Abstract
Surgical and intensive care patients are at a heightened risk for arterial embolization due to pre-existing conditions such as age, hypercoagulability, cardiac abnormalities and atherosclerotic disease. Most arterial emboli are clots that originate in the heart and travel to distant vascular beds where they cause arterial occlusion, ischemia, and potentially infarction. Other emboli form on the surface of eroded arterial plaque or within its lipid core. Thromboemboli are large clots that dislodge from the surface of athesclerotic lesions and occlude distal arteries causing immediate ischemia. Atheroemboli, which originate from fracturing the lipid core tend to cause a process of organ dysfunction and systemic inflammation, termed cholesterol embolization syndrome. The presentation of arterial emboli depends on the arterial bed that is affected. The most common manifestations are strokes and acute lower limb ischemia. Less frequently, emboli target the upper extremities, mesenteric or renal arteries. Treatment involves rapid diagnosis, which may be aided by precise imaging studies and restoration of blood flow. The type of emboli, duration of presentation, and organ system affected determines the treatment course. Long-term therapy includes supportive medical care, identification of the source of embolism and prevention of additional emboli. Patients who experienced arterial embolism as a result of clots formed in the heart should be anticoagulated. Arterial emboli from atherosclerotic disease of the aorta or other large arteries should prompt treatment to reduce the risk for atherosclerotic progression, such as anti-platelet therapy and the use of statin drugs. The use of anticoagulation and surgical intervention to reduce the risk of arterial embolization from atherosclerotic lesions is still being studied.
Keywords: Arterial embolism, atheroembolism, atherosclerosis, complication, diagnosis and management, thromboembolism
INTRODUCTION
Complications of arterial embolism are a leading cause of disability and death in the United States. Arterial embolism results when a mass of tissue or a foreign substance travels through the vascular tree, ultimately lodging in a distal artery where it obstructs blood flow. This obstruction leads to ischemia, organ dysfunction and potential infarction. Manifestations of this complex disease include medical and surgical emergencies such as stroke, acute limb ischemia, mesenteric ischemia and renal failure. Despite many advances in diagnosis and management, arterial embolic disease continues to challenge clinicians, while contributing greatly to morbidity and mortality.
The surgical and critical care population is especially vulnerable to arterial embolism. Many such patients are already at increased risk due to pre-existing comorbidities such as age and heart disease. The increased stress response, stasis, dehydration and inflammatory processes associated with surgery and critical illness further increase chances of thrombus formation in the heart or vascular tree.[1] Patients taking anticoagulating medications due to atrial fibrillation (AF), mechanical heart valves or other indications are commonly instructed to stop taking them perioperatively to reduce the risk of bleeding. Such factors put them at especially high risk for thrombus formation,[2] and it is these thrombi that are at the core of subsequent embolic events. Furthermore, since the majority of emboli occur in patients with significant underlying disease, the patient's underlying comorbidities increase the risk of therapeutic interventions, and may limit the options available for restoring blood flow to the ischemic area.
Considering the morbidity and mortality associated with arterial embolic phenomena, clinicians in the intensive care unit, as well as those providing care to perioperative patients need to be familiar with embolic disease to facilitate risk stratification, prevention, early recognition and appropriate treatment both in the immediate and long-term settings. This article aims to provide a review of arterial embolic disease and provide a diagnostic and management scheme for arterial embolism. While arterial embolic events can in certain cases be caused by entrained air, dislodged tissue (tumor for example), or clots crossing over from the venous system via a right to left shunt, this review will focus on the more common causes such as embolization from the heart and arterial tree.
Pathophysiology
Embolic vessel occlusion
The majority of arterial emboli originate in the left heart where they form secondary to structural or functional abnormalities.[3–5] Most other emboli originate from the arterial tree itself.[6] In general, emboli that originate more proximally in relation to the heart have more potential targets available to them. Thus clots originating in the heart or the aortic arch can potentially embolize to any arterial branch in the body. Conversely, atherosclerotic plaque formed in more distal arteries such as the carotids are far more likely to embolize to the brain - causing strokes or transient ischemic attacks (TIA's), while plaques in the infra-renal aorta are far more likely to cause lower extremity ischemia.[6] Retrograde embolization may occasionally occur during the late diastolic flow reversal seen with decreased heart rates. This process is believed to allow large descending aortic plaques to cause strokes.[7] The ultimate probability of an embolus reaching any specific arterial bed is determined by the relative amount of blood flow that bed receives and the anatomy of the arterial branches supplying that area.[8] Larger emboli tend to lodge at points of acute narrowing such as arterial bifurcations or areas of luminal stenosis,[6,9] whereas smaller emboli may travel distally to lodge in tiny arterioles.[10]
Although arterial embolic disease shares many features with arterial thrombosis, there are important distinctions. Differentiating an acute embolic event from an acute thrombotic event is in fact one of the conundrums faced by medical practitioners. Both processes typically affect individuals with risk factors for heart disease and peripheral arterial disease.[11] Often, embolic and thrombotic diseases may coexist in the same patient. In situations such as limb ischemia, making the correct diagnosis early may allow for expedited care. Acuity of presentation is perhaps the most important distinction between the two processes. Because arterial thrombosis is a gradual process that presents with ischemia in its late stages, patients often have time to develop collateral flow to the ischemic region. However, individuals with embolic ischemia usually do not have time to build collateral circulation and therefore often present with more sudden and severe symptoms such as threatened limb loss.[12]
Cardiac sources of arterial emboli
As noted above, most arterial emboli originate in the heart. In the past, valvular damage from rheumatic heart disease (RHD) was responsible for most cardiac embolic sources. However, as the incidence of RHD has decreased and the population ages, AF has become the most common source of arterial emboli.[13] The most frequent manifestations of cardiac embolic events are strokes and transient ischemic events.[5] Clots formed in the left side of the heart also account for 55-87% of emboli to peripheral arteries[9,14,15] and most emboli to the viscera.[5] Long-term systemic anticoagulation, typically with a vitamin K antagonist such as warfarin, greatly reduces the probability of cardiac thrombus formation in high risk patients. Unfortunately, many patients worldwide are not adequately treated, putting them at high risk for arterial thromboembolism.[5,16]
During AF stasis of blood in the left atrium predisposes to clot formation within the left atrial appendage. These thrombi can range in size from a few millimeters to 4cm and are found in 5 to 14% of patients with AF lasting more than two days.[5] Generally, all patients with AF due to valvular pathology such as mitral stenosis (except those with a strong counter-indication) should be anti-coagulated due to their high risk for stroke and other embolic events.[17] For patients with non-valvular AF the CHADS2 scoring system is the most commonly used model for predicting the annual risk of stroke. The scoring system is an acronym that assigns point values for various independent risk factors associated with embolic stroke. One point is assigned for each of the following: C - congestive heart failure, H - hypertension, A - age of 75 years or older, D – diabetes Mellitus. Two points are assigned for S – prior stroke, TIA or thromboembolism. This system's simplicity and wide clinical applicability allow it to be used routinely in making the decision to start anticoagulation.[18] A less commonly assessed factor is atrial clot morphology as visualized with echocardiography. A “mobile ball” configuration for example is much less common than a “fixed ball” or “mound” configuration, but when seen on echo, it carries a much higher risk of embolization than the other two.[4]
Patients that have paroxysmal AF have a risk of stroke approximately as high as those who persistently remain in AF and thus should be treated the same.[19] Even after successful conversion to a normal sinus rhythm, the risk of embolization persists for a number of days.[20] Since AF is often asymptomatic[21] patients presenting in normal sinus rhythm but with evidence of thromboembolism should have their risk factors for AF assessed with a detailed history, physical examination and potentially echocardiography.
