Abstract
Microalbuminuria is the strong predictor of diabetic nephropathy, which is the main cause of morbidity and mortality in patients with diabetes mellitus (DM). Microalbuminuria is also characterized by increased prevalence of arterial hypertension, proliferative retinopathy, and peripheral neuropathy. The study was planned to evaluate the effect of Gokshura-Punarnava Basti in the management of microalbuminuria in DM (Madhumeha). Eligible diabetic patients with urine albumin excretion between 30 and 300 mg in 24 h were randomly divided into two groups. Asthapana Basti (decoction enema) of Gokshura and Punarnava Kwatha (decoction), Kalka (paste), Taila (medicated oil), Madhu (honey), and Saindhava (rock salt) for 6 consecutive days and Anuvasana (unctuous enema) of Gokshura-Punarnava Taila on 1st and 8th day by traditional Basti Putaka method was given in study group. Tablet Enalapril 5 mg, twice daily for 30 days was given to the patients in control group. The primary outcome measures were percentage change in the presenting complaints of diabetes, urine microalbumin, Blood Sugar Level (BSL), and Blood Pressure (BP). Enalapril showed 33.33% improvement, where as Gokshura-Punarnava Basti showed 79.59% improvement in the presenting complaints of diabetes, urine microalbumin, BSL and BP. Gokshura-Punarnava Basti has shown superior results in the management of microalbuminuria in DM as compared to control drug.
Keywords: Basti, diabetes mellitus, Gokshura, Madhumeha, microalbuminuria, Punarnava1
Introduction
We are in the midst of an epidemic; the epidemic of diabetes and its micro-vascular complications; without aggressive intervention health-care costs will be devastating to millions of patients predicted to be affected by diabetes over the next decades. One of the early markers of not only diabetic nephropathy, but also vascular disease in patients with diabetes, is the presence of microalbuminuria. It is a leading cause of end stage renal disease and also the leading cause of diabetes mellitus (DM) related morbidity and mortality.[1,2]
Among the diabetic population, 20-40% of patients develop diabetic nephropathy.[3] In type II DM, usually microalbuminuria may be present at the time of diagnosis, which reflects its long asymptomatic period.
Madhumeha may be equated with the DM. Charaka explain it as a life-style disorder,[4] due to over indulgence in heavy and richly nutritious food, day-time sleep, lack of exercises, other sedentary habits and not doing seasonal purifications.[5] Even though, there is no direct reference regarding diabetic nephropathy being manifested as a complication of Madhumeha in classical texts, but by the help of modern science, it can be concluded that diabetes being a metabolic disorder, manifestation of nephropathy changes in a diabetic patient are a result of metabolic derangements, contributing to hyperglycemia.[6]
As diabetic nephropathy is a complication of Madhumeha, so proper treatment of Madhumeha will prevent and pacify its complications. Management of diabetes described in classics; can be administered in nephropathy. These include modalities like Shamana (palliative), Shodhana (purificatory), and Rasayana (rejuvenation), etc.
Gokshura (Tribulus terrestris Linn.) is considered one among the drugs of Mootra Virechaneeya Gana (diuretic drugs)[7] by Charaka. Hence, it acts as Anulomana (downward movement) of Apana Vata. This corrects the Gati (movement) of Vata there by influencing it in a positive way. Gokshura is also a proven anti-diabetic agent.[8] Its Bastishodhana[9] (cleansing) effect reduces Avarana of Kapha and Meda in the microcirculation of kidneys. Punarnava (Boerhaavia diffusa L. nom. Cons.) has Ushna Veerya (hot property), which corrects Srotosanga existing in the kidneys. The Ushna Veerya assists in the regeneration of renal tissues. It also acts as anti-inflammatory[10] and diuretic.[11]
Basti due to its purification property, eliminates the excess of deranged metabolic waste and in turn clears the Avarana of Vata and normalizes the functions of Vyana and Apana Vata. Thus, the normalized Vata helps to stop the depletion of vital Dhatus (body elements) through urine. Based on these qualities Gokshura-Punarnava Basti was selected for the present study. Angiotensin Converting Enzyme (ACE) inhibitor was taken as control drug.[12] The objective of the current work is to study the effect of Gokshura-Punarnava Basti in the management of microalbuminuria in DM (Madhumeha).
