Abstract
Fat embolism syndrome (FES) is an ill-defined clinical entity that arises from the systemic manifestations of fat emboli within the microcirculation. Embolized fat within capillary beds cause direct tissue damage as well as induce a systemic inflammatory response resulting in pulmonary, cutaneous, neurological, and retinal symptoms. This is most commonly seen following orthopedic trauma; however, patients with many clinical conditions including bone marrow transplant, pancreatitis, and following liposuction. No definitive diagnostic criteria or tests have been developed, making the diagnosis of FES difficult. While treatment for FES is largely supportive, early operative fixation of long bone fractures decreases the likelihood of a patient developing FES.
Keywords: Fat embolism, fat embolism syndrome, trauma
OVERVIEW
Over 150 years ago, Zenker described the first case of fat embolism syndrome (FES) in a patient suffering from crush injury.[1] While FES was later clinically diagnosed and often reported in the literature over the next 100 years, Gurd's clinical description of the FES renewed interest in studying this syndrome.[2,3]
Fat embolism is the presence of fat particles within the microcirculation, while FES is the systemic manifestation of fat emboli within the microcirculation. Common systemic manifestations include respiratory distress, altered mental status, and a rash.
FES is most often associated with orthopedic trauma. Rare cases of FES have been reported to occur following bone marrow transplantation, osteomyelitis, pancreatitis, alcoholic fatty liver, and even liposuction.[4] Since most cases of FES occur following orthopedic trauma, available research focused on FES in orthopedic trauma patients.
EPIDEMIOLOGY
Fat embolization occurs frequently following orthopedic trauma. Fat globules have been detected in the blood of 67% of orthopedic trauma patients in one study.[5] This number increased to 95% when the blood is sampled in close proximity to the fracture site.[6]
Hypoxemia may suggest fat embolization causing subclinical FES. Almost all patients monitored with continuous pulse oximetry following a long-bone fracture will have episodes of hypoxemia.[7]
Further embolization may occur during operative fixation. Intraoperative transesophageal echocardiogram studies have detected fat embolization in 41% of patients during the fixation of long-bone fractures.[8]
While almost all patients will have fat globules detected in the blood or develop transient hypoxia, the incidence of FES is much lower. In his initial study defining the clinical criteria for FES, Gurd reported the incidence of FES as 19% in a group of trauma patients.[9] As early operative fixation of long-bone fractures has become standard care, modern studies report an incidence of FES between 0.9% and 11%.[10–12]
PATHOPHYSIOLOGY
Fat particles enter the circulation and cause damage to capillary beds. While the pulmonary system is most frequently affected, fat embolism can occur in the microcirculation of the brain, skin, eyes, and heart can be involved.
The two leading theories for the formation of fat embolism are the mechanical theory and biochemical theory. The mechanical theory proposes that obstruction of the systemic vasculature by fat embolism occurs from the direct release of bone marrow into the venous system following trauma. An elevated intramedullary pressure following trauma leads to the release of fat through open venous sinusoids. The embolized fat obstructs capillary beds. While this accounts for embolisms within the pulmonary capillaries, the theory does not explain embolisms within the systemic capillaries beds. Patients have been shown to have systemic fat embolization without a patent foramen ovale.[13]
The biochemical theory provides an alternate explanation for fat embolization. This theory proposes that the inflammatory response to trauma causes the release of free fatty acids from the bone marrow into the venous system. The elevated free fatty acids as well as the inflammatory mediators cause damage to capillary beds. Elevated free fatty acid levels have been associated with hypoxemia.[14] Free fatty acids have also been shown to induce inflammation within the lungs.[15]
Regardless of the mechanism initiating fat embolism, the end result is an intense inflammatory response. Capillary beds develop increased permeability and inflammatory mediators damage surrounding tissues. In the lungs, this induces lung injury that is indistinguishable from ARDS.
CLINICAL PRESENTATION
Embolized fat droplets can travel to microvasculature throughout the body. FES is, therefore, a multiorgan disease and can damage any microcirculatory system within the body. Fat has been reported to embolize to the lungs, brain, skin, retina, kidneys, liver, and even the heart.[16]
Presenting signs are nonspecific and include tachypnea, tachycardia, and fever. Patient may have a petechial rash. Specific symptoms are dependent on the organ systems involved.
