Abstract
Despite the advances in the treatment of cancer, mortality is still high. Complementary and alternative medicine is emerging as a potent modality in cancer treatment. ‘Swarna Bhasma’ (SB), containing gold particles, is an ancient Indian medicine has shown its anticancer activity. This present study was conducted to detect the effect of SB on solid malignancies. A total of 43 patients were included in this study received SB for 1 year. Seventeen patients showed response. The response was best in rectal cancer group 70% (7/10). Nearly 41.02% patients survived for 1 year after treatment but after 5 years this came down to 15.38%.
Keywords: Cancer, complementary medicine, gold particle, Swarna Bhasma
Introduction
Incidence of cancer is ever increasing.[1] Despite modern diagnostic and treatment modalities (surgery, chemotherapy, and radiotherapy) mortality and morbidity is still high.[1] Studies have demonstrated the anticancer activity of some complementary and alternative medicines (CAM) like ‘Swarna Bhasma’ (SB) (made of gold particles).[2] We conducted a prospective clinical trial on 43 patients with various solid malignancies (lung, liver, pancreas, gall bladder, and colon) with SB.
Aims and Objectives
To assess the response of malignant tumors to SB.
To identify the side effects of SB.
Materials and Methods
Study area and duration
The study was conducted in the Calcutta Gastroenterology Research Centre (CGRI) situated in Kolkata, West Bengal, India. The study started from January 2005 and completed in February 2010. The study protocol was approved by the Institutional Ethics Committee (IEC) and consent was taken from each patient in this regard.
Study design
The study was prospective, single arm, observational study.
Inclusion criteria
The patients of malignancy [confirmed by histo-pathological examination of per rectal biopsy, cyto-pathological exam of computed tomography (CT) guided fine needle aspiration cytology (FNAC)] involving lung, liver, pancreas, gall bladder, and rectum of both sexes were included. In all these patients palliative care was advised by treating oncologist.
Exclusion criteria
-
(i)
Operable tumors
-
(ii)
Prior chemotherapy or radiotherapy
-
(iii)
Patients unwilling to have CAM.
Treatment
SB powder (50 mg/kg/day) per-orally prescribed for 1 year or till disease progression or appearance of intolerable side effects.
Follow-up
Patients were followed up monthly for 1 year, then 6 monthly for 5 years. Response measured by (i) radiological tumor response (by CT scan or ultrasonography) defined by Response Evaluation Criteria in Solid Tumors (RECIST), (ii) survival, and (iii) side effects of SB.
According to RECIST criteria, response was divided into four groups:
Complete Response (CR): Disappearance of all target lesions. Any pathological lymph nodes (whether target or nontarget) must have reduction in short axis to < 10 mm.
Partial Response (PR): At least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters.
Progressive Disease (PD): At least a 20% increase in the sum of diameters of target lesions, taking as reference the smallest sum on study (this includes the baseline sum if that is the smallest on study). In addition to the relative increase of 20%, the sum must also demonstrate an absolute increase of at least 5 mm. (Note: The appearance of one or more new lesions is also considered progression.)
Stable Disease (SD): Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum diameters while on study.[3]
Statistical analysis
Statistical analyses were performed with SPSS software version 11.0.1 for Windows (SPSS, Inc., Chicago, IL, USA), P values less than 0.05 were considered significant.
Result
Out of 43 patients, 4 patients did not complete the treatment course. Thirty-nine patients received complete treatment and followed up for 5 years. Among them, 10 lung, 8 liver, 6 pancreas, 5 gall bladder, and 10 rectum cancer were present. Male: female ratio was 24:15. Mean age was 53 years. Carcinoma rectum patients were confirmed by colonoscopic or direct per-rectal biopsy, whereas others were confirmed by CT guided FNAC. Staging was done following American Joint Commitee on Cancer (AJCC) staging system 2002. Operable patients were excluded from the study. Out of 39 patients, 17 showed response (CR or PR). Response measured by radiological tumor size regression following RECIST criteria.[3] For lung, pancreatic, and gall bladder malignancy CT was used, whereas ultrasonography (per cuteneous and endoscopic) was used for liver and rectal cancers. The response was best in rectal cancer group 70% (7/10) was statistically significant. Nearly, 41.02% patients survived for 1 year after treatment but after 5 years this came down to 15.38% [Table 1]. No statistically significant preferential response based on gender was noted. Lung, gall bladder, and liver showed 40%, 40%, and 37.5% response, respectively [Figure 1]. Poor response was noted in pancreas group (16.66%). No significant side effect was noted during the course of treatment that led to discontinuation.
Table 1.