Materials and Methods
Selection of patients
Total 100 patients suffering from DM with microalbuminuria were selected from Out Patient Department (OPD) and Indoor Patient Department (IPD) of Panchakarma Department, IPGT & RA, Jamnagar and Medicine OPD of G.G. Hospital, Jamnagar irrespective of religion, sex, occupation, caste, etc., after screening. The patients were divided into two groups by following the computer generated randomization plan.
Ethical clearance was obtained from the Institutional Ethics Committees vide PGT/7/Ethics/2009-2010/3494/15 dated 08/02/2010 and MCLJ/IEC/113/2010 dated 12/04/2010. This study is registered in Clinical Trial Registry of India with registration No. CTRI/2012/03/002496 dated 15/03/2012.
Inclusion criteria
Known type II diabetic patients with urine albumin between 30 and 300 mg in 24 h urine sample.
Hypertensive patients with Blood Pressure (BP) <200/120 mm of Hg.
Age in between 20 and 70 years irrespective of gender.
Patients eligible for Basti treatment.
Exclusion criteria
Diabetic patients with urine albumin >300 mg in 24 h urine sample.
Hypertensive patients with BP >200/120 mm of Hg.
Microalbuminuria induced due to other diseases such as pregnancy and chronic renal failure.
Patients with other severe complications of DM such as ketoacidosis and paralysis.
Plasma creatinine more than 2.5 mg/dl.
Any other serious systemic illness.
Patient contra-indicated for Basti.
Laboratory investigations
Routine hematological, biochemical and urine examination, urine microalbumin in 24 h sample and glomerular filtration rate (GFR) were carried out before and after the treatment.
Methodology
Selected patients were randomly divided into two groups. All of them were advised to continue their anti-diabetic and antihypertensive drugs. Diet control and regular exercise was advised to all patients. Protein restricted diet and proper life-style was advised in both groups. All patients in Basti group (BG) were explained the Pathyapathya during Basti.
Grouping
Study group (BG): Gokshura-Punarnava Basti
-
Asthapana Basti (decoction enema) – contents
- Gokshura-Punarnava decoction – 350 ml
- Gokshura-Punarnava oil[13] – 100 ml
- Honey – 75 ml
- Gokshura-Punarnava paste – 15 g
- Rock salt – 5 g.
-
Anuvasana Basti (unctuous enema)
- Gokshura-Punarnava oil – 120 ml.
Duration: Total days = 8* (*1st and 8th day Anuvasana Basti after food in morning, 2nd to 7th day Asthapana Basti before food between 9.00-10.30 am according to Yoga Basti pattern).
Basti was prepared by the classical method[14] and administered by traditional Putaka method.
Control group/Enalapril group
Drug: ACE inhibiter – Tablet Enalapril
Dose: 5 mg twice a day
Mode of administration: Oral
Duration: 30 days.
The drugs for Basti were procured from Pharmacy, Gujarat Ayurved University and the control drug (tablet enalapril) was provided from the dispensary of Civil Hospital.
Follow-up study
After completion of the therapy, patients were kept under observation to assess the immediate and short-term improvement for 1 month.
Criteria for assessment
Improvement observed in patients was assessed mainly on the basis of relief in percentage change in the presenting complaints of Madhumeha on the basis of the score decided in a previous study.[15]
Urine microalbumin, GFR, blood sugar level (BSL) and BP.
Statistical analysis
The data obtained in the study was subjected to statistical tests.
Subjective criteria
Percentage of improvement in each parameter of both the treated groups is calculated. Wilcoxon signed-rank test is applied to the statistical data for evaluating the difference in the before and after treatment scores. Chi-square test is applied for comparison between both groups.
Objective criteria
Obtained data of objective parameters between the groups was statistically determined by paired t-test, unpaired Student's t-test for both the groups with the level of significance set at P < 0.05.
Observations
Total 100 patients were registered in the present study with 50 in the study group that is, BG and 50 in the control group, that is, EG. Among them, total 97%, (49 in BG and 48 in EG) completed the treatment.
Maximum registered patients were belonging to 41-55 years age group (48%), average random BSL was more than 281 mg/dl (35%); positive family history of DM (75%) and having the history of diabetes since 5-10 year (54%) and the history of hypertension since 5 years (44%). Maximum patients had Vishamagni (48%), Krura Koshtha (50%), Vata Kapha Prakriti (47%), Madhyama Atura Bala (76%), and belonging to overweight criteria (66%). In maximum patients Kapha Dushti (50.11%) and Medovaha Sroto Dushti (45.03%) Lakshana were observed.
