Abstract
Pulmonary embolism (PE) is responsible for approximately 100,000 to 200,000 deaths in the United States each year. With a diverse range of clinical presentations from asymptomatic to death, diagnosing PE can be challenging. Various resources are available, such as clinical scoring systems, laboratory data, and imaging studies which help guide clinicians in their work-up of PE. Prompt recognition and treatment are essential for minimizing the mortality and morbidity associated with PE. Advances in recognition and treatment have also enabled treatment of some patients in the home setting and limited the amount of time spent in the hospital. This article will review the risk factors, pathophysiology, clinical presentation, evaluation, and treatment of PE.
Keywords: Pulmonary embolism, thrombosis, venous thromboembolism
INTRODUCTION
Venous thromboembolism (VTE) and PE is the third most common cause of cardiovascular death after myocardial infarction (MI) and cerebrovascular accidents (CVA).[1] Many PEs are likely undiagnosed and calculating the true incidence remains challenging. However, PE remains a significant cause of preventable in-hospital mortality.
RISK FACTORS
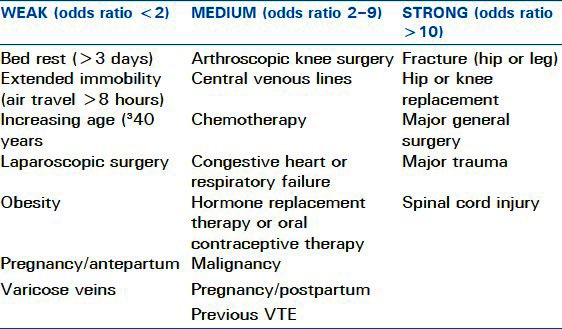
Most clinically significant PEs originate as VTEs in the lower extremities or pelvic veins. Less frequently, upper extremity thromboembolic events lead to PE. Various conditions lead to the generation of VTE. Virchow's triad of hypercoagulability, venous stasis, and vessel wall injury provides a model for understanding many of the risk factors. These factors are usually either inherited or acquired, as shown in Tables 1 and 2. Overall, major risk factors for thromboembolic events include recent immobilization, MI, CVA, surgery, and recent trauma. Additional major risk factors include prior VTE, advanced age, malignancy, known thrombophilia, and indwelling venous catheter. Moderate risk factors include family history of VTE, use of estrogen or hormone replacement therapy, smoking, pregnancy, and obesity.
Table 1.
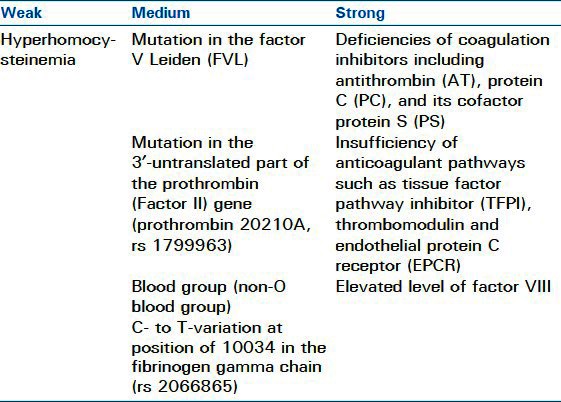
Table 2.
PATHOPHYSIOLOGY
PE occurs when deep venous thrombi detach and embolize to the pulmonary circulation. Pulmonary vascular occlusion occurs and impairs gas exchange and circulation. In the lungs, the lower lobes are more frequently affected than the upper, with bilateral lung involvement being common. Larger emboli wedge in the main pulmonary artery, while smaller emboli occlude the peripheral arteries. Peripheral PE can lead to pulmonary infarction, manifested by intra-alveolar hemorrhage. Pulmonary infarction occurs in about 10% of patients without underlying cardiopulmonary disease. Obstruction of the pulmonary arteries creates dead space ventilation as alveolar ventilation exceeds pulmonary capillary blood flow. This contributes to ventilation-perfusion mismatch, with vascular occlusion of the arteries increasing pulmonary vascular resistance. In addition, humoral mediators, such as serotonin and thromboxane, are released from activated platelets and may trigger vasoconstriction in unaffected areas of lung. As the pulmonary artery systolic pressure increases, right ventricular after load increases, leading to right ventricular failure. As the right ventricular failure progresses, impairment in left ventricular filling may develop. Rapid progression to myocardial ischemia may occur secondary to inadequate coronary artery filling, with potential for hypotension, syncope, electromechanical dissociation, or sudden death.
