Abstract
The objective of the present study was to design ophthalmic delivery systems based on polymeric carriers that undergo sol-to-gel transition upon change in temperature or in the presence of cations so as to prolong the effect of HP-β-CD Voriconazole (VCZ) in situ gelling formulations. The in situ gelling formulations of Voriconazole were prepared by using pluronic F-127 (PF-127) or with combination of pluronic F-68 (PF-68) and sodium alginate by cold method technique. The prepared formulations were evaluated for their physical appearance, drug content, gelation temperature (T gel), in vitro permeation studies, rheological properties, mucoadhesion studies, antifungal studies, and stability studies. All batches of in situ formulations had satisfactory pH ranging from 6.8 to 7.4, drug content between 95% and 100%, showing uniform distribution of drug. As the concentration of each polymeric component was increased, that is, PF-68 and sodium alginate, there was a decrease in T gel with increase in viscosity and mucoadhesive strength. The in vitro drug release decreased with increase in polymeric concentrations. The stability data concluded that all formulations showed the low degradation and maximum shelf life of 2 years. The antifungal efficiency of the selected formulation against Candida albicans and Asperigillus fumigatus confirmed that designed formulation has prolonged effect and retained its properties against fungal infection.
1. Introduction
In the ophthalmic drug delivery systems, protective barriers of eye lead to low absorption of drug and it leads to poor bioavailability of therapeutic drugs. The cul-de-sac normally holds 7–9 μL of tears but can retain up to approximately 20–30 μL without overflowing. The normal tear flow rate and film thickness are 1 μL/min and 4–9 μm. The normal pH of the tears is ~6.5–7.6. The drainage of instilled solutions (25–50 μL) away from the front of the eye is essentially completed at around 90 sec. Under normal conditions, the eye can accommodate only a very small volume without overflowing. Commercial eye drops have a volume of ~30 μL, which is about the volume of the conjunctival sac in humans; however, after a single blink, only an estimated 10 μL remains [1]. The poor bioavailability and less therapeutic response of convential eye drops occurs mainly due to the gravity induced lacrimal flow and normal tear turnover of the eye. Frequent dosing is usually associated with nonpatient compliance and tear drainage of the administered dose passes via the nasolacrimal duct into the gastrointestinal tract, leading to side effects. Due to this drug loss in front of the eye, very small drug is available to enter the cornea and inner tissue of eye. Its leads to very small corneal contact time (about 1-2 mins) in humans for instilled solution usually less than 10%. Therefore, only small amount of drug actually penetrates the cornea and reaches intraocular surface [2, 3].
An ideal ophthalmic drug delivery must be able to release the drug in sustained manner and remain in the front of eye for prolong period of the time. As a result, various attempts have been made to prolong the contact time of drug on the ocular surface and also to slow down the drug elimination [4], that is, development of viscous gel to prolong the precorneal drug retention [5, 6], microparticle suspension [7], or polymeric solution [8], inserts [9], and collagen shields [10]. However, these dosage forms also comprise some disadvantages such as discomfort especially in elderly patients, loss of device during sleep, or rubbing eye and poor compliance, as well as blurred vision.
The ophthalmic drug delivery based on in situ gel can overcome these problems. As in situ activated gel forming systems can be administered in drop form and create considerably fewer problems with vision and also provide better sustained properties than drops these in situ gelling systems consist of polymers that exhibit sol-to-gel phase transitions due to change in specific physicochemical parameters (pH, temperature, and ionic strength) in the environment, cul-de-sac in the case of eye [11]. There are different approaches used for triggering the in situ gel formation: physiological stimuli (e.g., temperature induced and pH induced), physical changes in biomaterials (e.g., diffusion of solvent and swelling), and chemical reactions (e.g., enzymatic, chemical, and photoinitiated polymerization).
Polymers such as pluronics (poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPOPEO) Triblock), polymer networks of poly(acrylic acid) (PAA), and polyacrylamide (PAAm) or poly(acrylamide-co-butyl methacrylate) are temperature-induced polymers which are liquid at room temperature (20°C–25°C) and undergo gelation when arrive in contact with body fluids (35°C–37°C), due to an increase in temperature [12, 13]. Certain polymers such as PAA (Carbopol, carbomer) or its derivatives, mixtures of poly(methacrylic acid) (PMA) and poly(ethylene glycol) (PEG), show change from sol to gel with change of pH [14, 15]. In presence of various ion such as k+, Ca+2, Mg+2, and Na+, certain ion sensitive polysaccharides such as carrageenan, gellan gum (Gelrite), pectin, and sodium alginate undergo phase transition [16, 17]. Poloxamer 407 (PF-127) is a nonionic surfactant composed of poly(ethylene oxide)-b(poly(propylene oxide)-b-poly(ethylene oxide) (PEO-PPO-PEO) showing amphiphilic behavior due to hydrophobic propylene oxide domains and hydrophilic ethylene oxide domains. Pluronic F127 exhibits sol to gel transition at 37°C when used at a higher concentration of (25%–30%) (w/v). By using different series of poloxamers, cross-linking agents, by changing pH and ionic strength gelation, temperature can be adjusted within the range of 33–36°C [18–20].
With most common treatments, amphotericin B and natamycin fungal ulcers tend to have very poor outcomes. Since 1960, no new medication has been approved by the FDA and there has been only a single randomized trial of antifungal therapy for fungal ulcers. There are studies that indicate that the newer triazoles, such as voriconazole, are more effective in vitro against filamentous fungi such as Aspergillus species, a common cause of fungal keratitis [21]. Voriconazole is a broad spectrum antifungal agent and is commonly used in fungal keratitis and also active against species that are known to be resistant to the other antifungal agents. It is a second-generation synthetic derivative of fluconazole. Voriconazole differs from fluconazole by the addition of a methyl group to the propyl back bone and by the substitution of a triazole moiety with a fluoropyrimidine group, resulting in increased activity of drug. Antifungal potency increased by the substitution of a triazole ring with a pyrimidine moiety, and the addition of a fluorine to this ring structure at the 5 position enhanced in vivo efficacy [22].
