Abstract
Background:
Extracorporeal membrane oxygenation (ECMO) therapy constitutes the last option for patients with acute respiratory distress syndrome (ARDS) refractory to conservative treatment. Since primary care centers are unable to provide this therapy, such patients need a transfer to a tertiary care center, which may be life-threatening without extracorporeal support.
Methods:
An ECMO transport team implanted an ECMO at the site of the primary care center with subsequent transport of the patient to the tertiary care center. Between September 2009 and March 2011, six patients with ARDS were treated by our ECMO transport team. Mean age was 39.5±12.0 years. All implantations were done percutaneously in a veno-venous configuration.
Results:
No complications occurred during the implant procedure and the subsequent transport. Four patients (67%) were successfully weaned from ECMO-therapy, and discharged from hospital.
Conclusion:
With a specialized ECMO transport team, ECMO-implantation can be achieved successfully in a peripheral hospital, and patients can be transported safely.
Keywords: Acute respiratory distress syndrome, extracorporeal life support, extracorporeal membrane oxygenation, interhospital transfer
INTRODUCTION
In the H1N1 pandemia in 2009/2010, acute respiratory distress syndrome (ARDS) developed in 5.5% of all cases. When patients developed H1N1 associated pneumonia, it progressed to ARDS in 37% to 50% of cases.[1–3] ARDS carries a high mortality, which ranges between 34% and 58%.[4,5] Extracorporeal membrane oxygenation has become a life-saving therapeutic option in severe ARDS which cannot be treated by aggressive ventilatory therapy.[6] The required technical equipment is only available in tertiary care centers. Therefore, patients with ARDS at a peripheral hospital have a lower chance of survival as compared to patients in a center hospital, unless they are transferred from the primary to the tertiary care hospital.[7] Interhospital transfer under conventional therapy carries a high risk for patients in such critical condition.[8] Installation of ECMO in the peripheral hospital with subsequent transportation to the center hospital offers those patients a better chance to survive. For this reason, an ECMO transport team was established consisting of a cardiac surgeon and a perfusionist. In case of a request by a peripheral hospital, this team can rapidly establish a veno-venous ECMO therapy on-site and then transfer the patient to the tertiary care center.
MATERIALS AND METHODS
Patient evaluation and preparation for the implant procedure
After receiving the request, the cardiac surgeon on duty assessed the patient's condition in the peripheral hospital and confirmed the indication for ECMO therapy. An ambulance car carried the cardiac surgeon, the perfusionist and the equipment to the peripheral hospital. On-site, the patient's condition was assessed once again. The pulmonary situation was evaluated including the ventilatory parameters and the respirator settings, and laboratory values were checked, especially red blood count, platelet count and the coagulation status. If available, transesophageal echocardiography (TEE) was installed.
Surgical technique
Thereafter, the patient was scrubbed in a sterile fashion, and the procedure was begun after administration of a heparine bolus (100 IU/kg). Both cannulae were inserted in Seldinger's technique, preferably via the right femoral vein for venous drainage and via the right internal jugular vein for venous return of oxygenated blood [Figure 1]. Ideally, the drainage cannula was placed under TEE guidance with its tip positioned at the confluence of the inferior vena cava with the right atrium [Figure 2]. For this purpose, a long cannula of 24 or 28 F (VFEM, Edwards Lifesciences, Horw, Switzerland) was used depending on the flow required. The cannula in the internal jugular vein was positioned in the superior vena cava without TEE control. Therefore, a short cannula of 16 or 18 F (Fem-Flex II, Edwards Lifesciences, Horw, Switzerland) was applied, according to the calculated flow. If there would have been any doubt concerning the positioning of the cannulae, fluoroscopy would have been performed. Once the cannulae were placed and connected to the ECMO system free of air bubbles, extracorporeal circulation was started. A completely heparin coated ECMO tubing set with a Quadrox PLS oxygenator was used together with a ROTAFLOW console (Maquet, Hirrlingen, Germany).
Figure 1.
