Abstract
Green microalgae for several decades have been produced for commercial exploitation, with applications ranging from health food for human consumption, aquaculture and animal feed, to coloring agents, cosmetics and others. Several products from green algae which are used today consist of secondary metabolites that can be extracted from the algal biomass. The best known examples are the carotenoids astaxanthin and β-carotene, which are used as coloring agents and for health-promoting purposes. Many species of green algae are able to produce valuable metabolites for different uses; examples are antioxidants, several different carotenoids, polyunsaturated fatty acids, vitamins, anticancer and antiviral drugs. In many cases, these substances are secondary metabolites that are produced when the algae are exposed to stress conditions linked to nutrient deprivation, light intensity, temperature, salinity and pH. In other cases, the metabolites have been detected in algae grown under optimal conditions, and little is known about optimization of the production of each product, or the effects of stress conditions on their production. Some green algae have shown the ability to produce significant amounts of hydrogen gas during sulfur deprivation, a process which is currently studied extensively worldwide. At the moment, the majority of research in this field has focused on the model organism, Chlamydomonas reinhardtii, but other species of green algae also have this ability. Currently there is little information available regarding the possibility for producing hydrogen and other valuable metabolites in the same process. This study aims to explore which stress conditions are known to induce the production of different valuable products in comparison to stress reactions leading to hydrogen production. Wild type species of green microalgae with known ability to produce high amounts of certain valuable metabolites are listed and linked to species with ability to produce hydrogen during general anaerobic conditions, and during sulfur deprivation. Species used today for commercial purposes are also described. This information is analyzed in order to form a basis for selection of wild type species for a future multi-step process, where hydrogen production from solar energy is combined with the production of valuable metabolites and other commercial uses of the algal biomass.
Keywords: Algae technology, green algae species, solar energy, hydrogen, biomass, stress, pharmaceuticals
Abbreviations:
- AA,
Arachidonic acid
- ALA,
α-linolenic acid
- ATP,
Adenosine triphosphate
- BHT,
Butylated hydroxytoluene
- C,
Carbon
- CO2,
Carbon dioxide
- Cyt bf,
Cytochrome bf
- DHA,
Docosahexaenoic acid
- DNA,
Deoxy ribonucleic acid
- EFA,
Essential fatty acid
- EPA,
Eicosapentaenoic acid
- ETA,
Eicosatrienoic acid
- GLA,
γ-linolenic acid
- LA,
Linoleic acid
- LHC,
Light harvesting complex
- MAA,
Mycosporine-like amino acid
- Mg,
Magnesium
- Mn,
Manganese
- N,
Nitrogen
- NADPH,
Nicotinamide adenine dinucleotide phosphate
- OEC,
Oxygen evolving complex
- P,
Phosphate
- PC,
Plastocyanin
- PSI,
Photosystem I
- PSII,
Photosystem II
- PQ,
Plastoquinone
- PUFA,
Polyunsaturated fatty acid
- QA,
Quinone A
- QB,
Quinone B
- RNA,
Ribonucleic acid
- RuBisCO,
Ribulose-1,5-bisphosphate carboxylase
- ROS,
Reactive oxygen species
- SQDG,
Sulfoquinovosyl diacylglycerol
- S,
Sulfur
- TAG,
Triacyl glycerol
- UV,
Ultraviolet
Introduction
Use of algae
The concept of culturing microalgae in the laboratory was introduced by Warburg (1919), who cultured Chlorella for the purpose of photosynthesis research. The first attempts for mass culturing of algae were performed in 1950s, with Chlorella pilot plants in Massachusetts and Tokyo (Richmond and Soeder, 1986). In Southeast Asia algae culturing developed commercially at an early stage; in 1977 there were 30 Chlorella factories in Taiwan. Algae represent a highly diverse group of organisms, which are able to grow under a variety of different conditions. Algae are found at low and high temperatures, low and high light intensities, different pH, high salt concentration, in water bodies or in desert crusts, or in symbiosis with animals (Barsanti et al., 2008).
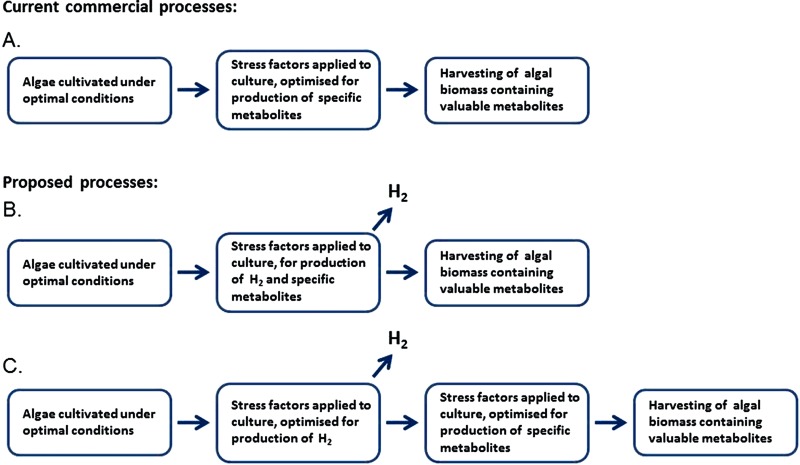
Over the last decades, more attention has been paid to the possibilities of growing algae commercially, and several different fields within use of algal biomass have unfolded. Algae are presently produced and sold as health food all over the world. Algal biomass is commonly used for aquaculture feed, as well as for other animal feed. Extracts from algae can be used for production of cosmetics and many different pharmaceutical products (Apt and Behrens, 1999; Luiten et al., 2003; Yamaguchi, 1997). The research on microalgal biotechnology has had a steady increase over the last decade (Plaza et al., 2009). During the same period, research has shown that the algae are able to produce fair amounts of the energy carrier hydrogen under sulfur (S) deprivation (Ghirardi et al., 2000; Melis et al., 2000). In the process described below, hydrogen is, in principle, produced from solar energy by direct and indirect biophotolysis under anaerobic conditions. This mechanism for handling sulfur deprivation prevents oxidative stress in the algae. It was proven already in 1948 that there were possibilities to manipulate the chemical composition of green microalgae by varying their different growth conditions (Spoehr and Milner, 1949). Since then, it has been shown that applying different forms of stress to the cells promote the production of secondary metabolites, some of which can have pharmaceutical and/or industrial value as thoroughly described in the following. Figure 1A shows the principle of a two-step process of producing algal biomass with aluable metabolites. The algae in the first step are grown under optimal conditions for an efficient production of biomass, followed by a second step where stress factors are applied in order to induce production of the valuable metabolites.
Figure 1. .
Production of valuable metabolites from algae in commercial use today (A), compared to the proposed processes where stress factors are applied to induce both hydrogen production and production of valuable metabolites simultaneously (B), or in sequence (C).
Food
Microalgae have most likely been used as a human nutrient source since ancient times. The first known report about a food source made from blue-green algae (cyanobacteria) was published in 1520 by Hernán Cortés, describing findings from Lake Texcoco in Mexico. The Aztecs who lived there are assumed to have used tecuitlatl, a cake made from Spirulina, as a major part of their diet. In Central Africa, the population around Lake Chad is still using dihé made from Spirulina as a food source, as they have done for probably hundreds of years (van Eykelenburg, 1980). The option of using algae as a food source today is dependent on many variables. Some requirements have to be fulfilled regarding, for example, content of protein and other nutrients, content of antioxidants and other health-promoting agents, taste and odor, contents of toxic compounds and general safety of oral intake by humans. Currently algal biomass is sold as health food in many parts of the world, produced from only a limited number of species.
Aquaculture
Microalgae are at the base of the aquatic food chain and are, in general, highly acknowledged for their nutritional value (Brown et al., 1997). The first reports of culturing microalgae for use as feed in aquaculture were published a 100 years ago (Allen and Nelson, 1910) and, since then, the use of microalgae for this purpose has developed rapidly. Today, microalgae are widely used as one of the most important feed sources for different groups of commercially important aquatic organisms in both freshwater and marine aquaculture (Duerr et al., 1998). Aquaculture is a growing industry, and the industry of cultivation of microalgae for this purpose is consequently increasing.
Secondary metabolites and stress
Green microalgae are known to contain a very high amount of nutrients, proteins in particular. Under optimal growth, the relative content of the various nutrients is fairly similar among species (Hu, 2004). However, during sub-optimal conditions, this changes. When algae apply stress reactions for handling more difficult environmental conditions, algal species use very different methods for managing the change in the environment. Depending on their ability to handle the various types of stress, the algae will produce different secondary metabolites in order to increase their chance of survival.
