Abstract
Herbal remedies have a long history of use for gum and tooth problems such as dental caries. The present microbiological study was carried out to evaluate the antimicrobial efficacy of three medicinal plants (Terminalia chebula Retz., Clitoria ternatea Linn., and Wedelia chinensis (Osbeck.) Merr.) on three pathogenic microorganisms in the oral cavity (Streptococcus mutans, Lactobacillus casei, and Staphylococcus aureus). Aqueous extract concentrations (5%, 10%, 25%, and 50%) were prepared from the fruits of Terminalia chebula, flowers of Clitoria ternatea, and leaves of Wedelia chinensis. The antimicrobial efficacy of the aqueous extract concentrations of each plant was tested using agar well diffusion method and the size of the inhibition zone was measured in millimeters. The results obtained showed that the diameter of zone of inhibition increased with increase in concentration of extract and the antimicrobial efficacy of the aqueous extracts of the three plants was observed in the increasing order – Wedelia chinensis < Clitoria ternatea < Terminalia chebula. It can be concluded that the tested extracts of all the three plants were effective against dental caries causing bacteria.
Keywords: Clitoria ternatea, dental caries, Terminalia chebula, Wedelia chinensis
Introduction
Evidence of the use of plants for medicinal purposes dates some 60,000 years back in both Western and Eastern cultures.[1] Medicines derived from plants have been a part of our traditional health care system. Indian civilization, as everyone is aware, is very ancient and rich in natural resources. The use of herbs- and plant-derived products for treating various diseases has been a common practice since ages. Herbal remedies have a long history of use for gum and tooth problems. It is well known that our ancestors have used unrefined sea salt, neem seed oil, twigs of mango or neem tree to clean their teeth. Even today, in many parts of rural India, people practice these ancient methods of tooth cleaning.[2]
There has been a change in thinking globally, with a growing tendency to “GO NATURAL”.[2] The World Health Organization estimates that 4 billion people (80% of the World's population) use herbal medicines for some aspect of primary healthcare.[1] This change is because the plant-based therapeutics are natural products, non-narcotic, easily bio-degradable, pose minimum environmental hazards, have less adverse effects, and are easily available and affordable.[3]
The upsurge in the prevalence of side effects of many synthetic antimicrobial agents and incidence of multidrug resistant bacteria has spurred scientists on the research for plant based antimicrobial agents. Most of the plants used for medicinal and dental purposes have been identified and their applications are well documented and described by different authors, but the antimicrobial efficacy of many plants is yet to be verified.[4]
Earlier studies established that the ripe fruit of Terminalia chebula Retz. is valuable in the prevention and treatment of oral diseases like gingivitis, stomatitis, etc., The extract of T. chebula reported to be potential anticariogenic mouthwash.[2] In view of its medicinal properties and applications in dentistry this plant was selected in the present work to examine the antimicrobial efficacy against dental pathogens.
Extensive studies by several authors have demonstrated that Clitoria ternatea Linn. to be an effective natural remedy for variety of ailments. It has powerful antimicrobial activity against various pathogens such as Escherichia coli, Vibrio cholerae, Staphylococcus aureus, etc.[5] Previous studies indicated that Wedelia chinensis (Osbeck.) Merr. has anti-inflammatory action and is a potential analgesic where its efficacy can be comparable to standard drugs such as aspirin, morphine etc[6] [Table 1]. A very limited published reports concerning the antimicrobial activity of C. ternatea and W. chinensis against dental pathogens are available. Therefore the present study focused on the antimicrobial efficacy of these plants.
Table 1.
The ethnobotanical and phytochemical data of three medicinal plants
The present microbiological study was aimed to evaluate the antimicrobial efficacy of three medicinal plants T. chebula Retz. [Figure 1a], C. ternatea Linn. [Figure 1b], and W. chinensis (Osbeck.) Merr. [Figure 1c] against pathogenic microorganisms present in the oral cavity (Streptococcus mutans, Lactobacillus casei, and Staphylococcus aureus) and to determine the zone of inhibition at different concentrations (5%, 10%, 25%, and 50%).
