Abstract
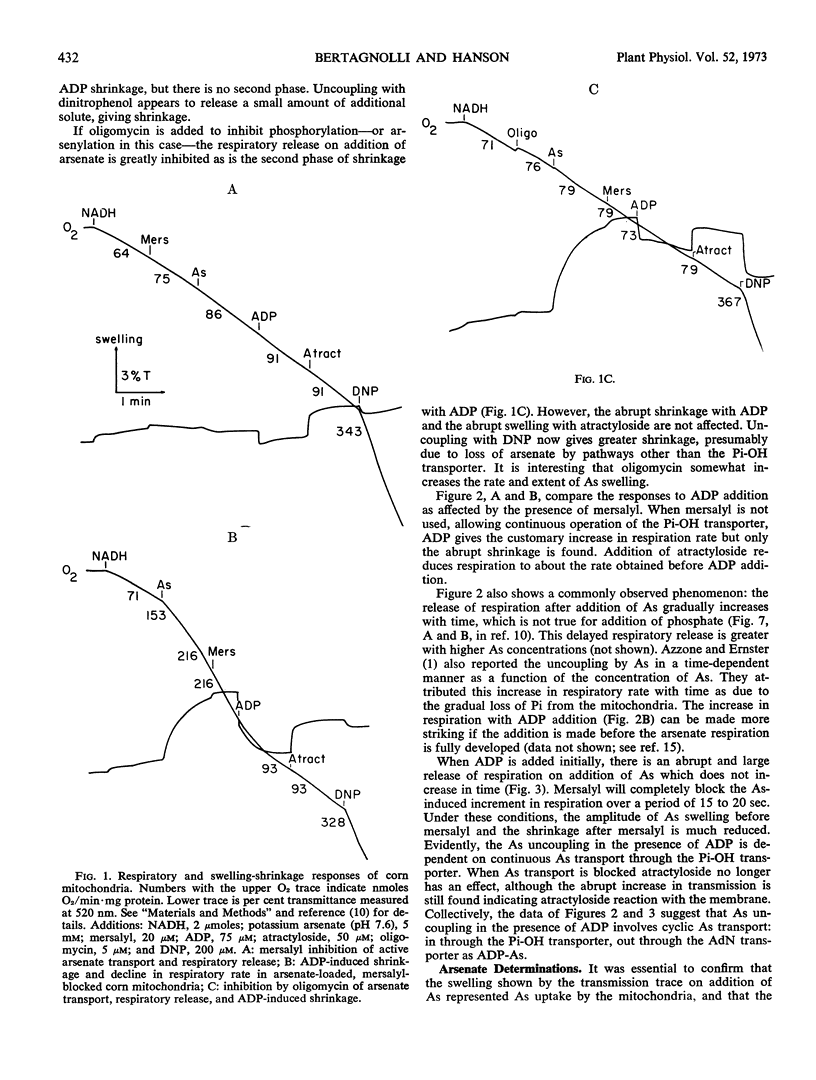
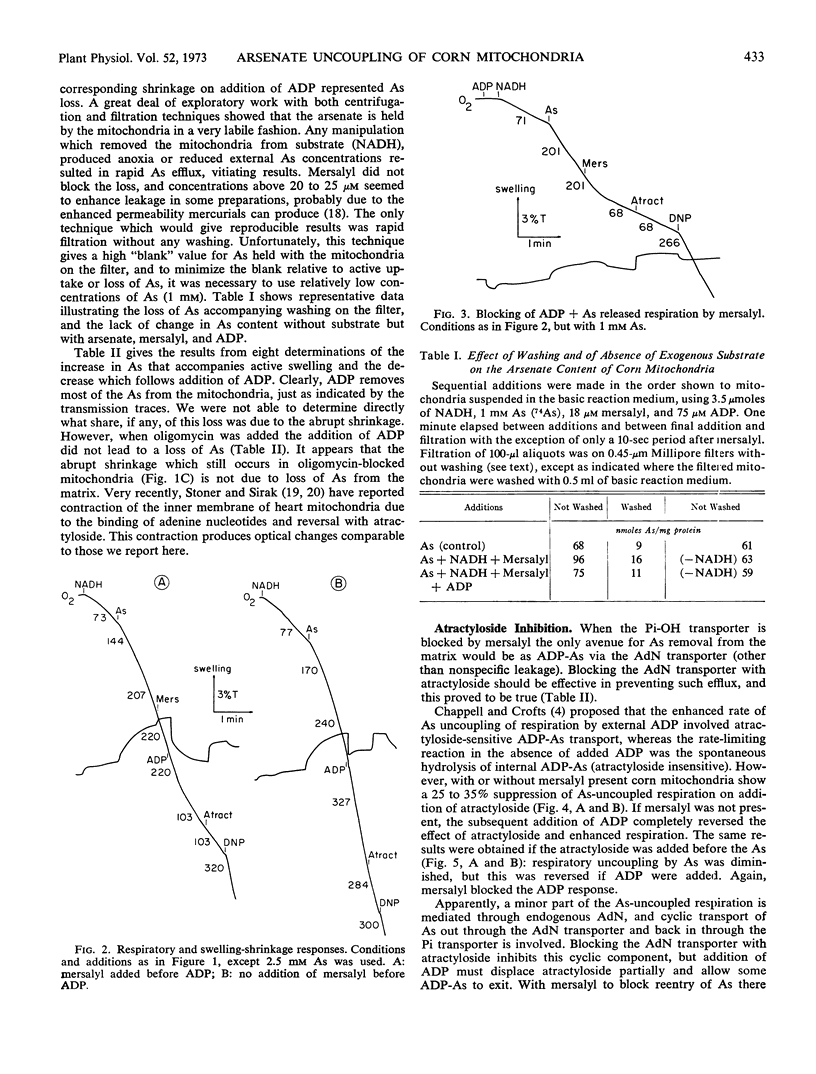
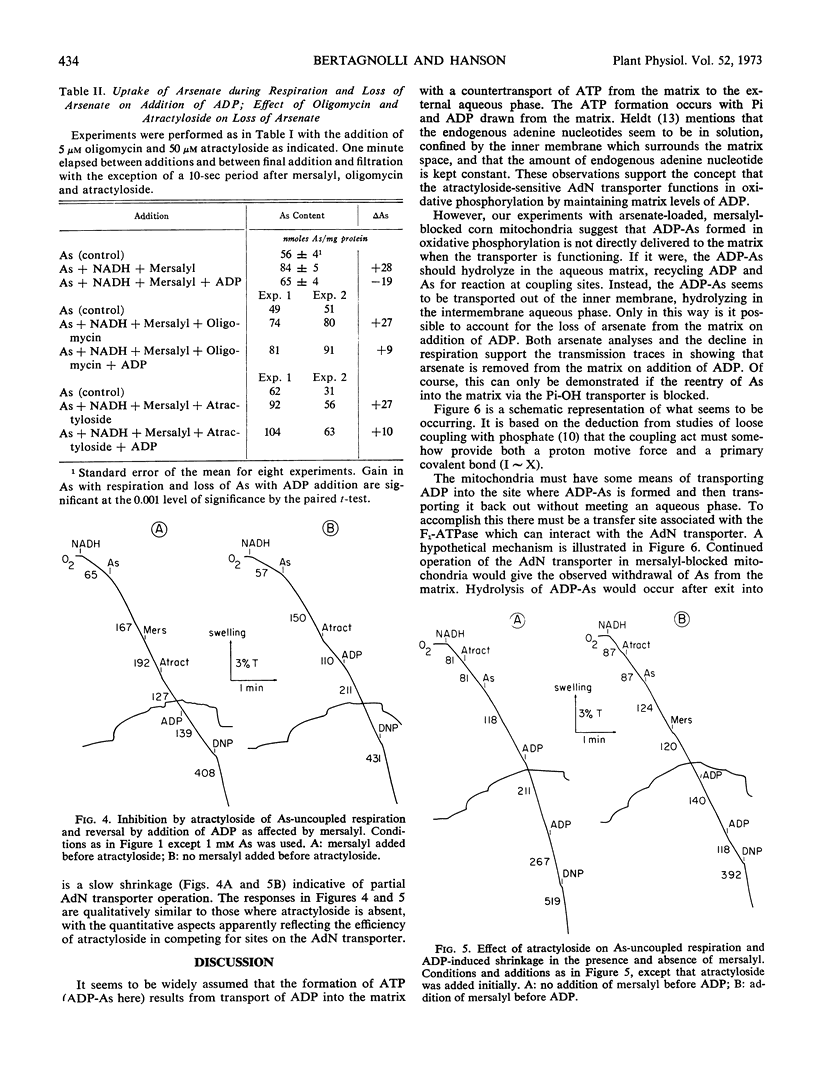
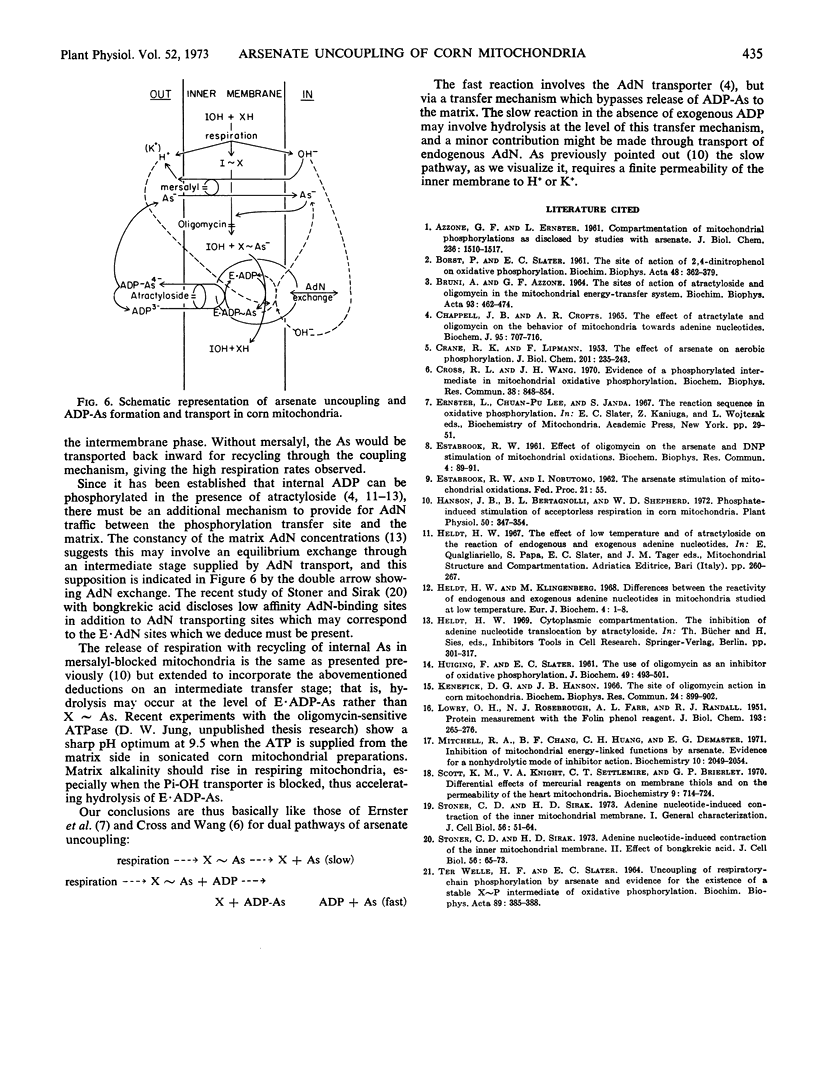
Arsenate uncouples mitochondrial respiration in a process stimulated by ADP, inhibited by oligomycin, and competitively inhibited by inorganic phosphate. If mersalyl is added to corn mitochondria to block further transport of accumulated arsenate, the uncoupled respiration continues unabated due to recycling of matrix arsenate. Addition of ADP now inhibits rather than promotes respiration and the mitochondria shrink. It is established by arsenate analyses that arsenate is removed from the matrix. Oligomycin or atractyloside block the removal by inhibiting ADP-arsenate formation or transport, respectively. It is deduced that ADP-arsenate is stable in the membrane and is transported outward for hydrolysis in the external aqueous phase. Hence, ADP-arsenate formed in oxidative phosphorylation is not directly released to the matrix, and a mechanism must exist for its direct transfer to the transporter.