CLINICAL PRESENTATION
Prompt recognition of a PE is crucial because of the high associated mortality and morbidity, which may be prevented with early treatment. Failure to diagnose PE is a serious management error since 30% of untreated patients die, while only 8% succumb with effective therapy.[5] Unfortunately, PE may be asymptomatic or present with sudden death. Characteristic signs and symptoms such as tachycardia, dyspnea, chest pain, hypoxemia, and shock are non-specific and are present in many other conditions, such as acute MI, congestive heart failure, or pneumonia. In the Prospective Investigation of Pulmonary Embolism Diagnosis II (PIOPED II) trial, patients with PE had a range of signs and symptoms. Common signs were tachypnea (54%) and tachycardia (24%). The most common symptoms were dyspnea, usually of onset within seconds, at rest or with exertion (73%), pleuritic pain (44%), calf or thigh pain (44%), calf or thigh swelling (41%), and cough (34%).[6] With only 24% of patients presenting with tachycardia, the majority of patients lacked one of the most common findings. Additionally, PIOPED II excluded many types of patients, such as those with chronic elevated creatinine levels or receiving dialysis, critically ill patients, or people with recent MI. Applicability is therefore limited. Therefore, a high index of suspicion and assessment of risk factors are critical for the recognition of pulmonary embolic events.
EVALUATION
Because of the variable nature of the presentation of PE, the evaluation largely depends on the likelihood of PE and the stability of the patient. There are scoring systems to assist in the determination of likelihood of PE and thromboembolic events. Diagnostic scoring systems such as the Wells criteria and Geneva score are often used [Tables 3 and 4].
Table 3.
Modified Wells Criteria
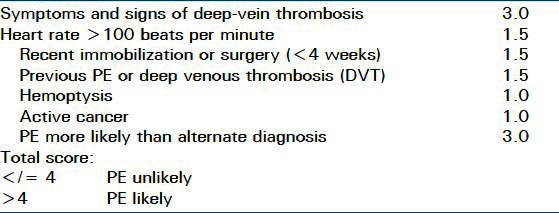
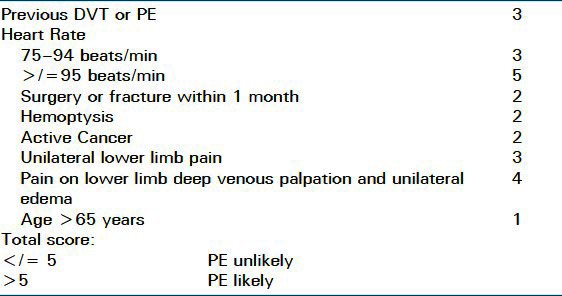
Table 4.
Revised Geneva Score
Beyond the Wells and Geneva systems, which clinicians use to help rule in thrombosis, the PE Rule-Out Criteria (PERC) can help rule-out PE in low-risk emergency department patients. PERC criteria are listed in Table 5.
Table 5.
PE Rule-out Criteria[9]

If a patient fulfills all of the PERC criteria and has a low probability of PE by Wells criteria and the gestalt opinion of the evaluating physician, then a PE may be ruled out.[9] In reality, very few patients meet these criteria and the PERC assessment is not reliable for the in-hospital setting. The aforementioned tools: Wells score, Geneva score, and PERC work best in assessing the need for further work-up of stable patients presenting to the emergency room; with inpatients and critically ill patients, such tools are not as reliable. The elements of diagnostic workup will vary depending on whether the patient is hospitalized and whether there is hemodynamic instability. In a patient with a suspected PE, diagnosis of proximal DVT in a symptomatic patient, or in an asymptomatic patient who has contraindications to CT angiography, is sufficient to rule in PE.[10] In a stable patient, presenting from an outpatient setting, who has not sustained recent trauma or surgery, a d-dimer test should be performed. If negative, and clinical suspicion is low, then the likelihood of PE is low and further workup is unnecessary. d-Dimer is a degradation product of cross-linked fibrin that is formed immediately after fibrin clots are degraded by plasmin and reflects a global activation of blood coagulation and fibrinolysis. Therefore, d-dimer is not a useful test in post-operative patients because it will be elevated due to coagulation and fibrinolysis. It is also elevated in trauma patients, hospitalized patients, and those with critical illness for similar reasons. Extremity venous ultrasound (US) is a quick, noninvasive modality that can detect DVT. Because it can be performed with a portable machine, venous US may negate the need for potentially dangerous transport of unstable, critically ill patients. Unfortunately, US testing often depends on the availability of technicians to perform the imaging and further evaluation by radiologists and other physicians with such skills. Recently, emergency room physicians and intensivists have been trained in US which results in faster detection of both symptomatic and asymptomatic extremity thromboses. When performed by trained ICU physicians, compression US studies of the extremities yielded a sensitivity of 86% and a specificity of 96% with a diagnostic accuracy of 95%.