The average duration required for Niruha Basti administration was 25.75 s and for Anuvasana Basti 16.75 s. Average retention time was observed 6.25 min in Asthapana and 5.48 h in Anuvasana.
Results
Effect of therapies on presenting complaints of diabetes
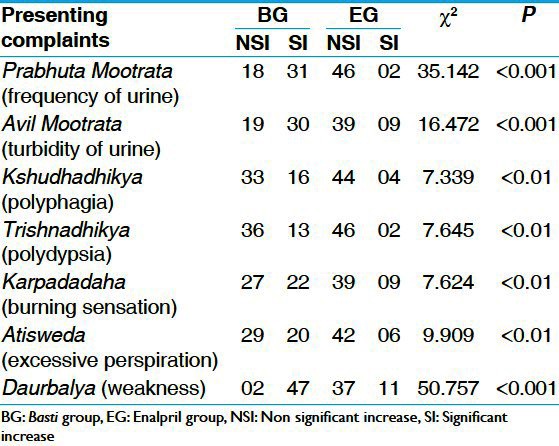
The effect of BG was significant in almost all complaints of Madhumeha. Highly significant (P < 0.001) improvement is found in Prabhuta Mootrata (frequency of urine), Avila Mootrata (turbidity of urine) and Daurbalya (weakness). Significant improvement (P < 0.01) was found in Trishnadhikya (polydypsia), Karapada Daha (burning sensation in palms and soles) and Atisweda (excessive perspiration) [Table 1].
Table 1.
Comparative effect of therapies on presenting complaints of Madhumeha
Effect of therapies on urine microalbumin
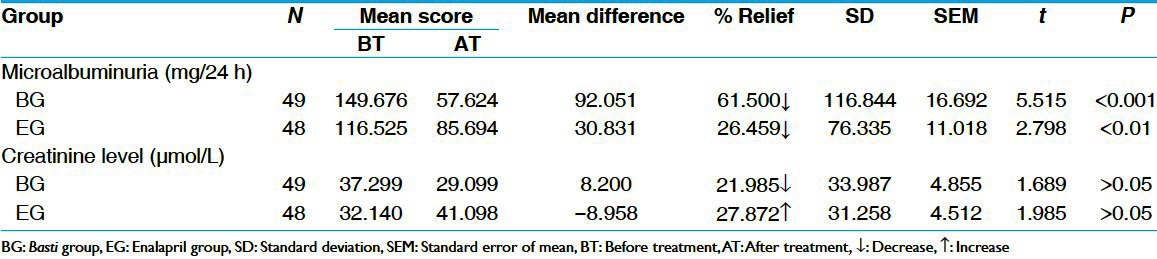
BG provided 61.50% of relief in urine microalbumin, whereas EG provided 26.46% relief. The results in BG were statistically highly significant (P < 0.001) whereas in EG, the result was statistically significant (P < 0.01) in reduction of urine microalbumin. In BG statistically insignificant decrease in urine creatinine was observed although in EG insignificant increase in urine creatinine was seen [Table 2].
Table 2.
Effect of therapies on urine microalbumin and creatinine level
Effect of therapies on GFR
BG and EG both were shown statistically insignificant (P > 0.05) results on GFR.
Effect of therapies on BP
BG shows 15.04% and EG shows 3.45% relief on systolic BP. On diastolic BP, BG shows 15.49% and EG shows 03.03% relief. On comparison of results within the groups there was a highly significant difference (P < 0.001) in systolic and diastolic BP [Table 3].
Table 3.