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AZZONE G. F., ERNSTER L. Compartmentation of mitochondrial phosphorylations as disclosed by studies with arsenate. J Biol Chem. 1961 May;236:1510–1517. [PubMed] [Google Scholar]
- BRUNI A., AZZONE G. F. THE SITES OF ACTION OF ATRACTYLOSIDE AND OLIGOMYCIN IN THE MITOCHONDRIAL ENERGY-TRANSFER SYSTEM. Biochim Biophys Acta. 1964 Dec 9;93:462–474. doi: 10.1016/0304-4165(64)90330-7. [DOI] [PubMed] [Google Scholar]
- CHAPPELL J. B., CROFTS A. R. THE EFFECT OF ATRACTYLATE AND OLIGOMYCIN ON THE BEHAVIOUR OF MITOCHONDRIA TOWARDS ADENINE NUCLEOTIDES. Biochem J. 1965 Jun;95:707–716. doi: 10.1042/bj0950707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE R. K., LIPMANN F. The effect of arsenate on aerobic phosphorylation. J Biol Chem. 1953 Mar;201(1):235–243. [PubMed] [Google Scholar]
- ESTABROOK R. W. Effect of oligomycin on the arsenate and DNP stimulation of mitochondrial oxidations. Biochem Biophys Res Commun. 1961 Feb 24;4:89–91. doi: 10.1016/0006-291x(61)90352-7. [DOI] [PubMed] [Google Scholar]