The pulmonary circulation is most commonly affected in FES, with up to 75% of patients experiencing respiratory depression.[17,18] The degree of respiratory dysfunction can range from mild hypoxia requiring supplemental oxygen to ARDS requiring prolonged mechanical ventilation. Patients may decompensate rapidly to respiratory failure, especially during manipulation of fractures whilemoving the patient or setting the fracture in the operating room. This must especially be taken into consideration while in the operating room; an anesthetized patient may develop acute hypoxia secondary to FES.
FES can cause nonspecific neurological symptoms. Symptoms are believed to arise from cerebral edema rather than ischemia, so symptoms are nonlateralizing.[19] Patients may become lethargic or restless. A change in Glasgow coma scale (GCS) may suggest the development of cerebral edema due to FES. In the setting of severe cerebral edema the patient may become unresponsive.[20]
Dermal involvement results in a petechial rash, reported in approximately 50% of patients.[21] This rash tends to be transient, lasting less than 24 h. While the torso is most commonly affected, the entire dermis and even mucosal membranes can be involved.
Microvascular injury of the retina results in hemorrhagic lesion of the retina, seen in 50% of patients.[22] These lesions are self-limited, disappearing within weeks.[23] Residual visual deficits are uncommon.
Patients may also develop thrombocytopenia or a decrease in hemoglobin in FES.
DIAGNOSIS
Since FES is a heterogeneous disease with no pathognomonic features, its diagnosis can be challenging. Gurd proposed a of clinical criteria for diagnosing FES in 1970 that he later modified with Wilson[4,5] [Table 1]. Schonfeld has suggested a scoring system to helpin diagnosis[24] [Table 2], while Lindeque proposed that FES can be diagnosed based on respiratory changes alone[25] [Table 3]. None of these criteria have been validated or have been universally accepted.
Table 1.
Gurd and Wilson's criteria for FES

Table 2.
Schonfeld's scoring system for FES
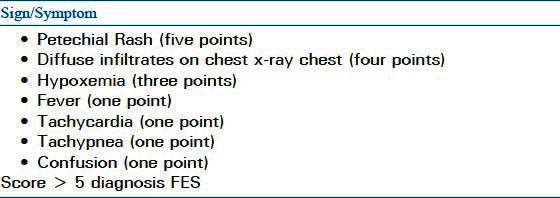
Table 3.
Lindeque's criteria for FES

In the acute setting respiratory distress is the most clinically significant feature of FES. Respiratory distress from FES is indistinguishable from ARDS seen in polytrauma patients. White has suggested defining FES as ARDS with additional organ involvement due to bone trauma.[26]
Laboratory and imaging tests can helpin the diagnosis, but are nonspecific. Patients will demonstrate hypoxemia while on room air on arterial blood gas. Chest x-ray will often show diffuse interstitial infiltrates while chest CT scan will show diffuse areas of vascular congestion and pulmonary edema.
Attempts at developing specific test for FES have been disappointing. Biologic markers such as lipase, free fatty acids, and phospholipase A2 have all been shown to be elevated in patients with FES; however, this elevation is nonspecific in patients with lung injury. Microscopic examination of blood, urine, or sputum may show fat globules, but again this finding is nonspecific.
Bronchoalveolar lavage (BAL) has been heavily investigated as a diagnostic tool for FES.[27–29] Lipid inclusions within macrophages can be quantified BAL. BAL though is invasive and time intensive. It has not become widely used in diagnosing FES.
With the absence of specific tests or criteria the diagnosis of FES is dependent on the clinical acumen of the treating physician. It should be clinically suspected in patients with respiratory distress or a petechial rash and are at high risk for developing FES.
TREATMENT
Pharmacologic interventions
Therapeutic treatments developed specifically for FES have been largely unsuccessful. Early experiments attempted to use dextrose to decrease free fatty acid mobilization or ethanol to decrease lipolysis; however, neither have shown clinical benefits.[30,31] Anticoagulation with heparin was found to be beneficial in animal models but is no longer commonly used in clinical practice due to the risk of bleeding and unproven benefits.[32–34]
Corticosteroidtherapy has been proposed as a potential therapy for FES by limiting free fatty acid levels, stabilizing membranes, and inhibiting complement mediated leukocyte aggregation. Meta-analysis of seven randomized trials using prophylactic corticosteroids in patients with long-bone fractures found that corticosteroids reduce the risk of FES by 77% (95% CI: 40–91%).[35] This same trial reported no difference in mortality, infection, or avascular necrosis in patients treated with corticosteroids compared to control patients. This meta-analysis, though, included only one recent trial. A 2004 randomized trial found no difference in incidence of FES between patients treated with methylprednisolone.[7] While still controversial, some clinicians administer corticosteroids to patients with long-bone fractures as FES prophylaxis. Methylprednisolone is the most commonly used steroid and dosages range from 6 to 90 mg/kg.