Other described cardiac sources of emboli are mitral and aortic valve disease, atrial myxomas (or other intra-cardiac masses), endocarditis, left ventricular thrombi (typically caused by impaired contractility) and prosthetic heart valves. Most of these diagnoses can be directly diagnosed or suggested by echocardiography.[16,22] The individual contribution of each of these cardiac pathologies to arterial embolic disease as a whole is difficult to determine given their relatively low prevalence. Furthermore, since these conditions frequently co-exist with each other (for example valvular disease and AF) separating each one's relative contribution to in a clinical setting is difficult. Dag et. al examined sources of peripheral emboli in 822 patients and attributed 80% of embolic sources to the heart. Further analysis determined that 76% were due to AF and valvular disease, 1.2% due to endocarditis, 1.2% due to cardiomyopathy and 1.5% due to cardiac tumors.[9] In another series of 397 patients 50% had AF, 8% had another cardiac conduction abnormality, 14% had valvular heart disease and 11% had cardiac insufficiency as a likely source.[15]
The aorta as the primary source of arterial emboli
Aortic atherosclerotic plaque is another leading source of embolic disease.[23] Although the findings of atherosclerosis were published as early as the 18th century by pathologists like Albrecht Haller (1708-1777),[6] and it has been suggested at least since 1862 that atherosclerotic plaque may contribute to arterial occlusions, the opportunity to observe these lesions in a living patient only presented itself recently with the arrival of modern imaging technology.[23] As late as 1989 up to 40% of patients with the clinical manifestations of embolic stroke were classified as having an undetermined cause if they did not have carotid disease or AF.[24] Transesophageal echocardiography (TEE), computerized tomography (CT), and magnetic resonance imaging (MRI) allowed the visualization of plaques in the aorta of living patients. This soon led to the identification of such plaques as a significant third cause of stroke and other embolic phenomena.[25]
Aortic plaque is an extension of atherosclerosis in other arteries, and thus has many of the same risk factors - namely age, male gender, family history, hypertension, hypercholesterolemia, smoking and diabetes. There is also an association with elevated levels of homocysteine, hypercoagulable states, elevated inflammatory markers such as C-reactive protein, and being Caucasian.[25] Overlap in risk factors for aortic plaque, carotid disease, valvular disease and AF results in the coexistence of these disease states in many patients. This has led some to question the role of aortic plaque in embolic events.[26,27] However, most analyses suggest that aortic plaque is an independent contributor to stroke and other emboli.[27–30]
The prevalence of aortic plaque was assessed by TEE in the Stoke Prevention: Assessment of Risk in a Community (SPARC) study. Aortic plaque in any location was found in almost 44% of a relatively unselected sample of 588 patients averaging 67 years in age.[26] Complex plaque, defined as plaque at least 4mm thick or having a mobile component, was seen in 7.6% of the population. This complex plaque is more likely to cause embolic events.[31] The amount of total and complex plaque increased as the aorta was evaluated from proximal to distal. Thus, the ascending aorta had the least and the descending aorta had the highest frequency of both regular plaque and complex plaque.
The American Heart Association classifies aortic plaque into stages I-VI based on histology.[32] Stages I through III represent earlier phases of lipid accumulation within the walls of arteries and may begin in childhood and adolescence. These stages typically remain clinically silent and the associated lesions do not show up on common imaging modalities. In contrast, the advanced stages, IV through VI, typically develop later in life and are visible on both CT and ultrasound examination. During these phases extra-cellular lipid continues to build up, leading to fibrosis, calcification and inflammation. These processes create complex atherosclerotic lesions that ultimately weaken the endothelial wall. As the endothelium weakens, turbulent blood flow can cause plaque erosion (also called rupture). Erosions expose the thrombogenic contents of the plaque to platelets and coagulation factors precipitating the formation the intra-luminal thrombus which defines stage VI plaque.[6]
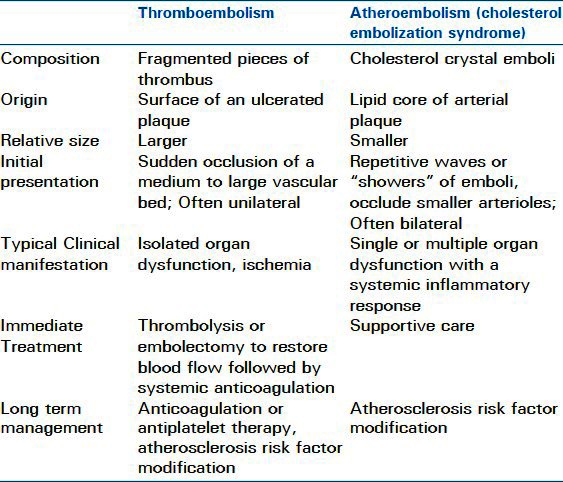
Aortic plaque can give rise to two different types of emboli – thromboemboli and atheroemboli (also called cholesterol embolization syndrome - CES) [Table 1]. Both types of emboli originate from the same source and can present simultaneously in the same patient, but they differ in composition and clinical manifestations. Thromboemboli, which are 20 to 45 times more common than atheroemboli,[6,23] are fragmented pieces of thrombus from the surface of an ulcerated plaque.[24] These fragments tend to be larger than atheroemboli and thus present with the sudden occlusion of a medium to large vascular bed.[6] In such circumstances organ ischemia is maximal at the onset of symptoms. Atheroemboli on the other hand are cholesterol crystals from the lipid core of arterial plaque. They are smaller particles that tend to be released in repetitive “showers.” Atheroemboli occlude smaller arterioles (typically under 200 micrometers in diameter) and trigger an inflammatory process that may present as fever and malaise. “Blue toe syndrome” is a classic example of an atheroembolic phenomenon. In general, the presentation of thromboembolism tends to be sudden and unilateral, while the presentation of atheroembolism tends to be subacute, bilateral and distal.[23] Either type of arterio-arterial embolus can occur spontaneously; however, embolic events may also be triggered by trauma, surgery or an intravascular procedure.