Figure 1.

(a) Terminalia chebula (fruit) (b) Clitoria ternatea (flower) (c) Wedelia chinensis (leaf)
Materials and Methods
Procurement of the plant material
The fruits of T. chebula, flowers of C. ternatea and leaves of W. chinensis were collected from Botanical garden, Tirupathi, Andhra Pradesh. The botanical identity was determined and authenticated at the Department of Botany, Sri Venkateswara University, Tirupathi, Andhra Pradesh, India.
Preparation of aqueous extract
The plant components were washed under tap water and rinsed in distilled water. They were air dried under room temperature for 4 days and grounded into fine powder with a mechanical grinder [Figure 2a-c]. The powder was weighed into 5, 10, 25, and 50 g using a digital weighing machine and stored in air tight sterile containers.
Figure 2.

(a) Powder form of Terminalia chebula (b) Powder form of Clitoria ternatea (c) Powder form of Wedelia chinensis
Cold aqueous extract was obtained by adding 100 ml of deionized distilled water to the pre-weighed amounts of the powdered plant extract. The constituents were stirred thoroughly and soaked separately in 250 ml conical flasks for 48 h at 4°C. Using a Whatman filter paper-1 crude aqueous extract concentrations of 5%, 10%, 25%, and 50% were prepared for all the three experimental plants [Figure 3]. The aqueous extracts thus prepared were used for antibacterial assay.[7,8]
Figure 3.

Different concentrations of plant extracts
Tested micro-organisms
Freeze dried forms of the micro-organisms S. mutans (MTCC No 3160), S. aureus (MTCC No 447), and L. casei (MTCC No 439) were obtained from Microbial type culture collection, Chandigarh, India.
Anti-microbial assay
Lyophilized forms of S. mutans, L. casei, and S. aureus were activated on respective culture media and 24 h-old sub cultures for each micro-organism was prepared by spread plate method. Agar well diffusion method prescribed by National Committee for Clinical Laboratory Standards (NCCLS 2000) was employed in antimicrobial susceptibility testing for the aqueous extract concentrations of each plant.[9] Agar media (100 ml) was sterilized in separate conical flasks, cooled and inoculated with 0.1 ml of the respective test bacterial suspension. After thorough mixing, the inoculated medium was transferred into sterilized Petri dishes and on solidification of agar medium, wells of about 6 mm diameter were punched into it with a sterilized cork borer. Prior to the addition of the test samples, wells were marked as 5, 10, 25, 50, and C (control). A total of 100 μl of aqueous plant extracts prepared at different concentrations, namely, 5% w/v, 10% w/v, 25% w/v, and 50% w/v were added to respective wells. Adding sterile distilled water alone to the wells served as control. The inoculated bacterial plates were incubated at 37°C and the diameter of inhibition zone was measured after 24 h of incubation. Similar protocol was followed for determining the antibacterial activity of all the three aqueous plant extracts.
Results
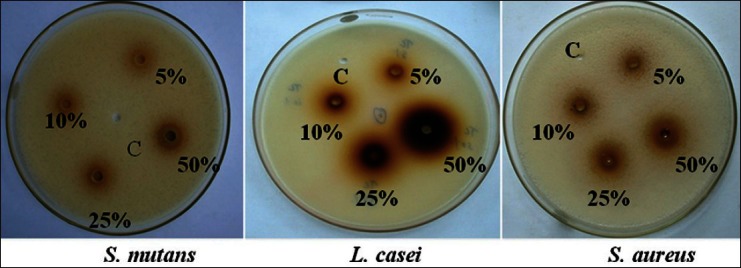
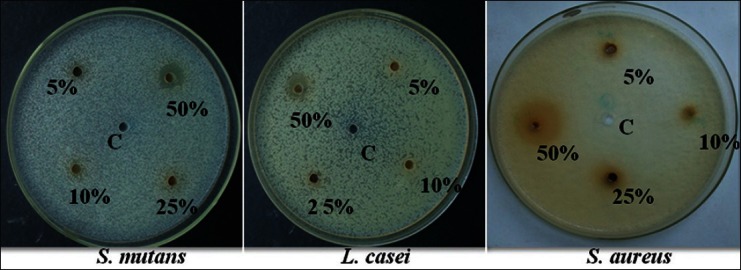
The results revealed that T. chebula exhibited highest antimicrobial efficacy than C. ternatea and W. chinensis. The results obtained showed that the diameter of zone of inhibition increased with increase in concentration of extract for Group I (T. chebula) Group II (C. ternatea), and Group III (W. chinensis) on S. mutans, L. casei, and S. aureus. The anti-microbial efficacy of the aqueous extracts with Group I, Group II, and Group III at 5%, 10%, and 25% were observed in the increasing order – W. chinensis < C. ternatea < T. chebula, whereas at 50% concentration it is in the order – C. ternatea < W. chinensis < T. chebula. The zones of inhibition observed at 5, 10, 25, and 50% against different organisms is placed at Table 2 [Figures 4-6].