Placement of inferior venal cava filters has been advocated as a method to reduce showering of emboli to the pulmonary vasculature. IVC filters as a prophylactic treatment to prevent FES have not been sufficiently studied.
Supportive treatment
Once a patient develops FES the only proven treatment is supportive care of the involved organ systems. Supplemental oxygen may be required to improve oxygenation. If ARDS develops the patient may require mechanical ventilation while recovering from lung injury. Patients may require intravenous fluid for resuscitation to avoid developing shock. In severe cases, in which a fat pulmonary embolism causes, right ventricular failure ionotropic support with dobutamine may be necessary.[36]
Patients with neurological manifestations require frequent neurological examinations and documentation of Glascow Coma Scale to assess for neurologic deterioration. Rapid deterioration may develop from increased cerebral edema.[20] Patients with FES and cerebral edema may benefit from placement of an intracranial pressure monitor in order to direct treatment of cerebral edema.[37]
Operative fixation
Early operative fixation of long-bone fractures is advocated to reduce the incidence of FES. In the long-bone, fractures were treated conservatively with prolonged immobilization, with the incidence of FES in these patients reported as 22%.[38] The movement of fracture ends prior to operative fixation have been shown to result in transient showering of fat embolism.[39] Cytokines remain persistently elevated in patients undergoing conservative treatment and then return to normal after operative fixation.[40]
The use of internal fixation devices for treatment of long bone fractures was accompanied by a reduction in the incidence of FES.[41] Several retrospective studies have also reported decreased incidence of FES with use of internal fixation devices.[11,42–47] Johnson et al. further demonstrated that patients undergoing fixation urgently had an incidence of ARDS of 7% compared to an incidence of ARDS of 39% in patients that had fixation delayed by more than 24 h.[43]
Increased intramedullary pressure during fixation increases the amount of fat emboli entering the circulation.[48] Care must be taken during operative fixation to limit intramedullary pressure.
While reaming may increase intramedullary pressure, reaming has not been shown to increase the incidence of FES. A randomized trial comparing pulmonary complications in patients undergoing fixation with reamed nailing and unreamed nailing found no difference between the two groups.[49] When assessed with transesophageal echocardiography, patients undergoing fixation with reamed and unreamed nails had visible pulmonary embolism.[50]
Surgical techniques to reduce embolization including drilling holes in the cortex to reduce intramedullary pressure, lavaging bone marrow prior to fixation to reduce marrow for embolization, venting of the femur, use of a bone-vacuum, and use of tourniquets to prevent embolization have been attempted. None of these have clearly been shown to reduce FES.[51–55]
Morbidity and mortality
With supportive care and early fixation FES has a favorable outcome. The most significant morbidity is associated with the development of ARDS. However, most patients can expect a complete recovery of pulmonary, neurologic, and retinal abnormalities. Mortality rates from FES in modern studies utilizing supportive measures and early operative fixation report the mortality from FES between 7% and 10%.[9,10]
CONCLUSION
FES most commonly presents with respiratory distress in orthopedic trauma patients. No specific diagnostic tests or criteria exist, so the syndrome is most often a diagnosis of exclusion. Therapy is most often directed at treatment of ARDS and support of other organ systems affected by fat embolization. Early operative fixation long-bone fractures have reduced the incidence of FES.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Scuderi CS. The present status of fat embolism. Bibliographic review. Int Surg Digest. 1934;18:195–215. [Google Scholar]