Table 1.
Differences between thromboemboli and atheroemboli
Organ specific manifestations of arterial emboli
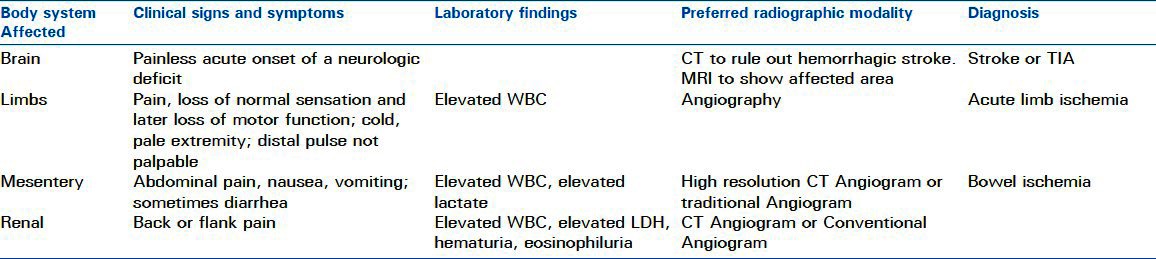
Whether an embolus originates in the heart, an artery or has yet another source, the location of the obstructed arterial bed determines clinical presentation. Arterial emboli commonly affect the brain and extremities. Less frequently they can wreak havoc in mesenteric and renal arteries. Rarely, small, but highly important vascular beds like the coronaries or ophthalmic arteries are affected. The next section will discuss arterial emboli to specific organs that are commonly affected. Atheroembolism will be covered separately since its presentation and treatment are distinct from thromboembolism. The manifestations and diagnosis of arterial embolism to specific organ systems is summarized in Table 2.
Table 2.
Clinical manifestations and diagnosis of arterial embolism
Neurologic manifestations of emboli
Strokes and transient ischemic attacks (TIA's) are the most important clinical manifestations of arterial emboli. Stoke is the 4th most common cause of death and the leading cause of disability in the United States.[33] Worldwide, 15 million people suffer stroke each year; approximately one third of these strokes are fatal.[34] Compared to thrombotic strokes, embolic strokes are on average more likely to cause disability or death because they result in the sudden occlusion of a main cerebral artery.[5,35] The actual extent of neurologic findings largely depends primarily on the location of the infarct. A secondary factor is the caliber of the vessel occluded which often determines the size of the infarcted territory.[5] Transient ischemic attacks (TIA's) are episodes of cerebral ischemia that result in a neurologic deficit which resolves in less than 24 hours (typically lasting no more than 12 minutes). TIAs by definition leave no sign of neuronal damage on subsequent imaging. Non-valvular AF is alone responsible for 45% of embolic strokes,[36] which translates into 15-20% of all strokes.[37] Valvular disease, plaque in the carotid arteries, aortic arch and descending aorta make up the bulk of the remaining embolic sources.
A clinical history and examination, showing the acute onset of a painless neurologic deficit, form the basis for the diagnosis of stroke or TIA. Patients presenting with symptoms of stroke must quickly have other potential diagnoses such as conversion disorder, hypertensive encephalopathy, hypoglycemia, complicated migraine, and post-seizure paresis ruled out.[38] Neurologic imaging is indicated primarily to rule out intracranial hemorrhage, which follows a different treatment algorithm.[39] The treatment of ischemic stroke, whether thrombotic or embolic, is supportive care and rapid consideration of techniques to restore blood flow to the affected area.[40]
Supportive care consists of avoiding further neurologic injury from hypoxia, hypotension, malignant hypertension, fever, hypoglycemia and hyperglycemia. This may require admission to the intensive care unit for large strokes causing intracranial hypertension or smaller strokes that affect areas of the brain involved in respiration and airway protection. Restoration of blood flow is achieved most commonly through the administration of intravenous thrombolytics such as t-PA.[40] Although this technique is widely used, its effectiveness diminishes with stroke duration and carries the risk of hemorrhagic conversion. Thrombolysis is typically indicated within four and a half hours of symptom onset.[41,42] Intra-arterial thrombolysis therapy may be considered within six hours of symptom onset.[42] Overall recanalization rates with t-PA are approximately 45%; however, they can be much lower when larger arteries are occluded, as is the case with many embolic strokes.[33] Other intra-arterial measures such as clot extraction are becoming increasingly available at certain centers.[18]
Immediate care for an embolic stroke is followed by risk factor modification, secondary stroke prevention and prevention of comorbidities. Recommendations include daily aspirin administration within 48 hours of stroke onset, treatment of elevated lipid levels, deep vein thrombosis prophylaxis, prevention of aspiration, provision of adequate nutrition, physical therapy, and smoking cessation.[5,40] Unlike in other embolic events, the use of systemic anticoagulation with heparin or low-molecular weight heparin in the immediate period (within 48 hours) after a stroke is not recommended due to the high risk of intracranial hemorrhage.[43] Systemic anticoagulation with a vitamin K antagonist can be started after 24 hours in patients with AF since they are at risk for recurrent cerebral embolization. However, this is an area of uncertainty, and waiting for two weeks may be advisable for patients with large strokes, poorly controlled hypertension or other risk factors for intracranial hemorrhage.[44]
Emboli to the limbs
Limb ischemia is the second most common cause of morbidity and mortality from arterial embolic disease. Although the incidence of peripheral arterial embolism has decreased due to wider use of anticoagulation and decreasing rates of RHD,[45] it is still a significant cause of morbidity and mortality. Emboli to the lower extremities are about four times as common as emboli to the upper extremities.[3] The introduction of the Fogarty Embolectomy Catheter in 1963 allowed minimally invasive technique and local anesthesia to be used for embolectomy.[3,45] This now standard treatment for arterial embolism[9,14–16,22,45] along with improved perioperative care, has greatly reduced both mortality and morbidity over the last several decades.[3,9,45]