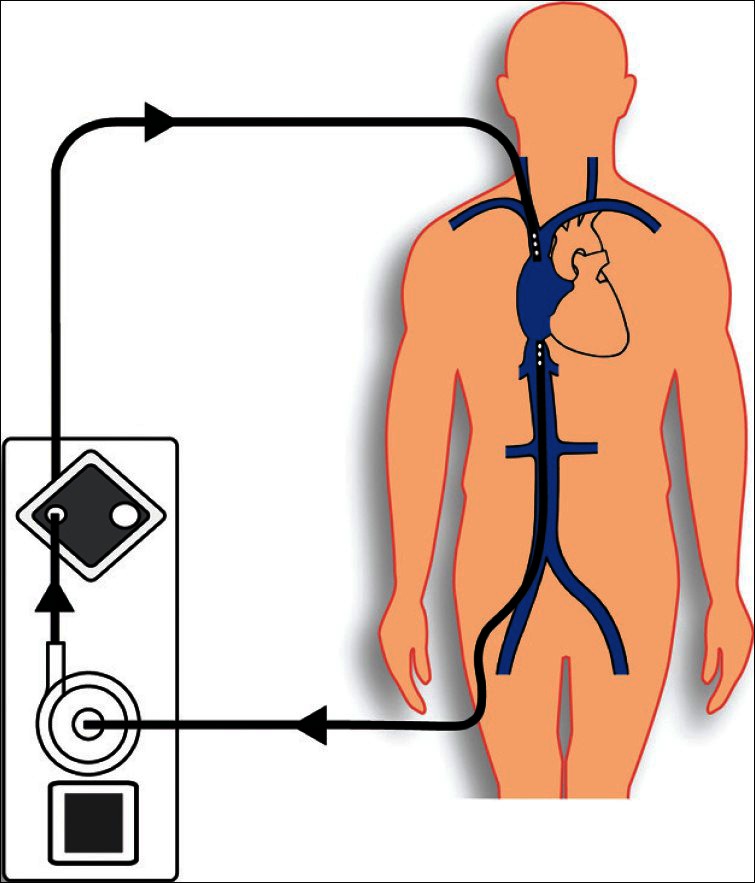
Scheme of veno-venous Extracorporeal membrane oxygenation setup. The cannulas were placed percutaneously using Seldinger's technique via the right internal jugular vein and the right femoral vein
Figure 2.
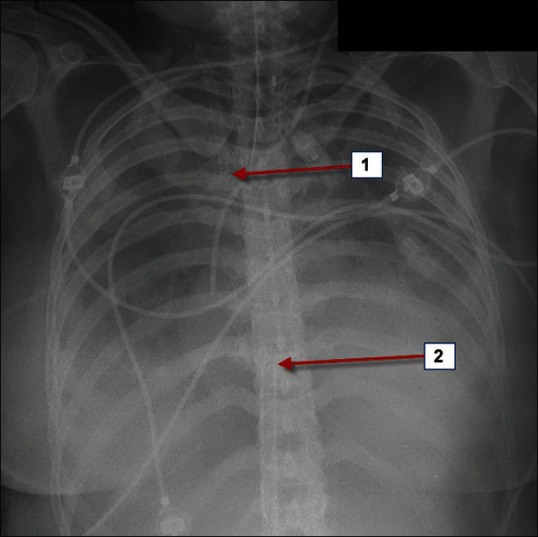
Optimal positioning of the inflow and outflow cannulae is the key for effective veno-venous Extracorporeal membrane oxygenation therapy. 1=outflow cannula via right internal jugular vein with its tip visible at the beginning of the superior vena cava. 2=tip of the inflow cannula at the junction of inferior vena cava to the right atrium via right femoral vein
Transportation on Extracorporeal membrane oxygenation therapy
Subsequently, Extracorporeal membrane oxygenation (ECMO) therapy was evaluated by means of hemodynamic monitoring, peripheral oxygen saturation and blood gas analysis. Extraordinary care was taken for fixation of the cannulae and the ECMO system, so that there was no risk for disconnection during the following transport. After a 30 minute period of stable ECMO therapy, the patient was transferred to the tertiary care center in an ambulance car. During transport the patient was monitored by electrocardiography, pulse oximetry and invasive arterial blood pressure measurement. The complex arrangement of patient, ECMO and accompanying medical staff in the car did not allow the usual speed of transportation. Upon arrival, the patient was transferred to the ICU, and therapy was continued by the intensivists.
Results of Extracorporeal membrane oxygenation transports
Between September 2009 and March 2011, six patients were supplied with an Extracorporeal membrane oxygenation (ECMO) at a primary care hospital and transferred to our center [Table 1]. Mean age was 39.5 years±12.0 years. Five patients were male and one female. All patients suffered from ARDS, which was indicated by a Murray score of more than 2.5 points.
