Abstract
Objective
To examine the feasibility of continuous glucose monitoring (CGM) use in very young children with type 1 diabetes (T1D).
Research Design and Methods
23 children less than 4 years of age with T1D were provided with a FreeStyle Navigator® (n=21) or a Paradigm® (n=2) CGM device. At baseline, mean age was 3.0 ± 0.8 years, mean HbA1c was 8.0 ± 0.8 %, 10 were using an insulin pump and 13 were on multiple daily injections. CGM use was evaluated over a 6-month period.
Results
Three children dropped out of the study before the end of 6 months. Among the 20 children who completed 6 months of follow-up, CGM use in month 6 was ≥6 days/week in 9 (45%), 4-<6 days/week in 2 (10%), and <4 days/week in 9 (45%). Skin reactions were minimal. Although there was no detectable change in mean HbA1c between baseline and 6 months (7.9% and 8.0%, respectively), there was a high degree of parental satisfaction with CGM as measured on the CGM Satisfaction Scale questionnaire. A high percentage of glucose values were in the hyperglycemic range and biochemical hypoglycemia was infrequent.
Conclusion
More than 40% of very young children were able to safely use CGM on a near daily basis after 6 months. CGM demonstrated frequent hyperglycemic excursions, with a large variability in glucose readings. Although improvement in glycemic control was not detected in the group as a whole, parental satisfaction with CGM was high.
Keywords: Continuous Glucose Monitoring, Young Children, T1D
Introduction
There are a number of factors that make glycemic control difficult in very young children with type 1 diabetes (T1D), including irregular patterns of eating, and unpredictable peaks and valleys in long-acting basal insulin. Young children are very sensitive to small changes in insulin doses and the ability to accurately and precisely deliver insulin by very small increments may only be possible via insulin pump therapy (1–3). Moreover, we have previously shown that parents have difficulty recognizing hypoglycemia in their pre-school children with T1D who often have blunted plasma epinephrine responses to falling blood glucose levels (4).
Tight glycemic control has been limited in young patients with T1D by the concern that recurrent episodes of hypoglycemia may have adverse consequences on the developing brain (5–7). Acute hypoglycemia has deleterious transient effects on multiple aspects of cognition (8–16) and it may play a role in the development of long term minor learning disabilities noted in children who have been diagnosed with T1D when they were < 5 years of age (17). In addition, there is increasing evidence that chronic hyperglycemia may adversely affect the developing brain (18). Hence the avoidance of frequent or severe hypoglycemia and persistent hyperglycemia may be critical, not only for the avoidance of the vascular complications of diabetes, but also for normal brain development and function.
Real-time continuous glucose monitoring (CGM) systems offer the potential to assist patients with T1D to optimize glycemic control more safely. Use of CGM in adult patients has been shown to significantly reduce elevated A1c levels without increasing the frequency of biochemical or symptomatic hypoglycemia (19) and to help maintain target A1c levels with less exposure to biochemical hypoglycemia in well-controlled patients (20). However, use of CGM has been less effective in improving glycemic control in school-aged and adolescent patients due in great measure to their inability to use these devices on a nearly daily basis.
Prior randomized trials have evaluated CGM in children ≥8 years (19, 21) but the use of technology in very young children has been limited (22, 23). Whether frequent CGM use is achievable with current devices in very young children and the benefits and difficulties that parents perceive in using these devices has not been established. Thus, this pilot study was undertaken to evaluate the feasibility of daily CGM use over a prolonged period of time in children less than 4 years old with T1D.
Methods
The study was conducted by the Diabetes Research in Children Network (DirecNet) at five clinical centers. The protocol and consent form were approved by the Institutional Review Board at each center. The parent or guardian gave written informed consent before enrollment. Major eligibility criteria included: 1) clinical diagnosis of T1D, 2) age <4 years and 3) current insulin regimen involving either use of an insulin pump or ≥2 daily insulin injections for at least one month.
The study protocol included an initial run-in period of 7 to 14 days during which a blinded CGM device was used to collect baseline data and to evaluate whether the child and parent were capable of long-term CGM use. A minimum of 4 out 7 days and at least 96 hours of successful glucose values including ≥24 hours overnight (10 p.m. to 6 a.m.) was necessary to continue in the 6-month study. In addition, at least 3 home fingerstick blood sugars per day were required. Of the 29 children who entered the run-in phase, 6 withdrew prior to its completion because of difficulty with sensor insertion (n=3), inability to keep the sensor in place (n=2), or parent frustration (n=1).