In 1985 Tawes et al. published a series of 739 patients with lower extremity thromboembolism treated over 20 years.[45] Comparing his experience with an earlier series by Blaisdell et al. published in 1978,[46] he demonstrateda reduction in mortality from 25% to 12% and a decrease in the amputation rate from 40% to 5%. He credits this improvement to earlier recognition and treatment, better medical management of co-existing comorbidities and postoperative anticoagulation with heparin. These findings have resonated in more recent literature as well. A multivariable analysis of 346 patients published by Hu in 2011 showed that duration of ischemia, severity of ischemia and the type of therapeuticintervention (embolectomy was the most effective) were the most important factors in determining outcome after acute arterial thromboembolism.[14] Duration of ischemia over six hours was found to increase the risk of limb loss considerably (odds ratio 40).[9] In another series, 12 hours of ischemia time increased the risk of amputation dramatically.[15] Modern rates of amputation or severe loss of function range from 5-23%.[9,14,15,45] Predictably, fasciotomy rates also vary (2. 3 to 14%) since many are performed expectantly.[9,16,22] There is a higher rate of amputations of lower extremities than upper extremities due to better collateral circulation around the site of occlusion in the upper limbs.[3,9]
Recent mortality rates due to arterial emboli range from 4 to 15%.[9,14,15,45] As expected, patients with arterial emboli have multiple risk factors for perioperative morbidity and mortality. One typical patient population studied had an average age of 69 years. Within this group 55% of patients had peripheral arterial disease, 47% had coronary artery disease, 28% had cerebrovascular disease, 46% had diabetes mellitus, 56% had HTN, 40% had hyperlipidemia and 69% used tobacco.[22] Other predictors of morbidity and mortality include coexisting bowel ischemia [relative risk (RR) - 6. 7], poor preoperative functional status (RR 4. 3), cardiac insufficiency (RR 2. 4) and renal disease (RR 2. 1).[15] Causes of death included myocardial infarction (MI) and other cardiac complications, pneumonia, renal failure and sepsis with multi-organ-system failure.[15,22] Morbidities outside of limb loss included heart failure, MI, stroke, respiratory failure, renal insufficiency, pulmonary emboli, bowel ischemia and infections.[15]
Patients with acute limb ischemia due to arterial embolism and arterial thrombosis from plaque rupture present with very similar clinical pictures. The classical complaints are pain, loss of normal sensation and later loss of motor function. The extremity often appears pale, is cold to the touch and a distal pulse is not palpable. In spite of these similarities in presentation, the treatment for these two conditions is significantly different. As already stated, embolectomy is the treatment for an embolic occlusion; whereas more complicated techniques such as arterial angioplasty, thrombolysis and possibly arterial bypass are the treatments for arterial thrombosis.[47] The gold standard to differentiate between these two scenarios is an angiogram, [Figure 1] but clinical clues may also help distinguish the two allowing for better triage of these patients. (1). Patients with embolic occlusions more frequently have anormal peripheral pulse on the contralateral side (71 vs. 31%), more frequently present with AF (32 vs. 3. 4%) or another embolic source (such as rheumatic mitral stenosis) and usually have more severe manifestations of limb ischemia (threatened limb loss 56 vs. 14%; gangrene 19 vs. 7%). Conversely, patients with thrombotic occlusions are more likely to have risk factors for peripheral arterial disease such as DM (45 vs. 20%), HTN (55 vs. 28%), hyperlipidemia (38 vs. 7%) and more commonly have a history of claudication (52 vs. 3%). (1)
Figure 1.
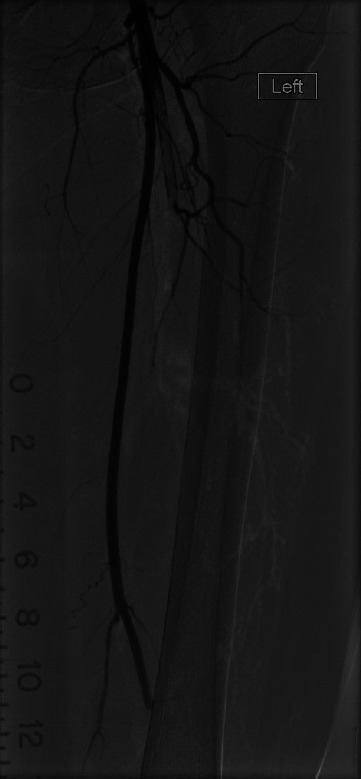
Emboli in left superficial femoral artery and profundafemoris artery
After embolectomy immediate anticoagulation is important for continued arterial patency and reduction of clot propagation. If peripheral artery angioplasty with or without stenting is required to maintain vessel patency, long-term aspirin or clopidogrel therapy is also recommended.[42] Long-term therapy after immediate reperfusion is dictated by the origin of the embolism. Hence, a search must be undertaken to identify this source of the embolism. Identification of the embolic source and long-term management are addressed later in this article.
Emboli to the mesenteric arteries
Bowel ischemia is a relatively uncommon but dreaded complication of arterial embolism. It requires early recognition and emergent treatment. However, even with modern care, the mortality rate for this condition is approximately 40%.[38,48] Most intestinal ischemia is non-thrombotic, but is instead caused by low flow states, mechanical obstruction of mesenteric vessels or disruption of the arterial supply by trauma, surgery or aneurysm.[38] Embolic obstruction makes up about 30% to 50% of the thrombotic causes or approximately10 to 15% of all bowel ischemia.[38,48] Most emboli to the mesentery lodge in the superior mesenteric artery. Its wide angle of origin and parallel course to the aorta makes it the most likely destination for emboli.[5] Most large thrombi will lodge in the first three to eight centimeters of the vessel, often leaving the proximal jejunum well perfused.[49]
The typical presentation may be non-specific, especially in the elderly population that is at highest risk for embolic occlusion. The most common complaints are abdominal pain (95% of cases), nausea (45%), vomiting (45%) and sometimes diarrhea (18%).[38,49] Laboratory tests are not very sensitive or specific, but can include leukocytosis, elevated amylase, elevated creatinine and elevated lactate. However, normal laboratory tests do not rule out bowel ischemia. Traditionally the diagnosis has been made with angiography, but in recent years, high resolution CT angiography has become the modality of choice.[5] In addition to having a high sensitivity (93%) and specificity (100%) for arterial occlusion, CT can demonstrate perforation or other abdominal pathology.[49] Diagnosis by surgery, with the use of intra-operative arterial duplex ultrasonography, can be an option when the patient displays peritoneal signs and time is critical.