Effect of therapies on blood pressure

Effect of therapies on BSL
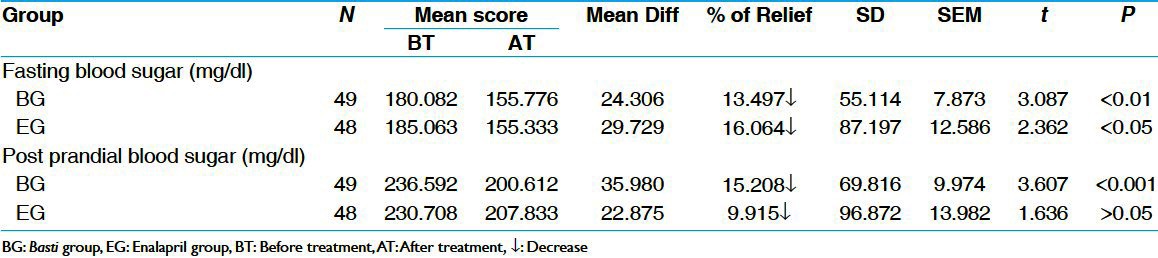
BG treated group shows highly significant (P < 0.01) decrease in fasting blood sugar by 13.50% and EG also shows a significant decrease (P < 0.05) in fasting blood sugar by 16.06%. In post-prandial blood sugar also BG showed statistically highly significant (P < 0.001) relief by 15.21% and EG showed statistically insignificant decrease (P > 0.05) by 9.92%. In comparison, no significant differences were observed in both fasting and post-prandial BSL when the values from BG were compared with EG [Table 4].
Table 4.
Effect of therapies on blood sugar level (paired ‘t’ test)
Effect of therapies on hematological and biochemical parameters
There was statistically insignificant change (P > 0.05) in all hematological and biochemical parameters in both the groups. However, there is a change from pre-test to post-test mean value of above parameters, which is within the physiological limit.
Overall effect of therapies
In BG, 40.81% showed moderate improvement, 38.78% showed mild improvement, whereas 20.41% patients were unchanged. In EG, 31.25% showed mild improvement, none of them got complete remission and marked improvement, 2.08% got moderate improvement, whereas 66.67% remained unchanged.
Discussion
Microalbuminuria comes under the heading of incipient nephropathy and it is not associated with significant clinical signs or any changes other than a very small increase in BP. However, symptoms like frothy urine can be seen in some patients with incipient nephropathy in OPD.
The study drug was adopted based on the expert practitioners’ views. Registered patients were administered trial therapy for 8 days and control drug for 1 month. Basti schedule was consisted of Anuvasana Basti on 1st and last day and consecutive Asthapana Basti for 6 days. The Yoga Basti schedule was not followed per se because; Anuvasana is not indicated in Bahudoshavastha like Prameha, where it may produce deleterious effects due to its Utkleshana (dislodgement of Dosha) property.[16] To attain unctuousness to the gastrointestinal tract and avoid vitiation of Vata due to Asthapana; Anuvasana Basti was scheduled for the 1st and last day of trial therapy. Continuous six Asthapana were given looking into the pathogenesis of the research question; that is, Kapha and Meda in excess causing Avarana to movement of Vata, Bahudoshatva and to attain cleansing effect. Though in classics, continuous Asthapana is contraindicated; however, still according to conditions (Avastha) it can be done.[17] As nephropathy patients are in a state of Bahudoshavastha, in such condition purification through Basti will be the best treatment. Therefore, the experiential (Anubhuta) combination of Basti was followed.
Mode of action of Gokshura-Punarnava Basti in microalbuminuria
In Avaranajanya Madhumeha, etiological factors mainly vitiates the Kapha and Pitta in turn affect the Jatharagni and Dhatwagni and disrupts metabolism and produces an excess of deranged quality Rasa, Meda, Kleda, Rakta, etc.[18] All these vitiated Dushyas obstructs the path of Vata (Avarana), which gets aggravated and changes its path and carries vital Dhatus toward Basti and excretes them out causing depletion.[19]
It could increase the digestive power (Agni); purifies each and every channel of the body and also helps to normalize the function of Rasavaha, Medovaha, Mootravaha Srotasa. Basti drugs after absorption conceivably helps in disintegration of pathogenesis as it possess anti-diabetic activity. It may alter the absorption of carbohydrate and fat from the intestine.