Metabolites are defined as, all organic compounds involved in the metabolism of living cells. Some metabolites are not part of the primary metabolic processes of growth, reproduction or general maintenance of the cell systems, but are produced for use in secondary cell functions. These are called secondary metabolites, and are often produced as a reaction to environmental stress. Their function can in some cases be, to increase the chance of survival or maintain the growth rate under specific conditions, but in other cases their function is not known. Numerous secondary metabolites are likely to be present in all algae; however, most of these are of no known practical or commercial interest. In this study the term “secondary metabolite” refers to compounds that can potentially be of pharmaceutical and/or other industrial interest.
Screening living organisms to identify new compounds with biological activities is referred to as bioprospecting. Lately there has been a particular interest in searching for new compounds in marine organisms, since the potential for new discoveries in the marine environment is particularly high (Hunt and Vincent, 2006). The identification of new compounds from marine organisms in general, from chemical structure to activity and function, has been extensively reviewed by Blunt and co-workers (Blunt et al., 2007, 2008, 2009; Blunt et al., 2003, 2004, 2005, 2006) and Mayer and Gustafson (2003, 2008). However, it has been found that in some cases the bioactive compounds identified from marine organisms originate from dietary intake of algae (Harrigan and Goetz, 2002; Proksch et al., 2002). General considerations on the reality of a future for pharmaceutical products from marine organisms can be found in Glaser and Mayer, (2009).
Energy
Hydrogen is an energy carrier with unique properties, like extremely low density, high energy content and with water being the only by-product after combustion. Technology for use of hydrogen as an energy carrier has rapidly developed during the last decades (Momirlan and Veziroglu, 2002; Seymour et al., 2008; Stiller et al., 2010). Currently all major car producers offer cars running on hydrogen as fuel. Gaffron and Rubin discovered in 1942 that the green microalgae Scenedesmus was able to produce hydrogen gas (Gaffron and Rubin, 1942), which opened up to a search for a way of using algae to convert solar energy into this useful energy carrier. Several methods for producing hydrogen from algae have been explored, as described in the Section “Hydrogen production”. Production of hydrogen is part of a survival mechanism used by the algae to cope with certain stress factors. Figures 1B and 1C illustrate a potential process where stress factors can be applied to induce production of hydrogen and valuable metabolites, either simultaneously or in sequence. Although a simultaneous production as shown in Figure 1B would involve one less step compared to process Figure 1C, it is unlikely that this can reach the same production efficiency, see discussion in Section “Summary and perspectives”.
Algae are also used as a source of biofuel such as biodiesel or bioethanol, and significant research has been performed over several years in order to make conversion of algal biomass to fuel a viable process. Several attempts have been made to produce algae for biofuel commercially. The topic of biofuel production from green algae is previously examined thoroughly, however, this study will focus on the other uses of algae mentioned above, and will not discuss aspects of the field of biofuel from algae. Extensive discussions on the issue of biofuel from algae can be found (Hu et al., 2008b; Khan et al., 2009; Mata et al., 2010; Posten and Schaub, 2009; Schenk et al., 2008; Vasudevan and Briggs, 2008).
Species
Clearly, many different phyla of algae have been used historically for different purposes, and many different phyla are in practical use today. However, this paper will only cover possibilities for use of green microalgae, as defined by Lewis and McCourt, (2004). This is due to the focus of this study on hydrogen production. A significantly higher number of green algae have the ability to produce hydrogen, compared to other types of algae (Boichenko and Hoffmann, 1994; Brand et al., 1989). Many species of green macroalgae also have potential use within some of the fields mentioned above. However, this study searches for species which are able to contribute to several stages of an overall process, including hydrogen production. Microalgae are here defined as single celled algae, or colony forming algae which in general form small colonies that are individually not visible to the eye.
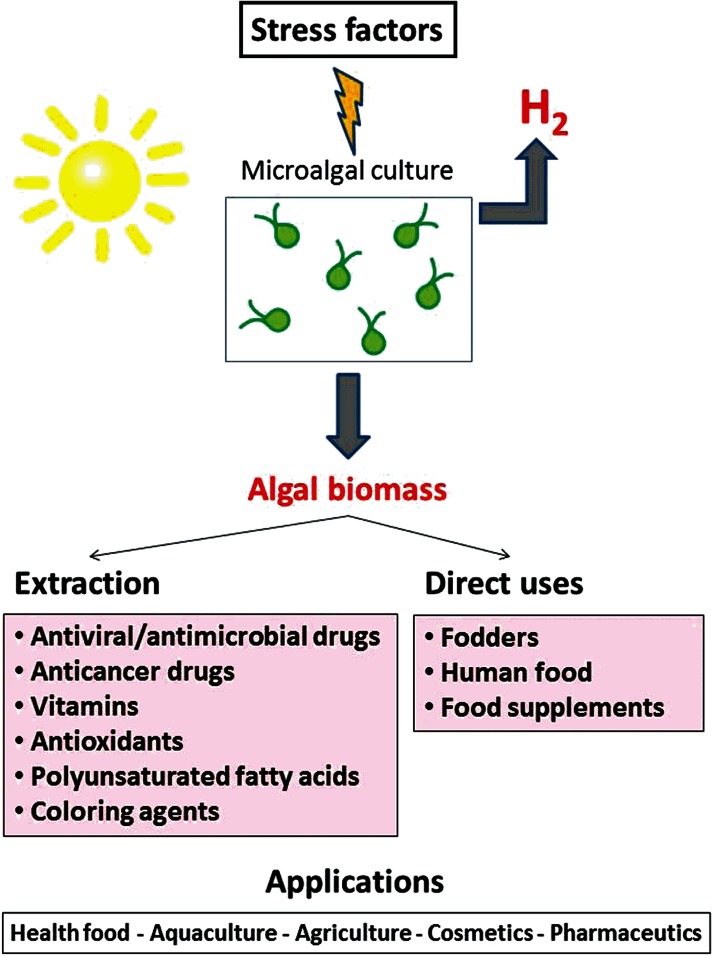
A combined multidisciplinary process for using solar energy to capture CO2 while producing hydrogen and different high value products has previously been presented (Skjånes et al., 2007). That paper summarizes, in an integrated manner, different technologies for use of algae, demonstrating the possibility of combining different areas of algae technology to produce hydrogen from solar energy and using the obtained algal biomass for various industrial applications, thus bringing added value to the hydrogen production processes. While the algae are cultivated under close-to-optimal conditions in a first stage, a second stage will apply stress by sulfur deprivation, which induces hydrogen production. After the hydrogen production phase, the microalgal biomass can be collected and used for different purposes: it can be used directly as health food for human consumption, as animal feed or in aquaculture. It is believed that in some cases after nutrient limitation, algal biomass may contain large amounts of valuable biomolecules, which can be extracted for pharmaceutical or industrial purposes. This concept is the starting point for the current study. A general overview of a proposed process, where certain stress factors are applied to an algal culture in order to induce production of hydrogen and produce several products with high commercial value, is presented in Figure 2. The multidisciplinary process described in Skjånes et al. (2007) has some resemblances to the biorefinery approach to algal biofuel production; see for example Subhadra (2010).
Figure 2. .
Overview of the combined process for production of hydrogen and bioactive metabolites. Green microalgae can be cultured under optimal growth conditions, followed by exposure to stress conditions (high light intensity, nutrient deprivation). The algal biomass can be harvested and used for different purposes, for example direct use as food supplement, aquaculture and animal fodders. Several valuable components can be extracted for the purpose of pharmaceutical industry, cosmetics or other types of industrial purposes.
In some cases, species have been selected as a result of their useful properties, such as growth rate or productivity of certain valuable metabolites. In other cases, the algal species have been selected since an already significant amount of research and experience allow for a simpler and less complicated further development of the process.
Desired properties for algae to be used in this combined process are to:
produce hydrogen during stress conditions like for example nutrient deprivation,
produce high content of nutrients for health food/animal feed purposes in a hydrogen production process,
produce metabolites with pharmaceutical or other industrial interest,
produce hydrogen and valuable metabolites either simultaneously or in sequence,
have fast growth rates under optimal conditions.
Aim
The main aim of this review has been to present the possibilities for using green algae species for hydrogen production from solar energy combined with the potential for additional products of pharmaceutical or other industrial value. The starting point for the review is a multidisciplinary process where several different areas of algal technology is combined, as previously described in Skjånes et al. (2007). This review will summarize stress factors and the algae’s ability for adaptation to stressful conditions, and the mechanisms that can be used to induce the production of valuable metabolites, which then will be linked to the stress factors that induce hydrogen production in green algae. The most important metabolites of potential commercial interest known to be produced in green algae will be described. Species that have a potential for production of certain valuable metabolites will be presented and correlated to species with the ability to produce hydrogen. Furthermore, the review will describe current commercial uses of algae for purposes such as health food and animal feed. In addition to the above mentioned issues, this review also aims at establishing a platform for selection of green algal species for use in a future process whereby hydrogen production from solar energy is combined with production of valuable metabolites, and/or with other commercial uses of the algal biomass.