Treatment of embolic mesenteric occlusion centers on early recognition and restoration or perfusion. Patients should be taken to surgery for emergent embolectomy and resection of any necrotic bowel.[5,38,48,49] When restoration of blood flow is not possible through embolectomy alone, a bypass from the aorta or iliac vessels may be undertaken. Frequently, a “second-look” surgery is necessary to confirm viability of the remaining intestine 24 to 36 hours later. Such an approach allows for more conservative resection of potentially salvageable bowel during the first procedure. In select patients, where infarcted or perforated bowel is unlikely, endovascular embolectomy or clot aspiration may be a justifiable option.[49] This may be more desirable in patients who are poor surgical candidates. Systemic thrombolysis is rarely undertaken in these situations as it may delay the restoration of blood flow to the ischemic area. After restoration of blood flow, systemic anticoagulation is recommended to maintain vessel patency and reduce the risk of further embolization.[38,49] Less commonly an intra-arterial vasodilator, such as papaverine may be used.
Emboli to the renal artery
Thromboemboli to the renal arteries are much less common than embolic strokes or embolic limb ischemia. Smaller thromboemboli and atheroemboli may often go unrecognized and stay clinically silent. However, renal emboli should always be considered when a patient develops new or worsening renal failure after an endovascular procedure in the suprarenal aorta.[6] Larger thrombi, such as those, originating in the heart, may cause occlusion of the main renal artery or its larger branches. Occlusions of larger vessels result in wedge shaped infarcts that may present with flank, back or generalized abdominal pain, which is sometimes accompanied by nausea.[50,51] Laboratory findings may include proteinuria, hematuria, eosinophiluria, elevated lactate dehydrogenase (LDH) and elevated creatinine.[5,50] An LDH value above 600 IU/L in the setting of flank or back pain is a sensitive and specific marker for renal infarction.[51] The definitive test for embolic occlusion of the renal artery is an angiogram, but high definition CT angiography is a sensitive,increasingly performed modality, especiallyfor more distal emboli.[5] Ultrasound is becoming more widely used in this setting as well due to the benefit of avoiding a contrast load in a patient who may already have compromised renal function.
No clear consensus exists about the optimal management of renal embolic disease. Systemic anticoagulation, fibrinolysis, and surgical embolectomy have all been used with success. Although early diagnosis and treatment are critical in all cases of arterial emboli, even delayed restoration of blood flow to the kidney has allowed for return of some renal function.[52] This may be due to collateral perfusion through ureteral, supra-renal and perirenal arteries or simply due to incomplete occlusion of the renal artery itself. A review of 41 cases of renal artery embolism recommended surgical embolectomy for patients who have occlusion of the main renal artery and no contraindication for surgery.[51] Fibrinolysis was recommended for patients that have more distal, intra-renal emboli or those who are poor surgical candidates. Systemic anticoagulation was reserved for patients who were considered to be too high risk for either surgery or fibrinolysis and those patients with distal emboli and no evidence of renal impairment.[51] Systemic anticoagulation is recommended in all patients with embolic renal occlusion after restoration of blood flow.
Atheroembolism
As with thromboembolism, the manifestations of atheroembolism (also called cholesterol embolism syndrome) depend on the affected vascular bed. However with cholesterol embolization syndrome (CES) multiple organs are often affected simultaneously. Many instances of CES may be missed due to their non-specific presentations. Showers of cholesterol crystals to the brain can result in confusion and memory loss rather than a focal neurologic deficit. Fundoscopic evaluation in these cases may reveal Hollenhorst plaques, which are yellow cholesterol particles at branch points of retinal arteries. Skin manifestations of CES are seen in 35-96% of cases. Typical skin findings (in order of decreasing frequency) include livedoreticularis, gangrene, cyanosis, ulceration, nodules and purpura.[53] Renal dysfunction from CES may be triggered acutely by a procedure or happen spontaneously as an acute, subacute, or chronic process. Gastrointestinal manifestations can present as slow bleeding from microscopic erosions and abdominal pain from an unclear source.
The diagnosis of CES should be suspected in a patient with atherosclerotic risk factors who presents with constitutional symptoms such as fever, myalgia, anorexia and fatigue as well as signs of inflammation and organ dysfunction. Laboratory studies may show an elevated erythrocyte sedimentation rate, hypereosinophilia, eosinophiluria, elevated C-reactive protein, hypocomplementemia, thrombocytopenia and leukocytosis.[6] Treatment in the acute setting is largely supportive. Anticoagulation is not advised, unless supported by another indication, since it may actually precipitate further cholesterol embolization.[53] Long-term management revolves around risk factor modification (as discussed below) to reduce additional atheroembolic events.
Determining the source of arterial embolism
Once the diagnosis of arterial embolism is established (or strongly suspected) and the immediate ischemic complications are addressed, it is important to identify the embolic source in order to prevent potential recurrence. Ultrasound evaluation, including echocardiography, and computerized tomographic angiography (CTA) are the most commonly used modalities for this search. In the setting of stroke carotid duplex evaluation is necessary to look for arterial stenosis or plaque. However, since the vast majority of emboli to all parts of the body originate in the heart, echocardiography has become a cornerstone of the evaluation of patients with suspected embolism.[54]
Atrial fibrillation, left ventricular (LV) thrombus (from LV dysfunction, LV aneurism or cardiomyopathy), cardiac tumors, rheumatic valve disease, endocarditis, mechanical cardiac valves, mitral valve prolapse, mitral annular calcification, atrial septal aneurysms, patent foramen ovale, calcified aortic stenosis, giant Lambl's excrescences, and aortic atherosclerotic plaque are all potential embolic sources that can be rapidly diagnosed by echocardiography. Transthoracic echocardiography (TTE) is in most cases the initial test of choice for detecting cardiac embolic sources.[54] In most cases TTE can identify the embolic source or suggest the responsible pathology indirectly. For example TTE may not directly show LV thrombus, but it may show a low ejection fraction or spontaneous echo contrast that suggests the presence of clot. The addition of echo contrast to TTE further increases the diagnostic accuracy in such cases.
Transesophageal echocardiography (TEE) can be helpful as a supplemental test if a cardiac or proximal aortic source is suspected but not identified by TTE.[54] TEE offers improved sensitivity and specificity when the diagnosis is uncertain. In one review of patients with peripheral embolic events, TTE demonstrated a direct cardiac source or a suspected cardiac source in 38% of patients while TEE identified a source or a potential source in 85%. This resulted in a change in management in 47% of patients that required TEE after TTE was indeterminate.[22] Conversely, another review of a similar group of patients TEE did not identify additional sources but did help rule out potential findings suggested by TTE.[16]
Echocardiography plays an important role in assessing valvular sources of embolism and should be performed in all cases of suspected infective endocarditis (IE). It may be useful both for the diagnosis of IE in patients with unexplained arterial embolism, and for the prediction of risk of embolism in patients with known IE. Transesophageal examination is necessary after a negative TTE if there is a high level of clinical suspicion for IE. TEE provides superior imaging in suspected prosthetic valve endocarditis, and when TTE provides inadequate imaging.[54] Signs of restricted leaflet motion, abnormal central regurgitation, loss of physiologic regurgitant jets in mechanical valves, or direct visualization of thrombus formation can help identify the embolic source. Because of its better sensitivity, TEE should be performed in patients with a prosthetic valve and an embolic event, if TTE is negative.