The Gokshura-Punarnava Basti was found effective in decreasing Prameha symptoms by virtue of Kledaharana, Kapha-Pitta Nirharana[20] and Basti Nanakarmakartwat.[21] Basti itself is a procedure having rejuvenation effect. Furthermore, due to correction of digestive fire and Sroto Shodhana adds to the effect of drug properties. That in turn may help to form the Dhatus in proper proportion with appropriate qualities. Ruksha quality of Punarnava and honey may help to reduce the Kleda. Drugs such as Punarnava and sesame oil with their Ushna Veerya may act over Vata and vitiated Kapha by combating their Sheeta Guna. Maximum drugs are having Katu Vipaka that reduces vitiated Kapha and may cause Sroto Shodhana. All these factors probably help in reduction of Bahudrava Shlesma and in turn reduction of vitiated Meda and Kleda. Thus, once these factors get normalized in the body they in turn make clear the path of Vata, which stops the depletion of vital Dhatus and restore normal physiology. Thus, pathology gets alleviated. Drug used in Basti on virtue of its properties are proven as an anti-diabetic and Tridoshashamaka effect. Researches proved chloroform extract of B. diffusa has significant anti-diabetic activity[22,23] and hypoglycemic function of T. terrestris is primarily concentrated in the phytochemicals known as sapopin.[24]
T. terrestris and B. diffusa acts as ACE inhibitors[25] and anti-oxidant.[26] B. diffusa is clinically proved as a useful and safe drug in patients of nephrotic syndrome[27] and its action as kidney regeneration.[28] Antioxidant action of study drug and procedure may prevent further formation of reactive oxygen species, exerting antioxidant properties.
No adverse effects (Vyapada) were observed during and after the study. Insignificant variations (increase or decrease) were observed in hematological and bio-chemical parameters (excluding BSL). This proves that this treatment is safe.
Conclusion
Microalbuminuria in DM can be considered as a complication of Madhumeha. There is no direct correlation of diabetic microalbuminuria as such in Ayurveda. Gokshura-Punarnava Basti was found to be more effective in reducing microalbuminuria in DM as compared to enalapril. A significant decrease was seen in BP and BSL in BG. The variations in biochemical and hematological parameters were within the physiological limits, hence Gokshura-Punarnava Basti can be safely practiced in cases of microalbuminuria.
References
- 1.Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nephropathy in type 1 (insulin-dependent) diabetes: An epidemiological study. Diabetologia. 1983;25:496–501. doi: 10.1007/BF00284458. [DOI] [PubMed] [Google Scholar]
- 2.Genuth SM. The case for blood glucose control. Adv Intern Med. 1995;40:573–623. [PubMed] [Google Scholar]
- 3.John L, Rao PS, Kanagasabapathy AS. Prevalence of diabetic nephropathy in non-insulin dependent diabetics. Indian J Med Res. 1991;94:24–9. [PubMed] [Google Scholar]
- 4.Agnivesha . In: Charaka, Dridhabala, Charaka Samhita, Chikitsa Sthana, Prameha Chikitsa, 6/4. Reprint ed. Jadavaji Trikamji Acharya., editor. Varanasi: Chaukhamba Krishnadas Academy; 2006. p. 445. [Google Scholar]
- 5.Ibidem. Charaka Samhita, Sutra Sthana, Kiyantah Shirsiya, 17/78-80;103-4 [Google Scholar]
- 6.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–21. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 7.Agnivesha . In: Charaka, Dridhabala, Charaka Samhita, Sutra Sthana, Shadvirechana Shatashritiya, 4/15. Reprint ed. Jadavaji Trikamji Acharya., editor. Vol. 33. Varanasi: Chaukhamba Krishnadas Academy; 2006. [Google Scholar]
- 8.El-Tantawy WH, Hassanin LA. Hypoglycemic and hypolipidemic effects of alcoholic extract of Tribulus alatus in streptozotocin-induced diabetic rats: A comparative study with T. terrestris (Caltrop) Indian J Exp Biol. 2007;45:785–90. [PubMed] [Google Scholar]