Voriconazole is a lipophilic drug with a low pH-dependent aqueous solubility (maximum 2.7 mg/mL at pH 1.2). Due to low solubility of voriconazole, it is encapsulated in β-cyclodextrin derivative in order to increase the solubility and stability of Voriconazole in aqueous solutions, while maintaining its lipophilicity and high corneal permeability [23, 24]. Hydroxypropyl-β-cyclodextrin (HP-β-CD), a cyclic oligosaccharide with outer hydrophilic surface and a lipophilic cavity, is capable of forming inclusion complexes with many lipophilic drugs. Solubility enhancement studies of indomethacin conducted using SBE-β-CD and HP-β-CD have revealed the better potential of HP-β-CD as a solubility enhancing agent [25]. Ciprofloxacin ophthalmic formulations prepared using HP-β-CD demonstrated better stability and biological activity than the ophthalmic solution without HP-β-CD [26]. Voriconazole has a good penetration through the cornea into the aqueous humour and does not affect intraocular safety when administered topically [27].
The objective of present study was to develop and evaluate a temperature triggered in situ ophthalmic gel system for voriconazole.
2. Experimental
2.1. Materials
Voriconazole and hydroxypropyl-β-cyclodextrin were received as gift samples from Matrix Laboratories, Hyderabad (India), pluronics (F-127), pluronics (F-68), sodium alginate, and mucin from porcine stomach type II were purchased from Sigma-Aldrich Pvt. Ltd., (India), and Hi Media Sabouraud Dextrose Agar was obtained from Deep Scientific Laboratories, Chandigarh, (India). All other chemicals used were of analytical reagent grade. Freeze-dried strains Candida albicans (MTCC 227) and Aspergillus fumigatus (MTCC 2544) were obtained from MTCC, IMTECH Chandigarh, India; Fresh whole eyeballs of goat were obtained from local butcher's shop (Zirakpur, Punjab, India) within one hour of slaughtering of animal.
2.2. Preparation of Voriconazole In Situ Gels
In situ gelling liquids were prepared using different concentrations of pluronic F-68 and sodium alginate with fixed concentration of pluronic F-127. Voriconazole (0.15 w/v) was weighed separately and dissolved in the distilled water with (1.5%w/v) HP-β-CD. Sodium alginate solutions of different concentrations (0.5%, 1%, and 1.5%) were prepared by dispersing the required amount in distilled water with continuous stirring until completely dissolved. The voriconazole solution was added to the alginate solution under constant stirring until uniform, clear solution was obtained. Further, to this mixture pluronic F-127 (15% w/v) and different concentrations of pluronic F-68 (14%, 15%, and 16%) were added. Benzalkonium chloride (0.01% w/v) was added as a preservative to the previous solutions. Sufficient amount of sodium chloride was added to the mixture to maintain the isotonicity. Finally, the volume was adjusted with distilled water up to 100 mL. Partially the dissolved pluronic solutions were stored overnight in a refrigerator at 4°C for hydration and stirred periodically until clear homogenous solutions were obtained. Nine batches of formulation were prepared by using different concentrations of sodium alginate and PF-68 as shown in Table 2.
Table 2.
Effect of the addition of different concentrations of sodium alginate and P-68 to voriconazole in situ gelling formulation on viscosity of gel, bioadhesion component, and force of bioadhesion.
| Formulations | Viscosity of gel at 100 rpm (cP) | Viscosity with bioadhesive component at 100 rpm (cP) | Force of bioadhesion (dyne/cm2) |
|---|---|---|---|
| VG1 | 235.7 ± 6.66 | 1857 ± 6.42 | 17.24 |
| VG2 | 249.3 ± 4.93†† | 1963 ± 6.55†† | 18.78 |
| VG3 | 262.3 ± 7.02†† | 2047 ± 4.16†† | 19.96 |
| VG4 | 274.3 ± 5.03†† | 2186 ± 2.08†† | 22.08 |
| VG5 | 306.3 ± 6.11†† | 2236 ± 4.58†† | 22.38 |
| VG6 | 329.3 ± 10.5†† | 2345 ± 3.21†† | 23.82 |
| VG7 | 355.7 ± 8.50†† | 2478 ± 4.01†† | 25.59 |
| VG8 | 351.7 ± 28.3†† | 2576 ± 3.11†† | 27.59 |
| VG9 | 414.3 ± 10.0†† | 2698 ± 3.79†† | 28.28 |
Values are mean ± SE of 3 gel viscosities in each group.
†Statistically significant difference at P < 0.05.
††Statistically significant difference at P < 0.01.
†††Statistically significant difference at P < 0.001 from control (VGI containing 15% PF-127, 14% PF-68, 0.5% sodium alginate) as determined by one-way ANOVA followed by Dunnett's test.
2.3. Physicochemical Evaluation of In Situ Gelling Formulations
2.3.1. Measurement of Gelation Temperature
At room temperature, ten milliliters of cold sample solution (pluronic containing formula) were put into a beaker (25 mL) and placed in a low temperature water bath. A thermometer was immersed into the sample solution for constant monitoring. The solution was heated with stirring at 200 rpm using a magnetic bar (9 × 25 mm). The temperature at which the magnetic bar stopped moving due to gelation was reported as the gelation temperature (T gel). Each sample was measured in triplicate [20, 28].
2.3.2. Drug Content Uniformity
Drug content of Voriconazole in situ gelling formulations was determined by accurately dissolving (1 mL) weighed quantity of formulation in 100 mL simulated tears fluid. The formulation was shaken for 2-3 min to completely dissolve, until it gives a clear gel solution. The solution was filtered through Millipore membrane filter (0.45 µm) and drug content was analyzed by UV-Vis Spectrophotometry at 260 nm. The experiments were done in triplicate and the mean ± SD was reported [29].
2.3.3. Rheological Studies
It is the important factor to determine the residence time of drug in the eye by considering the viscosity of the instilled formulation. The prepared solutions were allowed to gel at physiological temperature and then the viscosity determination was carried out by using Brookfield viscometer (Brookfield DV+Pro, Brookfield Engineering Laboratories, Middleboro, MA, USA). By plotting graph of shear rate versus shear stress, the flow pattern was checked.
2.3.4. Bioadhesion Strength
To quantify mucin-polymer mucoadhesive strength of gel formulation, a simple viscometric method was used [30]. Viscosities of 15% (w/v) porcine gastric mucin dispersions in STF were measured with a Brookfield viscometer in the absence (ηm) or presence (ηt) of different formulations at a shear rate of a shear rate of 100 rpm at 37°C. For homogenous distribution throughout the sample, viscometric measurements were performed after exactly 3 min of applying the shear force. Viscosity components of mucoadhesion (ηb) were calculated from the equation ηt = ηm + ηp + ηb, where ηp is the viscosity of corresponding pure polymer solution. The force of mucoadhesion (F) was calculated from the equation F = ηb · σ, where σ is the rate of shear/sec.