There are specific cases when TEE is clearly superior to TTE. TEE is clinically indicated to exclude thrombus in the left atrium prior to cardioversion. TEE is also necessary to fully define underlying cardiac pathology before invasive surgical procedures on the heart such as left atrial ligation or valve repair. Additionally TEE can be helpful in patients with AF who experience recurrent embolic events in spite of appropriate anticoagulation. In such circumstances TEE may show residual clot in the heart prompting adjustment of therapy[54] or reveal yet another source such as aortic arch atherosclerosis. For example, the presence of large thrombus (>0.8 cm2) on a prosthetic valve favors surgery or thrombolysis rather than anticoagulation due to a high risk of embolization.[42]
When the embolic source is not found in the heart or aorta with echocardiography, CTA can be used to visualize the entirety of the aorta and other large arteries.[6] CTA can show areas of large atherosclerotic plaque, dissections or aneurysms that are hidden from ultrasound evaluation by air containing structures like the trachea and bowel gas. Although CTA as well as traditional angiography allow a more comprehensive evaluation of the arterial tree TEE is more sensitive at identifying thoracic aortic plaque[6] Magnetic resonance imaging angiography is a newer modality that has not yet been adequately compared to TEE.
Long-term management of arterial embolism
Embolism from a cardiac source
In February of 2012 the American College of Chest physicians published evidence-based clinical practice guidelines addressing the short and long term antithrombotic therapy and prevention of thrombosis in a variety of patient populations at risk for thrombotic and embolic events.[42] These guidelines form the basis of preventing arterial embolization from a cardiac source in patients with AF, rheumatic valve disease, prosthetic valves, endocarditis, and other cardiac conditions. A complete synopsis of these guidelines is outside the scope of this article but a brief summary is provided below.
According to the guidelines, patients with AF, including those with paroxysmal AF and those undergoing rhythm control with an antiarrhythmic agent, should be anticoagulated if they have a CHADS2 score of 1 or more. Long-term anticoagulation is also recommended for patients with mitral stenosis who have additional risk factors for embolism (such as AF, a left atrial diameter > 55 mm, or existing thrombus) as well as patients with mechanical mitral or aortic valves. A limited 3 month course of anticoagulation is recommended for patients with bioprosthetic mitral valves, but not bioprosthetic aortic valves. The guidelines also make recommendations for patients with AF who require antiplatelet agents like aspirin and clopidogrel for indications such as coronary artery disease and coronary stents. These recommendations are based on assessments of the relative risk for bleeding, coronary thrombosis and embolism. Of note dabigatran is now the recommended agent for outpatient management of non-valvular AF, while a vitamin K antagonist (VKA) such as warfarin is still the preferred agent for most other outpatient indications with a goal INR of 2. 0-3. 0.
Arterial emboli from a non-cardiac source
Long-term management of embolic events due to aortic embolization includes risk factor modification, medical therapy and rarely surgical intervention. Risk factor modification includes smoking cessation, blood pressure control, control of blood sugars in the setting of diabetes mellitus, and lipid management.[6] These measures may retard further atherosclerotic progression and consequently reduce the risk of further thromboembolism or atheroembolism. The goals of blood pressure control are a systolic pressure less than 140 and a diastolic pressure less than 90mm Hg. This can be achieved through diet, weight loss and blood pressure medications. Lipid goals are low density lipoprotein cholesterol (LDL) of less than100 mg/dl and at least a 30% total reduction of LDL. The overall non-high density lipoprotein cholesterol also should be decreased to a level based on the patient's triglyceride concentration. This can also be achieved with a combination of life-style modification and medical therapy.[55]
Medical therapy for aortic embolism includes antiplatelet therapy, statin therapy (which may already be indicated for risk factor modification), and potentially anticoagulation. Guidelines from the American College of Cardiology and the American Heart Association recommend antiplatelet therapy with aspirin (75-325 mg daily) or clopidogrel (75 mg daily) in patients with symptomatic atherosclerotic disease of the lower extremities.[56] Although there is no large study evaluating this recommendation in patients with arterial embolism from aortic disease, anti-platelet therapy is advisable since many of these patients also have peripheral occlusions. The use of anticoagulation is not currently advised for patients with atheromas of the aorta, even when there is a large and mobile component. Small studies have shown mixed results. The Aortic Arch-Related Cerebral Hazard (ARCH) trial is currently comparing the use of anti-platelet therapy to full VKA anticoagulation in patients who have aortic arch atheroma.
For embolic events from the carotid arteries endarterectomy and stenting have been used to decrease the risk of further embolic strokes. These techniques have been tried on a limited basis for embolic disease originating in the abdominal aorta and iliac arteries. However at this time there is insufficient evidence to recommend them for general use.
SUMMARY
Acute arterial embolism is a heterogeneous disease whose presenting features, treatment and outcome depend on the source of the embolism, the type of embolism and the target organ affected. Most emboli originate from the heart, typically as a consequence of AF. Other embolic sources are diseased or prosthetic heart valves, left ventricular mural thrombus, intra-cardiac tumors and atherosclerotic lesions in the vascular tree. Emboli from atherosclerotic plaque can take two forms. The more common and larger form is thromboembolism which is a dislodged clot from the surface of the vascular plaque. The less frequent and smaller form is atheroemboli which are cholesterol crystals form the core of the lesion. The former tend to block larger arteries causing abrupt arterial occlusion, while the latter lodge in distal arterioles triggering an inflammatory reaction.
Arterial emboli manifest with organ dysfunction, demanding immediate recognition and therapy. The two most common sites for embolic events are the brain resulting in strokes and the lower extremities. Strokes present as a painless neurologic deficit, while acute embolic limb occlusion presents with a cold, painful limb. Less frequent targets are upper extremities, mesenteric vessels and the renal arteries. Except in the case of strokes, there are no strictly defined time limits for attempting reperfusion, but in general, the longer reperfusion is delayed, the greater the ischemic damage to the organ. Ischemia can ultimately lead to infarction and consequently increases morbidity and risk of mortality.