Analysis of current knowledge, stress and adaptation mechanisms
Many algae have the ability to survive harsh environmental conditions due to different adaptation strategies (Barsanti et al., 2008; Seckbach, 2007). Many mechanisms for adaptation lead to changes in the algae’s physiology, and as a consequence, the algae will produce different secondary metabolites as part of their adaptation strategies. In order to explore the possibilities within use of the algae’s production of metabolites with valuable properties, it is important to elucidate how the algae react during different forms of stress.
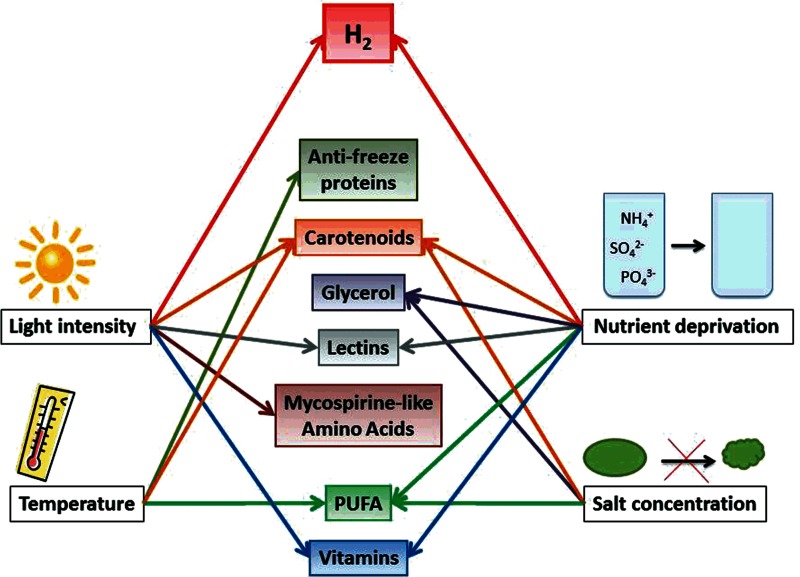
Light intensity
Algae can be found in areas where the light intensity can become very high, as for example in deserts, and in areas where the light intensities in addition vary considerably during the year, as for example in the Arctic region. These conditions require the cells to develop defense mechanisms against damaging effects of irradiant stress (Barsanti et al., 2008). Photodamage of the photosynthetic systems is a continuous process that occurs during light conditions in all photosynthetic eukaryotes. There are several theories explaining the mechanisms involved, some of which are summarized by Nishiyama et al., 2006; Takahashi and Badger, 2011; Tyystjärvi, 2008.
High light intensity can cause strong damaging effects in the cell due to over-excitation of the photochemical apparatus. When the solar energy absorbed by the antenna pigments exceeds the capacity of the photosynthetic system to process the energy, this will lead to generation of long lived excited triplet state chlorophyll which can interact with oxygen to cause the formation of reactive oxygen species (ROS). ROS can cause a great deal of damage to the cell; possible effects are briefly described in the Section “Antioxidants in general”. One of these effects from ROS is the inhibition of protein synthesis. Although it was previously shown that the biosynthesis of the important protein D1 is fairly constant both under low and high light intensities (Vasilikiotis and Melis, 1994), it is now clear that light induced ROS cause inhibition of synthesis of the D1 protein in photosystem II (PSII) (Nishiyama et al., 2011).
As mentioned above, there are several theories that have been proposed regarding the mechanisms for photodamage to PSII. The photodamage to PSII is most likely not caused by ROS, but rather as a direct consequence of the exposure of the PSII complex to solar energy. Some of the most recent studies show that the primary reaction in the photodamage of PSII is caused by absorption of light by Mn containing oxygen evolving complex (OEC), which leads to formation of high valent Mn species by disruption of the Mn complex and release of Mn ions. This damage is particularly sensitive to UV wavelengths (Takahashi et al., 2010; Wei et al., 2011). Absorption of visible light leads to an oxidized chlorophyll in the PSII reaction center, and without a supply of electrons from the OEC, this strong oxidant can cause damage to the D1 protein (Nishiyama et al., 2011). The damaged D1 protein is removed by proteolytic degradation, and replaced with newly synthesized protein. The electron acceptor QA of PSII may, under high light, be converted to a stable reduced form, thereby inhibiting further electron transport to photosystem I (PSI). When PSII is closed in this way, excitation leads to increased damage to the reaction center (Melis, 1999). Some studies indicate that the evidence demonstrating that the Mn complex is the primary site for photoinhibition does not exclude the theory that the chlorophyll reaction center may in some cases be the primary site (Oguchi et al., 2011).
Photoinhibition increases when the cell is under stress conditions that limit growth, for example CO2 limitation (Baroli and Melis, 1998), other nutrient limitation (Grossman, 2000), pH-, salt- or temperature stress (Morgan-Kiss et al., 2006; Neale and Melis, 1989). One of the reasons for this is that slower growth decreases an electron sink, thereby increasing the oxidative stress.
ROS are toxic products of the metabolism, but also have important roles in regulation and signaling (McCord, 2000). Nevertheless, photodamage in PSII is likely to occur under normal circumstances, and algal photosynthetic systems are dependent on efficient repair mechanisms to prevent lethal damage to the cells. Photoinhibition has been found to be strictly proportional to light intensity (Tyystjärvi, 2008). Mechanisms for adaptation to high light intensities are essential for survival of algae during stressful irradiant conditions. Adaptation mechanisms that balance the energy input with the energy output through CO2 assimilation and other metabolic pathways are important. One method used by algae exposed to high light intensities involves reduction of the antenna size, thereby limiting the amount of energy absorbed. Some potential mechanisms for dissipation of excess energy in photosynthesis have been summarized by Niyogi, 2000.
The primary defense system for radiant stress is considered to be the thermal dissipation mechanisms, a system that leads the excess energy away from the reaction centers and dissipates the energy as heat. This mechanism protects the photosystem at an early stage by preventing the formation of ROS, since the triplet state chlorophyll formed by the oxidative stress can be inactivated before ROS formation using the xanthophyll cycle. The triplet state chlorophyll can, in this mechanism, be deactivated by excitation energy transfer, directly or indirectly, to the light harvesting carotenoid violaxanthin, which is subsequently converted to the energy quenching carotenoid zeaxanthin via the intermediate antheraxanthin. This is referred to as energy- and delta pH dependent quenching (qE), and is induced by the rapid decrease in lumen pH that occurs under high light electron transport. The xanthophyll cycle is a reversible reaction and when the light intensity conditions return to normal, the zeaxanthin is converted back to the light harvesting violaxanthin (Jahns et al., 2009). It was shown that, if Dunaliella was prevented from performing the qE quenching mechanism, alternative quenching mechanisms were able to compensate and offer sufficient protection towards excess light (Thaipratum et al., 2009).
Oxidative damage can also occur as a consequence of uneven distribution of absorbed light between PSII and PSI. Reorganization of the antennae complexes leads to a redistribution of the excitation energy between PSII and PSI; this mechanism is referred to as state transition quenching (qT) and is regulated by the redox state of the PQ pool (Nield et al., 2004). Light harvesting proteins become phosphorylated under high irradiance conditions in state 1, when the PQ pool is reduced. The proteins then dissociate from PSII and migrate to the stromal lamellae, where they are incorporated into the peripheral antenna of PSI; the system is then converted to state 2. This method enables the algae to respond within minutes to changes in illumination.
Another possible form of quenching is called inactive PS II mediated quenching, where functional PSII centers are protected by inactivated reaction centers, which can dissipate the excess energy as heat. This mechanism has been observed in plants when less than 30% of the PSII reaction centers remain active (Chow et al., 2002).
The secondary defense system targets to quench the ROS before they cause damage to the photosystems. This is accomplished by producing antioxidants, a group of molecules thoroughly described in the Section “Analysis of current knowledge, potential products from algae”. All algae produce a number of antioxidants that are able to scavenge these free radicals, carotenoids being the most common. The carotenoids of the xanthophyll cycle described above have been shown in plants to have additional roles as antioxidants in the photo protection (Havaux et al., 2007).
Other reactions to prevent oxidative damage of the photosystems include releasing the reductive pressure of the electron transport chain. Excess electrons can be used by alternative electron sinks, for example to reduce oxygen (photorespiration) (Niyogi, 2000), or to use the assimilatory reaction of CO2 reduction resulting in storage materials like starch and lipids (Hu, 2004; Richmond and Soeder, 1986; Rodolfi et al., 2009). As described in the Section “Nutrient limitation”, lack of nutrients like C, N, P or S prevents growth, thereby removing one of the photosynthesis electron sinks; a situation which can lead to increased photoinhibition also at low light intensities that would normally not cause damage. In some cases, reductive pressure can be released in the form of hydrogen gas.
Light stress can be used as a mechanism for inducing the production of antioxidants such as carotenoids, vitamins, butylated hydroxytoluene (BHT), and others. These compounds can be extracted and used for a number of applications, as described in the Section “Analysis of current knowledge, potential products from algae”.