2.3.5. In Vitro Drug Permeation
The in vitro drug permeation studies were carried out by putting the in situ gelling formulation on Millipore membrane filter (0.15 mm) between the donor and receptor compartments of an all-glass modified Franz diffusion cell. To simulate the corneal epithelial barrier, the Millipore membrane filter was used, as isolated cornea will not remain viable beyond 4 hr. The receptor compartment of an all-glass modified Franz diffusion cell was filled with 10 mL freshly prepared simulated tear fluid (pH 7.0), and all air bubbles were expelled from the compartment. An aliquot (1 mL) of test solution was placed on the Millipore membrane filter, and the opening of the donor cell was sealed with a glass cover slip. The receptor fluid was kept at 37 ± 0.5°C with constant stirring using a Teflon-coated magnetic stir bead. Permeation study was continued for 10 hr, and samples were withdrawn from receptor and analyzed for Voriconazole content by measuring absorbance at 260 nm in a spectrophotometer (UV-Vis Spectrophotometer 2701 A Systronics, Mumbai, India).
Drug permeation experiments were also carried out using freshly excised goat cornea. Goat whole eyeballs were transported from the local butcher shop to the laboratory in cold (4°C) normal saline within 1 hr of slaughtering of the animal. The cornea was carefully excised along with 2 to 4 mm of surrounding scleral tissue and was washed with cold normal saline till the washing was free from proteins. Freshly excised cornea was fixed between clamped donor and receptor compartments in such a way that its epithelial surface faced the donor compartment. For the analysis of Voriconazole withdrawn from receptor compartment, the same procedure was adopted as mentioned earlier. Results were expressed as cumulative percentage of drug released versus time.
2.3.6. Release Kinetics Study
To study the drug release kinetics, data obtained from in vitro permeation studies were fitted in various kinetic models: zero order as the cumulative percent of drug permeated versus time, first order as the log cumulative percentage of drug remaining versus time, and Higuchi's model as the cumulative percent drug permeated versus square root of time. The release mechanism of voriconazole from in situ gel was determined by fitting the data into the Korsmeyer-Peppas model as the log cumulative percentage of drug released versus log time, and the exponent “n” was calculated from the slope of the straight line. If n < 0.45, then the diffusion mechanism is Fickian; if 0.5 < n < 0.8, the non-Fickian and n > 1 show super case II transport. The drug permeation data was plotted according to zero order, first-order kinetics, Higuchi equation, and Korsmeyer-Peppas equation [31].
2.3.7. Corneal Hydration (HL%)
Wet corneal weight (W a) was noted after removal of cornea from donor compartment after experiment. Each corneal sample soaked in methanol (1 mL) and dried overnight at 90°C and reweighed (W b). The percentage corneal hydration level (HL %) was calculated by the formula corneal hydration = [1 − (W a/W b )]∗100 [32].
2.3.8. Antifungal Studies
The antifungal efficiency and prolonged effect of selected sustained release in situ gel of voriconazole formulations were carried out on Candida albicans and Asperigillus fumigatus species. The nutrient agar medium was prepared by dissolving saboured dextrose in hot distilled water and media was autoclaved at 121°C for 15 min. By using diffusion method test organisms were previously seeded (10 CFU/mL) in the nutrient agar medium [33]. The aliquot test samples were poured into petri dish containing nutrient agar medium using micropipette. The plates were left for 30 min and then incubated at 25°C for 24 hr. The diameters of zone of inhibition for Candida albicans and Aspergillus fumigatus were measured after 24 hr and 120 hr, respectively.
2.3.9. Stability of In Situ Gel
Stability studies were carried out on in situ gelling formulations according to ICH guidelines [34]. All formulations were stored in closed amber glass bottles and placed at humidity chamber with a relative humidity of 75 ± 5% and temperatures of 40 ± 2°C or at room temperature. Samples were withdrawn at time 0, 3 weeks, 6 weeks, 3 months, and 6 months and analyzed for drug concentration. The formulations were evaluated at periodic intervals for pH, clarity, and drug content. The degradation rate constant was determined from the plot of logarithm of the remaining drug versus time.
2.4. Statistical Analysis
All values presented in the study are average of triplicate experiments for the same time points. Differences in viscosities and in vitro permeability profile of voriconazole in situ gel under different conditions were tested statistically using one-way analysis of variance (ANOVA) followed by Dunnett's test at different level of significance. (†Statistically significant difference at P < 0.05; ††statistically significant difference at P < 0.01; †††statistically significant difference at P < 0.001 from control.)
3. Results and Discussion
Pluronic F127 became one of the most extensively investigated temperature-responsive materials due to its unique thermoreversible gelation properties, but the phase transition temperature strongly depended on pluronic F127 concentration [35]. Pluronic F68 is incorporated into pluronic F127 in order to modulate the phase transisition temperature for ophthalmic drug delivery system [36]. Sodium alginate is a natural hydrophilic polysaccharide containing two types of monomers, b-d-mannuronic acid (M) and a-l-guluronic acid (G). Alginate is not easily eroded by tear fluid as it transforms into stable gel upon exposure to divalent cations and it has also mucoadhesive property [11, 37].
Pluronic 127 (15%, w/v) was selected as the basis of formulation because below this concentration it loses its sol-gel transition properties and it is used in combination with pluronics 68 and sodium alginate in different concentrations. Sodium alginate was combined in formulation for the additive effect of mucoadhesive property.
3.1. Clarity, Drug Content, and pH
The physicochemical properties of the Voriconazole formulations are shown in Table 1. The drug content, clarity, and pH of the formulations were found to be satisfactory and the formulations were liquid at both room temperature and refrigerated temperature conditions. For ophthalmic delivery, clarity of formulation is the main concern because acceptability of formulation is based on it. All gels of Voriconazole formulation batches were observed clear and transparent.
Table 1.