Table 2.
Zone of inhibition for Group I, Group II and Group III
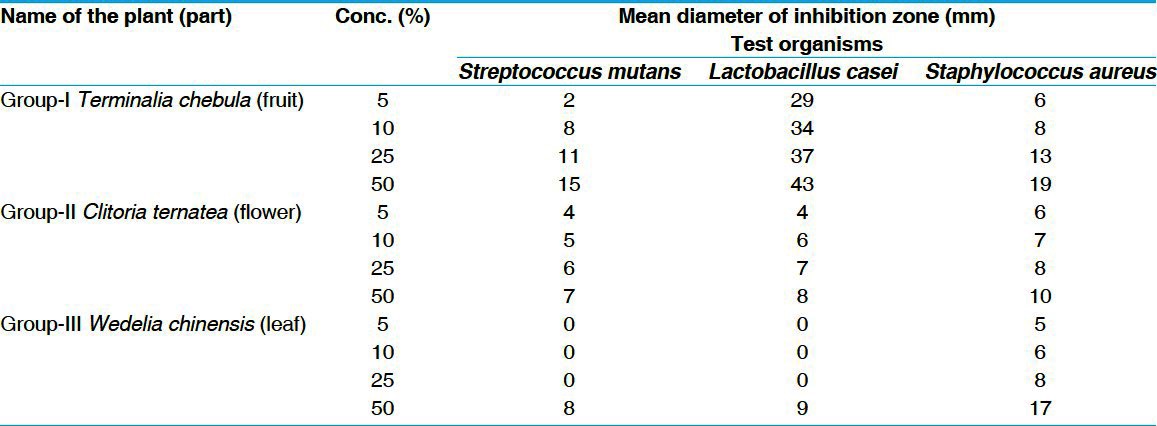
Figure 4.
Zone of inhibition for Group I
Figure 6.
Zone of inhibition for Group III
Figure 5.