- HUIJING F., SLATER E. C. The use of oligomycin as an inhibitor of oxidative phosphorylation. J Biochem. 1961 Jun;49:493–501. doi: 10.1093/oxfordjournals.jbchem.a127334. [DOI] [PubMed] [Google Scholar]
- Hanson J. B., Bertagnolli B. L., Shepherd W. D. Phosphate-induced Stimulation of Acceptorless Respiration in Corn Mitochondria. Plant Physiol. 1972 Sep;50(3):347–354. doi: 10.1104/pp.50.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Klingenberg M. Differences between the reactivity of endogenous and exogenous adenine nucleotides in mitochondria as studied at low temperature. Eur J Biochem. 1968 Mar;4(1):1–8. doi: 10.1111/j.1432-1033.1968.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Kenefick D. G., Hanson J. B. The site of oligomycin action in corn mitochondria. Biochem Biophys Res Commun. 1966 Sep 22;24(6):899–902. doi: 10.1016/0006-291x(66)90334-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitchell R. A., Chang B. F., Huang C. H., DeMaster E. G. Inhibition of mitochondrial energy-linked functions by arsenate. Evidence for a nonhydrolytic mode of inhibitor action. Biochemistry. 1971 May 25;10(11):2049–2054. doi: 10.1021/bi00787a013. [DOI] [PubMed] [Google Scholar]