Temperature
Several green algae are able to tolerate very low temperatures, examples are algae growing on snow and ice like Chlamydomonas nivalis and Chloromonas nivalis. However, for mesophilic algae, cold stress can have considerable inhibiting effects. One important factor causing cold stress is increased rigidity in the membrane systems, when temperature is reduced. Membrane fluidity is essential for electron transport by mobile carriers; optimal photosynthetic function relies on the correct folding of the complex membrane associated proteins of the photosynthetic electron transport chain. Other effects caused by cold stress include decreased enzyme reaction rates, which can lead to problems like oxidative stress (Morgan-Kiss et al., 2006).
Psychrophilic strains have a number of adaptation mechanisms that help the organisms to tolerate the cold. A necessary factor for algae to adapt to lower temperature is to maintain membrane fluidity by incorporating unsaturated fatty acids in the membranes. The amount of unsaturated lipids in the membranes and the extent of their unsaturation represent a major factor for avoiding membrane rigidity. Other adaptation mechanisms include cold shock and antifreeze proteins that bind to ice crystals and prevent cell damage.
At suboptimal temperatures, enzymes will in general have decreased reaction rates. One mechanism for adapting to cold environment is to produce more enzymes to compensate for lower specific activity, as already shown for Ribulose-1,5-bisphosphate carboxylase (RuBisCO) in psychrophilic Chloromonas species (Devos et al., 1998). Psychrophilic algae can also have increased amounts of ATP synthase, which may compensate for the reduced molecular diffusion rates. Elevated contents of ATP in the cells have been observed, and may be related to decline of energy consumption (Napolitano and Shain, 2005). Improved catalytic efficiencies can be obtained by increasing turnover numbers or by decreasing the substrate concentration required for optimal activity. Enzymes from psychrophiles may also exhibit a shift in optimal activity towards lower temperatures (Morgan-Kiss et al., 2006).
Another mechanism for cold adaptation involves differential energy partitioning. As absorption of light is an temperature independent process, it must be coordinated with the temperature dependent formation and utilization of ATP and NADPH. Low temperature can cause an imbalance between the energy that is absorbed by the photosystems and the energy that is consumed by the metabolic processes due to decreased metabolic rates. The problem with excess energy absorbed can be solved by reducing the antenna size, thereby limiting the amount of energy absorbed, photosystem transition from state 1 to state 2, or dissipating excess energy non-photochemically as heat. The situation can also be solved by increasing the sink capacity, as for example increasing the amount of Calvin cycle enzymes (Huner et al., 1998). High amounts of potential energy sinks like starch, lipids and secondary carotenoids have been observed in psychrophilic green algae (Leya et al., 2009; Remias et al., 2009).
Algae living in certain cold ecosystems, in particular on snow and ice surfaces, are often exposed to high irradiation, including high UV levels, which they need to handle in order to survive, see also the Section “Light intensity”. More than 100 species of green algae are identified as dominant organisms on snow, causing red, yellow, green and grey snow patches; Chlamydomonas nivalis being the best studied example (Morgan-Kiss et al., 2006). The red color in the algal cells is a result of increased astaxanthin production providing an UV-screening effect (Remias et al., 2005). This alga is also known to produce phenolic compounds as a photo protective response (Duval et al., 2000). In addition to have antioxidant activity, these substances have been attributed to chemotherapeutic, antimicrobial and anticancer activities (Blunden, 1993).
Two psychrophilic strains of Chlorella showed production of unsaturated fatty acids and antifreeze proteins. One strain showed an increased ability for adaptation when pre-cultivation temperatures were lower, while the other species showed the same reaction pattern during cold stress regardless of pre-cultivation temperature. This indicates that the adaptation mechanisms towards cold may vary widely between psychrophilic strains within the same genera (Hu et al., 2008a). Two different strains of Chlamydomonas raudensis, one psychrophilic and one mesophilic, showed different adaptive responses to irradiance stress at suboptimal temperatures (Szyszka et al., 2007); confirming that psychrophilic reaction patterns are not species specific. Algae have a general tendency to survive temperatures below their optimum for growth better than temperatures above optimum (Ukeles, 1961). This is most likely because enzymes can be denatured and therefore irreversibly inactivated by heat, while low temperatures often cause only a reversible inhibition.
Thermotolerant green algae are highly uncommon; photosynthetic organisms in hot springs are usually dominated by prokaryotes (Barsanti et al., 2008). However, some green algae have tolerance to temperatures up to 42°C, like Chlorella sorokiniana (de-Bashan et al., 2008; Sakai et al., 1995). Mutants with higher saturation of chloroplast membrane lipids had a higher tolerance for higher temperature (Sato et al., 1996). Under elevated temperatures, a large increase in astaxanthin content has been detected in the mesophilic Haematococcus sp. (Tjahjono et al., 1994), and in Chlorococcum sp. a similar astaxanthin increase was shown to lead to a relative decrease of β-carotene content (Liu and Lee, 2000). This can be explained by temperature derived increase in oxidative stress, leading to increased need for secondary carotenoids, or temperature dependent enzymatic reactions. Heat-shock response in Chlorella has been shown to involve cytochrome f in a programmed cell death process (Zuppini et al., 2009).
Temperature induced stress responses can be used for production of useful metabolites such as unsaturated fatty acids, antifreeze proteins, astaxanthin and other antioxidants like phenols, which have showed medical effects.
Osmotic stress
Green microalgae are found in freshwater, brackish water and seawater, but also in highly saline environments; some species can tolerate a wide range of different salinities (Chen and Jiang, 2009; Strizh et al., 2004). The most studied halophilic green algae are Dunaliella spp, which are widely distributed in high salinity environments (Hadi et al., 2008; Kaçka and Dönmez, 2008; Oren et al., 2008).
Osmotic changes caused by for example variation in salinity can inflict hypo- or hyperosmotic stress on the cells by impact on the cellular water potential and loss/uptake of ions through the cell membrane. Stress reactions, as measured by decreased growth rates, can vary considerably, but stress measured as survival rate often show that algae can survive salt stress over a much wider range of salinities than the case is for growth rates (Kirst, 1989). A consequence of high salinity can for example be impaired electron transfer between antenna pigments, electron transfer on the water splitting side of PSII and impaired photo activated electron flow of PSI (Satoh et al., 1983). Mechanisms involved in osmotic acclimation include water flux, which is a result of most changes in salinity and, in some cases, can be a sufficient way of preventing negative effects on the cell. Ion transport, which can be passive or active, leads to uptake or release of ions to adjust salt concentration inside the cells. Some species will also produce vacuoles to sequester the excessive ions (Stoynova-Bakalova and Toncheva-Panova, 2003). The organisms can also produce one or several organic osmolytes that can be present in high concentrations without inhibiting enzymatic activities (Oren, 2007). This can sometimes be a more long-term adjustment strategy as a response to large changes in salt concentration. One example of an organic osmolyte is glycerol, which is produced in large amounts in for example Dunaliella sp under salt stress (Hadi et al., 2008; Kaçka and Dönmez, 2008). It has been hypothesized that marine strains are able to maintain the glycerol molecules within the cell, which allow them to have a higher internal concentration of this important osmolyte, thereby being able to tolerate higher external salt concentrations. In fresh water strains, glycerol can diffuse more easily across the cell membrane. In this case, glycerol will be produced at a stable rate to provide equilibrium and is continuously excreted (León and Galván, 1994), leading to a lower salinity tolerance of the cell. In some marine algae, like Chlamydomonas pulsatilla and Dunaliella salina, there is a correlation between glycerol synthesis and degradation of starch; this is seen in particular as a consequence of high salinity shock during light exposure (Goyal, 2007; Hellebust and Lin, 1989; Kaplan et al., 1980). A variety of other osmolytes such as mannitol, proline and sucrose are produced by halotolerant green algae (Oren, 2007). A summary of osmotic responses to changes of salinity can be found in Chen and Jiang, (2009). Strains with a high ability to adapt to variations in salt concentrations in the environment are able to increase their energy yielding processes, which improve the ability to pump ions out of the cell and increase the tolerance to high salinity (Alyabyev et al., 2007).
Salinity tolerance and salinity optimum are strongly dependent on light and temperature conditions, in addition to nutrient limitation (Cho et al., 2007; Coesel et al., 2008). Photoinhibition can for example increase under high salt concentrations (Neale and Melis, 1989). Salinity stress usually leads to a decrease in growth rate. In some cases, increased salinity can lead to an increase in metabolites like palmitic and oleic acids, carotenoids like lutein and β-carotene, as shown in Botryococcus braunii (Rao et al., 2007) or astaxanthin, as shown in Haematococcus (Orosa et al., 2001). These products will in this case function as an energy sink to relieve the reductive pressure. Dunaliella spp. show an optimal production of β-carotene as a photo protective response under high salt concentrations (Ben-Amotz and Avron, 1983), as well as increased production of unsaturated fatty acids in microsomes (Azachi et al., 2002). High salinity tolerance is an advantage in commercial production since it can be used as a protection against contamination of other species.
