Abstract
Type-2 diabetes mellitus is a persistent health problem that requires innovative strategies to improve health and needs a multifactorial approach for the treatment. Saptarangyadi Ghanavati, a new formulated Ayurvedic compound consists of herbs with anti-diabetic potential, in addition to a folklore herb Saptarangi (Salacia chinensis) has been evaluated. In a total of 67 patients, 36 patients were of newly detected type-2 diabetes mellitus and 31 patients were of chronic type-2 diabetes mellitus and they were divided into group A and group B, respectively. Group A consisted newly detected subjects of type-2 diabetes and were not taking any regular medication and group B consisted of chronic cases of type-2 diabetes mellitus, who were taking modern ant-diabetic medication, but their blood-glucose level was not controlled to desired level. Patients in group A were administered Saptarangyadi Ghanavati each of 200 mg, 5 Vatis, 3-times a day-after breakfast, lunch, and dinner. Patients in group B were administered Saptarangyadi Ghanavati, in the same dose in addition to the concomitant anti-diabetic (Allopathic) medication. Serum insulin investigation, both fasting and post-prandial levels were evaluated in six patients of group B, showed a highly significant increase in first-phase insulin response. Glycosylated hemoglobin (serum HbA1c) evaluated in six patients of group A showed statistically significant reduction. There was also statistically significant reduction in the fasting blood sugar (FBS) and post prandial blood sugar (PPBS) parameters, both in newly detected cases as well as chronic cases of type-2 diabetes mellitus.
Keywords: Anti-diabetic, insulin response, glycosylated hemoglobin, Saptarangyadi Ghanavati, type-2 diabetes mellitus
Introduction
Nowhere is the diabetes epidemic more pronounced than in India, as World Health Organization (WHO) report shows that 32 million people had diabetes in the year 2000.[1] Type-2 diabetes is the result of a progressive impairment of pancreatic β-cell function in the setting of worsening insulin resistance. Studies in high-risk populations have demonstrated that during progression to diabetes, β-cells have declining function and lose the first phase of insulin secretion, resulting in less than adequate suppression of hepatic glucose production following meals. In addition, oscillations of insulin secretion become unmatched from their normal coupling with glucose. Several mechanisms are thought to be responsible for impaired β-cell function, including glucose toxicity and lipotoxicity and potentially contribute to β-cell loss.[2]
Type-2 diabetes mellitus is one of the most prevalent life style disorders in today's era.[3] Ayurveda, the science of life mentions Apathyanimittaja Prameha[4] which resembles type-2 diabetes mellitus in terms of etiology, pathogenesis and presentation of the disease. Therefore, the treatment regime prescribed in Ayurveda for Apathyanimittaja Parmeha has been adopted in the present study, aiming to counteract the complex metabolic derangement of type-2 diabetes mellitus and to explore the potential of a new Ayurvedic compound drug formulation to provide safe and cost effective treatment for type-2 diabetes mellitus.
Aims and Objectives
The present research was aimed to study the anti-diabetic potential of Saptarangyadi Ghanavati in newly detected cases of type-2 diabetes mellitus and in chronic uncontrolled cases of type-2 diabetes mellitus, whose blood glucose levels are not controlled up to the desirable limits with modern drugs.
Materials and Methods
For clinical study, the patients with signs and symptoms of Apathyanimittaja Prameha attending the OPD of Kaya Chikitsa Department, I.P.G.T. and R.A. Hospital, G.A.U., Jamnagar were included for the study.
Inclusion criteria
Diagnosed patients of type-2 diabetes mellitus or the patients preliminarily type-2 diabetes mellitus, diagnosed on the basis of signs and symptoms of the disease confirmed by Fasting Blood Sugar (FBS) and Post Prandial Blood Sugar (PPBS).
Patients between the age group of 35 and 70 years were included.
Exclusion criteria
Patients of Sahaja Prameha and IDDM (type-1 diabetes mellitus).
Patients below 35 and above 70 years of age.