Treatment of thromboembolic events consists of immediate reperfusion through thrombolysis, embolectomy and sometimes arterial bypass. This is usually followed by systemic anticoagulation to prevent further embolization or clot propagation in partially reperfused areas. Atheroembolic events generally do not require reperfusion or anticoagulation, but due warrant antiplatelet therapy. Long-term, management requires an attempt to identify the origin of the embolus and prevention of further embolic events. This can take the form of risk factor modification, long-term systemic anticoagulation or antiplatelet therapy, the use of statins and rarely surgical intervention to correct the pathology responsible for the embolic event.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kline JA, Runyon MS. Rosen's Emergency Medicine. Philadelphia: Mos by and imprint of Elsevier; 2009. Pulmonary embolism and deep venous thrombosis. [Google Scholar]
- 2.Beldi G, Beng L, Siegel G, Bisch-Knaden S, Candinas D. Prevention of perioperative thromboembolism in patients with atrial fibrillation. Br J Surg. 2007;94:1351–5. doi: 10.1002/bjs.5835. [DOI] [PubMed] [Google Scholar]
- 3.Clagett GP, Sobel M, Jackson MR, Lip GY, Tangelder M, Verhaeghe R. Antithrombotic therapy in peripheral arterial occlusive disease: The Seventh ACCPConference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(Suppl 3):609S–26. doi: 10.1378/chest.126.3_suppl.609S. [DOI] [PubMed] [Google Scholar]
- 4.Abe Y, Asakura T, Gotou J, Iwai M, Watanabe Y, Sando M, et al. Prediction of embolism in atrial fibrillation: Classification of left atrial thrombi by transesophageal echocardiography. Jpn Circ J. 2000;64:411–5. doi: 10.1253/jcj.64.411. [DOI] [PubMed] [Google Scholar]
- 5.Menke J, Lüthje L, Kastrup A, Larsen J. Thromboembolism in atrial fibrillation. Am J Cardiol. 2012;105:502–10. doi: 10.1016/j.amjcard.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Saric M, Kronzon I. Aortic atherosclerosis and embolic events. Curr Cardiol Rep. 2012;14:342–9. doi: 10.1007/s11886-012-0261-2. [DOI] [PubMed] [Google Scholar]
- 7.Harloff A, Simon J, Brendecke S, Assefa D, Helbing T, Frydrychowicz A, et al. Complex plaques in the proximal descending aorta: An underestimated embolic source of stroke. Stroke. 2012;41:1145–50. doi: 10.1161/STROKEAHA.109.577775. [DOI] [PubMed] [Google Scholar]
- 8.Burrowes KS, Clark AR, Tawhai MH. Blood flow redistribution and ventilation-perfusion mismatch during embolic pulmonary arterial occlusion. Pulm Circ. 2011;1:365–76. doi: 10.4103/2045-8932.87302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dag O, Kaygin MA, Erkut B. Analysis of risk factors for amputation in 822 cases with acute arterial emboli. Sci World J. 2012;2012:673483. doi: 10.1100/2012/673483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tunick PA, Kronzon I. Protruding atherosclerotic plaque in the aortic arch of patients with systemic embolization: A new finding seen by transesophageal echocardiography. Am Heart J. 1990;120:658–60. doi: 10.1016/0002-8703(90)90024-r. [DOI] [PubMed] [Google Scholar]
- 11.Mutirangura P, Ruangsetakit C, Wongwanit C, Sermsathanasawadi N, Chinsakchai K. Clinical differentiation between acute arterial embolism and acute arterial thrombosis of the lower extremities. J Med Assoc Thai. 2009;92:891–7. [PubMed] [Google Scholar]
- 12.Goldman L. Approach to the patient with possible cardiovascular disease. In: Goldman L, editor. Cecil Medicine. Philadelphia: Saunders Elsevier; 2007. [Google Scholar]
- 13.Campbell WB, Ridler BM, Szymanska TH. Two-year follow-up after acute thromboembolic limb ischaemia: The importance of anticoagulation. Eur J Vasc Endovasc Surg. 2000;19:169–73. doi: 10.1053/ejvs.1999.0999. [DOI] [PubMed] [Google Scholar]
- 14.Hu HD, Chang Q, Chen Z, Liu C, Ren YY, Cai YC, et al. [Management and prognosis of acute arterial embolism: A multivariable analysis of 346 patients] Zhonghua Yi Xue Za Zhi. 2011;91:2923–6. [PubMed] [Google Scholar]
- 15.Becquemin JP, Kovarsky S. Arterial emboli of the lower limbs: Analysis of risk factors for mortality and amputation. Association Universitaire de Recherche en Chirurgie. Ann Vasc Surg. 1995;9(Suppl):S32–8. doi: 10.1016/s0890-5096(06)60449-4. [DOI] [PubMed] [Google Scholar]
- 16.Gossage JA, Ali T, Chambers J, Burnand KG. Peripheral arterial embolism: Prevalence, outcome, and the role of echocardiography in management. Vasc Endovascular Surg. 2006;40:280–6. doi: 10.1177/1538574406291820. [DOI] [PubMed] [Google Scholar]
- 17.Chiang CW, Lo SK, Ko YS, Cheng NJ, Lin PJ, Chang CH. Predictors of systemic embolism in patients with mitral stenosis. A prospective study. Ann Intern Med. 1998;128:885–9. doi: 10.7326/0003-4819-128-11-199806010-00001. [DOI] [PubMed] [Google Scholar]
- 18.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 19.Singer DE, Albers GW, Dalen JE, Fang MC, Go AS, Halperin JL, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines 8th Edition. Chest. 2008;133(Suppl 6):546S–92. doi: 10.1378/chest.08-0678. [DOI] [PubMed] [Google Scholar]
- 20.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 21.Israel CW, Grönefeld G, Ehrlich JR, Li YG, Hohnloser SH. Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: Implications for optimal patient care. J Am Coll Cardiol. 2004;43:47–52. doi: 10.1016/j.jacc.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Tofigh AM, Karvandi M, Coscas R. Current incidence of peripheral arterial embolism and role of echocardiography. Asian Cardiovasc Thorac Ann. 2008;16:439–43. doi: 10.1177/021849230801600602. [DOI] [PubMed] [Google Scholar]
- 23.Kronzon I, Tunick PA. Aortic atherosclerotic disease and stroke. Circulation. 2006;114:63–75. doi: 10.1161/CIRCULATIONAHA.105.593418. [DOI] [PubMed] [Google Scholar]
- 24.Tunick PA, Kronzon I. Atheromas of the thoracic aorta: Clinical and therapeutic update. J Am Coll Cardiol. 2000;35:545–54. doi: 10.1016/s0735-1097(99)00604-x. [DOI] [PubMed] [Google Scholar]