The 23 children who successfully completed the run-in phase entered the 6-month study and were provided with a FreeStyle Navigator® (n=21) or Paradigm® (n=2) CGM device, blood glucose meters and strips. The parents were asked to attempt to use the CGM device on a daily basis and were given written instructions on how to use the sensor glucose data provided by CGM and fingerstick readings to make management decisions. Follow-up visits were conducted at 1, 4, 8, 13, 19 and 26 weeks and phone calls with a parent were made between each visit to review downloaded glucose data and adjust diabetes management. HbA1c level was measured using the DCA Vantage analyzer (Siemens Healthcare Diagnostics, Indianapolis) at baseline and at each follow-up visit (except at the 1-week visit). The CGM Satisfaction Scale questionnaire (24, 25) was completed by the parent or guardian at 26 weeks.
Severe hypoglycemia and other adverse events were monitored throughout the study. A skin assessment at the sensor insertion sites was conducted at each visit. Skin reactions were classified as none, mild (present, but not necessarily well defined or immediately noticeable to the subject), moderate (clearly defined or bothers the subject intermittently) or severe (extremely noticeable and bothersome to subject and may indicate infection, risk of infection or potentially life-threatening allergic reaction). Because the children were developmentally too young to independently recognize and react to hypoglycemia, in the absence of seizure or coma, hypoglycemia was only considered severe if there were associated signs or symptoms of neuroglycopenia including temporary impairment of cognition, incoherent, disoriented and/or combative behavior. If plasma glucose measurements were not available during such an event, neurological recovery attributable to the restoration of plasma glucose to normal was considered sufficient evidence that the event was induced by a low plasma glucose concentration.
Statistical Methods
The number of participants in this pilot study was a convenience sample not based on statistical principles. The amount of unblinded CGM use was determined from downloads of the CGM devices. CGM was considered to be used on a day when there was at least one sensor glucose value; there were at least 12 hours of sensor glucose data on 86% of days with at least one sensor value. CGM usage was summarized for every 4–5 week period after the baseline visit and in the last 4 weeks of the study just prior to the 26-week visit. Glycemic control was assessed with HbA1c levels and the proportion of sensor values in the following ranges: 71 mg/dL to 180 mg/dL, ≤60 mg/dL, ≤70 mg/dL and >250 mg/dL. Glucose variability was assessed with coefficient of variation (standard deviation divided by mean glucose). All CGM glucose indices were calculated giving equal weight to each of the 24 hours of a calendar day. Summary values are reported as the mean (±SD) or median and percentiles as appropriate for the distribution. No p-values were calculated in these descriptive analyses. Due to the small sample size, subgroup analyses are not reported. Analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC).
Results
The average age of the 23 children who successfully completed the run-in phase was 3.0 ±0.8 years (range 1.0 to 3.9) with 2 (9%) being 1-<2 years, 9 (39%) 2-<3 years and 12 (52%) 3-<4 years, and 35% were female (Table 1). At baseline, median duration of diabetes was 0.7 years (25th, 75th percentile: 0.4, 1.3) years and mean (±SD) HbA1c was 8.0 ± 0.8%. Ten (43%) of the children were using an insulin pump and the other 13 were on multiple daily injections of insulin (MDI); 4 children switched from MDI to an insulin pump in the middle of the study (at 8, 10, 16 and 19 weeks).
Table 1.
Baseline Characteristics (n=23 who completed the baseline visit)
| N=23 | |
|---|---|
| Age years mean ± SD | 3.0 ± 0.8 |
| [range] | [1.0 to 3.9] |
| 1–<2 years | 2 (9%) |
| 2–<3 years | 9 (39%) |
| 3–<4 years | 12 (52%) |
| Female | 8 (35%) |
| Race/Ethnicity | |
| White (Non-Hispanic) | 22 (96%) |
| African American | 1 (4%) |
| Insulin Modality | |
| MDI | 13 (57%) |
| Pump | 10 (43%) |
| Duration Diabetes (years) | |
| median (25th, 75th percentile) | 0.7 (0.4, 1.3) |
| [range] | [0.2 to 2.6] |
| Parent Education Level | |
| ≤12 | 1 (4%) |
| Associates | 7 (30%) |
| Bachelors | 7 (30%) |
| Masters | 3 (13%) |
| Professional | 5 (22%) |
| HbA1c (site DCA) at Baseline Visit | |
| mean ± SD | 8.0 ± 0.8 |
| [range] | [6.8 to 9.4] |
| 6.8%–<7.0% | 3 (13%) |
| 7.0%–<8.0% | 10 (43%) |
| 8.0%–9.4% | 10 (43%) |
| # Severe Hypoglycemia Events in the Last 6 Months Prior to Enrollment1 | |
| None | 22 (96%) |
| 1 event | 1 (4%) |
| Self-reported Home Blood Glucose Meter Measurements Per Day | |
| median (25th, 75th percentile) | 9 (8, 10) |
| 4–7 | 4 (17%) |
| 8–10 | 14 (61%) |
| 11–14 | 5 (22%) |
Frequency of severe hypoglycemia in the 6 months prior to the study was reported by parents.
