Abstract
A number of plant species were surveyed to obtain pure leaf epidermal tissue in quantity. Commelina communis L. and Tulipa gesnariana L. (tulip) were chosen for further work. Chlorophyll a/b ratios of epidermal tissues were 2.41 and 2.45 for C. communis and tulip, respectively. Phosphoenolpyruvate carboxylase, ribulose-1,5-diphosphate carboxylase, malic enzyme, and NAD+ and NADP+ malate dehydrogenases were assayed with epidermal tissue and leaf tissue minus epidermal tissue. In both species, there was less ribulose 1,5-diphosphate than phosphoenolpyruvate carboxylase activity in epidermal tissue whether expressed on a protein or chlorophyll basis whereas the reverse was true for leaf tissue minus epidermal tissue. In both species, malic enzyme activities were higher in epidermal tissue than in the remaining leaf tissue when expressed on a protein or chlorophyll basis. In both species, NAD+ and NADP+ malate dehydrogenase activities were higher in the epidermal tissue when expressed on a chlorophyll basis; however, on a protein basis, the converse was true. Microautoradiography of C. communis epidermis and histochemical tests for keto acids suggested that CO2 fixation occurred predominantly in the guard cells. The significance and possible location of the enzymes are discussed in relation to guard cell metabolism.
Full text
PDF


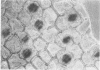
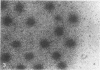
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BABSON A. L., SHAPIRO P. O., WILLIAMS P. A., PHILLIPS G. E. The use of a diazonium salt for the determination of glutamic-oxalacetic transaminase in serum. Clin Chim Acta. 1962 Mar;7:199–205. doi: 10.1016/0009-8981(62)90010-4. [DOI] [PubMed] [Google Scholar]
- Chen T. M., Brown R. H., Black C. C. Photosynthetic CO(2) Fixation Products and Activities of Enzymes Related to Photosynthesis in Bermudagrass and Other Plants. Plant Physiol. 1971 Feb;47(2):199–203. doi: 10.1104/pp.47.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Gutierrez M. Metabolic Activities in Extracts of Mesophyll and Bundle Sheath Cells of Panicum miliaceum (L.) in Relation to the C(4) Dicarboxylic Acid Pathway of Photosynthesis. Plant Physiol. 1972 Dec;50(6):728–732. doi: 10.1104/pp.50.6.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREELAND R. O. The green pigment and physiology of guard cells. Science. 1951 Jul 27;114(2952):94–95. doi: 10.1126/science.114.2952.94. [DOI] [PubMed] [Google Scholar]
- Geiger P. J., Bessman S. P. Protein determination by Lowry's method in the presence of sulfhydryl reagents. Anal Biochem. 1972 Oct;49(2):467–473. doi: 10.1016/0003-2697(72)90450-2. [DOI] [PubMed] [Google Scholar]
- Highkin H. R., Boardman N. K., Goodchild D. J. Photosynthetic Studies on a Pea-mutant Deficient in Chlorophyll. Plant Physiol. 1969 Sep;44(9):1310–1320. doi: 10.1104/pp.44.9.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humble G. D., Raschke K. Stomatal opening quantitatively related to potassium transport: evidence from electron probe analysis. Plant Physiol. 1971 Oct;48(4):447–453. doi: 10.1104/pp.48.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas J. E. Photophosphorylation Can Provide Sufficient Adenosine 5'-Triphosphate to Drive K Movements during Stomatal Opening. Plant Physiol. 1972 Apr;49(4):649–650. doi: 10.1104/pp.49.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke K. Saturation Kinetics of the Velocity of Stomatal Closing in Response to CO(2). Plant Physiol. 1972 Feb;49(2):229–234. doi: 10.1104/pp.49.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarth G. W. MECHANISM OF THE ACTION OF LIGHT AND OTHER FACTORS ON STOMATAL MOVEMENT. Plant Physiol. 1932 Jul;7(3):481–504. doi: 10.1104/pp.7.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmer C., Kanai R., Pallas J. E., Jr, Black C. C., Jr Detection of high levels of phosphoenolpyruvate carboxylase in leaf epidermal tissue and its significance in stomatal movements. Life Sci II. 1973 Feb 22;12(4):155–159. [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]