- 25.Kronzon I, Tunick PA. Atheromatous disease of the thoracic aorta: Pathologic and clinical implications. Ann Intern Med. 1997;126:629–37. doi: 10.7326/0003-4819-126-8-199704150-00008. [DOI] [PubMed] [Google Scholar]
- 26.Meissner I, Khandheria BK, Sheps SG, Schwartz GL, Wiebers DO, Whisnant JP, et al. Atherosclerosis of the aorta: Risk factor, risk marker, or innocent bystander? A prospective population-based transesophageal echocardiography study. J Am Coll Cardiol. 2004;44:1018–24. doi: 10.1016/j.jacc.2004.05.075. [DOI] [PubMed] [Google Scholar]
- 27.Tunick PA, Rosenzweig BP, Katz ES, Freedberg RS, Perez JL, Kronzon I. High risk for vascular events in patients with protruding aortic atheromas: A prospective study. J Am Coll Cardiol. 1994;23:1085–90. doi: 10.1016/0735-1097(94)90595-9. [DOI] [PubMed] [Google Scholar]
- 28.Jones EF, Kalman JM, Calafiore P, Tonkin AM, Donnan GA. Proximal aortic atheroma. An independent risk factor for cerebral ischemia. Stroke. 1995;26:218–24. [PubMed] [Google Scholar]
- 29.Di Tullio MR, Sacco RL, Homma S. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N Engl J Med. 1996;335:1464. doi: 10.1056/NEJM199611073351913. [DOI] [PubMed] [Google Scholar]
- 30.Amarenco P, Duyckaerts C, Tzourio C, Hénin D, Bousser MG, Hauw JJ. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:221–5. doi: 10.1056/NEJM199201233260402. [DOI] [PubMed] [Google Scholar]
- 31.Stern A, Tunick PA, Culliford AT, Lachmann J, Baumann FG, Kanchuger MS, et al. Protruding aortic arch atheromas: Risk of stroke during heart surgery with and without aortic arch endarterectomy. Am Heart J. 1999;138:746–52. doi: 10.1016/s0002-8703(99)70191-2. [DOI] [PubMed] [Google Scholar]
- 32.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–74. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 33.Mullen MT, Pisapia JM, Tilwa S, Messé SR, Stein SC. Systematic review of outcome after ischemic stroke due to anterior circulation occlusion treated with intravenous, intra-arterial, or combined intravenous+intra-arterial thrombolysis. Stroke. 2012;43:2350–5. doi: 10.1161/STROKEAHA.111.639211. [DOI] [PubMed] [Google Scholar]
- 34.Sanoski CA. Current approaches to anticoagulation for reducing risk of atrial fibrillation-related stroke. J Pharm Pract. 2012:1–10. doi: 10.1177/0897190012452309. [DOI] [PubMed] [Google Scholar]
- 35.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The An Ticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 36.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke. 1991;22:983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 37.Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008;92:17–40. doi: 10.1016/j.mcna.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Endean ED, Barnes SL, Kwolek CJ, Minion DJ, Schwarcz TH, Mentzer RM., Jr Surgical management of thrombotic acute intestinal ischemia. Ann Surg. 2001;233:801–8. doi: 10.1097/00000658-200106000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: A guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001–23. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 40.Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 41.Del Zoppo GJ, Saver JL, Jauch EC, Adams HP., Jr American Heart Association Stroke Council. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: A science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945–8. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 141(Suppl 2):7S–47. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paciaroni M, Agnelli G, Micheli S, Caso V. Efficacy and safety of anticoagulant treatment in acute cardioembolic stroke: A meta-analysis of randomized controlled trials. Stroke. 2007;38:423–30. doi: 10.1161/01.STR.0000254600.92975.1f. [DOI] [PubMed] [Google Scholar]
- 44.Lansberg MG, O’Donnell MJ, Khatri P, Lang ES, Nguyen-Huynh MN, Schwartz NE, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(Suppl 2):e601S–36. doi: 10.1378/chest.11-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tawes RL, Jr, Harris EJ, Brown WH, Shoor PM, Zimmerman JJ, Sydorak GR, et al. Arterial thromboembolism. A 20-year perspective. Arch Surg. 1985;120:595–9. doi: 10.1001/archsurg.1985.01390290073012. [DOI] [PubMed] [Google Scholar]
- 46.Blaisdell FW, Steele M, Allen RE. Management of acute lower extremity arterial ischemia due to embolism and thrombosis. Surgery. 1978;84:822–34. [PubMed] [Google Scholar]
- 47.Dale WA. Differential management of acute peripheral arterial ischemia. J Vasc Surg. 1984;1:269–78. [PubMed] [Google Scholar]
- 48.Sise MJ. Mesenteric ischemia: The whole spectrum. Scand J Surg. 2010;99:106–10. doi: 10.1177/145749691009900212. [DOI] [PubMed] [Google Scholar]
- 49.Renner P, Kienle K, Dahlke MH, Heiss P, Pfister K, Stroszczynski C, et al. Intestinal ischemia: Current treatment concepts. Langenbecks Arch Surg. 2010;396:3–11. doi: 10.1007/s00423-010-0726-y. [DOI] [PubMed] [Google Scholar]
- 50.Lessman RK, Johnson SF, Coburn JW, Kaufman JJ. Renal artery embolism: Clinical features and long-term follow-up of 17 cases. Ann Intern Med. 1978;89:477–82. doi: 10.7326/0003-4819-89-4-477. [DOI] [PubMed] [Google Scholar]
- 51.Fort J. Renal artery embolism: Prospective study of 41 patients based on a diagnostic and therapeutic algorithm. Open Urol Nephrol J. 2008:9–15. [Google Scholar]
- 52.Fort J, Camps J, Ruiz P, Segarra A, Gomez M, Matas M, et al. Renal artery embolism successfully revascularized by surgery after 5 days’ anuria. Is it never too late? Nephrol Dial Transplant. 1996;11:1843–5. [PubMed] [Google Scholar]
- 53.Saric M, Kronzon I. Cholesterol embolization syndrome. Curr Opin Cardiol. 2011;26:472–9. doi: 10.1097/HCO.0b013e32834b7fdd. [DOI] [PubMed] [Google Scholar]
- 54.Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance American College of Chest Physicians. J Am Soc Echocardiogr. 2011;24:229–67. doi: 10.1016/j.echo.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011update: A guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 124:2458–73. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 56.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]