CGM Use
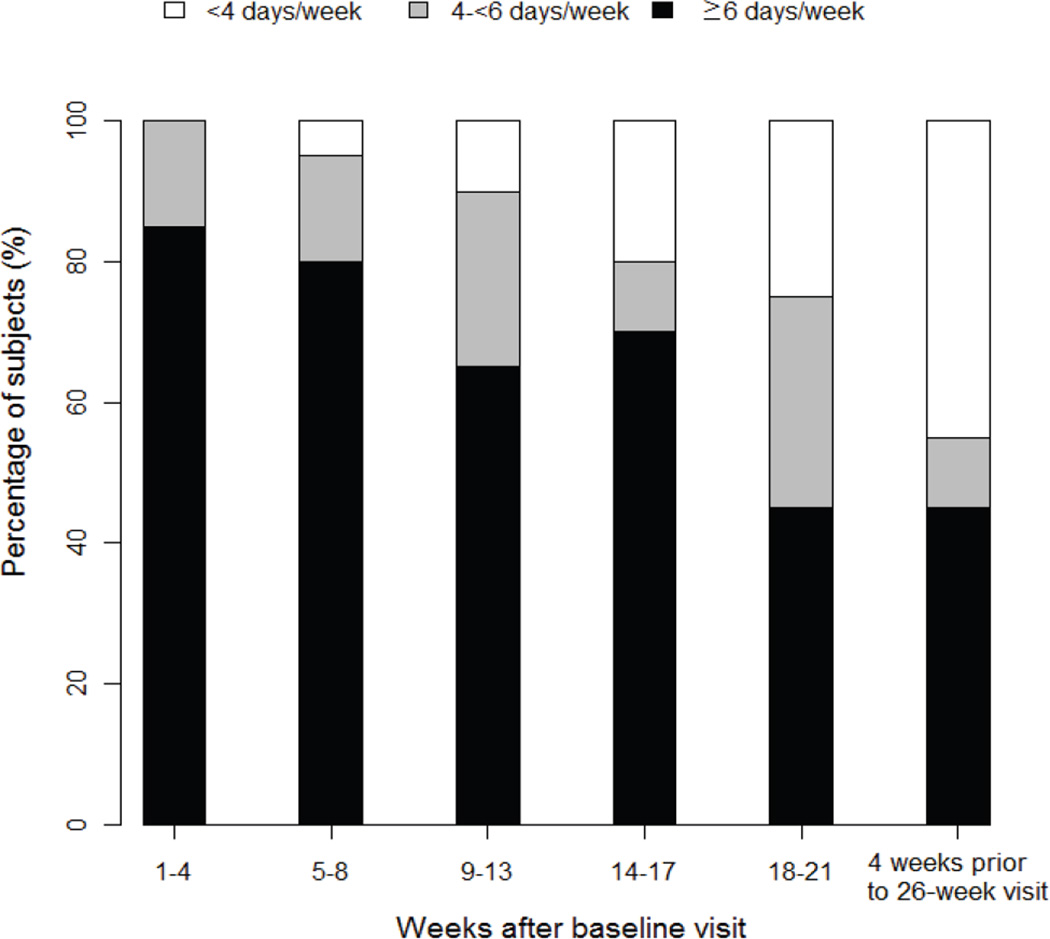
Two children dropped out of the study during the first week due to difficulty with sensor insertion and one dropped out after 19 weeks due to personal issues of the parent unrelated to the study. For the other 20 children who completed the entire 26 weeks, median sensor use was 6.5 (25th, 75th percentile: 6.0, 7.0) days per week in the first 4 weeks. Sensor use declined throughout the study with median use of 4.7 (2.6, 6.6) days per week during 22–26 weeks. As shown in Figure 1, 85% of subjects used the sensor ≥ 6 days per week and 15% of subjects used the sensor 4-≤6 days a week during the first 4 weeks. However, during the last 4 weeks of the study, CGM was being used ≥6 days/week in 9 (45%) children, 4-<6 days/week in 2 (10%) and <4 days/week in 9 (45%).