- 2.Gauss H. The pathology of fat embolism. Arch Surg. 1924;9:592–605. [Google Scholar]
- 3.Wilson JV, Salisbury CV. Fat embolism in war surgery. Br J Surg. 1943;31:384–92. [Google Scholar]
- 4.Gurd AR. Fat embolism: An aid to diagnosis. J Bone Joint Surg Br. 1970;52:732–7. [PubMed] [Google Scholar]
- 5.Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56B:408–16. [PubMed] [Google Scholar]
- 6.llardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: A pro- spective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14:955–62. [PubMed] [Google Scholar]
- 7.Wong MW, Tsui HF, Yung SH, Chan KM, Cheng JC. Continuous pulse oximeter monitoring for inapparent hypoxemia after long bone fractures. J Trauma. 2004;56:356–62. doi: 10.1097/01.TA.0000064450.02273.9B. [DOI] [PubMed] [Google Scholar]
- 8.Pell AC, Christie J, Keating JF, Sutherland GR. The detection of fat embolism by transoesophageal echocardiography during reamed intramedullary nailing. A study of 24 patients with femoral and tibial fractures. J Bone Joint Surg Br. 1993;75:921–5. doi: 10.1302/0301-620X.75B6.8245083. [DOI] [PubMed] [Google Scholar]
- 9.Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132:435–9. doi: 10.1001/archsurg.1997.01430280109019. [DOI] [PubMed] [Google Scholar]
- 10.Fabian TC, Hoots AV, Stanford DS, Patterson CR, Mangiante EC. Fat embolism syndrome: Prospective evaluation in 92 fracture patients. Crit Care Med. 1990;18:42–6. [PubMed] [Google Scholar]
- 11.Riska EB, Myllynen P. Fat embolism in patients with multiple injuries. J Trauma. 1982;22:891–4. doi: 10.1097/00005373-198211000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Duis HJ, Nijsten MW, Klasen HJ, Binnendijk B. Fat embolism in patients with an isolated fracture of the femoral shaft. J Trauma. 1988;28:383–90. doi: 10.1097/00005373-198803000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Nijsten MW, Hammer JP, Dius HJ, Posma JL. Fat embolism and patent foreamen ovale. Lancet. 1989;1:1271. doi: 10.1016/s0140-6736(89)92370-2. [DOI] [PubMed] [Google Scholar]
- 14.Nixon JR, Brock-Utne JG. Free fatty acid and arterial oxygen changes following major injury. A correlation between hypoxemia and increased free fatty acid level. J Trauma. 1978;18:23–6. doi: 10.1097/00005373-197801000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Parker FB, Wax SD, Kusajima K, Webb WR. Hemodynamic and pathological findings in experimental fat embolism. Arch Surg. 1974;108:70–4. doi: 10.1001/archsurg.1974.01350250060017. [DOI] [PubMed] [Google Scholar]
- 16.Bokhari SI, Alpert JS. Probable acute coronary syndrome secondary to fat embolism. Cardiol Rev. 2003;11:156–9. doi: 10.1097/01.crd.0000053906.65644.a3. [DOI] [PubMed] [Google Scholar]
- 17.Burger LW, Dines DE, Linscheid RL. Fat embolism syndrome and the adult respiratory distress syndrome. Mayo Clinic Proc. 1974;49:107–9. [PubMed] [Google Scholar]
- 18.McCarthy B, Mammen E, Leblanc LP, Wilson RF. Subclinical fat embolism: A prospective study of 50 patients with extremity fractures. J Trauma. 1973;13:9–16. [PubMed] [Google Scholar]
- 19.Butteriss DJ, Mahad D, Soh C, Walls T, Weir D, Birchall D. Reversible cytotoxic cerebral edema in cerebral fat embolism. AJNR Am J Neuroradiol. 2006;27:620–3. [PMC free article] [PubMed] [Google Scholar]
- 20.Meeke RI, Fitzpatrick GJ, Phelan DM. Cerebral edema and fat embolism syndrome. Intensive Care Med. 1987;13:291–2. doi: 10.1007/BF00265121. [DOI] [PubMed] [Google Scholar]
- 21.Gossling HR, Pellegrin VD. Fat embolism syndrome. A review of the pathophysiology and physiologic basis of treatment. Clin Orthop Relat Res. 1982;56:408–16. [PubMed] [Google Scholar]