Table 1.
Patient data and outcome of Extracorporeal membrane oxygenation therapy
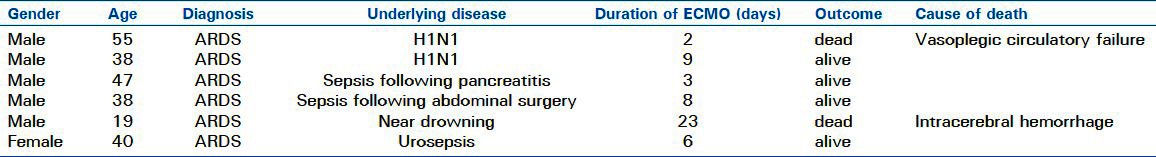
Two patients developed ARDS due to H1N1-pneumonia, two patients due to sepsis because of gastrointestinal disease, one patient due to urosepsis and one due to near-drowning. All patients were refractory to conventional ventilatory therapy. In none of the patients, we encountered complications during implantation. Transport of the patients from the primary to the tertiary care center was safe, and no complications occurred during transportation. The mean distance was 25 km with a range from 12 to 55 km.
Outcome of Extracorporeal membrane oxygenation therapy
In the following stay at the tertiary care center, four patients (67%) were successfully weaned from ECMO, all surviving up to date. Mean follow-up time for the surviving patients was 374 days (38-559 days). Two patients died on ECMO support, one because of vasoplegic circulatory failure two days after ECMO implantation, the other due to intracranial hemorrhage 23 days after ECMO implantation [Table 1]. Thirty day mortality was 33%, as was mortality to hospital discharge.
DISCUSSION
Acute respiratory distress syndrome is associated with a high mortality rate, even with state-of-the-art intensive care treatment.[4–6] If conventional therapy fails, ECMO therapy is the last chance for survival.[9,10] When ECMO therapy is initiated as an ultima ratio therapy, it is often too late to safe the patient. It is therefore desirable to start ECMO treatment at an earlier stage, which gives the lungs a better chance to recover since protective lung ventilation can be instituted.[11–13]
The goal of interhospital transfer is to offer patients better treatment options at high volume centers with specialized know-how.[8,14] Interhospital transfer may be dangerous for a patient in a critical condition.[8] Critically ill patients, who were admitted to tertiary intensive care units after interhospital transfer had significantly higher ICU mortality rates than patients admitted directly via the emergency department.[15,16] This emphasizes the necessity of an individual risk-benefit evaluation for every individual patient who is considered for interhospital transfer.
If one considers the potential risk of an incident during interhospital transport, it appears self-evident that a high level of safety has to be warranted. This includes the quality of the equipment as well as the expertise of the medical staff.[17,18]
It has been shown that the assignment of a specialized retrieval team and the availability of state-of-the-art equipment are associated with better outcomes.[19]
If these prerequisites are not given, the transport inevitably increases the risk for such patients who are already at high risk to die. On the other hand, if those patients are not transferred to the tertiary care center, survival might be even less likely. Therefore, the benefit must outweigh the risk of the interhospital transport, in order to improve patient outcome. This is, however, difficult to calculate for an individual patient.
There is an increasing number of observational case series which show that interhospital transfer under ECMO therapy can be performed safely with low complication rates.[20–24] Our report compares favorably with the experience of those centers. The CESAR trial was the first well-designed, controlled, prospectively randomized study which was able to show a significant improvement in survival of patients with severe ARDS at 6 months following interhospital transport to a tertiary care center for consideration for ECMO therapy.[7]
Considering the improval of outcome following ECMO therapy for ARDS over the recent years, a big effort should be undertaken to provide this treatment to a growing number of patients. While conventional therapy of ARDS is associated with a mortality rate of 34-58%,[4,5] recent studies, such as The Australia and New Zealand ECMO influenza investigators, have shown a markedly reduced mortality to 21% following ECMO therapy.[25] However, the expansion of ECMO therapy requires the amplification of resources such as medical staff, ICU beds and ECMO equipment. This is only feasible if hospital administration is willing to provide appropriate financial and logistic support.