Physiochemical characterization of in situ gels of Voriconazole, mean ± SD; n = 3.
| Formulations | Concentration | Gelation temperature (°C) | Drug content % |
pH | |
|---|---|---|---|---|---|
| *PF-68 | *Na alginate | ||||
| VG1 | 14 | 0.5 | 37.33 ± 0.73 | 96.98 ± 1.34 | 6.8 |
| VG2 | 14 | 1 | 36.50 ± 0.35 | 96.34 ± 2.00 | 6.9 |
| VG3 | 14 | 1.5 | 35.70 ± 0.36 | 93.33 ± 1.26 | 7.0 |
| VG4 | 15 | 0.5 | 33.80 ± 0.40 | 92.34 ± 2.15 | 6.9 |
| VG5 | 15 | 1 | 32.60 ± 0.30 | 94.91 ± 0.372 | 7.1 |
| VG6 | 15 | 1.5 | 30.83 ± 0.65 | 99.01 ± 2.79 | 7.4 |
| VG7 | 16 | 0.5 | 29.63 ± 0.51 | 97.09 ± 1.90 | 6.9 |
| VG8 | 16 | 1 | 28.36 ± 0.41 | 92.34 ± 0.66 | 6.8 |
| VG9 | 16 | 1.5 | 24.36 ± 0.41 | 91.41 ± 0.51 | 7.2 |
*PF 68—Pluronic 68 or Poloxamer 118.
*Na alginate—sodium alginate.
All the formulations should have satisfactory pH ranging from 6.8 to 7.4, which is acceptable for ocular delivery. Drug content values ranging from 91.4 ± 0.51 to 99.01 ± 2.79% were showing uniform distribution of drug.
3.2. Gelation Temperature (T gel)
T gel is the temperature at which the liquid phase makes a transition to gel. The basic prerequisite for in situ gelling system is that the gel formulation should be a free flowing liquid at room temperature for ease to be administrated at site of application in eye where it becomes gel as in nature at physiological temperature of human eye, that is, 37°C [38].
The gelation temperature (T gel) for the the formulations was found in between range of 24.36 ± 0.41 to 37.33 ± 0.73 (Table 1). The minimum gelation temperature was observed for batch VG9, that is, 24.36 ± 0.41. This might be effect of higher concentration of PF-68 (16%) and with combination of sodium alginate (1.5%). The result also suggests that the increased concentration of P-68 in in situ gel decreases the gelation temperature of formulation. The earlier literature reported that the addition of pluronic PF-68 in formulation can lead to micellar entanglement and changing the PEO/PPO ratio [39]. In micellation, on one hand, the in aqueous solution forms an aggregate with the hydrophilic head, and on the other, aqueous solvent is sequestered with the hydrophobic single-tail regions in the micelle centre. The formation of micelles might increase the viscosity of vehicles and end up in forming a gel.
Sodium alginate is also responsible for decreasing gelation temperature. As the concentration of sodium alginate increases, that is, 1.5% T gel decreases as shown in batches (VG3, VG6, VG9), it reduced due to more entangled nature of the polymeric networks [40].
3.3. Rheological Viscosity
The viscosity values of all formulations were shown in Table 2. The result suggests that all the formulations provide pseudoplastic behavior as shown in Figure 1. Among all the formulations, VG9 formulation provides maximum viscosity and statistically significant (P < 0.01) value, that is, 2698 ± 3.79 cps at 100 rpm (37°C). Viscosity is an important factor to determine the residence time of drug in the eye. In ocular drug delivery system, the ophthalmic products should not disturb the pseudoplastic character of precorneal tear film. The ocular shear rate is about 0.03 s−1 during interblinking periods and 4250–28500 s−1 during blinking. So, the viscoelastic fluids having high viscosity under low shear rates and low viscosity under high shear rates called as pseudo plastic fluid are often preferred [41]. All the formulations exhibited pseudo plastic behavior; that is, with increase in shear rate, a decrease in viscosity was observed. This might be due to that gel formation (T gel) decreases as concentration of pluronics (P-68) and sodium alginate increases as a result of micellar enlargement and packing. This result also contributes that due to these micelle entanglements, they cannot separate easily from each other, which leads to the high viscosity of gels containing high concentrations of pluronics (P-68) and sodium alginate.
Figure 1.
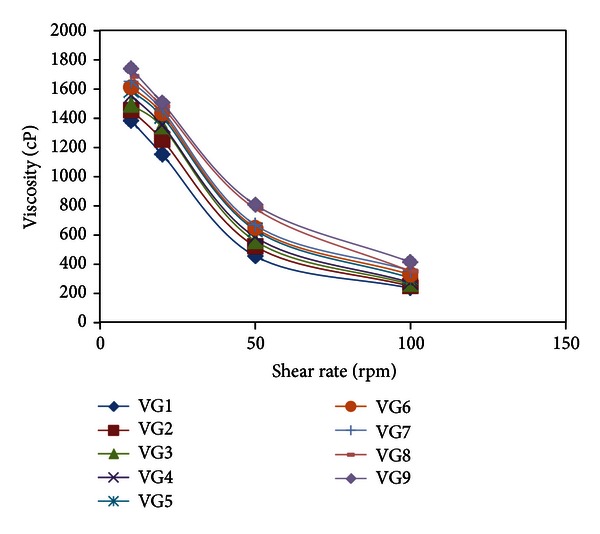
Viscosity profile of all Voriconazole in situ gels at different shear rates (rpm).
3.4. Bioadhesive Strength
The force of bioadhesion is an important and crucial physicochemical parameter for in situ forming ophthalmic gels since it prevents the formulation from rapid drainage, and hence, prolongs its residence time. The values of all bioadhesive forces were depicted in Table 2. The force of bioadhesion of formulation VG9 was observed maximum (28.28 dynes/cm2), that is, statistically significant (P < 0.01) compared with other formulation. The rest of formulation provides the bioadhesion force in between range of 17.24 to 27.59 dynes/cm2. The Voriconazole in situ gel formulation VG9 exhibits maximum bioadhesion force due to combination effect of sodium alginate and PF-68. As the viscosity of VG9 was maximum, that is, (2698 ± 3.79 cps), that might contribute to higher force of bioadhesion as compared to other formulations. Increasing the mucoadhesive polymer, sodium alginate concentration in the formulation significantly increased the mucoadhesive force of the formulation. Addition of PF-68 enhanced the bioadhesive force, since the pluronic with a hydrophilic oxide group could bind to oligosaccharide chains. The higher the concentration of PF-68, the greater the bioadhesive force of pluronic gel.
3.5. In Vitro Drug Permeation
The in vitro permeation studies of Voriconazole in situ gel were carried out through the Millipore membrane filter paper (0.15 µm) and freshly excised goat cornea, clamped between donor and receptor compartments of all glass modified Franz diffusion cell. Formulation VG3 containing higher concentration of sodium alginate with respect to formulation VG1 and VG2 showed lesser amount of drug through Millipore membrane filter at the end of 1 hr and same release pattern was obtained at 10 hr (Table 3).