Patients suffering from any serious systemic disorders such as uncontrolled hypertension, tuberculosis, carcinoma, and HIV were excluded for present study.
Laboratory investigations
Hemoglobin, Total Leucocyte Count (TLC), differential leucocyte count, Erythrocyte Sedimentation Rate (ESR).
Urine for routine and microscopic examination.
Biochemical investigations: FBS, PPBS, lipid profile, Serum insulin (S. insulin) and Serum Hemoglobin A1c (S. HbA1c).
Treatment protocol
Group A: Patients with newly diagnosed type-2 diabetes mellitus, not taking any medication were administered Saptarangyadi Ghanavati.
Group B: Patients with concomitant anti-diabetic (Allopathic) medication, whose blood glucose is not well under control. These patients were administered the test drug Saptarangyadi Ghanavati in the integrated manner with ongoing conventional medicine.
Drug, dose and duration
Drug: Saptarangyadi Ghanavati.
Dose: Ghanavati 200 mg of each, 5 Vati thrice a day, after breakfast, lunch, and dinner.
Anupana: Luke warm water.
Duration: 2 months (for both groups).
The patients under both the groups were provided a proper diet chart planned according to the classics and keeping glycemic index of the dietary substances and calorie requirement of the patients. Simultaneously they were asked to maintain a routine of 30 min walk in the morning and in the evening hours, 7 days a week.
There was 1 month of follow-up, after completion of 2 months of the treatment.
Statistical analysis
Evaluation of the data through statistical estimation within the group and comparison between the groups AT (After Treatment) were assessed using paired and unpaired Student's t test, respectively. The statistical estimations particularly sample means, SD (Standard Deviation), SEM (Standard Error of Mean), calculated t value and P (Probability) values were obtained by applying the standard formulae. For comparison of the subjective parameters, Chi-square test was used. P < 0.05 was considered as statistically significant.
Observations and Results
A total of 75 patients, consisting of 39 patients newly detected and 36 chronic cases of type-2 diabetes mellitus, were registered in group A and group B, respectively. In group A, 36 and in group B, 31 patients completed the study. In the clinical study maximum number (40%) of patients belonged to the age group of 46-55 years and 52% were males. Majority of them belonged to Hindu religion (88%), married (98.6%), house wives (42.6%), educated (84%), and were from middle class (42.6%) of the society. Positive family history for type-2 diabetes was found in 42% of the patients.
The symptoms reported included, Prabhuta Mutrata (88%), Alasya (67%), Avilamutrata (55%), Pipasadhikya (82%), Kshudadhikya (46%), Atisveda (51%), Pindikodveshtana (76%), and Shrama (73%) [Graph 1].
Graph 1.
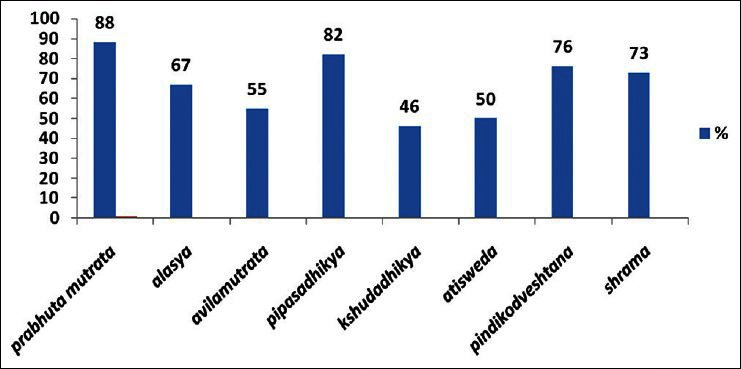
Symptoms reported in the patients of Apathyanimittaja Prameha (type-2 Diabetes mellitus)
Mean FBS and PPBS values were 179 mg/dl and 244 mg/dl in group A, respectively. In group B, mean FBS and PPBS values were 180 mg/dl and 241 mg/dl, respectively, before the commencement of the treatment. In group A, mean serum cholesterol and serum triglyceride values were 199 mg/dl and 163 mg/dl, respectively. In group B, S. cholesterol and S. triglyceride were having mean values of 197 mg/dl and 216 mg/dl, respectively. In group A, mean value of S. HDL (Serum High Density Lipoprotein) was 45 mg/dl and in group B mean value for S. HDL was 42 mg/dl. Mean values of S. insulin (fasting) and S. insulin (post prandial) in six patients of group B were 1.75 AIU/mL and 4.57 AIU/mL, respectively. Mean S. HbA1c value in six patients of group A was 9.45%. In group A, 3+ urine sugar was present in 12.8% of the patients, followed by 2+ in 7.6%, and 1+ in 12.8% patients. In group B, 4+ urine sugar was found in 2.8% of the patients, followed by 3+ in 16.7%, 2+ in 2.8%, and 1+ urine sugar in 19.4% of the patients.