[11] In this same trial, median time delay between the ordering of formal vascular study (FVS) and the FVS result was 13.8 hours, which leads to significant delays in treatment. In another study, after 10 minutes of training in two point compression ultrasound, emergency medicine physicians were able to detect lower extremity thrombosis with a sensitivity of 100% and a specificity of 99%.[12] Besides the limitations of time delays due to lack of available personnel to perform such imaging, there are certain patient limitations that may limit study accuracy and feasibility; obesity, edema, and dressings often impede good imaging quality.[13] The other consideration in obtaining lower extremity ultrasound for diagnosis of PE is that it is not a reliable modality to check for pelvic venous thrombosis and there are also many instances in which patients have PEs, but no evidence of extremity thrombosis. In one study, the use of ultrasonography alone for suspected PE had a sensitivity of only 29%.[14] Furthermore, false positive results may result in potentially dangerous and unnecessary anticoagulation in patients who do not actually have PEs. Helical CT angiography has largely replaced ventilation-perfusion (V/Q) lung scanning for diagnosis because it is rapid, permits direct visualization of PE, and can demonstrate other diagnoses in patients without PE. In patients with a contraindication to intravenous contrast dye, a V/Q scan is used. A normal lung scan effectively excludes the diagnosis of PE, but only 25% of patients with suspected PE have a normal scan. In one of the largest studies evaluating V/Q scanning, the PIOPED II trial, V/Q scans could effectively rule-out PE in patients with a very low probability scan and a very low clinical likelihood of PE.[15] Other additional tests such as EKG or echocardiography may help with the diagnosis. However, EKG findings are often non-specific, as tachycardia is frequent. Evidence of right heart strain on EKG or echocardiography may support the diagnosis as well as provide information about the severity of the PE. One particular finding on echocardiogram, known as the McConnell's sign, is strongly suggestive of PE. In those patients with suspected PE and right ventricular dysfunction, the finding of normal wall motion of the RV apex but akinesia of the mid-RV free wall has a positive predictive value of 71% for the diagnosis of PE and a negative predictive value of 96%.[16]
TREATMENT
In hemodynamically stable patients, without contraindications to systemic anticoagulation, parenteral anticoagulation with subsequent conversion to vitamin K antagonists is the mainstay of therapy. Early initiation is paramount as patients may quickly decompensate. Patients who present to the emergency room with acute PE have decreased mortality if anticoagulation treatment commences in the emergency room, rather than waiting until after admission.[17] Supportive care of hypoxemia and hemodynamic instability should be instituted. Hemodynamically unstable patients may benefit from fibrinolytic therapy. However, the role for fibrinolysis is limited, with significant bleeding occurring in up to 13% of patients. The use of bolus thrombolytics during cardiopulmonary arrest may have some benefit when PE is strongly suspected[18,19] and the patient does not respond to resuscitation. Mechanical thrombolysis with catheter-directed embolectomy and fibrinolytic therapy can also be used. Systemic heparin, either in the form of unfractionated heparin or low-molecular-weight heparin (LMWH), is the mainstay of treatment. LMWH is advantageous in ease of administration, monitoring, lower potential for heparin-induced thrombocytopenia. However, it is not an appropriate choice for patients with renal failure or for patients at significant risk of bleeding, because of its longer half-life and lack of reversibility. Newer options for anticoagulation include oral factor Xa inhibition with agents under investigation, such as rivaroxaban.[20,21] If patients continue to have PEs despite therapeutic anticoagulation, experience bleeding events, or have other contraindications to anticoagulation, permanent or temporary inferior vena cava filters (IVCF) may be used. IVCF can prevent further pulmonary emboli in patients with lower extremity deep venous thrombosis, but do not reduce mortality. Propagating iliofemoral venous thrombus while on anticoagulation is another indication for IVCF placement.