Our mobile ECMO team was established in 2009 at the time of the H1N1 pandemia, when the demand for transport of patients on ECMO support markedly increased. According to the recommendations in the position article of the ECLS working group of the European Association for Cardio-Thoracic Surgery,[26] the team essentially consists of a cardiac surgeon, who places the cannulae, and a perfusionist who runs the ECMO. In addition, an intensivist or anesthesiologist is needed who takes care of the ventilation and drug management during transportation. All members of an ECMO transport team need to have extensive experience in ECMO therapy, especially regarding the implant procedure and the required hardware. This needs special emphasis since in such a setting the implant procedure is not performed in a familiar surrounding with possible experienced backup. Members of the ECMO transport team need to be able to identify and adequately handle possible complications of the implant procedure, such as shunt flow due to inappropriate cannula placement or vascular injury.
For transportation, a particularly equipped ambulance car serving as mobile intensive care unit should be available.[26] Not only should it offer enough space for all the medical personel, the devices and equipment, but also have a connection for power supply. This safes energy of the ECMO battery and prevents an ECMO standstill due to low battery charge which may be encountered on transports of a greater distance. As yet, we have used a regular ambulance car which normally provides power supply. However, space is very limited, and it is hard to arrange patient, medical staff and machinery in the car.
All material which is needed for connecting the patient to the ECMO has to be taken from the specialized center to the peripheral hospital. Guide wires, surgical instruments, cannulae and introduction sets are highly sophisticated tools which cannot be expected to be available in a hospital of primary care.
Transesophageal echocardiography (TEE) for correct positioning of the cannulae is usually done by a cardiologist of the peripheral hospital. If TEE is not available, the location of the cannulae is controlled by fluoroscopy. Optimal positioning of the cannulae is crucial for the effectiveness of ECMO-therapy and needs to be verified before starting the transport. Thereafter protection of the cannulae from displacement is of utmost importance for the subsequent transport.
CONCLUSION
Emergency on-site veno-venous ECMO implantation in a peripheral hospital and the subsequent patient transport to the tertiary center appears to be feasible with low risk and promising outcome. A dedicated ECMO transport team and professional equipment is a precondition for its success. The costs for material and man power as well as logistic challenges are considerable.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bagdure D, Curtis DJ, Dobyns E, Glodé MP, Dominguez SR. Hospitalized children with 2009 pandemic influenza A (H1N1): Comparison to seasonal influenza and risk factors for admission to the ICU. PLoS One. 2010;5:e15173. doi: 10.1371/journal.pone.0015173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai L, Gu L, Cao B, Zhai XL, Lu M, Lu Y, et al. Clinical features of pneumonia caused by 2009 influenza A(H1N1) virus in Beijing, China. Chest. 2011;139:1156–64. doi: 10.1378/chest.10-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Champunot R, Tanjatham S, Kerdsin A, Puangpatra P, Wangsai S, Treebuphachatsakul P, et al. Impact of pandemic influenza (H1N1) virus-associated community-acquired pneumonia among adults in a tertiary hospital in Thailand. Jpn J Infect Dis. 2010;63:251–6. [PubMed] [Google Scholar]
- 4.Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30:51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 5.Bersten AD, Edibam C, Hunt T, Moran J. Australian and New Zealand Intensive Care Society Clinical Trials Group. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med. 2002;165:443–8. doi: 10.1164/ajrccm.165.4.2101124. [DOI] [PubMed] [Google Scholar]
- 6.Hamid IA, Hariharan AS, Shankar NR. The advent of ECMO and pumpless extracorporeal lung assist in ARDS. J Emerg Trauma Shock. 2011;4:244–50. doi: 10.4103/0974-2700.82212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009;374:1351–63. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 8.Fromm RE, Jr, Dellinger RP. Transport of critically ill patients. J Intensive Care Med. 1992;7:223–33. doi: 10.1177/088506669200700503. [DOI] [PubMed] [Google Scholar]