Effect of the therapies
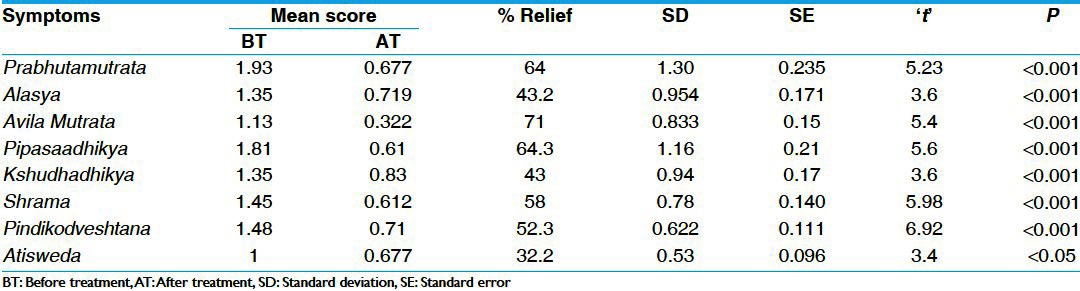
There was statistically highly significant (P < 0.001) reduction of 69% and 64% in Prabhuta Mutrata in group A and group B, respectively. In Alasya, there was statistically highly (P < 0.001) significant reduction of 59% and 43% in group A and group B, respectively. In Avila Mutrata group A and group B showed statistically highly (P < 0.001) significant reduction of 82% and 71%, respectively. In Pipasadhikya, group A and group B showed statistically highly (P < 0.001) significant reduction of 63% and 64%, respectively. In Kshudhadhikya, group A showed reduction of 53% and group B showed reduction of 43%, both were statistically highly significant (P < 0.001). In Alasya, there was statistically highly significant (P < 0.001) reduction of 52% and 58% in group A and group B, respectively. In Atisveda there was statistically highly significant (P < 0.001) reduction of 30% in group A and statistically significant reduction (P < 0.05) of 32% in group B. There was statistically highly significant (P < 0.001) reduction of 67% and 52% in Pindikodveshtana, in group A and group B, respectively [Tables 1 and 2].
Table 1.
Effect of therapy on cardinal symptoms in group A (n=36)
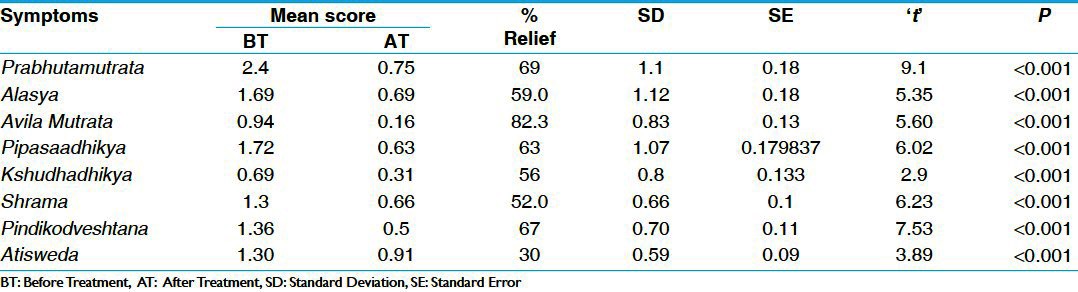
Table 2.