Green algae are also found in desert crusts, for example Desmococcus olivaceus, Chlamydomonas sp, Chlorococcum humicola, Chlorella vulgaris, Palmellococcus miniatus, along with cyanobacteria, diatoms and euglenoids (Barsanti et al., 2008). These algae have high tolerance to dry conditions, high irradiation and fluctuating temperatures. There is a strong link between desiccation and osmotic stress, as described above, and increased salinity has been used experimentally to mimic effects of drying (Satoh et al., 1983).
Valuable metabolites that can be produced during osmotic stress include for example glycerol, carotenoids and unsaturated fatty acids, as mentioned above. These products have many applications and are described in the Section “Analysis of current knowledge, potential products from algae”.
pH
Green microalgae can be found in many different pH environments; a limited number of species are able to grow and photosynthesize under very low pH. The most studied species of green algae isolated from acidic environments is Chlamydomonas acidophila. This acidophilic alga can grow at pH as low as 1.5, with an upper limit of pH 7 (Gerloff-Elias et al., 2005). Growth rates in this organism under low pH are comparable with growth rates of mesophilic organisms under neutral pH, showing that the algae are well adapted to the acidic conditions and are not inhibited by low pH. There are several strategies for algae to handle an acidic environment. Both acidophilic and acidotolerant species are dependent on being able to maintain a close to neutral pH in the cytosol in order to prevent damage to intracellular systems (Gerloff-Elias et al., 2006). Maintaining a neutral internal pH when the external pH is low requires specific ATP driven H+ pumps, which is an energy demanding process. The H+ can be transported both into vacuoles and also out of the cells. Vacuolar proton pumps remove the H+ from the cytosol at a rate proportional with the H+ concentration. The plasma membrane, however, removes the protons at a constant rate, triggered by internal acidification, as has been found in the acidophilic green alga Eremosphaera viridis (Bethmann and Schönknecht, 2009). H+ influx into the vacuoles was compensated by cation release, while H+ efflux out of the cell was compensated by anion efflux. At very low pH, more than 50% of the synthesized ATP was in this case used for H+ pumping. One example of a mechanism applied to handle the increased energy demand has been suggested to be cyclic electron transport around PSI that leads to increased proton potential across the thylakoid membrane, which then is used for ATP production (Gerloff-Elias et al., 2005). However, the suggestion that there is a cyclic electron transport around PSI for this purpose has been disputed (Langner et al., 2009). Another strategy is increased metabolism. Photosynthetic rates can be significantly higher under low pH than under neutral pH, possibly as a compensation for increased respiration rates. In Chlamydomonas acidophila, modifications in the electron transport at low pH leads to increased PSII excitation pressure in the light, partly compensated by higher electron transport capacity of PSII and increased nonphotochemical quenching (Gerloff-Elias et al., 2005). Increased fatty acid saturation in the membranes and acid tolerant cell wall proteins are also mechanisms related to low pH adaptation (Tatsuzawa et al., 1996). Low external pH is, like some other forms of stress, shown to induce production of heat shock proteins (Gerloff-Elias et al., 2006). Effects of exposure to pH stress in Chlamydomonas applanata include reduction of cell volume, increase in pyrenoidal volume, reduction of starch reserves, and production of mucilage leading to palmelloid colonies (Visviki and Santikul, 2000). Haematococcus has shown increased astaxanthin production at low pH when exposed to light stress and N-deprivation (Orosa et al., 2001). In Chlorococcum sp, the content of the dominant secondary carotenoid canthaxanthin increases when pH is below optimum for growth (Liu and Lee, 2000).
Nutrient limitation
Limitation of photosynthetic growth in nature is very often caused by limiting access of nutrients, in particular the major nutrients nitrogen (N), phosphorus (P) and sulfur (S). Inhibition of growth is a natural consequence of lack of nutrients since important building blocks of the cell contain these elements. One example is the high amount of N and P in DNA and RNA, where they serve as important building blocks in the structure. N is also essential in proteins, each amino acid building block contains at least one atom of this major element. P is also essential for transporting energy in the form of ATP, and is a component of phospholipids that make up the cellular membranes. Pollutants containing phosphate is a common cause of eutrophication and algal blooms, showing that P is often a limiting factor for algal growth in natural freshwater environments. S is also an essential component of proteins, since the important amino acids cysteine and methionine, among others, contain this element. In particular disulphide bridges made up of cysteine–cysteine disulphide covalent bonds are important in protein assembly and structure. In green algae, N is mostly taken up in the form of ammonium (NH4 +) or nitrate (NO3 −), P in the form of phosphate (PO4 3−) whereas S is taken up in the form of sulfate (SO4 2−).
Furthermore, green algae are dependent on trace metals that are important components of proteins, often as enzyme cofactors. A trace metal that is important in photosynthesis in algae, is for example iron (Fe), being a component in cytochromes, and thereby vital for electron transport. Fe is also an important part of enzymes like hydrogenase (Capon et al., 2009). Other important trace metals in photosynthesis are manganese (Mn), which is a component of the water splitting complex at PSII, and magnesium (Mg), which is a component of chlorophyll. Some algae also depend on vitamins from the environment, in particular vitamin B. As an example, out of 154 species of Chlorophyta, that were examined for Vitamin B12 dependency, 105 were able to synthesize this vitamin themselves, while 49 species were showed to require B12 from the environment (Croft et al., 2005). As described in the Section “Analysis of current knowledge, potential products from algae”, most algae are able to produce Vitamins A, C, D and E, some species also in amounts that have economical potential.
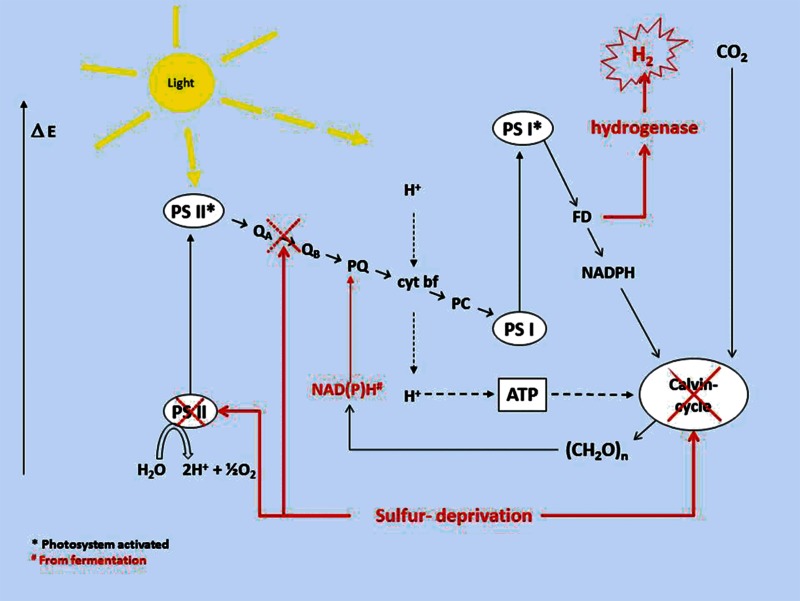
When the algal cells are deprived of any major nutrients, the growth cycle ceases and there is a shift in production of cell metabolites. The cell starts producing enzymes that enhance uptake mechanisms for the limiting nutrient. At the same time, certain proteins and lipids that are not essential during the deprivation are degraded, which is, in many cases, a way of releasing the limiting nutrient that can be used for essential processes in the cell (Pollock et al., 2005; Schreiner et al., 1975; Yildiz et al., 1994; Zhang et al., 2004). During sulfur deprivation, the important enzyme RuBisCO is specifically degraded, and the Calvin cycle stops (Zhang et al., 2002b).
Another general response to nutrient limitation is a decrease in photosynthetic activity, which is vital for the cells survival. Chlamydomonas reinhardtii has shown a 75% decrease of oxygen evolution in cells being starved from P after 4 days, and a similar decrease in oxygen evolution after 1 day of S deprivation (Wykoff et al., 1998). The same study also showed the following changes after deprivation of these major nutrients: Inhibited electron flow in PSII caused by photodamage which left 30% of the PSII inactivated, formation of non-reducing QB preventing e-transfer from PSII to PSI, and transition of the photosystems from state 1 to state 2, as described in the Section “Light intensity”. Light saturation occurs at lower light intensities in nutrient deprived algae compared to algae supplied with sufficient quantities of nutrients, and there is a correlation between the cells’ reactions to nutrient deprivation and light intensity. Some algae have the ability for intracellular storage of P in the form of polyphosphate bodies located in the cytosol (Olsen et al., 1983). Algae do not have a similar storage system for S, a fact that explains the longer interval before inhibition of oxygen evolution of P starved cells compared to cells exposed to S starvation. The sulfolipid sulfoquinovosyl diacylglycerol (SQDG) located in the chloroplast membranes is associated with PSII (Sato et al., 1995), and has been shown to degrade faster than RuBisCO during sulfur deprivation. The degradation of this sulfolipid is believed to function as a source of sulfur during the first few hours of sulfur deprivation, before the degradation of RuBisCO starts (Sugimoto et al., 2007).