Response and survival rate after Swarna Bhasma treatment
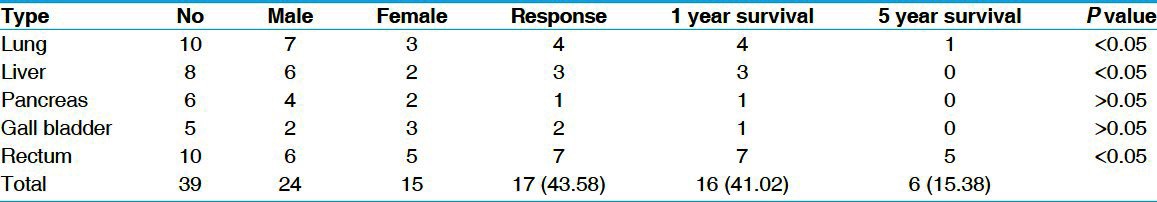
Figure 1.
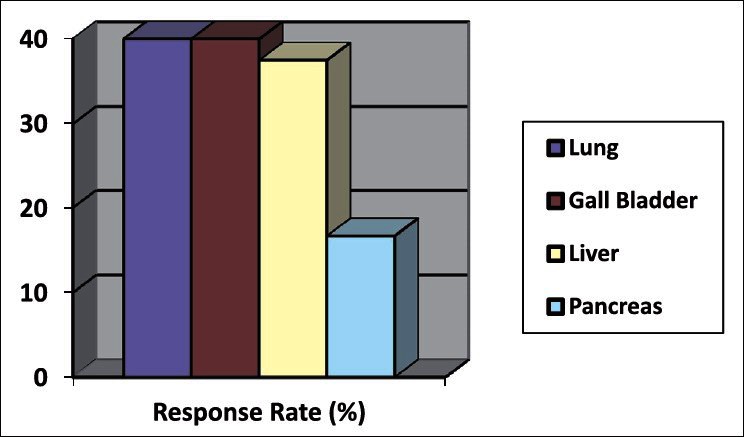
Response rate of different malignancies
Discussion
Cancer treatment is an intensively researched area in the field of modern medicine. Despite this, outcome is not a gold standard. Need exists for an effective weapon. To fill this link CAM is an emerging as a potent modality.[3–8] After review of published literature, it is evident that CAM is mostly used as palliative care. This study with SB is unique in its kind as it is being used as primary therapy.
SB (“Swarna” meaning gold, “Bhasma” meaning ash) is an ancient Indian Ayurvedic medicine used for rejuvenation and revitalization during old age.[3,9,10] Few recent laboratory studies established the pharmacological effects of gold nanoparticle (main ingredient of SB). Gold-silica nanoshell has been used in Nanoshell-Assisted Photo-Tthermal therapy (NAPT) to kill tumor cells by near Infra Red (IR) light.[4] It also showed analgesic property.[5] Gold-EGFR conjugates have been used to detect the cancer cells by exploiting the light scattering properties of gold nanoconjugates.[6] Its use in chronic lymphocytic leukemia showed good results.[7] In this study SB showed fare results in several solid tumors. But the sample size of this study is not adequate enough to have statistically significant results. Thus further randomized blinded studies with large sample size are to be contemplated to establish the effect of SB in malignancy.
Conclusion
Swarna Bhasma can be a potential drug for anticancer therapy. Randomized control trials with large sample size is required to establish this statement.
References
- 1.Source. [last accessed on Feb 2012]. p. 10. Available from: http://www.iarc.fr/en/media centre/iarcnews/2010/globocany2008.php .
- 2.Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM. Gold nanoparticles: A new X-ray contrast agent. Br J Radiol. 2006;79:248–53. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- 3.Eisenhauera EA, Therasseb P, Bogaertsc J, Schwartzd LH, Sargente D, Fordf R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Pitsillides CM, Joe EW, Wei X, Anderson RX, Lin CP. Selective cell targeting with lightabsorbing microparticles and nanoparticles. Biophys J. 2003;84:4023–32. doi: 10.1016/S0006-3495(03)75128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA. 2003;100:13549–54. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajaj S, Vohora SB. Analgesic activity of gold preparations used in Ayurveda and Unani-Tibb. Indian J Med Res. 1998;108:104–11. [PubMed] [Google Scholar]
- 7.El-Sayed IH, Huang X, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: Applications in oral cancer. Nano Lett. 2005;5:829–4. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 8.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–7. [PubMed] [Google Scholar]
- 9.Mohaptra S, Jha CB. Physicochemical characterization of Ayurvedic bhasma (Swarna makshika bhasma): An approach to standardization. Int J Ayurveda Res. 2010;1:82–6. doi: 10.4103/0974-7788.64409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paul W, Sharma CP. Blood compatibility studies of Swarna bhasma (gold bhasma), an Ayurvedic drug. Int J Ayurveda Res. 2011;2:14–22. doi: 10.4103/0974-7788.83183. [DOI] [PMC free article] [PubMed] [Google Scholar]