Effect of therapy on cardinal symptoms in group B (n=31)
In FBS parameters, there was statistically significant (P < 0.01) reduction of 12% in group A and 10% in group B, which was also statistically significant (P < 0.05). In PPBS, there was statistically highly significant (P < 0.001) reduction of 24% in group A and 18% in group B, which is statistically significant (P < 0.05) [Table 3]. There was statistically insignificant reduction in S. cholesterol, S. triglyceride, and S. HDL in both the groups. There was insignificant increase in S.creatinine and S.urea in group A and group B. Similarly there was insignificant reduction (P > 0.05) in both SGOT (Serum Glutamic Oxaloacetic Transminase) and SGPT (Serum Glutamic Pyruvate Transminase) in group A and B respectively [Tables 4 and 5]. There was statistically insignificant (P > 0.05) increase of 59% fasting S. insulin and statistically significant (P < 0.05) increase of 87% in post-prandial S. insulin levels [Table 6]. There was statistically significant (P < 0.05) reduction of 40% in S. HbA1c [Table 7]. Group B showed statistically significant (P < 0.05) reduction of 53% in urine sugar and Group A showed statistically insignificant (P > 0.05) reduction of 38.4% in urine sugar [Table 8].
Table 3.
Effect of therapies on blood sugar level
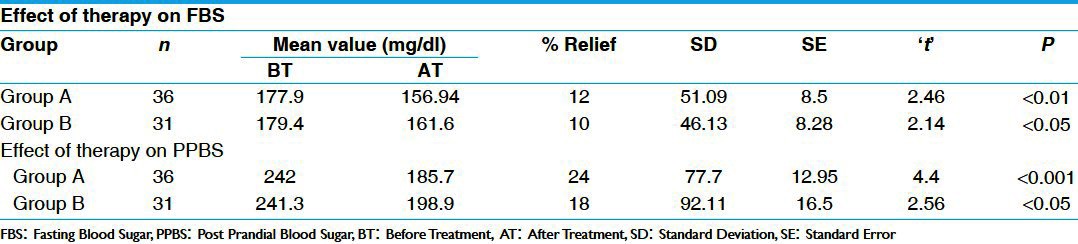
Table 4.
Effect of therapy on serum bio.chemical parameters in Group A (n=36)
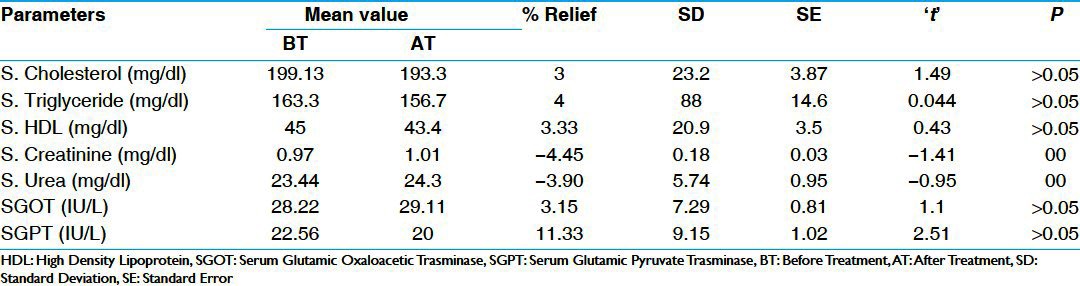
Table 5.