When the Calvin cycle is inhibited, oxidative stress will consequently occur, unless the cells provide alternative pathways to dissipate the excess reducing power. In response to nutrient deprivation, many algae respond by creating energy- and carbon sinks by producing large amounts of starch, carotenoids and lipids. Many of these have useful properties in terms of medical effects or industrial use. They are described thoroughly in the Section “Analysis of current knowledge, potential products from algae”.
A well-studied species is the halotolerant green algae Dunaliella salina, which is able to produce large amounts of carotenoids. Many algae have shown the ability for increased accumulation of carotenoids during nutrient deprivation, some are listed in Table 1. These pigments are deposited as hydrophobic globules in the cytoplasm and are thought to function as a “sunscreen” to dissipate excess energy as heat, and thereby protecting the reaction centers. This reaction to nutrient deprivation is commonly seen and is particularly well studied in the astaxanthin producing Haematococcus (He et al., 2007; Imamoglu et al., 2009; Jin et al., 2006), and β-carotene producing Dunaliella (Coesel et al., 2008; Salguero et al., 2003). Major nutrient deficiency, like lack of N, P or S, leads to significant increase of these protective pigments. Another common reaction to nutrient deficiency is increased cellular content of lipids, although this has been mostly explored as a reaction to N-deprivation (Griffiths and Harrison, 2009; Illman et al., 2000; Wang et al., 2009; Zhekisheva et al., 2002). As an example, a high number of green algal species will increase their content of lipids from ∼15–30% to ∼25–65% when deprived of N, as summarized by Griffiths and Harrison, (2009). Other species will, on the other hand, maintain or decrease their lipid content, as is the case with for example Chlorella sorokiniana and some Dunaliella and Tetraselmis species (Becker, 2004b; Griffiths and Harrison, 2009). In some cases, the nutrient deprivation can lead to an increase of polyunsaturated fatty acid (PUFA) content, as for example production of arachidonic acid (AA) by Parietochloris incisa (Solovchenko et al., 2008). An increased production of starch is also often seen as a reaction to nutrient limitation (Cao et al., 2001; Libessart et al., 1995; Matagne et al., 1976; Rigano et al., 2000). A parallel increase of lipids and carotenoids and/or starch can sometimes be observed (Solovchenko et al., 2009; Timmins et al., 2009b; Wang et al., 2009; Zhekisheva et al., 2002).
Table 1. .
Algal species which have been studied and shown ability to produce hydrogen and/or metabolites with pharmaceutical/ industrial interest and algae which are being used today for commercial purposes.
| Genus | Species† | Hydrogen production‡ | Hydrogen –S‡ | Health food | Aquaculture/ animal feed | Valuable metabolites§,# |
|---|---|---|---|---|---|---|
| Ankistrodesmus | spp. | FS (Turker et al., 2003) | α-linolenic acid (Ben-Amotz et al., 1985) | |||
| Hexadecatetraenoic acid (Ben-Amotz et al., 1985) | ||||||
| angustus | D (Brand et al., 1989) | |||||
| braunii | D (Kessler, 1973) | Astaxanthin (Borowitzka, 1988) | ||||
| falcatus | D (Bishop et al., 1977) | |||||
| spiralis | Mycosporine-like amino acids (Xiong et al., 1999) | |||||
| Botryococcus | sppbraunii | α-linolenic acid (Chiang et al., 2004) | ||||
| Linoleic acid (Chiang et al., 2004) | ||||||
| Butylated hydroxytoluene (Babu and Wu, 2008) | ||||||
| Lutein (Rao et al., 2006) | ||||||
| β-carotene (Borowitzka, 1988) | ||||||
| Extracellular alkadienes (wax for cosmetics) (Mendes et al., 2003) | ||||||
| Brachiomonas | submarina | Glycerol (Ahmadand Hellebust, 1986) | ||||
| Carteria | spp | BP (de Pauw and Persoone, 1988, Lavens and Sorgeloos, 1996) | ||||
| crusifera | D (Brand et al., 1989) | |||||
| eugametos | D (Brand et al., 1989) | |||||
| Chlamydobotrys | stellata | D (Boichenko et al., 1992) | ||||
| Chlamydocapsa | spp | Lutein (Leya et al., 2009) | ||||
| Cantaxanthin (Leya et al., 2009) | ||||||
| Astaxanthin (Leya et al., 2009) | ||||||
| Chlamydomonas | spp. | BL, BP, FZ, MR, BS, BP, | Glycerol (Miyasaka et al., 1998) | |||
| FS (Lavens and Sorgeloos, 1996, Opuszynski, 1981) | Vitamin B (Vilchez et al., 1997) | |||||
| α-linolenic acid (Poerschmann et al., 2004) | ||||||
| Hexadecatetraenoic acid (Poerschmann et al., 2004) | ||||||
| acidophila | Lutein (Garbayo et al., 2008) | |||||
| β-carotene (Garbayo et al., 2008) | ||||||
| Violaxanthin (Garbayo et al., 2008) | ||||||
| applanata | D (Brand et al., 1989) | Haemagglutinin (Chu et al., 2004) | ||||
| asymmetrica | Haemagglutinin (Chu et al., 2004) | |||||
| chlamydogama | D (Brand et al., 1989) | |||||
| debaryana | D (Healey, 1970) | |||||
| dorsoventralis | D (Brand et al., 1989) | Haemagglutinin (Chu et al., 2004) | ||||
| dysosmos | D (Healey, 1970) | |||||
| elliptica | D (Brand et al., 1989) | |||||
| eugametos | D (Kessler, 1974) | Vitamin B (Uhlik and Gowans, 1974) | ||||
| euryale | D (Skjånes et al., 2008) | D (Skjånes et al., 2008) | ||||
| hindakii | D (Brand et al., 1989) (noctigama) | |||||
| hydra | D (Brand et al., 1989) | |||||
| intermedia | D (Kessler, 1974) | |||||
| moewusii | D (Healey, 1970) | D (Meuser et al., 2009) | α-linolenic acid (Arisz et al., 2000) | |||
| Linoleic acid (Arisz et al., 2000) | ||||||
| Hexadecatetraenoic acid (Arisz et al., 2000) | ||||||
| nivalis | Astaxanthin (Remias et al., 2005) | |||||
| Phenolic antioxidants (Duval et al., 2000) | ||||||
| Mycosporine-like amino acids (Duval et al., 2000) | ||||||
| noctigama | D (Winkler et al., 2002) | D (Skjånes et al., 2008) | Haemagglutinin (Chu et al., 2004) | |||
| pulsatilla | Glycerol (Ahmadand Hellebust, 1986) | |||||
| pulvinata | Haemagglutinin (Chu et al., 2004) | |||||
| reinhardtii | D (Kessler, 1974) | D (Melis et al., 2000) | Transgenic proteins (Griesbeck et al., 2005) | |||
| Toxic fatty acids (McCracken et al., 1980, Spruell, 1984) | ||||||
| Glycerol (León and Galván, 1999) | ||||||
| Vitamin C (Borowitzka, 1988) | ||||||
| Vitamin E (Borowitzka, 1988) | ||||||
| simplex | Haemagglutinin (Chu et al., 2004) | |||||
| texensis | D (Brand et al., 1989) | |||||
| ulvaensis | Haemagglutinin (Chu et al., 2004) | |||||
| vectensis | D (Skjånes et al., 2008) | D (Skjånes et al., 2008) | ||||
| Chlorella | spp. | D (Chader et al., 2009, Timmins et al., 2009a) | NP (Pulz and Gross, 2004, Yamaguchi, 1997) | MR, BS, FS, BL, ML, FZ (Apt and Behrens, 1999, de Pauw and Persoone, 1988, Duerr et al., 1998, Pulz and Gross, 2004, Turker et al., 2003) | Vitamin C (Running et al., 2002) | |
| Vitamin B (Borowitzka, 1988) | ||||||