- 9.Anonymus. I. New Delhi: Department of AYUSH, Ministry of Health and Family Welfare, Govt. of India; 2001. The Ayurvedic Pharmacopoeia of India; p. 52. Part I. [Google Scholar]
- 10.Sushruta . In: Sushruta Samhita, Sutra Sthana, Annapanvidhi, 46/255. 9th ed. Jadavaji Trikamji Acharya., editor. Varanasi: Chaukhamba Orientalia; 2007. p. 232. [Google Scholar]
- 11.Anonymus. I. New Delhi: Department of AYUSH, Ministry of Health and Family Welfare, Govt. of India; 2001. The Ayurvedic Pharmacopoeia of India; p. 128. Part I. [Google Scholar]
- 12.Pedersen MM, Christensen CK, Hansen KW, Christiansen JS, Mogensen CE. ACE-inhibition and renoprotection in early diabetic nephropathy. Response to enalapril acutely and in long-term combination with conventional antihypertensive treatment. Clin Invest Med. 1991;14:642–51. [PubMed] [Google Scholar]
- 13.Sharangadhara Murthy PHC. Sharangadhara Samhita, Madhyama Khanda, Sneha Kalpana, 9/1-2, translator. 2nd ed. Varanasi: Chaukhamba Sanskrit Series Office; 2007. p. 199. [Google Scholar]
- 14.Agnivesha . In: Charaka, Dridhabala, Charaka Samhita, Siddhi Sthana, Bastisutriya, 3/23. Reprint ed. Jadavaji Trikamji Acharya., editor. Varanasi: Chaukhamba Krishnadas Academy; 2006. p. 693. [Google Scholar]
- 15.Pawar A. Jamnagar: I.P.G.T. and R.A., Dept. of Kaya Chikitsa, Thesis Submitted to Gujarat Ayurveda University; 2003. A comparative study of the role of Basti therapy and Pramehaghna drugs in the management of Madhumeha. [Google Scholar]
- 16.Sushruta . In: Sushruta Samhita, Chikitsa Sthana, Anuvasanottara Basti 37/77. 9th ed. Jadavaji Trikamji Acharya., editor. Varanasi: Chaukhamba Orientalia; 2007. p. 536. [Google Scholar]
- 17.Agnivesha . In: Charaka, Dridhabala, Charaka Samhita, Siddhi Sthana, Panchakarmiya Siddhi, 2/24-28. Reprint ed. Jadavaji Trikamji Acharya., editor. Varanasi: Chaukhamba Krishnadas Academy; 2006. p. 690. [Google Scholar]
- 18.Ibidem. Charaka Samhita, Sutra Sthana, Kiyanta Sirsiya, 17/79-80; 103 [Google Scholar]
- 19.Ibidem. Charaka Samhita, Nidana Sthana, Prameha Nidana, 4/8. :213. [Google Scholar]
- 20.Ibidem. Charaka Samhita, Chikitsa Sthana, Prameha Chikitsa, 6/51; 452 [Google Scholar]
- 21.Sushruta . In: Sushruta Samhita, Chikitsa Sthana, Netrabastipraman Pravibhaga Chikitsa, 35/3. 9th ed. Jadavaji Trikamji Acharya., editor. Varanasi: Chaukhamba Orientalia; 2007. p. 525. [Google Scholar]
- 22.Rao KN, Boini KM, Nammi S. Effect of chronic administration of Boerhavia diffusa Linn. leaf extract on experimental diabetes in rats. Trop J Pharm Res. 2004;3:305–9. [Google Scholar]
- 23.Pari L, Amarnath Satheesh M. Antidiabetic effect of Boerhavia diffusa: Effect on serum and tissue lipids in experimental diabetes. J Med Food. 2004;7:472–6. doi: 10.1089/jmf.2004.7.472. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Qu W, Wang Y, Wan H, Tian C. Hypoglycemic effect of saponin from Tribulus terrestris. Zhong Yao Cai. 2002;25:420–2. [PubMed] [Google Scholar]
- 25.Somanadhan B, Varughese G, Palpu P, Sreedharan R, Gudiksen L, Smitt UW, et al. An ethnopharmacological survey for potential angiotensin converting enzyme inhibitors from Indian medicinal plants. J Ethnopharmacol. 1999;65:103–12. doi: 10.1016/s0378-8741(98)00201-3. [DOI] [PubMed] [Google Scholar]
- 26.Amin A, Lotfy M, Shafiullah M, Adeghate E. The protective effect of Tribulus terrestris in diabetes. Ann N Y Acad Sci. 2006;1084:391–401. doi: 10.1196/annals.1372.005. [DOI] [PubMed] [Google Scholar]
- 27.Singh RP, Shukla KP, Pandey BL, Singh RG, Ushana, Singh RH. Recent approaches in clinical and experimental evaluation of diuretic action of Punarnava (B. diffusa) with special reference to nephrotic syndrome. J Res Educ Indian Med. 1992;1:26–36. [Google Scholar]
- 28.Mishra J, Singh R. The effect of indigenous drug Boerhaavia diffusa on kidney regeneration. Indian J Pharmacol. 1980;12:59–64. [Google Scholar]