Figure 1. CGM Usage during the Study.
(limited to children who completed the 26 week visit, N=20)
CGM was considered to be used on a day when there was at least one sensor glucose value.
Metabolic/Glycemic Measures
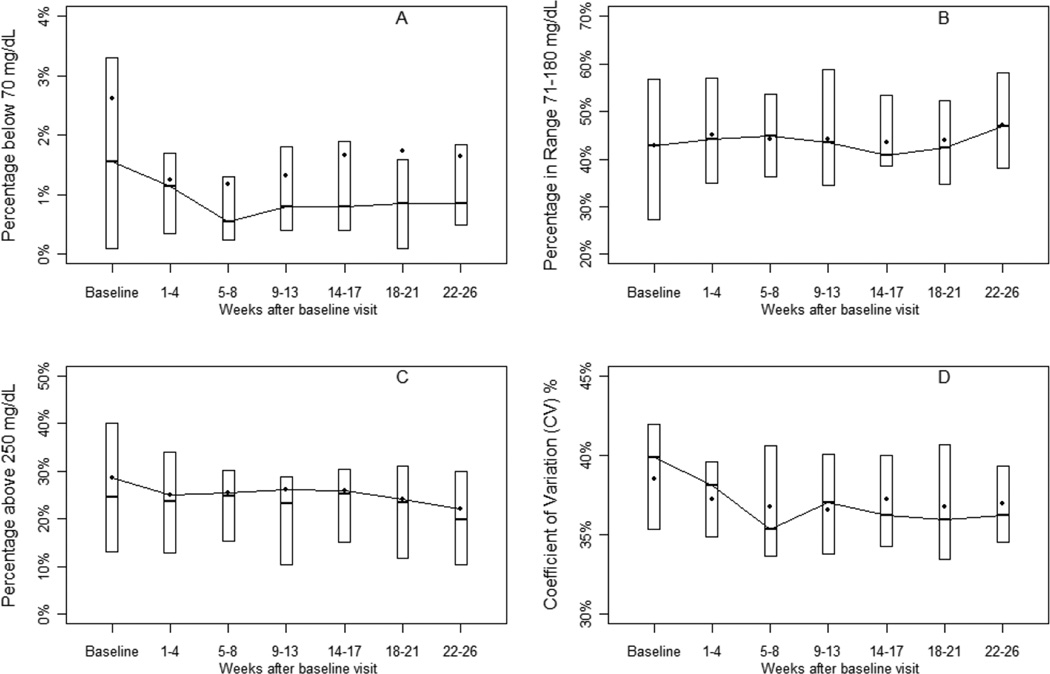
Mean HbA1c for the 20 children who completed the study was 7.9 ± 0.8% at baseline and 8.0 ± 0.8% at 26 weeks. Mean HbA1c increased 0.5 ± 0.7% in the 3 children whose baseline HbA1c was ≤7.0%, increased 0.4 ± 0.7% in the 9 children whose baseline HbA1c was 7.0-<8.0%, and decreased by 0.4 ± 0.7% in the 8 children whose baseline HbA1c was >8.0%. CGM sensor glucose values remained steady over the 6-month period as shown in figure 2.
Figure 2. Glycemic Indices throughout the study (N=20.
% of values ≤70mg/dL (A), % of values in the target range 71–180 mg/dL (B), % of values >250 mg/dL (C) and CV (D). The bottom and top of each box represent the 25th and 75th percentile, respectively. The horizontal line inside the box represents the median and the dot represents the mean.
A listing of each individual’s data including baseline clinical characteristics, CGM use and major outcomes is available in the online supplemental table (Table A1).
CGM Satisfaction
Parents reported high overall satisfaction on the CGM Satisfaction Scale at 26 weeks with an average item score of 4.1 on a 5-point Likert scale (Table 2). Mean item scores were more favorable than "Neutral" (above 3.0) on all 43 items. It is particularly noteworthy that 100% of parents responded that use of CGM helped them learn how to treat hypoglycemia better, clarified how everyday habits affected blood glucose levels, helped adjusting insulin dose through the night; and all but one parent responded that the use of CGM made them more sure about making diabetes decisions, prevented hypoglycemia, helped them relax knowing the unwanted changes in blood glucose will be detected quickly, helped keeping diabetes under control on sick days, showed patterns in blood glucose not seen before, prevented problems rather than fixing them, and felt safer knowing that they can be warned about hypoglycemia before it happens.