| Extract w antimicrobial and anticancer activity (Ördög et al., 2004) | ||||||
| ellipsiodea | Violaxanthin (Cha et al., 2008) | |||||
| Antheraxanthin (Cha et al., 2008) | ||||||
| Zeaxanthin (Cha et al., 2008) | ||||||
| Extract w antimicrobial activity (Chu et al., 2004) | ||||||
| Extract w apoptose inducing effect (Cha et al., 2008) | ||||||
| Haemagglutinin (Chu et al., 2004) | ||||||
| emersonii | Canthaxanthin (Bhosale and Bernstein, 2005) | |||||
| fusca | D (Kessler, 1974) | Lutein (Del Campo et al., 2000) | ||||
| Astaxanthin (Borowitzka, 1988) | ||||||
| Canthaxanthin (Borowitzka, 1988) | ||||||
| Extract w antimicrobial activity (Chu et al., 2004) | ||||||
| Haemagglutinin (Chu et al., 2004) | ||||||
| homosphaera | D (Kessler, 1974) | α-linolenic acid (Ahlgren et al., 1992) | ||||
| kessleri | D (Kessler, 1974) (Parachlorella) | Extract w antimicrobial activity (Chu et al., 2004) | ||||
| Haemagglutinin (Chu et al., 2004) | ||||||
| luteovirtidis | Haemagglutinin (Chu et al., 2004) | |||||
| Mycosporine-like amino acids (Karsten et al., 2007) | ||||||
| minutissima | BL, PL (Duerr et al., 1998) | Eicosapentaenoic acid (Seto et al., 1984) | ||||
| Extract w antimicrobial and anticancer activity (Ördög et al., 2004) | ||||||
| Mycosporine-like amino acids (Xiong et al., 1999) | ||||||
| protothecoides | D (Bishop et al., 1977) | Lutein (Chen, 1998, Shi et al., 2006) | ||||
| Extract w antimicrobial activity (Chu et al., 2004) | ||||||
| Haemagglutinin (Chu et al., 2004) | ||||||
| Linoleic acid (Day et al., 2009) | ||||||
| Vitamin C (Running et al., 2002) | ||||||
| pyrenoidosa | D (Brand et al., 1989) | D (Skjånes et al., 2008) | Extract w antimicrobial/ antifungal activity (Abedin and Taha, 2008, Chu et al., 2004) | |||
| Haemagglutinin (Chu et al., 2004) | ||||||
| Polysaccharide w immunostim effect (Pugh et al., 2001, Yang et al., 2006) | ||||||
| Polysaccharide w antitumor activity (Sheng et al., 2007) | ||||||
| Lutein (Wu et al,. 2007) | ||||||
| Inhibition of dioxin maternal transfer (Nakano et al., 2005) | ||||||
| Vitamin B (Becker, 2004b) | ||||||
| Vitamin E (Becker, 2004b) | ||||||
| saccharophila | Haemagglutinin (Chu et al., 2004) | |||||
| salina | D (Chader et al., 2009) | D (Chader et al., 2009) | ||||
| sorokiniana | D (Bishop et al., 1977) | D (Chader et al., 2009) | Fatty acid extract w/medical activity (Chou et al., 2008) | |||
| Sulfoxidation biocatalyst activity (Daligault et al., 2006) | ||||||
| Lutein (Matsukawa et al., 2000) | ||||||
| α /β-carotene (Matsukawa et al., 2000) | ||||||
| Vitamin E (Matsukawa et al., 2000) | ||||||
| Haemagglutinin (Chu et al., 2004) | ||||||
| Mycosporine-like amino acids (Xiong et al., 1999) | ||||||
| sphaerica | Mycosporine-like amino acids (Karsten et al., 2007) | |||||
| stigmatophora | Polysaccharide w anti-innflammatory and immunosuppressive activity (Guzman et al., 2003) | |||||
| Extract w effect on the central nervous system (Laguna et al., 1993) | ||||||
| vacuolata | D (Bishop et al., 1977) | Extract w antimicrobial activity (Chu et al., 2004) | ||||
| vulgaris | D (Healey, 1970) | D (Timmins et al., 2009a) | NP (Pulz and Gross, 2004, Raja et al., 2008, Rodriguez-Garcia and Guil-Guerrero, 2008) | FS, HF (Gouveia et al., 2002, Janczyk et al., 2009, Raja et al., 2008) | Lutein (Cha et al., 2008) | |
| Canthaxanthin (Gouveia et al., 1996, Mendes et al., 2003) | ||||||
| Astaxanthin (Gouveia et al., 1996, Mendes et al., 2003) | ||||||
| Vitamin C (Borowitzka, 1988) | ||||||
| Vitamin E (Borowitzka, 1988) | ||||||
| Glycoprotein w anti-cancer activity (Hasegawa et al., 2002, Tanaka et al., 1998) | ||||||
| Sulfonated polysaccharide, antioxidant (Mohamed, 2008) | ||||||
| Haemagglutinin (Chu et al., 2004) | ||||||
| Eicosatrienoic acid (Becker, 2004b) | ||||||
| α-linolenic acid (Piorreck et al., 1984) | ||||||
| Linoleic acid (Piorreck et al., 1984) | ||||||
| Extract w toxin-protective effect (Kim et al., 2009a) | ||||||
| xanthella | Haemagglutinin (Chu et al., 2004) | |||||
| zofingiensis | Lutein (Bhosale and Bernstein, 2005, Del Campo et al., 2004) | |||||
| Astaxanthin (Bhosale and Bernstein, 2005, Del Campo et al., 2004) | ||||||
| Canthaxanthin (Pelah et al., 2004) | ||||||
| Haemagglutinin (Chu et al., 2004) | ||||||
| Chlorococcum | sp | D (Boichenko and Hoffmann, 1994) | BL, BP, FZ, MR, BS BP (Lavens and Sorgeloos, 1996) | Astaxanthine (Leya et al., 2009, Liu and Lee, 2000, Yuan et al., 2002) | ||
| Adonixanthin (Yuan et al., 2002) | ||||||
| Canthaxanthin (Yuan et al., 2002) | ||||||
| Lutein (Leya et al., 2009) | ||||||
| y-linolenic acid (Ohta et al., 1995) | ||||||
| citriforme | Lutein (Del Campo et al., 2000) | |||||
| humicolum | D (Boichenko and Hoffmann, 1994) | |||||
| littorale | D (Ueno et al., 1999) | Ethanol (Ueno et al., 1998) | ||||
| submarinum | D (Kamp et al., 2008) | D (Kamp et al., 2008) | Glycerol (Blackwell and Gilmour, 1991) | |||
| vacuolatum | D (Kessler, 1974) | |||||
| Chlorogonium | elongatum | D (Kreuzberg, 1984) | ||||
| Chloromonas | nivalis | Lutein (Leya et al., 2009) | ||||
| Zeaxanthin (Leya et al., 2009) | ||||||
| Chlorosarcinopsis | ereme | D (Boichenko and Hoffmann, 1994) | ||||
| Chodatella | balatonica | D (Boichenko and Hoffmann, 1994) | ||||
| Coelastrella | striolata | Canthaxanthin (Abe et al., 2007) | ||||
| Astaxanthin (Abe et al,. 2007) | ||||||
| β-carotene (Abe et al., 2007) | ||||||
| Coelastrum | microporum | FS (Opuszynski, 1981) | ||||
| proboscideum | D (Bishop et al., 1977) | Lutein (Del Campo et al., 2000) | ||||
| Coccomyxa | lacustris | D (Boichenko and Hoffmann, 1994) | ||||
| Cosmarium | botrytis | D (Brand et al., 1989) | ||||
| turpinii | D (Brand et al., 1989) | |||||
| Desmococcus | olivaceus | Extract w antimicrobial and anticancer activity (Ördög et al., 2004) | ||||
| Desmodesmus | spp. | D (Timmins et al., 2009a) | D (Timmins et al., 2009a) | |||
| subspicatus | D (Skjånes et al., 2008) | D (Skjånes et al., 2008) | ||||
| quadricauda | D (Healey, 1970) (Scenedesmus) | |||||
| Dictyococcus | pseudovarians | D (Boichenko and Hoffmann, 1994) | ||||
| Dunaliella | sp. | NP (Finney et al., 1984, Pulz and Gross, 2004, Yamaguchi, 1997) | BP, BS, MR, PL (Apt and Behrens, 1999, D'Souza and Loneragan, 1999, Lavens and Sorgeloos, 1996, Pulz and Gross, 2004) | Glutathione (Li et al., 2004) | ||
| Glycerol (Kaçka and Dönmez, 2008) | ||||||
| bardawil | NP (Mokady et al., 1989) | FS (Shpigel et al., 2006) | β-carotene (Rabbani et al., 1998) | |||
| Extract w anti-tumor effect (Fujii et al., 1993) | ||||||
| Hexadecatetraenoic acid (Fried et al., 1982) | ||||||
| Linoleic acid (Fried et al., 1982) | ||||||
| α-linolenic acid (Fried et al., 1982) | ||||||