- Scott K. M., Knight V. A., Settlemire C. T., Brierley G. P. Differential effects of mercurial reagents on membrane thiols and on the permeability of the heart mitochondrion. Biochemistry. 1970 Feb 17;9(4):714–724. doi: 10.1021/bi00806a003. [DOI] [PubMed] [Google Scholar]
- Stoner C. D., Sirak H. D. Adenine nucleotide-induced contraction of the inner mitochondrial membrane. I. General characterization. J Cell Biol. 1973 Jan;56(1):51–64. doi: 10.1083/jcb.56.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner C. D., Sirak H. D. Adenine nucleotide-induced contraction on the inner mitochondrial membrane. II. Effect of bongkrekic acid. J Cell Biol. 1973 Jan;56(1):65–73. doi: 10.1083/jcb.56.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TER WELLEH, SLATER E. C. UNCOUPLING OF RESPIRATORY-CHAIN PHOSPHORYLATION BY ARSENATE AND EVIDENCE FOR THE EXISTENCE OF A STABLE X-P INTERMEDIATE OF OXIDATIVE PHOSPHORYLATION. Biochim Biophys Acta. 1964 Aug 26;89:385–388. doi: 10.1016/0926-6569(64)90238-x. [DOI] [PubMed] [Google Scholar]