Table 2.
| Mean score |
(%) favorable 3 | |
|---|---|---|
| Overall mean score | 4.1 | 79% |
| Benefit subscale4 | 4.4 | 89% |
| Lack of Hassle subscale4 | 3.9 | 72% |
| Question | Mean | N (%) favorable3 |
| Makes me feel safer knowing that I will be warned about low blood sugar before it happens | 4.8 | 19 (95%) |
| ►I don’t recommend this for other children with diabetes | 4.7 | 18 (90%) |
| Has made me worry less about having low blood sugars | 4.7 | 18 (90%) |
| Shows patterns in blood sugars that we didn’t see before | 4.6 | 19 (95%) |
| Helps to keep low blood sugars from happening | 4.6 | 19 (95%) |
| Helps with keeping diabetes under control on sick days | 4.6 | 19 (95%) |
| Helps me to relax, knowing that unwanted changes in blood sugar will be detected quickly | 4.5 | 19 (95%) |
| Helps in adjusting doses of insulin needed through the night | 4.5 | 20 (100%) |
| Helps prevent problems rather than fixing them after they’ve happened | 4.5 | 19 (95%) |
| I want to use this device when it is approved for sale | 4.5 | 17 (85%) |
| Has taught me new things about diabetes that I didn’t know before | 4.4 | 18 (90%) |
| ►Is more trouble than it is worth | 4.4 | 16 (80%) |
| Teaches me how eating affects blood sugar | 4.4 | 18 (90%) |
| Makes it clearer how some everyday habits affect blood sugar levels | 4.4 | 20 (100%) |
| Helps me to be sure about making diabetes decisions | 4.3 | 19 (95%) |
| Has helped me to learn how to treat low sugars better | 4.3 | 20 (100%) |
| ►Has caused more family arguments | 4.3 | 17 (85%) |
| ►Skips too many readings to be useful | 4.3 | 18 (90%) |
| Makes adjusting insulin easier | 4.2 | 16 (80%) |
| Allows more freedom in daily life | 4.2 | 15 (75%) |
| ►Gives a lot of results that don’t make sense | 4.1 | 16 (80%) |
| ►Makes it harder to sleep | 4.1 | 15 (75%) |
| ►Causes more embarrassment about feeling different from others | 4.1 | 15 (75%) |
| ►The feedback from the device is not easy to understand or useful | 4.1 | 16 (80%) |
| ►Causes me to be more worried about controlling blood sugars | 4.0 | 16 (80%) |
| Has helped me to learn about the right amount of exercise | 4.0 | 14 (70%) |
| Has helped my family to get along better about diabetes | 4.0 | 14 (70%) |
| ►Causes too many hassles in daily life | 4.0 | 13 (65%) |
| ►Is too hard to get working right | 4.0 | 15 (75%) |
| Makes it easier to complete other diabetes self care duties | 3.9 | 13 (65%) |
| ►Causes our family to talk about blood sugars too much | 3.9 | 15 (75%) |
| Has helped to adjust pre-meal insulin doses | 3.9 | 16 (80%) |
| ►Makes me think about diabetes too much | 3.9 | 14 (70%) |
| ►Has been harder or more complicated than expected | 3.9 | 12 (60%) |
| ►Causes others to ask too many questions about diabetes | 3.8 | 14 (70%) |
| ►Interferes a lot with sports, playing outside, etc | 3.8 | 14 (70%) |
| ►Causes too many interruptions during the day | 3.8 | 14 (70%) |
| Has made it easier to accept doing blood sugar tests | 3.6 | 12 (60%) |
| ►Alarms too often for no good reason | 3.5 | 13 (65%) |
| Has helped to control diabetes better even when not wearing it | 3.5 | 9 (45%) |
| ►Shows more “glitches” and “bugs” than it should | 3.5 | 12 (60%) |
| ►Sometimes gives too much information to work with | 3.4 | 11 (55%) |
| ►Is uncomfortable or painful | 3.4 | 9 (45%) |
Scoring on a 5 point Likert scale with a higher value denoting more favorable response towards CGM use.
►indicates a negatively worded item, the scoring of which has been reversed so that a higher score denotes a more favorable response towards CGM.
A favorable response is defined as agree/strongly agree with positively worded item or disagree/strongly disagree with negatively worded item.
Benefits of CGM and Lack of Hassles of CGM (25). A higher value denotes more satisfaction for both subscales (more perceived benefit or fewer hassles).