Table 3.
In vitro permeation of Voriconazole from in situ gels through millipore membrane filter and freshly excised goat cornea.
| % Drug permeation* | |||||
|---|---|---|---|---|---|
| Formulations |
Millipore paper | Goat cornea | Corneal hydration (%) | ||
| t 1 | t 10 | t 1 | t 4 | ||
| VG1 | 22.01 ± 0.21 | 64.98 ± 0.65 | 16.89 ± 0.27 |
30.82 ± 0.27 | 77.03 ± 1.9 |
| VG2 | 20.27 ± 0.23†† | 63.82 ± 1.07†† | 14.61 ± 0.15†† | 28.58 ± 0.12†† | 79.80 ± 0.6 |
| VG3 | 18.47 ± 0.10†† | 59.01 ± 0.35†† | 12.82 ± 0.12†† | 27.46 ± 0.19†† | 78.71 ± 2.3 |
| VG4 | 21.98 ± 0.19†† | 60.32 ± 0.80†† | 15.84 ± 0.08†† | 29.31 ± 0.26†† | 75.69 ± 0.5 |
| VG5 | 19.89 ± 0.08†† | 58.13 ± 0.27†† | 13.64 ± 0.41†† | 27.80 ± 0.46†† | 74.90 ± 1.7 |
| VG6 | 18.24 ± 0.13†† | 52.96 ± 0.38†† | 12.14 ± 0.52†† | 25.94 ± 0.72†† | 76.33 ± 3.1 |
| VG7 | 20.69 ± 0.21†† | 58.30 ± 0.39†† | 15.04 ± 0.43†† | 27.37 ± 1.70†† | 77.05 ± 0.9 |
| VG8 | 15.10 ± 0.12†† | 51.47 ± 0.47†† | 13.57 ± 0.46†† | 27.60 ± 0.47†† | 79.02 ± 1.7 |
| VG9 | 12.64 ± 0.18†† | 49.84 ± 0.49†† | 9.93 ± 0.10†† | 19.74 ± 0.21†† | 75.90 ± 0.6 |
*Mean ± SD, n = 3.
t 1—cumulative percent drug after 1 hr; t 10—cumulative percent drug after 10 hr; t 4—cumulative percent drug after 4 hr.
†Statistically significant difference at P < 0.05.
††Statistically significant difference at P < 0.01.
†††Statistically significant difference at P < 0.001 from control (VGI containing 15% PF-127, 14% PF-68, 0.5% sodium alginate) as determined by one-way ANOVA followed by Dunnett's test.
The formulation VG6 containing 15% w/v of PF-68 and a comparitively higher concentration (1.5%) of sodium alginate, showed a cumulative release of 53% at the end of 10 hr which indicated that VG6 provided a sustained release. The results are shown in Figure 2 showing statistically significant values (P < 0.01), which indicates that the in vitro permeation from in situ gel of Voriconazole (VG1 to VG9) was sustained for 10 hr. The drug permeated from the formulation VG9 showed a lesser cumulative percantage release in comparison to the other formulations and hence demonstrated the maximum sustained release. The effect of PF-68 on the release rate of VCZ from the pluronic-based in situ gelling formulations showed little effect on the release rate of VCZ from in situ gelling formulations. The addition of PF-68 (16%) resulted in a decrease in the drug release rate compared to PF-68 (14%) and PF-68 (15%). The results indicate that as the concentration of PF-68 and sodium alginate increases, T gel decreases due to micellar entanglement, leading to higher viscosity of the gel which functioned as an increasingly resistant barrier to drug release. Due to increase in the number and size of micelles within the gel structure, it leads to the enhanced resistance resulting in a more entangled system and more rigid gel and also attributed to the increase in viscosity.
Figure 2.
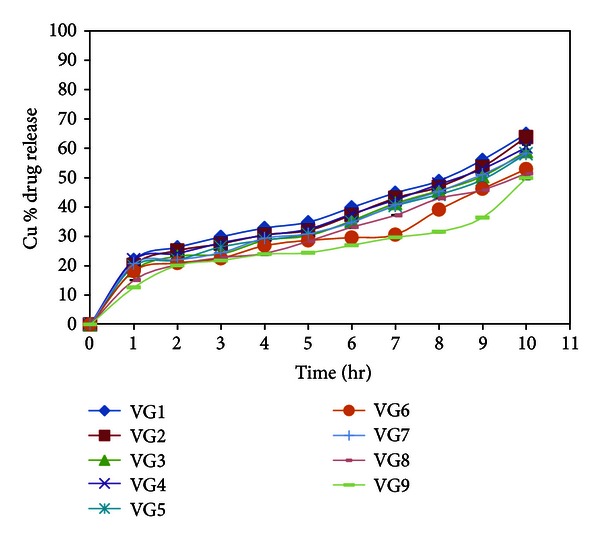
In vitro permeation profile of Voriconazole from in situ gelling systems through Millipore membrane filter.
The in vitro drug permeation studies of in situ gel formulations of voriconazole through excised goat corneas are shown in Table 4 and Figure 3. To mimic real life conditions, excised goat corneas were used for permeation studies and the experiment was conducted for 4 hr considering cornea viability, and the drug permeation from in situ gels ranged between 30.82 and 19.74%, which was less than the permeation observed with the Millipore membrane filter in 4 hr. In goat corneas permeation studies, the formulation VG9 showed 19.74 ± 0.21% release at the end of 4 hour which is less as compared to other formulations.
Table 4.