SUMMARY
VTE and PE remain preventable causes of morbidity and mortality. By combining patient presentation, clinical suspicion, and scoring systems, diagnosis may be streamlined and unnecessary treatment may be minimized. More physicians have training and access to portable ultrasound devices, which may prevent delays in recognition and treatment of VTE. In-hospital patients, especially those who are critically ill, continue to pose diagnostic dilemmas. In such patients, clinical scoring systems and imaging may be inconclusive. The improved accuracy of helical CT scans has improved our recognition of PE in many of these patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Weitz JI. Pulmonary embolism. In: Goldman L, Schafer AI, editors. Goldman's Cecil Medicine. Philadelphia, PA: Elsevier; 2011. [Google Scholar]
- 2.Rosendaal FR, Reitsma PH. Genetics of venous thrombosis. J Thromb Haemost. 2009;1:301–4. doi: 10.1111/j.1538-7836.2009.03394.x. [DOI] [PubMed] [Google Scholar]
- 3.Moheimani F, Jackson DE. Venous thromboembolism: Classification, risk factors, diagnosis, and management. ISRN Hematol. 2011;2011:124610. doi: 10.5402/2011/124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho WK. Deep vein thrombosis–risks and diagnosis. Aust Fam Physician. 2010;39:468–74. [PubMed] [Google Scholar]
- 5.Meyer NJ. Pulmonary embolic disorders: Thrombus, Air, and Fat. In: Hall JB, Schmidt GA, Wood LD, editors. Principles of Critical Care. New York: McGraw-Hill; 2005. [Google Scholar]
- 6.Stein PD, Beemath A, Matta F, Weg JG, Yusen RD, Hales CA, et al. Clinical characteristics of patients with acute pulmonary embolism: Data from PIOPED II. Am J Med. 2007;120:871–9. doi: 10.1016/j.amjmed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: Increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83:416–20. [PubMed] [Google Scholar]
- 8.Le Gal G, Righini M, Roy PM, Sanchez O, Aujesky D, Bounameaux H, et al. Prediction of pulmonary embolism in the emergency department: The revised Geneva score. Ann Intern Med. 2006;144:165–71. doi: 10.7326/0003-4819-144-3-200602070-00004. [DOI] [PubMed] [Google Scholar]
- 9.Kline JA, Courtney DM, Kabrhel C, Moore CL, Smithline HA, Plewa MC, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6:772–80. doi: 10.1111/j.1538-7836.2008.02944.x. [DOI] [PubMed] [Google Scholar]
- 10.Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379:1835–46. doi: 10.1016/S0140-6736(11)61904-1. [DOI] [PubMed] [Google Scholar]
- 11.Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139:538–42. doi: 10.1378/chest.10-1479. [DOI] [PubMed] [Google Scholar]
- 12.Crisp JG, Lovato LM, Jang TB. Compression ultrasonography of the lower extremity with portable vascular ultrasonography can accurately detect deep venous thrombosis in the emergency department. Ann Emerg Med. 2010;56:601–10. doi: 10.1016/j.annemergmed.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Tapson VF, Carroll BA, Davidson BL, Elliott CG, Fedullo PF, Hales CA, et al. The diagnostic approach to acute venous thromboembolism. Clinical practice guideline. American Thoracic Society. Am J Respir Crit Care Med. 1999;160:1043–66. doi: 10.1164/ajrccm.160.3.16030. [DOI] [PubMed] [Google Scholar]
- 14.Turkstra F, Kuijer PM, van Beek EJ, Brandjes DP, ten Cate JW, Büller HR. Diagnostic utility of ultrasonography of leg veins in patients suspected of having pulmonary embolism. Ann Intern Med. 1997;126:775–81. doi: 10.7326/0003-4819-126-10-199705150-00005. [DOI] [PubMed] [Google Scholar]
- 15.Gottschalk A, Stein PD, Sostman HD, Matta F, Beemath A. Very low probability interpretation of V/Q lung scans in combination with low probability objective clinical assessment reliably excludes pulmonary embolism: Data from PIOPED II. J Nucl Med. 2007;48:1411–5. doi: 10.2967/jnumed.107.040998. [DOI] [PubMed] [Google Scholar]
- 16.McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78:469–73. doi: 10.1016/s0002-9149(96)00339-6. [DOI] [PubMed] [Google Scholar]
- 17.Smith SB, Geske JB, Maguire JM, Zane NA, Carter RE, Morgenthaler TI. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. 2010;137:1382–90. doi: 10.1378/chest.09-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flores J, de Tena JG, de Pablo R, Daguerre M. Successful outcome of cardiopulmonary arrest with systemic thrombolysis. Eur J Intern Med. 2008;19:e38–9. doi: 10.1016/j.ejim.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Janata K, Holzer M, Kürkciyan I, Losert H, Riedmüller E, Pikula B, et al. Major bleeding complications in cardiopulmonary resuscitation: The place of thrombolytic therapy in cardiac arrest due to massive pulmonary embolism. Resuscitation. 2003;57:49–55. doi: 10.1016/s0300-9572(02)00430-6. [DOI] [PubMed] [Google Scholar]
- 20.Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, et al. EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 21.Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, et al. EINSTEIN–PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–97. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]