Adverse Events
Severe hypoglycemia occurred in only one subject, a 3.5 years old at enrollment, who reported one severe hypoglycemic (SH) event in the 6 months prior to the study and experienced four SH events during the study (one during the run-in phase while a blinded CGM was worn and three after the run-in phase). Of the three SH events that occurred after the run-in, the child was using the CGM during one hypoglycemic event and was not using CGM during the other two events.
No severe skin reactions were reported over the 6-month period of the study. At the 26-week visit, 2 out of 20 children had evidence of a mild skin reaction and one a moderate reaction. Moderate skin reactions were reported at least once during the study by 8 (35%) of the 23 children and mild reactions at least once by an additional 8 (35%). In 8 children, skin reactions were reported as a reason for reduced CGM use during the study.
Discussion
In this pilot study, we examined the feasibility of CGM in very young children with T1D over a prolonged period of time. The results of the study show that CGM can be safely used in this age group. More than 40% of children in the study were using CGM on a daily or near-daily basis after 6 months. This is similar to the use reported in a randomized controlled trial (RCT) in an older cohort, where 46% of 8–14 year olds used the CGM device at least 6 days a week after 6 months (26). It should be noted that in both of these studies the results reflect a select group of families; in both studies a run-in period excluded participants who were unable to obtain a minimum amount of CGM usage prior to enrollment. Furthermore, this analysis excludes 6 children who failed to wear a sensor during the run-in phase of the study and 3 additional children who dropped out in the middle of the study. Overall, 30% of the children who enrolled in the study were successful in wearing a sensor 6 or more days a week at the 6 month study conclusion.
After 6 months, CGM was still being used ≥ 4 days per week by more than half of the children and parental satisfaction with using CGM was remarkably high, in fact higher than it was for parents of older children in the JDRF CGM RCT (mean score 4.1 versus 3.8 (27). However, there was lack of improvement in HbA1c or in glycemic indices measured with CGM.
Ninety percent of parents reported that using the CGM “made them worry less about having low blood sugars” and 95% of them reported “Helps me to relax, knowing that unwanted changes in blood sugar will be detected quickly”. Although CGM appeared to enhance parent quality of life particularly with respect to avoidance of hypoglycemia, this did not translate into more aggressive management in an attempt to achieve tighter glucose control. Even with the use of CGM, glucose values in most of these very young children with T1D exceeded 250 mg/dL for more than 4 hours a day whereas glucose levels ≤70 mg/dL were present for only a few minutes a day. For some parents, it is possible that observing downward trends of the CGM glucose levels might have actually made them less aggressive in trying to achieve tighter glycemic control, which might explain why HbA1c levels rose in most of the children with baseline HbA1c levels <7.5%.
This pilot study has demonstrated that a CGM device can be successfully worn by very young children with T1D but that a better understanding of the barriers to better glycemic control are needed. The results emphasize the importance of ultimately having an artificial pancreas to improve glycemic control in these children.
Supplementary Material
Acknowledgements
Abbott Diabetes Care (Alameda, CA) provided the FreeStyle Navigator® and the FreeStyle blood glucose Meter and test strips. Medtronic Minimed (Northridge, CA) provided the both Paradigm® and sensors at a discounted price.
Financial Disclosures
Financial support: This research was supported by the following NIH/NICHD HD41890-10; HD41906-10; HD41908-10; HD41915; HD41918; HD56526.
Appendix
Writing Committee:
Eva Tsalikian, MD; Larry Fox, MD; Stuart Weinzimer, MD; Bruce Buckingham, MD; Neil H. White, MD; Roy Beck, MD, PhD; Craig Kollman, PhD; Dongyuan Xing, MPH; Katrina Ruedy, MSPH, and the Diabetes Research in Children Network (DirecNet) Study Group
The DirecNet Study Group:
Clinical Centers: (Listed in alphabetical order with clinical center name, city, and state. Personnel are listed as (PI) for Principal Investigator, (I) for co-Investigator and (C) for Coordinators.) (2) Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, IA: Eva Tsalikian, MD (PI); Michael J. Tansey, MD (I); Julie Coffey, MSN (C); Joanne Cabbage (C); (3) Nemours Children’s Clinic, Jacksonville, FL: Nelly Mauras, MD (PI); Larry A. Fox, MD (I); Kim Englert, RN (C); Joe Permuy, ARNP (C) (4) Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, CA: Bruce A. Buckingham, MD (PI); Darrell M. Wilson, MD (I); Paula Clinton, RD, CDE (C); Kimberly Caswell, APRN (C); (5) Department of Pediatrics, Yale University School of Medicine, New Haven, CT: Stuart A. Weinzimer, MD (PI); William V. Tamborlane, MD (I); Jennifer Sherr, MD (I); Amy Steffen, BS (C); Kate Weyman, MSN (C); (6) Washington University, St. Louis, MO: Neil H. White, MD (PI); Ana Maria Arbelaez, MD, (I); Lucy Levandoski, PA-C (C); Angie Starnes, RN, BSN, CDE (C); Coordinating Center: Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD; Katrina J. Ruedy, MSPH; Craig Kollman, PhD; Dongyuan Xing, MPH; National Institutes of Health: Gilman D. Grave, MD, PhD; Karen K. Winer, MD; Ellen Leschek, MD; Data and Safety Monitoring Board: Mark Sperling, MD; Dorothy M. Becker, MBBCh; Patricia Cleary, MS; Carla Greenbaum, MD; Antoinette Moran, MD.