Kinetic profiles of in vitro drug release from in situ gels through Millipore membrane filter and freshly excised goat cornea.
| Formulations | (R 2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Zero order | 1st order | Higuchi | Korsmeyer-Peppas | ||||||
| Millipore goat | Membrane cornea | Millipore goat | Membrane cornea | Millipore goat | Membrane cornea | Millipore goat | Membrane cornea |
Mechanism of drug release |
|
| VG1 | 0.9286 | 0.8887 | 0.9326 | 0.9176 | 0.9505 | 0.9866 | 0.9126 | 0.9474 | Fickian |
| VG2 | 0.9320 | 0.9006 | 0.9227 | 0.9128 | 0.9362 | 0.9414 | 0.8981 | 0.7705 | Non-Fickian |
| VG3 | 0.9461 | 0.9207 | 0.9227 | 0.9269 | 0.9452 | 0.9346 | 0.8981 | 0.8073 | Non-Fickian |
| VG4 | 0.9298 | 0.8840 | 0.9438 | 0.9077 | 0.9506 | 0.9738 | 0.9337 | 0.8922 | Fickian |
| VG5 | 0.9338 | 0.9102 | 0.9402 | 0.9195 | 0.9478 | 0.9388 | 0.9015 | 0.7878 | Fickian |
| VG6 | 0.9011 | 0.9250 | 0.8985 | 0.9325 | 0.9183 | 0.9428 | 0.9077 | 0.8304 | Fickian |
| VG7 | 0.9376 | 0.8828 | 0.9435 | 0.9059 | 0.9560 | 0.9755 | 0.896 | 0.8947 | Fickian |
| VG8 | 0.9587 | 0.9089 | 0.9535 | 0.9179 | 0.9620 | 0.9366 | 0.9451 | 0.7789 | Non-Fickian |
| VG9 | 0.8812 | 0.9311 | 0.8632 | 0.9440 | 0.9034 | 0.9750 | 0.9023 | 0.890 | Non-Fickian |
Figure 3.
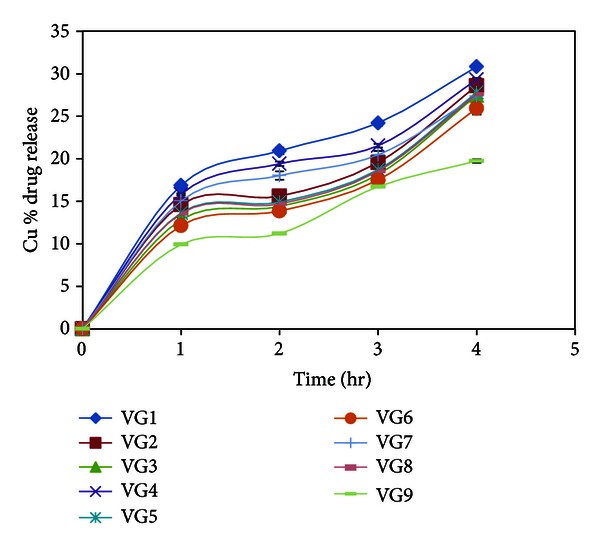
In vitro permeation profile of Voriconazole from in situ gelling systems through freshly excised goat cornea.
The rank order of drug release was VG1 > VG2 > VG4 > VG3 > VG7 > VG5 > VG6 > VG8 > VG9 for Millipore membrane filter as well as in goat cornea. Cornea (made of epithelium (lipophilic), stroma (hydrophilic), and endothelium (less lipophilic than epithelium)) acts as a lipophilic-hydrophilic barrier and the drug will have to partition through the barrier for corneal penetration while Millipore membrane filter acts as a mechanical barrier to drug diffusion. Accordingly, permeation through the cornea would be lower compared to that across the Millipore filter paper.
3.6. Release Kinetics Study
Release kinetic models are shown in Table 4. The table indicates that the correlation coefficient of release data fits more into the Higuchi model for most number of cases than any other available models (using Millipore membrane and goat cornea as permeation medium). The release profiles of in situ gelling formulations were treated with the Korsmeyer-Peppas equation, and slope values n > 0.89 were indicating anamolous drug release involving a combination of both Fickian and non-Fickian diffusions through the Millipore membrane filter and excised goat cornea.
3.7. Corneal Hydration
The corneal hydration level of normal mammalian cornea is between 75% and 80% [42]. The drug concentration, pH, and addition of preservatives and/or polymers in Voriconazole in situ gelling formulations did not show any corneal damage as the corneal hydration value for all corneas remained in the normal range of 75% to 80% (Table 3).
3.8. Microbiological Assay
The antifungal efficiency of the selected controlled release voriconazole formulation VG9 was evaluated against organisms including Candida albicans and Aspergillus fumigatus. The mean diameters of zone of inhibition with Candida albicans and Aspergillus fumigatus are depicted in Table 5. For the preparation of Control 2, the same set of procedures was followed as employed in case of the formulation VG9, except for the use of HP-β-CD. This was done to check the antifungal potential of voriconazole alone in absence of HP-β-CD. The microbiological assay studies conducted using agar diffusion method indicated that HP-β-CD based Voriconazole in situ gel formulation VG9 inhibited the growth of Candida albicans and Aspergillus fumigatus, while the control solutions of VG9 formulation without drug (Control 1) and VG9 without HP-β-CD (Control 2) did not inhibit the fungal growth. The inhibition zones were evaluated after 24 hours, and reduction in the growth of microorganisms was clearly observed. The zone of inhibition increased significantly (P < 0.01) as the amount of Voriconazole diffused from the in situ gel was increased.
Table 5.
A comparative study of anti-fungal activity of voriconazole in situ gel against Candida albicans and Aspergillus fumigatus.
| S.NO. | Solution | Mean of diameter of zone of inhibition (mm) ± SE |
Range of zone size (mm) |
Coefficient of variance (%) |
|---|---|---|---|---|
| Candida albicans | Test | 32.33 ± 0.16 | 32.11–32.48 | 0.49 |
| Control 1 | 10.04 ± 0.03 | 10.01–10.12 | 0.30 | |
| Control 2 | 12.05 ± 0.04 | 12.01–12.09 | 0.04 | |
| Aspergillus fumigates | Test | 68.19 ± 0.56 | 68.01–69.01 | 0.81 |
| Control 1 | 10.02 ± 0.01 | 10.01–10.14 | 0.15 | |
| Control 2 | 12.07 ± 0.02 | 12.05–12.09 | 0.17 |
*Test—VG9 formulation.
*Control 1—VG9 formulation without drug.
*Control 2—VG9 formulation without HP-β-CD.
3.9. Stability Studies
Finally, accelerated stability studies at elevated temperature and humidity revealed no significant changes in pH and clarity of in situ gelling formulations. The Voriconazole concentrations in all formulations at accelerated and room temperature are shown in Figure 4. The degradation rate constants (kcal) and shelf life (t90) were found to range between 2.61 and 2.12 days−1 and 889–694 days. The stability studies concluded that all formulations showed the lowest degradation and maximum stability of 2 years.
Figure 4.