Effect of therapy on serum bio.chemical parameters in Group B (n=31)
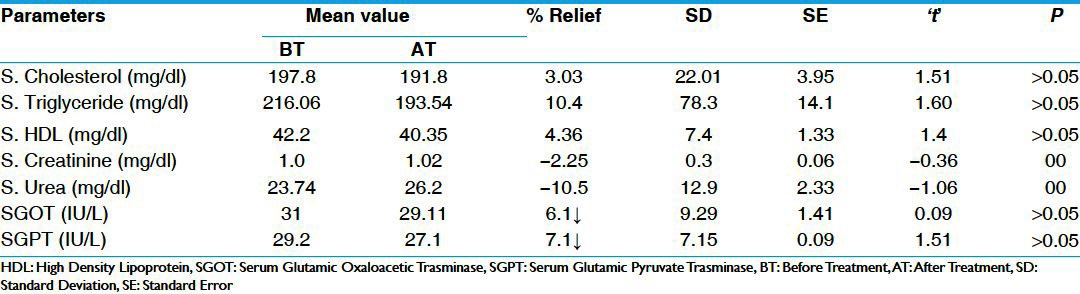
Table 6.
Effect of therapy on serum insulin in group B (n=6)
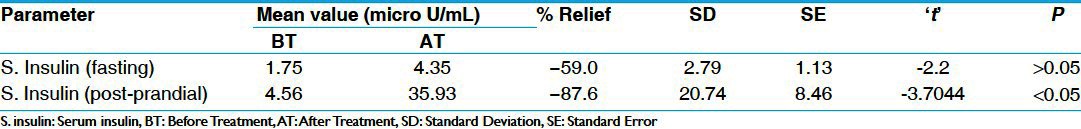
Table 7.
Effect of therapy on serum HbA1c in group A (n=6)

Table 8.
Effect of therapies on urine glucose level
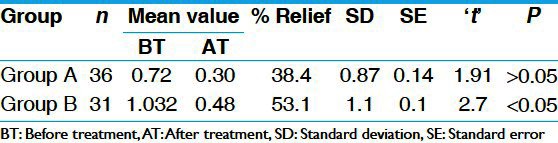
Overall effect of the therapy
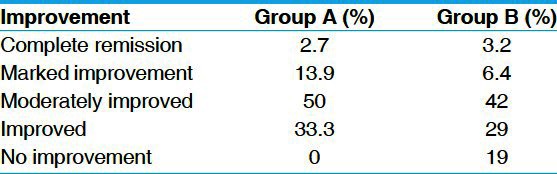
In group A, marked improvement of symptoms was found in 50% of the patients followed by improvement in 33.3%, marked improvement in 13.9% and complete remission in 2.7% of the patients. In group B, moderate improvement was found in 42% of the patients, followed by improvement in 9% of the patients, marked improvement in 6.4% of the patients, complete remission in 3.2% of the patients and no improvement in 19% of the patients [Table 9].
Table 9.
Overall effect of the therapy
Discussion
Type-2 diabetes mellitus and Apathyanimittaja Prameha have a similarity in terms of etiology, etiopathogenesis as well as presentation of the disease. The cardinal symptoms mentioned in the Ayurvedic texts such as Prabhuta Mutrata (polyuria), Avila Mutrata (turbid urine or the urine with high specific gravity) are also invariably found in almost all the diagnosed cases of type-2 diabetes mellitus. Secondly, Ayurvedic texts mention Prameha as one of the first disease as a manifestation of obesity, which is the most prominent predisposing factor in the incident of type-2 diabetes mellitus. Life style and diet style factors such as sedentary habits, high sugar content food articles such as simple carbohydrates, milk products, and sweets, which make an individual prone for the incidence of type-2 diabetes mellitus, are also mentioned in Ayurvedic texts as predisposing factors for Apathyanimittaja Prameha. Thus in this study, the treatment regime both in the form of lifestyle modifications as well as pharmacological intervention using the Ayurvedic herbs mentioned for their Medohara, Pramehahara, Rasayana actions were selected, in addition to a folklore herb (Salacia chinensis).
A total of 75 patients, consisting of 39 patients newly detected and 36 chronic cases of type-2 diabetes mellitus, were registered in this study and were kept in group A and group B, respectively. A total of 67 patients completed the treatment, 36 in group A and 31 in group B.