Contributor Information
Eva Tsalikian, University of Iowa, Iowa City, IA, 52242.
Larry Fox, Nemours Children’s Clinic, Jacksonville, FL 32256.
Stuart Weinzimer, Yale University, New Haven, CT, 06520.
Bruce Buckingham, Stanford University, Stanford, CA, 94305.
Neil H. White, Washington University, St. Louis, MO, 63110.
Beck Beck, Jaeb Center for Health Research, Tampa, FL, 33647.
Craig Kollman, Jaeb Center for Health Research, Tampa, FL, US, 33647.
Dongyuan Xing, Jaeb Center for Health Research, Tampa, FL, US, 33647.
Katrina Ruedy, Jaeb Center for Health Research, Tampa, FL, US, 33647.
References
- 1.DiMeglio LA, Pottorff TM, Boyd SR, France L, Fineberg N, Eugster EA. A randomized, controlled study of insulin pump therapy in diabetic preschoolers. JPediatr. 2004;145:380–384. doi: 10.1016/j.jpeds.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 2.Fox LA, Buckloh LM, Smith SD, Wysocki T, Mauras N. A Randomized Controlled Trial of Insulin Pump Therapy in Young Children With Type 1 Diabetes. Diabetes Care. 2005;28:1277–1281. doi: 10.2337/diacare.28.6.1277. [DOI] [PubMed] [Google Scholar]
- 3.Weinzimer SA, Ahern JH, Doyle EA, et al. Persistence of benefits of continuous subcutaneous insulin infusion in very young children with type 1 diabetes: a follow-up report. Pediatrics. 2004;114:1601–1605. doi: 10.1542/peds.2004-0092. [DOI] [PubMed] [Google Scholar]
- 4.Diabetes Research in Children Network (DirecNet) Study Group. Blunted Counterregulatory Hormone Responses to Hypoglycemia in Young Children and Adolescents With Well-Controlled Type 1 Diabetes. Diabetes Care. 2009;32:1954–1959. doi: 10.2337/dc08-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis EA, Jones TW. Hypoglycemia in children with diabetes: incidence, counterregulation and cognitive dysfunction. J Pediatr Endocrinol Metab. 1998;11:177–182. doi: 10.1515/jpem.1998.11.s1.177. [DOI] [PubMed] [Google Scholar]
- 6.Northam EA, Anderson PJ, Werther GA, Warne GL, Andrewes D. Predictors of change in the neuropsychological profiles of children with type 1 diabetes 2 years after disease onset. Diabetes Care. 1999;22:1438–1444. doi: 10.2337/diacare.22.9.1438. [DOI] [PubMed] [Google Scholar]
- 7.Warren RE, Frier BM. Hypoglycaemia and cognitive function. Diabetes Obes Metab. 2005;7:493–503. doi: 10.1111/j.1463-1326.2004.00421.x. [DOI] [PubMed] [Google Scholar]
- 8.Fanelli C, Paramore D, Hershey T, Terkamp C, Ovalle F, Craft S, et al. Impact of nocturnal hypoglycemia on hypoglycemic cognitive dysfunction in type 1 diabetes. Diabetes. 1998;47:1920–1927. doi: 10.2337/diabetes.47.12.1920. [DOI] [PubMed] [Google Scholar]
- 9.Hannonen R, Tupola S, Ahonen T, Riikonen R. Neurocognitive functioning in children with type-1 diabetes with and without episodes of severe hypoglycaemia. Dev Med Child Neurol. 2003;45:262–268. doi: 10.1017/s0012162203000501. [DOI] [PubMed] [Google Scholar]
- 10.Haumont D, Dorchy H, Pelc S. EEG abnormalities in diabetic children: influence of hypoglycemia and vascular complications. Clin Pediatr. 1979;18:750–753. doi: 10.1177/000992287901801205. [DOI] [PubMed] [Google Scholar]
- 11.Hershey T, Craft S, Bhargava N, White NH. Memory and insulin dependent diabetes mellitus (IDDM): effects of childhood onset and severe hypoglycemia. J Int Neuropsychol Soc. 1997;3:509–520. [PubMed] [Google Scholar]
- 12.Hershey T, Lillie R, Sadler M, White NH. A prospective study of severe hypoglycemia and long-term spatial memory in children with type 1 diabetes. Pediatric Diabetes. 2004;5:63–71. doi: 10.1111/j.1399-543X.2004.00045.x. [DOI] [PubMed] [Google Scholar]