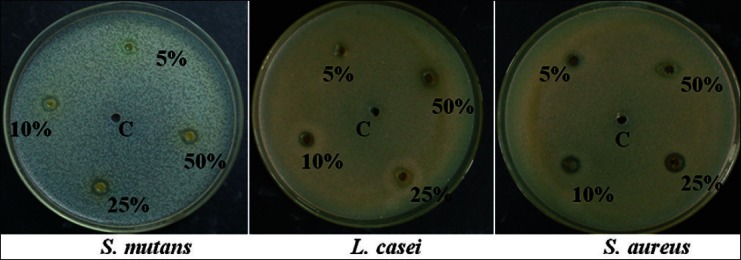
Zone of inhibition for Group II
Discussion
Oral microbes start to colonize on an infant's mouth soon after birth. S. salivarius, S. mitis, and S. oralis have been identified as the first and most dominant oral microbes to colonize the oral cavities of newborn infants.[10] With the eruption of primary teeth, the number and complexity of the micro-flora in the oral environment increase. The species colonizing the teeth after eruption include Streptococcus sanguis, Staphylococcus spp., Veillonella spp., Neisseria spp., Actinomyces spp., and Lactobacilli spp.[10]
The emergence of multi drug-resistant bacterial and fungal strains has increased substantially in the recent years and is posing a serious therapeutic problem worldwide. One of the methods to reduce the resistance to antibiotics is by utilizing antibiotic resistant inhibitors produced from medicinal plants. Researchers stated that plant extracts show target sites other than those used by antibiotics, which will be active against drug-resistant pathogens.[11] Plants contain phytochemicals such as alkaloids, tannins, essential oils, flavanoids, etc., which have pronounced antimicrobial activity and other properties. The phytoconstituents present in the plants exhibit anti-cariogenic effects through various modes of action, including bactericidal effects on oral bacteria, prevention of adherence of bacteria to the tooth surfaces, inhibition of glucan production, and inhibition of amylases.[12]
T. chebula commonly called as “Black myrobalan” belongs to the family Combretaceae is native to India, China, Malaysia, Vietnam, Sri Lanka, Pakistan, and Tibet. T. chebula is called as “King of Medicine” in Tibet and is listed at the top of the list in Ayurvedic materia medica due to its extraordinary power of healing.[13] The plant is used as a rejuvenative drink, laxative (unripe), astringent (ripe), anti-helminthic, expectorant, carminative, appetite stimulant. It is used to treat asthma, sore throat, hiccup, piles, leprosy, skin disorders, urinary tract infections, wound infections, anemia, chronic intermittent fever, heart disease, diarrhea, anorexia, cough, etc.[13] The ripe fruit is used in prevention and treatment of dental caries, spongy and bleeding gums, gingivitis, and stomatitis.[14,15] Tannic acid, gallic acid, and chebulic acid are the major constituents of the ripe fruit of T. chebula. The amount of tannic acid in the aqueous extract of T. chebula is 13% and few authors reported that tannic acid is bacteriostatic or bactericidal to some Gram positive and Gram negative pathogens.[13] Tannic acid can be well adsorbed to the hydroxyapatite of the tooth or to the salivary mucins, alternatively it can bound to the anionic groups on the surface of the bacterial cells, which resulted in protein denaturation and ultimately bacterial cell death.[16] According to earlier studies[14] the aqueous extract of T. chebula prevents plaque formation on the surface of tooth by inhibiting sucrose-induced adherence and glucan-induced aggregation, two processes which foster the colonization of S. mutans on the surface of tooth. The antioxidant property present in the extract may be useful in treatment of diabetic patients having dental caries.[16]
C. ternatea commonly called as “Butterfly pea” belongs to the family Fabaceae. It is a perennial herbaceous plant with elliptical and obtuse leaves. It grows as a vine or creeper, doing well in moist neutral soil. The most striking feature is its vivid deep blue flowers. This plant is native to tropical equatorial Asia, but has been introduced to Africa, Australia, and America. It is found throughout India in tropical areas especially in the southern India. It is a major ingredient in “Medya-Rasayana” (brain tonic) a rejuvenating recipe used for treatment of neurological disorders.[17] It is used as brain-tonic, nervine tonic, and laxative. The juice of flowers is reported to be used in insect bites and skin diseases. The roots are useful in asthma, burning sensation, ascites, inflammation, leucoderma, leprosy, hemicrania, amentia, pulmonary tuberculosis and reported as bitter, refrigerant, ophthalmic, laxative, diuretic, cathartic, aphrodisiac, tonic.[5] Major phytoconstituents found in C. ternatea are the pentacyclic triterpenoids such as taraxerol and taraxerone. High calcium concentration in the plant showed that it can be exploited as a source of calcium in herbal drink.
W. chinensis commonly called as “Chinese Wedelia” belongs to the family Asteraceae. It is a tender, procumbent perennial hairy herb with branches usually less than 50 cm long. It is a reputed herbal medicine in Ayurvedic, Siddha, and Unani system of medicine.[6] It is distributed in tropical and warm temperature regions in India, Burma, Sri Lanka, China, and Japan. The leaves of the plant can be used in treatment of dermatological disorders, cough, headache, hair loss, dying of hair, lice, nervous weakness, anemia, digestive disorders etc., The juice of the leaves is used as a snuff in cephalalgia and as a home remedy in osteochondritis, multiple sclerosis and juvenile arthritis, etc.[18] W. chinensis contains wedelolactone and demethylwedelolactone (Coumestans derivatives) possessing potent anti-hepatotoxic effect and is incorporated as a major ingredient in a number of potent anti-hepatotoxic phyto-pharmaceutical formulations.