Saptarangyadi Ghanavati provided significant relief in almost all the cardinal symptoms. On comparison, both the groups were equally significant in all the symptoms except Prabhuta Mutrata, in which group A showed better effect then group B. This can be because of the less chronicity (average chronicity <9 months) in group A as compared to group B (average chronicity >5 years). In the FBS parameters, group A showed statistically significant (P < 0.01) reduction of 12% and group B showed a reduction of 10%, which was also statistically significant (P < 0.05). Reduction of fasting blood glucose can be attributed β-cell protective and regenerative effect of the drugs like Karvellaka[5] and Methika[6] in the combination which might have improved the basal insulin secretion and thus, might have reduced the hepatic gluconeogenesis also. This assumption is further supported by an increase in fasting S. insulin levels, to some extent. In PPBS parameter, group A showed statistically highly significant (P < 0.001) reduction of 24% and group B showed a reduction of 18%, which was also statistically significant (P < 0.05). Reduction of post-prandial blood glucose can be attributed insulin secretagogue effect of the drugs like Karvellaka,[7] Methika,[8] and Guduchi[9] of the combination, which might have improved the response also. This assumption is further supported by an increase (statistically significant, P < 0.05) in post-prandial S. insulin levels. Both fasting as well as post-prandial levels of S. insulin were carried out in six patients in group B to evaluate β-cell regenerative effect (if any) and β-cell supportive (by improving the incretin response) effect of the combination. Fasting S. insulin levels showed a statistically insignificant increase, whereas post-prandial S. insulin showed a statistically significant increase. In chronic diabetic state, there is β-cell destruction, which reduces the basal insulin secretion, but acute insulin response to the oral glucose intake is not that much impaired. Saptarangyadi Ghanavati had shown an increase in the fasting S. insulin level, which was not statistically significant. However, this increase may be because of the slight regenerative effect of Saptarangyadi Ghanavati, which is supported by significant reduction in the fasting blood glucose as a result of improvement in basal insulin secretion.
Significant increase in post-prandial S. insulin levels can be attributed to the combined effect of improvement in the basal insulin secretion (supported by significant reduction in FBS levels) as well as improvement in the response (supported by significant reduction in PPBS levels) because of the insulotropic effect of the ingredients of Saptarangyadi Ghanavati like Karvellaka,[7] Methika,[8] Guduchi.[9] There was statistically significant reduction (P < 0.05) in S. HbA1c levels carried out in a selected number (n − 6) of the patients in group A. This reduction can be attributed to the multifactorial, i.e., Pramehaghna (Guduchi,[10] Methika,[11] Karvellaka,[12] etc.), Medohara (Karvellaka,[13] Methika,[14] etc.), Rasayana (Triphala,[15] Guduchi[16], etc.) effect of the ingredients of the combination. Significant reduction in S.HbA1c levels shows good glycemic control for the long term as well as significant improvement in the lipid profile besides reduction in oxidative stress related to hyperglycemia.
After 2 months of the treatment, in group A serum creatinine (S. creatinine) and serum urea (S. urea) had statistically insignificant (P > 0.05) increase of 4.45% and 3.9%, respectively. In group B, S. urea and S. creatinine showed a statistically insignificant increase of 2.25% and 10.5%, respectively. These insignificant increases in the S. urea and S. creatinine indicate that there is no harm in the renal functions and thus Saptarangyadi Ghanavati does not cause any renal impairment.
Conclusion
The treatment regime mentioned for Apathyanimittaja Prameha can be a worth for the management of the type-2 diabetes by countering its complex pathology. The Pramehaghna (antidiabetic), Medohara (antihyperlipidemic), and Rasayana (anti-oxidant) property of the Ayurvedic drugs not only ensures good glycemic control when supported by Pathya and Apathya mentioned for Prameha but also will delay its complications.