- 9.Park PK, Napolitano LM, Bartlett RH. Extracorporeal membrane oxygenation in adult acute respiratory distress syndrome. Crit Care Clin. 2011;27:627–46. doi: 10.1016/j.ccc.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Patroniti N, Zangrillo A, Pappalardo F, Peris A, Cianchi G, Braschi A, et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: Preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011;37:1447–57. doi: 10.1007/s00134-011-2301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamid IA, Hariharan AS, Shankar NR. The advent of ECMO and pumpless extracorporeal lung assist in ARDS. J Emerg Trauma Shock. 2011;4:244–50. doi: 10.4103/0974-2700.82212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park PK, Napolitano LM, Bartlett RH. Extracorporeal membrane oxygenation in adult acute respiratory distress syndrome. Crit Care Clin. 2011;27:627–46. doi: 10.1016/j.ccc.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Tiruvoipati R, Botha J, Peek G. Effectiveness of extracorporeal membrane oxygenation when conventional ventilation fails: Valuable option or vague remedy? J Crit Care. 2012;27:192–8. doi: 10.1016/j.jcrc.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Koppenberg J, Taeger K. Interhospital transport: Transport of critically ill patients. Curr Opin Anaesthesiol. 2002;15:211–5. doi: 10.1097/00001503-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Hill AD, Vingilis E, Martin CM, Hartford K, Speechley KN. Interhospital transfer of critically ill patients: Demographic and outcomes comparison with nontransferred intensive care unit patients. J Crit Care. 2007;22:290–5. doi: 10.1016/j.jcrc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Flabouris A, Hart GK, George C. Outcomes of patients admitted to tertiary intensive care units after interhospital transfer: Comparison with patients admitted from emergency departments. Crit Care Resusc. 2008;10:97–105. [PubMed] [Google Scholar]
- 17.Fromm RE, Jr, Dellinger RP. Transport of critically ill patients. J Intensive Care Med. 1992;7:223–33. doi: 10.1177/088506669200700503. [DOI] [PubMed] [Google Scholar]
- 18.van Lieshout EJ, de Vos R, Binnekade JM, de Haan R, Schultz MJ, Vroom MB. Decision making in interhospital transport of critically ill patients: National questionnaire survey among critical care physicians. Intensive Care Med. 2008;34:1269–73. doi: 10.1007/s00134-008-1023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiegersma JS, Droogh JM, Zijlstra JG, Fokkema J, Ligtenberg JJ. Quality of interhospital transport of the critically ill: Impact of a Mobile Intensive Care Unit with a specialized retrieval team. Crit Care. 2011;15:R75. doi: 10.1186/cc10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peris A, Cianchi G, Biondi S, Bonizzoli M, Pasquini A, Bonacchi M, et al. Extracorporeal life support for management of refractory cardiac or respiratory failure: Initial experience in a tertiary centre. Scand J Trauma Resusc Emerg Med. 2010;18:28. doi: 10.1186/1757-7241-18-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciapetti M, Cianchi G, Zagli G, Greco C, Pasquini A, Spina R, et al. Feasibility of inter-hospital transportation using extra-corporeal membrane oxygenation (ECMO) support of patients affected by severe swine-flu(H1N1)-related ARDS. Scand J Trauma Resusc Emerg Med. 2011;19:32. doi: 10.1186/1757-7241-19-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Ancona G, Capitanio G, Chiaramonte G, Serretta R, Turrisi M, Pilato M, et al. Extracorporeal membrane oxygenator rescue and airborne transportation of patients with influenza A (H1N1) acute respiratory distress syndrome in a Mediterranean underserved area. Interact Cardiovasc Thorac Surg. 2011;12:935–7. doi: 10.1510/icvts.2010.260448. [DOI] [PubMed] [Google Scholar]
- 23.Philipp A, Arlt M, Amann M, Lunz D, Müller T, Hilker M, et al. First experience with the ultra compact mobile extracorporeal membrane oxygenation system Cardiohelp in interhospital transport. Interact Cardiovasc Thorac Surg. 2011;12:978–81. doi: 10.1510/icvts.2010.264630. [DOI] [PubMed] [Google Scholar]
- 24.Haneya A, Philipp A, Foltan M, Mueller T, Camboni D, Rupprecht L, et al. Extracorporeal circulatory systems in the interhospital transfer of critically ill patients: Experience of a single institution. Ann Saudi Med. 2009;29:110–4. doi: 10.4103/0256-4947.51792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies A, Jones D, Bailey M, Beca J, Bellomo R, et al. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302:1888–95. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 26.Beckmann A, Benk C, Beyersdorf F, Haimerl G, Merkle F, Mestres C, et al. Position article for the use of extracorporeal life support in adult patients. Eur J Cardiothorac Surg. 2011;40:676–80. doi: 10.1016/j.ejcts.2011.05.011. [DOI] [PubMed] [Google Scholar]