Because of the various therapeutic properties and easy availability, these three plants were selected for the present study to evaluate the antimicrobial efficacy on pathogenic oral micro-organisms in different concentrations [Table 1]. The process of extraction of the experimental plants employed is ‘Maceration’ method as per Taiga and Friday[7,8] where the parts of the plants are dried, pulverized, weighed in different concentrations, and soaked in 100 ml of distilled water at 4°C for 48 h to get the required quantity of crude extract concentrations. Aqueous extract was used as water is a high polarity solvent, readily available and almost all the compounds of the plant dissolve in it without affecting its biological properties.[4]
According to NCCLS 2000 standards, the anti-microbial efficacy of any agent can be evaluated by the following methods: Broth dilution method, agar dilution method, disc diffusion method, agar well diffusion method, and ditch plate method. The present study employed Agar well diffusion method[9,16] to evaluate the antimicrobial efficacy of three test plants since it is more reliable and acceptable. The results obtained showed that diameter of zone of inhibition increased with increase in concentration of extract for Group I (T. chebula) Group II (C. ternatea), and Group III (W. chinensis) on S. mutans, S. aureus, and L. casei. The antimicrobial efficacy of the aqueous extracts of Group I, Group II, and Group III were observed in the increasing order – W. chinensis < C. ternatea < T. chebula. The results with Group 1 (T. chebula) are in accordance with the previous studies conducted.[11,13-15]
In one study,[13] the antimicrobial potential of T. chebula fruit extracts (acetone, ethanol, methanol, cold and hot aqueous) were evaluated against five micro-organisms (S. mutans, S. aureus, Lactobacillus acidophilus, C. albicans and Saccharomyces cerevisia) using agar well diffusion method. The zones of inhibition against S. aureus and S. mutans coincide with those formed in the present study conducted on T. chebula. In another study,[15] the zones of inhibition formed with T. chebula on S. aureus are smaller when compared with the zones of the present study. This difference may be due to the method of antibacterial assay performed (disc diffusion method) or in the extract preparation of the plant. Studies[14] evaluated anti-bacterial potential of the aqueous extract of T. chebula at different concentrations (6-30%) on S. mutans. The diameter of zone of inhibition increased with increase in concentration of extract and is nearly equal to the zones obtained in the present study.
The literature showing anti-microbial effects of C. ternatea and W. chinensis on dental pathogens is scanty. The present study is a preliminary evaluation to explore the anti-microbial properties of these experimental plants.
Conclusions
At 5% concentration, C. ternatea showed greater anti-microbial efficacy against S. mutans and with the remaining three concentrations T. chebula showed greater antimicrobial efficacy against S. mutans. At all the four concentrations, T. chebula showed greater antimicrobial efficacy against L. casei and S. aureus. W. chinensis showed anti-microbial efficacy against S. mutans L. casei at 50% concentration only.
Since the tested extracts of all three plants were effective against pathogenic micro-organisms present in the oral cavity, purification and toxological studies of these plants and in vivo trials should be carried out. The anti-microbial efficacy can be enhanced if the phyto constituents of these plant extracts are purified using different solvents like ethanol, methanol, acetone, etc., Anti-bacterial activity of these medicinal herbs, if translated into clinical practice would lead to the development of indigenous, chemical free, cost effective, and holistic oral hygiene aids, which can be incorporated into various oral hygiene formulations like dentrifices, mouth rinses, gum paints, etc., With continued growth of biotechnology and increasing tools for validation of the bioactive compounds, the potential is high that one day our food will serve as medicine.