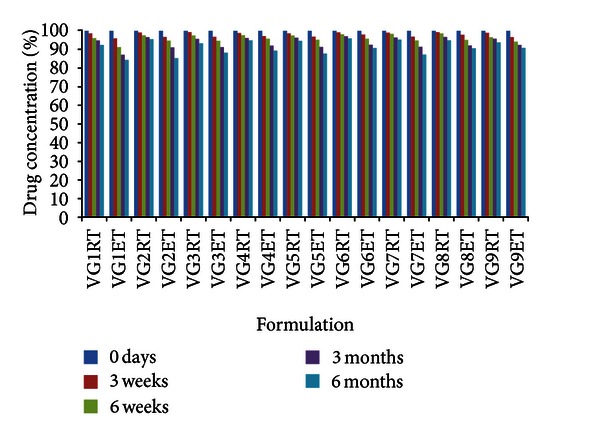
Stability of Voriconazole in situ gels under accelerated temperature and room temperature. Mean ± SD (n = 3). RT: room temperature (30°C); ET: elevated temperature (40°C).
4. Conclusions
In conclusion, we suggest that the in situ gelling formulations of HP-β-CD based Voriconazole can be a promising vehicle for topical ocular administration of antifungal against Candida albicans and Aspergillus fumigatus. Its application could reduce the necessity for repeated drug administration at frequent intervals due to the sustained release of the formulation, thereby potentially lowering corneal toxicity and increasing patient compliance.
Conflict of Interests
The authors report no conflict of interests.
Acknowledgment
The authors are grateful to Dr. Madhu Chitkara, Vice Chancellor, Chitkara University, for providing excellent infrastructure facility for the literature review and research.
References
- 1.Saettone MF, Giannaccini B, Guiducci A, Marca F, Tota G. Polymer effects on ocular bioavailability, II: the influence of benzalkonium chloride on the mydriatic response of tropicamide in different polymeric vehicles. Int J Pharm. 1985;25(73):83 pages. [Google Scholar]
- 2.Shell JW. Ocular drug delivery systems—a review. Cutaneous and Ocular Toxicol. 1982;1:49–63. [Google Scholar]
- 3.Robinson JR. Ocular drug delivery: mechanisms of corneal drug transport & mucoadhesive delivery systems. STP Pharma. 1989;12:839–846. [Google Scholar]
- 4.Le Bourlais C, Acar L, Zia H, Sado PA, Needham T, Leverge R. Ophthalmic drug delivery systems—recent advances. Progress in Retinal and Eye Research. 1998;17(1):33–58. doi: 10.1016/s1350-9462(97)00002-5. [DOI] [PubMed] [Google Scholar]
- 5.Patton TF, Robinson JR. Ocular evaluation of polyvinyl alcohol vehicle in rabbits. Journal of Pharmaceutical Sciences. 1975;64(8):1312–1316. doi: 10.1002/jps.2600640811. [DOI] [PubMed] [Google Scholar]
- 6.Sattone MF, Ginnaccini B, Tenzggi A, Tellini PN. Vehicle effect on ophthalmic bioavailibility: the influence of different polymers on the activity of pilocarpine in rabbits and man. Journal of Pharmacy and Pharmacology. 1982;34:464–466. doi: 10.1111/j.2042-7158.1982.tb04762.x. [DOI] [PubMed] [Google Scholar]
- 7.Hui HW, Robinson JR. Ocular delivery of progesterone using a bioadhesive polymer. International Journal of Pharmaceutics. 1985;26(3):203–213. [Google Scholar]
- 8.Gunny RH, Ibrahim AA, Duri P, Wilson CG, Washington N. Design and evaluation of controlled release system for the eye. Journal of Controlled Release. 1987;6:367–373. [Google Scholar]
- 9.Ding S. Recent developments in ophthalmic drug delivery. Pharmaceutical Science & Technology Today. 1998;1:328–3235. [Google Scholar]
- 10.Taravella MJ. Collagen shield delivery of ofloxacin to the human eye. Journal of Cataract and Refractive Surgery. 1999;25(4):562–565. doi: 10.1016/s0886-3350(99)80056-x. [DOI] [PubMed] [Google Scholar]
- 11.Séchoy O, Tissié G, Sébastian C, Maurin F, Driot JY, Trinquand C. A new long acting ophthalmic formulation of Carteolol containing alginic acid. International Journal of Pharmaceutics. 2000;207(1-2):109–116. doi: 10.1016/s0378-5173(00)00539-1. [DOI] [PubMed] [Google Scholar]
- 12.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. European Journal of Pharmaceutics and Biopharmaceutics. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 13.Bromberg LE, Ron ES. Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Advanced Drug Delivery Reviews. 1998;31(3):197–221. doi: 10.1016/s0169-409x(97)00121-x. [DOI] [PubMed] [Google Scholar]
- 14.Soppimath KS, Aminabhavi TM, Dave AM, Kumbar SG, Rudzinski WE. Stimulus-responsive "smart" hydrogels as novel drug delivery systems. Drug Development and Industrial Pharmacy. 2002;28(8):957–974. doi: 10.1081/ddc-120006428. [DOI] [PubMed] [Google Scholar]
- 15.Ritter Jones M, Messersmith PB. In situ forming biomaterials. Oral and Maxillofacial Surgery Clinics of North America. 2002;14(1):29–38. doi: 10.1016/s1042-3699(02)00015-8. [DOI] [PubMed] [Google Scholar]
- 16.Bhardwaj TR, Kanwar M, Lal R, Gupta A. Natural gums and modified natural gums as sustained-release carriers. Drug Development and Industrial Pharmacy. 2000;26(10):1025–1038. doi: 10.1081/ddc-100100266. [DOI] [PubMed] [Google Scholar]
- 17.Guo JH, Skinner GW, Harcum WW, Barnum PE. Pharmaceutical applications of naturally occurring water-soluble polymers. Pharmaceutical Science and Technology Today. 1998;1(6):254–261. [Google Scholar]
- 18.Schmolka IR. A review of block polymer surfactants. Journal of the American Oil Chemists’ Society. 1977;54(3):110–116. [Google Scholar]
- 19.Gilbert JC, Richardson JL, Davies MC, Palin KJ, Hadgraft J. The effect of solutes and polymers on the gelation properties of pluronic F-127 solutions for controlled drug delivery. Journal of Controlled Release. 1987;5(2):113–118. [Google Scholar]
- 20.Choi HG, Jung JH, Ryu JM, Yoon SJ, Oh YK, Kim CK. Development of In situ-gelling and mucoadhesive acetaminophen liquid suppository. International Journal of Pharmaceutics. 1998;165(1):33–44. [Google Scholar]