- 13.Hershey T, Lillie R, Sadler M, White NH. Severe hypoglycemia and long-term spatial memory in children with type 1 diabetes mellitus: a retrospective study. J Int Neuropsychol Soc. 2003;9:740–750. doi: 10.1017/S1355617703950077. [DOI] [PubMed] [Google Scholar]
- 14.Hershey T, Perantie DC, Warren SL, Zimmerman EC, Sadler M, White NH. Frequency and timing of severe hypoglycemia affects spatial memory in children with type 1 diabetes. Diabetes Care. 2005;28:2372–2377. doi: 10.2337/diacare.28.10.2372. [DOI] [PubMed] [Google Scholar]
- 15.Northam EA, Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA. Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care. 2001;24:1541–1546. doi: 10.2337/diacare.24.9.1541. [DOI] [PubMed] [Google Scholar]
- 16.Rovet JF, Ehrlich RM. The effect of hypoglycemic seizures on cognitive function in children with diabetes: A 7-year prospective study. J Pediatr. 1999;134:503–506. doi: 10.1016/s0022-3476(99)70211-8. [DOI] [PubMed] [Google Scholar]
- 17.Bjorgaas M, Gimse R, Vik T, Sand T. Cognitive function in type 1 diabetic children with and without episodes of severe hypoglycaemia. Acta Paediatr. 1997;86:148–153. doi: 10.1111/j.1651-2227.1997.tb08856.x. [DOI] [PubMed] [Google Scholar]
- 18.Lin A, Northam EA, Rankins D, Werther GA, Cameron FJ. Neuropsychological profiles of young people with type 1 diabetes 12 yr after disease onset. Pediatr Diabetes. 2010;11:235–243. doi: 10.1111/j.1399-5448.2009.00588.x. [DOI] [PubMed] [Google Scholar]
- 19.The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 20.The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32:1378–1383. doi: 10.2337/dc09-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diabetes Research in Children Network Study Group. Continuous glucose monitoring in children with type 1 diabetes. J Pediatr. 2007;151:388–393. doi: 10.1016/j.jpeds.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandrud LM, Xing D, Kollman C, Block JM, Kunselman B, Wilson DM, et al. The Medtronic Minimed Gold continuous glucose monitoring system: an effective means to discover hypo- and hyperglycemia in children under 7 years of age. Diabetes Technol Ther. 2007;9:307–316. doi: 10.1089/dia.2007.0026. [DOI] [PubMed] [Google Scholar]
- 23.Patton SR, Williams LB, Edger SJ, Crawford MJ, Dolan L, Powers SW. Use of continuous glucose monitoring in young children with type 1 diabetes: implications for behavioral research. Pediatric Diabetes. 2011;12:18–24. doi: 10.1111/j.1399-5448.2010.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diabetes Research in Children Network (DirecNet) Study Group. Youth and parent satisfaction with clinical use of the Glucowatch G2 Biographer in the management of pediatric type 1 diabetes. Diabetes Care. 2005;28:1929–1935. doi: 10.2337/diacare.28.8.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Validation of measures of satisfaction with and impact of continuous and conventional glucose monitoring. Diabetes Technol Ther. 2010;12:679–684. doi: 10.1089/dia.2010.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2009;32:1947–1953. doi: 10.2337/dc09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tansey M, Laffel L, Cheng J, et al. Satisfaction with continuous glucose monitoring in adults and youths with type 1 diabetes. Diabet Med. 2011 doi: 10.1111/j.1464-5491.2011.03368.x. In Press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.