies in humans (8,9) have shown vasoconstriction and altered FMD with PM2.5 but typically on an acute time scale. Stimulation of the angiotensin-1 receptor (32) or uncoupling of endothelial NO synthase (33) or generation of highly reactive oxygen species via nicotinamide adenine dinucleotide phosphate (reduced) oxidase and Toll-like receptor pathways (34) and Rho kinase activation (35) have been proposed as the relevant vascular mechanisms of PM2.5. We found that long-term exposure to PM2.5 might produce chronic changes in the brachial artery that negatively affects its ability to react to shear stress, primarily from NO-mediated endothelial dysfunction.
Insights into potential mechanisms of PM2.5 exposure on endothelial function are further suggested by a subgroup analysis analyzing antihypertensive medication use. Although limited by subgroup sizes, we found that the use of ACE inhibitors, but not other classes of antihypertensive agents (including angiotensin receptor blockers) or lipid-lowering drugs, seemed to abrogate the association between long-term PM2.5 concentrations and FMD%. Elevated endothelial kinins and NO from ACE inhibition could play a role in mitigating PM2.5-induced endothelial dysfunction (36,37). Recently, we found that the genetic variation in the renin-angiotensin-aldosterone pathway seemed to modify the effect of traffic-related air pollution on increased left ventricular mass in the MESA cohort (38).
Study strengths and limitations
Major strengths of our study include availability of vascular functional measurements in a large multiethnic cohort with high-quality control standards and excellent data on covariates. We took advantage of the specialized monitoring and sophisticated exposure models from MESA Air to predict spatially resolved, individual-specific estimates at each participant’s home (15).
Our study has several limitations. Most importantly, this is a cross-sectional evaluation of images collected on 1 occasion; important information might be gained by a longitudinal study of vascular function. Our findings also may not be generalizable to either normal younger individuals or those with recognized clinical disease. It should be noted that although our long-term exposure estimates are derived from sophisticated exposure modeling methods, they are subject to measurement error and may not fully reflect an individual’s long-term exposure because information on specific microenvironments, time-activity patterns, or periods of exposure >1 year before testing is lacking. Future MESA Air exposure metrics will be able to incorporate individual-level data on pollutant infiltration efficiencies and time spent indoors, which will improve these estimates.
FMD is a commonly applied marker for NO-mediated endothelial dysfunction (39). We found consistent associations between PM2.5 and reduced NO-mediated endothelial dysfunction, whether expressed as FMD% (with or without adjustment for 1/BAD) or the simple change in FMDmm. Because MESA did not include nitroglycerin administration, we could not rule out NO-independent endothelial dysfunction in this analysis.
Conclusions
This is the first epidemiological study to suggest that long-term exposure to PM2.5 is associated with decreased endothelial function in a conduit artery independent of cardiovascular risk factors. This finding provides a clue that long-term perturbations of the endothelium–smooth muscle complex from PM2.5 exposure lead to chronic functional changes. These functional changes may partly explain the risk of cardiovascular events previously associated with these environmental exposures.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the MESA Air staff, and the participants of the MESA study for their valuable contributions. They also thank Karen Jansen and Cynthia Curl for their editorial assistance.
This research was supported by contracts N01-HC-95159 through N01-HC-95167 and HL 077612 (MESA LUNG) from the National Heart, Lung and Blood Institute; grants P50ES015915, K23ES19575, K24ES013195, and R01-016932 from the National Institute of Environmental Health Sciences; and STAR Grant RD831697 from the U.S. Environmental Protection Agency. All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations and Acronyms
- ACE
angiotensinconverting enzyme
- BAD
baseline arterial diameter
- BMI
body mass index
- FMD
flow-mediated dilation
- IQR
interquartile range
- MAD
maximum arterial diameter
- NO
nitric oxide
- PM2.5
particulate matter <2.5 μm in aerodynamic diameter
- SBP
systolic blood pressure
Footnotes
APPENDIX For supplementary tables, please see the online version of this article.