References
- 1.Gossell-Williams M, Simon OR, West ME. The past and present use of plants for medicines. West Indian Med J. 2006;55:217–8. doi: 10.1590/s0043-31442006000400002. [DOI] [PubMed] [Google Scholar]
- 2.Carounanidy U, Satyanarayanan R, Velmurugan A. Use of an aqueous extract of Terminalia chebula as an anticaries agent: A clinical study. Indian J Dent Res. 2007;18:152–6. doi: 10.4103/0970-9290.35823. [DOI] [PubMed] [Google Scholar]
- 3.Kannan P, Ramadevi SR, Hopper W. Antibacterial activity of Terminalia chebula fruit extract. Afr J Microbiol Res. 2009;3:180–4. [Google Scholar]
- 4.Erturk O. Antibacterial and antifungal effects of alcoholic extracts of 41 medicinal plants growing in Turkey. Czech J Food Sci. 2010;28:53–60. [Google Scholar]
- 5.Gupta GK, Chahal J, Bhatia M. Clitoria ternatea (L.): Old and new aspects. J Pharm Res. 2010;3:2610–4. [Google Scholar]
- 6.Meena AK, Rao MM, Meena RP, Panda P, Renu Pharmacological and phytochemical evidences for the plants of Wedelia genus: A review. Asian J Pharm Reviews. 2011;1:7–12. [Google Scholar]
- 7.Taiga A. Efficacy of selected plant extracts in the control of fungal dry rot of white yam (Dioscorea rotundata) tubers in Kogi State. Am-Eurasian J Sustain Agric. 2009;3:310–3. [Google Scholar]
- 8.Taiga A, Friday E. Variations in phytochemical properties of selected fungicidal aqueous extracts of some plant leaves in Kogi state, Nigeria. Am-Eurasian J Sustain Agric. 2009;3:407–9. [Google Scholar]
- 9.Owhe-Ureghe UB, Ehwarieme DA, Eboh DO. Antibacterial activity of garlic and lime on isolates of extracted carious teeth. Afr J Biotechnol. 2010;9:3163–6. [Google Scholar]
- 10.Law V, Seow WK, Townsend G. Factors influencing oral colonization of Mutans streptococci in young children. Aus Dent J. 2007;52:93–100. doi: 10.1111/j.1834-7819.2007.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 11.Prabhat Ajaybhan, Navneet, Chauhan A. Evaluation of antimicrobial activity of six medicinal plants against dental pathogens. Report and Opinion. 2010;2:37–42. [Google Scholar]
- 12.Parimala Devi B, Ramasubramaniaraja R. Dental caries and medicinal plants-an overview. J Pharm Res. 2009;2:1669–75. [Google Scholar]
- 13.Aneja KR, Joshi R. Evaluation of antimicrobial properties of fruit extracts of Terminalia chebula against dental caries pathogens. Jundishapur J Microbiol. 2009;2:105–11. [Google Scholar]
- 14.Jagtap AG, Karkera SG. Potential of the aqueous extract of Terminalia chebula as an anticaries agent. J Ethnopharmacol. 1999;68:299–306. doi: 10.1016/s0378-8741(99)00058-6. [DOI] [PubMed] [Google Scholar]
- 15.Kumar M, Agarwal RC, Dey S, Rai VK, Johnson B. Antimicrobial activity of aqueous extract of Terminalia chebula. Int J Curr Pharm Res. 2009;1:56–60. [Google Scholar]
- 16.Jagadish L, Anand Kumar VK, Kaviyarasan V. Effect of triphala on dental bio-film. Indian J Sci Technol. 2009;2:30–3. [Google Scholar]
- 17.Patil Amol P, Patil Vijay R. Clitoria ternatea Linn: An overview. Int J Pharm Res. 2011;3:20–3. [Google Scholar]
- 18.Sureshkumar S, Sivakumar T, Chandrasekar MJ, Suresh B. Investigating the anti-inflammatory and analgesic activity of leaves of Wedelia chinensis (Osbeck) Merr. In standard experimental animal. Iran J Pharm Sci. 2006;2:123–9. [Google Scholar]