Acknowledgment
The authors are thankful to Dr. Ashok B.K. and Dr. Sulakshan for their invaluable support.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Steppel JH, Horton ES. Beta-cell failure in the pathogenesis of type 2 diabetes mellitus. Curr Diab Rep. 2004;4:169–75. doi: 10.1007/s11892-004-0019-3. [DOI] [PubMed] [Google Scholar]
- 3.Pappachan MJ. Increasing prevalence of life style diseases: High time for action. Indian J Med Res. 2011;134:143–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Sushruta . In: Sushruta Samhita, Chikitsa Sthana, 11/3. Vaidya Jadavji Trikamji Acharya., editor. Varanasi: Chaukhamba Surbharati Prakashana; 2008. p. 451. [Google Scholar]
- 5.Singh N, Gupta M. Regeneration of beta cells in islets of Langerhans of pancreas of alloxan diabetic rats by acetone extract of Momordica charantia (Linn.) (bitter gourd) fruits. Indian J Exp Biol. 2007;45:1055–62. [PubMed] [Google Scholar]
- 6.Saxena A, Vikram NK. Role of selected Indian plants in management of type 2 diabetes: A review. J Altern Complement Med. 2004;10:369–78. doi: 10.1089/107555304323062365. [DOI] [PubMed] [Google Scholar]
- 7.Yibchok-anun S, Adisakwattana S, Yao CY, Sangvanich P, Roengsumran S, Hsu WH. Slow acting protein extract from fruit pulp of Momordica charantia with insulin secretagogue and insulinomimetic activities. Biol Pharm Bull. 2006;29:1126–31. doi: 10.1248/bpb.29.1126. [DOI] [PubMed] [Google Scholar]
- 8.Zahedi Asl S, Farahnaz S, Ghasemi A, Zaree B. The effect of carbon tetrachloride extract of Trigonella foenum graecum seeds on glycogen content of liver in streptozotocin-induced diabetic rats. Int J Endocrinol Metab. 2007;5:70–5. [Google Scholar]
- 9.Rajalakshmi M, Eliza J, Priya CE, Nirmala A, Daisy P. Anti-Diabetic effect of Tinospora cordifolia stem extracts on steptozotocin- induced diabetic rats. Afr J Pharm Pharmacol. 2009;3:171–80. [Google Scholar]
- 10.Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 11.Raju J, Gupta D, Rao AR, Yadava PK, Baquer NZ. Trigonella foenum graecum (fenugreek) seed powder improves glucose homeostasis in alloxan diabetic rat tissues by reversing the altered glycolytic, gluconeogenic and lipogenic enzymes. Mol Cell Biochem. 2001;224:45–51. doi: 10.1023/a:1011974630828. [DOI] [PubMed] [Google Scholar]
- 12.Matheka DM, Kiama TN, Alkizim FO, Bukachi F. Glucose-lowering effects of Momordica charantia in healthy rats. Afr J Diabet Med. 2011;19:15–9. [Google Scholar]
- 13.Arayne MS, Sultana N, Mirza AZ, Zuberi MH, Siddiqui FA. In–vitro hypoglycemic activity of methanolic extract of some indigenous plants. Pak J Pharm Sci. 2007;20:261–8. [PubMed] [Google Scholar]
- 14.Toshiaki H, Kohji Y, Yoshikatsu S, Kazunaga Y. Effect of fenugreek seed extract in obese mice fed a high-fat diet. Biosci Biotechnol Biochem. 2005;69:1186–8. doi: 10.1271/bbb.69.1186. [DOI] [PubMed] [Google Scholar]
- 15.Agnivesha . In: Charaka, Dridhabala, Charaka Samhita, Chikitsa Sthana, Karprachitiya Rasayanapadam 1-3/42-47. Vaidhya Jadavaji Trikamji Acharya., editor. Varanasi: Chaukhamba Surbharati Prakashana; 2008. p. 385. [Google Scholar]
- 16.Singh SS, Pandey SC, Srivastava S, Gupta VS, Patro B, Ghosh AC. Chemistry and Biochemical properties of Tinospora cordifolia. Indian J Pharmacol. 2003;35:83–91. [Google Scholar]