- 21.Prajna NV, Mascarenhas J, Krishnan T, et al. Comparison of natamycin and voriconazole for the treatment of fungal keratitis. Archives of Ophthalmology. 2010;128(6):672–678. doi: 10.1001/archophthalmol.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hariprasad SM, Mieler WF, Lin TK, Sponsel WE, Graybill JR. Voriconazole in the treatment of fungal eye infections: a review of current literature. British Journal of Ophthalmology. 2008;92(7):871–878. doi: 10.1136/bjo.2007.136515. [DOI] [PubMed] [Google Scholar]
- 23.Davies N. Biopharmaceutical considerations in topical ocular drug delivery. Clinical and Experimental Pharmacology and Physiology. 2000;27:558–562. doi: 10.1046/j.1440-1681.2000.03288.x. [DOI] [PubMed] [Google Scholar]
- 24.Dupuis A, Tournier N, Le Moal G, Venisse N. Preparation and stability of voriconazole eye drop solution. Antimicrobial Agents and Chemotherapy. 2009;53(2):798–799. doi: 10.1128/AAC.01126-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halim Mohamed MA, Mahmoud AA. Formulation of indomethacin eye drops via complexation with cyclodextrins. Current Eye Research. 2011;36(3):208–216. doi: 10.3109/02713683.2010.536294. [DOI] [PubMed] [Google Scholar]
- 26.Nijhawan R, Agarwal SP. Development of an ophthalmic formulation containing ciprofloxacin-hydroxypropyl-b-cyclodextrin complex. Bollettino Chimico Farmaceutico. 2003;142(5):214–219. [PubMed] [Google Scholar]
- 27.Klont RR, Eggink CA, Rijs AJ, Wesseling P, Verweij PE. Successful treatment of Fusarium keratitis with cornea transplantation and topical and systemic voriconazole. Clinical Infectious Diseases. 2005;40(12):110–112. doi: 10.1086/430062. [DOI] [PubMed] [Google Scholar]
- 28.Singhare DS, Khan S, Yeole PG. Temperature induced In situ gel of lidocaine hydrochloride for periodontal anaesthsia. Indian Drugs. 2005;42(8):519–524. [Google Scholar]
- 29.Shastri D, Prajapati S, Patel L. Thermoreversible mucoadhesive ophthalmic In situ hydrogel: design and optimization using a combination of polymers. Acta Pharmaceutica. 2010;60(3):349–360. doi: 10.2478/v10007-010-0029-4. [DOI] [PubMed] [Google Scholar]
- 30.Hassan EE, Gallo JM. A simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strength. Pharmaceutical Research. 1990;7(5):491–495. doi: 10.1023/a:1015812615635. [DOI] [PubMed] [Google Scholar]
- 31.Costa P, Lobo SMJ. Modeling and comparison of dissolution profiles. European Journal of Pharmaceutical Sciences. 2001;13:123–133. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 32.Pathak MK, Chhabra G, Pathak K. Design and development of a novel pH triggered nanoemulsified in-situ ophthalmic gel of fluconazole: ex-vivo transcorneal permeation, corneal toxicity and irritation testing. Drug Development and Industrial Pharmacy. 2013;39(5):780–790. doi: 10.3109/03639045.2012.707203. [DOI] [PubMed] [Google Scholar]
- 33.Bauer HW, Kirby WMM, Slerris JC, Truck M. Antibiotic susceptibility testing by a standardized single disc method. American Journal of Clinical Pathology. 1996;45:493–496. [PubMed] [Google Scholar]
- 34.Q1A (R2): stability testing of new drug substances and products. Proceedings of the International Conference on Harmonization (ICH '03); 2003; Geneva, Switzerland. [Google Scholar]
- 35.Edsman K, Carlfors J, Petersson R. Rheological evaluation of poloxamer as an In situ gel for ophthalmic use. European Journal of Pharmaceutical Sciences. 1998;6(2):105–112. doi: 10.1016/s0928-0987(97)00075-4. [DOI] [PubMed] [Google Scholar]
- 36.Wei G, Xu H, Ding PT, Li SM, Zheng JM. Thermosetting gels with modulated gelation temperature for ophthalmic use: the rheological and gamma scintigraphic studies. Journal of Controlled Release. 2002;83(1):65–74. doi: 10.1016/s0168-3659(02)00175-x. [DOI] [PubMed] [Google Scholar]
- 37.Smart JD, Kellaway IW, Worthington HEC. An in-vitro investigation of mucosa-adhesive materials for use in controlled drug delivery. Journal of Pharmacy and Pharmacology. 1984;36(5):295–299. doi: 10.1111/j.2042-7158.1984.tb04377.x. [DOI] [PubMed] [Google Scholar]
- 38.Kim EY, Gao ZG, Park JS, Li H, Han K. rhEGF/HP-β-CD complex in poloxamer gel for ophthalmic delivery. International Journal of Pharmaceutics. 2002;233(1-2):159–167. doi: 10.1016/s0378-5173(01)00933-4. [DOI] [PubMed] [Google Scholar]
- 39.Qi H, Li L, Huang C, Li W, Wu C. Optimization and physicochemical characterization of thermosensitive poloxamer gel containing puerarin for ophthalmic use. Chemical and Pharmaceutical Bulletin. 2006;54(11):1500–1507. doi: 10.1248/cpb.54.1500. [DOI] [PubMed] [Google Scholar]
- 40.Smart JD, Kellaway IW, Worthington HEC. An in-vitro investigation of mucosa-adhesive materials for use in controlled drug delivery. Journal of Pharmacy and Pharmacology. 1984;36(5):295–299. doi: 10.1111/j.2042-7158.1984.tb04377.x. [DOI] [PubMed] [Google Scholar]
- 41.Liu Z, Li J, Nie S, Liu H, Ding P, Pan W. Study of an alginate/HPMC-based In situ gelling ophthalmic delivery system for gatifloxacin. International Journal of Pharmaceutics. 2006;315(1-2):12–17. doi: 10.1016/j.ijpharm.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 42.Maurice DM, Riley MV. Biochemistry of the Eye. London, UK: Graymore; 1970. Ocular Pharmacokinetics; pp. 6–16. [Google Scholar]