- 22.Adams CB. The retinal manifestations of fat embolism. Injury. 1971;2:221–4. doi: 10.1016/s0020-1383(71)80055-4. [DOI] [PubMed] [Google Scholar]
- 23.Chuang EL, Miller FS, 3rd, Kalina RE. Retinal lesions following long bone fractures. Ophthalmology. 1985;92:370–4. doi: 10.1016/s0161-6420(85)34023-x. [DOI] [PubMed] [Google Scholar]
- 24.Schonfeld SA, Ploysongsang Y, DiLisio R, Crissman JD, Miller E, Hammerschmidt DE, et al. Fat embolism prophylaxis with corticosteroid: A prospective study in high-risk patients. Ann Intern Med. 1983;99:438–43. doi: 10.7326/0003-4819-99-4-438. [DOI] [PubMed] [Google Scholar]
- 25.Lindeque BG, Schoeman HS, Dommissen GF, Boeyens MC, Vlok AL. Fat embolism syndrome: A double blind therapeutic study. J Bone Joint Surg Br. 1987;69:128–31. doi: 10.1302/0301-620X.69B1.3818718. [DOI] [PubMed] [Google Scholar]
- 26.White T, Petrisor BA, Bhandari M. Prevention of fat embolism syndome. Injury. 2006;37S:S59–67. doi: 10.1016/j.injury.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 27.Mimoz O, Edouard A, Beydon L, Quillard J, Verra F, Fleury J, et al. Contribution of bronchoalveolar lavage to the diagnosis of posttraumatic pulmonary fat embolism. Intensive Care Med. 1995;21:973–80. doi: 10.1007/BF01700658. [DOI] [PubMed] [Google Scholar]
- 28.Chastre J, Fagon JY, Soler P, Fichelle A, Dombret MC, Huten D, et al. Bronchoalveolar lavage for rapid diagnosis of the fat embolism syndrome in trauma patients. Ann Intern Med. 1990;113:583–8. doi: 10.7326/0003-4819-113-8-583. [DOI] [PubMed] [Google Scholar]
- 29.Vedrinne JM, Guillaume C, Gagnieu MC, Gratadour P, Fleuret C, Motin J. Bronchoalveolar lavage in trauma patients for diagnosis of fat embolism syndrome. Chest. 1992;102:1323–7. doi: 10.1378/chest.102.5.1323. [DOI] [PubMed] [Google Scholar]
- 30.Stoltenberg JJ, Gustilo RB. The use of methylprednisolone and hypertonic glucose in the prophylaxis of fat embolism syndrome. Clin Orthop Relat. 1979:211–21. [PubMed] [Google Scholar]
- 31.Schier MR, Wilson RF, James RE, Riddle J, Mammen EF, Pedersen HE. Fat embolism prophylaxis: A study of four treatment modalities. J Trauma. 1977;17:621–9. [PubMed] [Google Scholar]
- 32.Saldeen T. Intravascular coagulation in the lungs in experimental fat embolism. Acta Chir Scand. 1969;135:653–62. [PubMed] [Google Scholar]
- 33.Burhop KE, Selig WM, Beeler DA, Malik AB. Effect of heparin on increased pulmonary microvascular permeability after bone marrow aspiration in awake sheep. Am Rev Respir Dis. 1987;136:134–41. doi: 10.1164/ajrccm/136.1.134. [DOI] [PubMed] [Google Scholar]
- 34.Laterre PF, Wittebole X, Dhainaut JF. Anticoagulant therapy in acute lung injury. Crit Care Med. 2003;31:S329–36. doi: 10.1097/01.CCM.0000057912.71499.A5. [DOI] [PubMed] [Google Scholar]
- 35.Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism in patient with long bone fractures? A meta-analysis. Can J Surg. 2009;52:386–93. [PMC free article] [PubMed] [Google Scholar]
- 36.Kerbaul F, Rondelet B, Motte S, Fesler P, Hubloue I, Ewalenko P, et al. Effects of norepinephrine and dobutamine on pressure load induced heart failure. Crit Care Med. 2004;32:1035–40. doi: 10.1097/01.ccm.0000120052.77953.07. [DOI] [PubMed] [Google Scholar]
- 37.Sie MY, Toh KW, Rajeev K. Cerebral fat embolism: An indication for an ICO monitor? J Trauma. 2003;55:1185–6. doi: 10.1097/01.TA.0000100833.57027.1F. [DOI] [PubMed] [Google Scholar]
- 38.Riska EB, von Bonsdorff H, Hakkinen S, Jaroma H, Kiviluoto O, Paavilainen T. Prevention of fat embolism by early internal fixation of fractures in patients with multiple injuries. Injury. 1976;8:110–6. doi: 10.1016/0020-1383(76)90043-7. [DOI] [PubMed] [Google Scholar]
- 39.Tachakra SS, Potts D, Idowu A. Early operative fracture management of patients with multiple injuries. Br J Surg. 1990;77:1194. doi: 10.1002/bjs.1800771040. [DOI] [PubMed] [Google Scholar]