REFERENCES
- 1.Dockery DW, Pope CA, 3rd, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–9. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 2.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343:1742–9. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 3.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 4.Mills NL, Tornqvist H, Robinson SD, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–6. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- 5.O’Neill MS, Veves A, Zanobetti A, et al. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–20. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- 6.Rundell KW, Hoffman JR, Caviston R, Bulbulian R, Hollenbach AM. Inhalation of ultrafine and fine particulate matter disrupts systemic vascular function. Inhal Toxicol. 2007;19:133–40. doi: 10.1080/08958370601051727. [DOI] [PubMed] [Google Scholar]
- 7.Dales R, Liu L, Szyszkowicz M, et al. Particulate air pollution and vascular reactivity: the bus stop study. Int Arch Occup Environ Health. 2007;81:159–64. doi: 10.1007/s00420-007-0199-7. [DOI] [PubMed] [Google Scholar]
- 8.Peretz A, Sullivan JH, Leotta DF, et al. Diesel exhaust inhalation elicits acute vasoconstriction in vivo. Environ Health Perspect. 2008;116:937–42. doi: 10.1289/ehp.11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–6. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 11.Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2009;120:502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrington DM, Fan L, Drum M, et al. Brachial flow-mediated vasodilator responses in population-based research: methods, reproducibility and effects of age, gender and baseline diameter. J Cardiovasc Risk. 2001;8:319–28. doi: 10.1177/174182670100800512. [DOI] [PubMed] [Google Scholar]
- 13.Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2010;300:H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szpiro AA, Sampson PD, Sheppard L, Lumley T, Adar SD, Kaufman JD. Predicting intra-urban variation in air pollution concentrations with complex spatio-temporal dependencies. Environmetrics. 2010;21:606–31. doi: 10.1002/env.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampson PD, Szpiro AA, Sheppard L, Lindström J, Kaufman JD. Pragmatic estimation of a spatio-temporal air quality model with irregular monitoring data. Atmospheric Environment. 2011;45 [Google Scholar]
- 16.Wilton D, Szpiro A, Gould T, Larson T. Improving spatial concentration estimates for nitrogen oxides using a hybrid meteorological dispersion/land use regression model in Los Angeles, CA and Seattle, WA. Sci Total Environ. 2010;408:1120–30. doi: 10.1016/j.scitotenv.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 17.Cohen MA, Adar SD, Allen RW, et al. Approach to estimating participant pollutant exposures in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Environ Sci Technol. 2009;43:4687–93. doi: 10.1021/es8030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kronmal RA. Spurious correlation and the fallacy of the ratio standard revisited. J Royal Stat Soc Series A Stat Soc. 1993;156:379–92. [Google Scholar]
- 19.Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation. 2004;109:613–9. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 20.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 21.Briet M, Collin C, Laurent S, et al. Endothelial function and chronic exposure to air pollution in normal male subjects. Hypertension. 2007;50:970–6. doi: 10.1161/HYPERTENSIONAHA.107.095844. [DOI] [PubMed] [Google Scholar]
- 22.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–8. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 23.Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–6. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 24.Celermajer DS, Adams MR, Clarkson P, et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334:150–4. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- 25.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–7. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 26.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26:631–40. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 27.Pope CA, 3rd, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–41. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–58. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 29.Samet JM, Zeger SL, Dominici F, et al. The National Morbidity, Mortality, and Air Pollution Study. Part II: Morbidity and mortality from air pollution in the United States. Res Rep Health Eff Inst. 2000;94:5–70. discussion 71–9. [PubMed] [Google Scholar]
- 30.Dominici F, McDermott A, Zeger SL, Samet JM. Airborne particulate matter and mortality: timescale effects in four US cities. Am J Epidemiol. 2003;157:1055–65. doi: 10.1093/aje/kwg087. [DOI] [PubMed] [Google Scholar]
- 31.Lenters V, Uiterwaal CS, Beelen R, et al. Long-term exposure to air pollution and vascular damage in young adults. Epidemiology. 2010;21:512–20. doi: 10.1097/EDE.0b013e3181dec3a7. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Carter JD, Dailey LA, Huang YC. Pollutant particles produce vasoconstriction and enhance MAPK signaling via angiotensin type I receptor. Environ Health Perspect. 2005;113:1009–14. doi: 10.1289/ehp.7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knuckles TL, Lund AK, Lucas SN, Campen MJ. Diesel exhaust exposure enhances venoconstriction via uncoupling of eNOS. Toxicol Appl Pharmacol. 2008;230:346–51. doi: 10.1016/j.taap.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Kampfrath T, Maiseyeu A, Ying Z, et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res. 2011;108:716–26. doi: 10.1161/CIRCRESAHA.110.237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Q, Yue P, Ying Z, et al. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler Thromb Vasc Biol. 2008;28:1760–6. doi: 10.1161/ATVBAHA.108.166967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson TJ, Elstein E, Haber H, Charbonneau F. Comparative study of ACE-inhibition, angiotensin II antagonism, and calcium channel blockade on flow-mediated vasodilation in patients with coronary disease (BANFF study) J Am Coll Cardiol. 2000;35:60–6. doi: 10.1016/s0735-1097(99)00537-9. [DOI] [PubMed] [Google Scholar]
- 37.Linz W, Wohlfart P, Scholkens BA, Malinski T, Wiemer G. Interactions among ACE, kinins and NO. Cardiovasc Res. 1999;43:549–61. doi: 10.1016/s0008-6363(99)00091-7. [DOI] [PubMed] [Google Scholar]
- 38.Van Hee VC, Adar SD, Szpiro AA, et al. Common genetic variation, residential proximity to traffic exposure, and left ventricular mass: the Multi-Ethnic Study of Atherosclerosis. Environ Health Perspect. 2010;118:962–9. doi: 10.1289/ehp.0901535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57:363–9. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.