- 40.Schoffel U, Bonnaire F, van Specht BU, Kuner EH. Monitoring the response to injury. Injury. 1991;22:377–82. doi: 10.1016/0020-1383(91)90099-z. [DOI] [PubMed] [Google Scholar]
- 41.Meek RN, Vivoda EE, Pirani S. Comparison of mortality of patients with multiple injuries according to type of fracture treatment—a retrospective age- and injurymatched series. Injury. 1986;17:2–4. doi: 10.1016/0020-1383(86)90003-3. [DOI] [PubMed] [Google Scholar]
- 42.Goris RJ, Gimbrere JS, van Niekerk JL, Schoots FJ, Booy LH. Early osteosynthesis and prophylactic mechanical ventilation in the multitrauma patient. J Trauma. 1982;22:895–903. doi: 10.1097/00005373-198211000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Johnson KD, Cadambi A, Seibert GB. Incidence of adult respiratory distress in patients with multiple musculoskeletal injuries: Effect of early operative stabilization of fractures. J Trauma. 1985;25:375–84. doi: 10.1097/00005373-198505000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Svenningsen S, Nesse O, Finsen V, Hole A, Benum P. Prevention of fat embolism syndrome in patients with femoral fractures—immediate or delayed operative fixation? Ann Chir Gynaecol. 1987;76:163–6. [PubMed] [Google Scholar]
- 45.Talucci RC, Manning J, Lampard S, Bach A, Carrico CJ. Early intramedullary nailing of femoral shaft fractures: A cause of fat embolism syndrome. Am J Surg. 1983;146:107–11. doi: 10.1016/0002-9610(83)90269-6. [DOI] [PubMed] [Google Scholar]
- 46.Bone LB, Johnson KD, Weigelt J, Scheinberg R. Early versus delayed stabilization of femoral fractures: A prospective randomized study. J Bone Joint Surg. 1989;71:336–40. [PubMed] [Google Scholar]
- 47.Brundage SI, McGhan R, Jurkovich GJ, Mack CD, Maier RV. Timing of femur fracture fixation: Effect on outcome in patients with thoracic and head injuries. J Trauma. 2002;52:299–307. doi: 10.1097/00005373-200202000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Christie J, Robinson CM, Pell AC, McBirnie J, Burnett R. Transcardiac echocardiography during invasive intramedullary procedures. J Bone Joint Surg Br. 1995;77:450–5. [PubMed] [Google Scholar]
- 49.Anwar IA, Battistella FD, Neiman R, Olson SA, Chapman MW, Moehring HD. Femur fractures and lung complications: Aprospective randomized study of reaming. Clin Orthop Relat Res. 2004;422:71–6. [PubMed] [Google Scholar]
- 50.Coles RE, Clements FM, Lardenoye JW, Wermeskerken GV, Hey LA, Nunley JA, et al. Transesophageal echocardiography in quantification of emboli during femoral nailing: Reamed versusunreamed techniques. J South Orthop Assoc. 2000;9:98–104. [PubMed] [Google Scholar]
- 51.Martin R, Leighton RK, Petrie D, Ikejiani C, Smyth B. Effect of proximal and distal venting during intramedullary nailing. Clin Orthop Relat Res. 1996;332:80–9. doi: 10.1097/00003086-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Dalgorf D, Borkhoff CM, Stephen DJ, Finkelstein J, Kreder HJ. Venting during prophylactic nailing for femoral metastases: Current orthopedic practice. Can J Surg. 2003;46:427–31. [PMC free article] [PubMed] [Google Scholar]
- 53.Byrick RJ, Bell RS, Kay JC, Waddell JP, Mullen JB. High-volume, high-pressure pulsatile lavage during cemented arthroplasty. J Bone Joint Surg Am. 1989;71:1331–6. [PubMed] [Google Scholar]
- 54.Hagley SR. The fulminant fat embolism syndrome. Anaesth Intensive Care. 1983;11:162–6. doi: 10.1177/0310057X8301100214. [DOI] [PubMed] [Google Scholar]
- 55.Pitto RP, Koessler M, Kuehle JW. Comparison of fixation of the femoral component without cement and fixation with use of a bone-vacuum cementing technique for the prevention of fat embolism during total hip arthroplasty. A prospective, randomized clinical trial. J Bone Joint Surg Am. 1999;81:831–43. doi: 10.2106/00004623-199906000-00010. [DOI] [PubMed] [Google Scholar]