| primolecta | y-linolenic acid (Ohta et al., 1995) | |||||
| Pheophoride-like comp w anti-virus activity (Ohta et al., 1995) | ||||||
| salina | NP (Pulz and Gross, 2004, Raja et al., 2008) | NS (Raja et al., 2008) | β-carotene (Coesel et al., 2008, Hejazi and Wijffels, 2003) | |||
| Zeaxanthin (Bhosale and Bernstein, 2005) | ||||||
| Extract w protective effect against fibrosarcoma (Raja et al., 2007) | ||||||
| Extract w antimicrobial activity (Herrero et al., 2006) | ||||||
| Glycerol (Hadi et al., 2008) | ||||||
| α-linolenic acid (Ben-Amotz et al., 1985) | ||||||
| tertiolecta | BL, FS (Carballo-Cárdenas et al., 2003) | Lutein (Barbosa et al., 2005) | ||||
| β-carotene (Barbosa et al., 2005) | ||||||
| Vitamin C (Barbosa et al., 2005) | ||||||
| Vitamin E (Carballo-Cárdenas et al., 2003) | ||||||
| Extract w central nervous system effect (Villar et al., 1992) | ||||||
| Hexadecatetraenoic acid (D'Souza and Loneragan, 1999, Gouveia and Oliveira, 2009) | ||||||
| α-linolenic acid (D'Souza and Loneragan, 1999, Gouveia and Oliveira, 2009) | ||||||
| Linoleic acid (Gouveia and Oliveira, 2009) | ||||||
| Eudorina | elegans | D (Brand et al., 1989) | FS (Opuszynski, 1981) | |||
| Golenkinia | sp. | D (Boichenko and Hoffmann, 1994) | ||||
| Gonium | sociale | D (Brand et al., 1989) | ||||
| Haematococcus | spp. | D (Boichenko and Hoffmann, 1994) | NP (Lorenz et al., 2000, Raja et al., 2008) | NS (Lorenz et al., 2000, Pulz and Gross, 2004) | Astaxanthin (Ceron et al., 2007, He et al., 2007, Jin et al., 2006, Kang et al., 2005, Lorenz et al., 2000) | |
| droebakensis | D (Brand et al., 1989) | |||||
| lacustris | Canthaxanthin (Chattopadhyay et al., 2008) | |||||
| Astaxanthin (Chattopadhyay et al., 2008) | ||||||
| pluvalis | D (Boichenko and Hoffmann, 1994) | NP (Pulz and Gross, 2004, Raja et al., 2008) | FS (Choubert et al., 2006, Raja et al., 2008) | Astaxanthin (Domínguez-Bocanegra et al., 2004, Kang et al., 2005, Zhekisheva et al., 2005) | ||
| Halochlorococcum | sp. | D (Boichenko and Hoffmann, 1994) | ||||
| saccatum | D (Greenbaum et al., 1983) | |||||
| Kirchneriella | lunaris | D (Bishop et al., 1977) | ||||
| obesa | D (Boichenko and Hoffmann, 1994) | |||||
| Lobochlamys | culleus | D (Meuser et al., 2009) | D (Meuser et al., 2009) | |||
| segnis | D (Winkler et al., 2002) | |||||
| Micromonas | sp | BL, BP (Lavens and Sorgeloos, 1996, Raja et al., 2008) | ||||
| pusilla | BL (Martinez-Fernandez and Southgate, 2007, Martinez-Fernandez et al., 2006) | α-Linolenic acid (Martinez-Fernandez et al., 2006) | ||||
| Stearidonic acid (Martinez-Fernandez et al., 2006) | ||||||
| Neoxanthin (Egeland et al., 1995) | ||||||
| Prasonoxanthin (Egeland et al., 1995) | ||||||
| Micromonal (pigment) (Egeland et al., 1995, Latasa et al., 2004) | ||||||
| Monoraphidium | Astaxanthin (Fujii et al., 2008) | |||||
| Muriella | aurantiaca | Lutein (Del Campo et al., 2000) | ||||
| Muriellopsis | sp. | Lutein (Blanco et al., 2007, Del Campo et al., 2000) | ||||
| α-Linolenic acid (Blanco et al., 2007) | ||||||
| Nannochloris | sp. | BP, MR, SC (de Pauw and Persoone, 1988) | Eicosapentaenoic acid (Ben-Amotz et al., 1985) | |||
| atomus | Eicosapentaenoic acid (Reitan et al., 1994) | |||||
| α-Linolenic acid (Reitan et al., 1994) | ||||||
| Arachidonic acid (Reitan et al., 1994) | ||||||
| Linoleic acid (Reitan et al., 1994) | ||||||
| oculata | MR, BS (Duerr et al., 1998) | |||||
| Neochloris | minuta | D (Boichenko and Hoffmann, 1994) | ||||
| oleaobundans | BP (Barnhart, 2006, Gatenby et al., 2003) | Linoleic acid (Gatenby et al., 2003) | ||||
| Linolenic acid (Gatenby et al., 2003) | ||||||
| Neospongiococcum | spp. | Astaxanthin (Borowitzka, 1988) | ||||
| Canthaxanthin (Borowitzka, 1988) | ||||||
| gelatinosum | Lutein (Del Campo et al., 2000) | |||||
| Oocystis | sp. | D (Skjånes et al., 2008) | BL, BS (Myrand and de la Noüe, 1983) | α-Linolenic acid (Patil et al., 2007) | ||
| Linoleic acid (Patil et al., 2007) | ||||||
| elliptica | FS (Opuszynski, 1981) | |||||
| parva | D (Boichenko and Hoffmann, 1994) | |||||
| Pandorina | morum | D (Brand et al., 1989) | FS (Elliott et al., 2008, Opuszynski, 1981) | |||
| Parachlorella | kessleri | D (Kessler, 1974) | ||||
| (Chlorella) | ||||||
| Parietochloris | incisa | Arachidonic acid (Zhang et al., 2002a) | ||||
| Pediastrum | boryanum | D (Boichenko and Hoffmann, 1994) | FS (Opuszynski, 1981) | |||
| duplex | FS (Opuszynski, 1981) | |||||
| Platymonas | striata | Lutein (Egeland et al., 1997) | ||||
| subcordiformis | D (Guan et al., 2004) | D (Guan et al., 2004) | PL, BL, BP, AL, BS, MR (Lavens and Sorgeloos, 1996, Tang et al., 2006) | Lutein (Egeland et al., 1997) | ||
| (Tetraselmis) | Violaxanthin (Egeland et al., 1997) | |||||
| tetrahele | Lutein (Egeland et al., 1997) | |||||
| Neoxanthin (Egeland et al., 1997) | ||||||
| Prototheca | moriformis | Vitamin C (Running et al., 2002) | ||||
| Pseudokirchneriella | subcapitata | D (Skjånes et al., 2008) | BS, MR (Belgis and Guido, 2003) | α-Linolenic acid (Patil et al., 2007) | ||
| Linoleic acid (Patil et al., 2007) | ||||||
| Pseudospongiococcum | protococcoides | D (Boichenko and Hoffmann, 1994) | ||||
| Pyramimonas | sp | BL, BP (Lavens and Sorgeloos, 1996, Raja et al., 2008) | Docosahexaenoic acid (Tzovenis et al., 2009) | |||
| Lutein (Egeland et al., 1997) | ||||||
| amylifera | β-carotene (Egeland et al., 1997) | |||||
| Siphonaxanthin (Egeland et al., 1997) | ||||||
| cf. cordata | Sterols (Ponomarenko et al., 2004) | |||||
| obovata | β-carotene (Egeland et al., 1997) | |||||
| urceolata | Lutein (Egeland et al., 1997) | |||||
| Scenedesmus | sp | FS, FZ, MR, BR (de Pauw and Persoone, 1988, Pulz and Gross, 2004, Turker et al., 2003) | Extract w antimicrobial and anticancer activity (Shon et al., 2004, Ördög et al., 2004) | |||
| Mycosporine-like amino acids (Xiong et al., 1999) | ||||||
| acutiformis | D (Soeder and Hegewald, 1988) | |||||
| acutus | NP (Kim et al., 2007) | FS (Kim et al., 2007) | α-linolenic acid (Ahlgren et al., 1992) | |||
| Vitamin B (Borowitzka, 1988) | ||||||
| Vitamin C (Borowitzka, 1988) | ||||||
| Vitamin E (Borowitzka, 1988) | ||||||
| almeriensis | Lutein (Ceron et al., 2008, Sánchez et al., 2008) | |||||
| armatus | D (Soeder and Hegewald, 1988) | Lutein (Tukaj et al., 2003) | ||||
| bicellularis | D (Soeder and Hegewald, 1988) | |||||
| communis | D (Soeder and Hegewald, 1988) | |||||
| komarekii | D (Soeder and Hegewald, 1988) | |||||
| obliquus | D (Gaffron and Rubin, 1942) | Astaxanthin (Qin et al., 2008) | ||||
| Vitamin B (Borowitzka, 1988) | ||||||
| Vitamin C (Borowitzka, 1988) | ||||||
| Vitamin E (Borowitzka, 1988) | ||||||
| Hexadecatetraenoic acid (Becker, 2004b) | ||||||
| α-Linolenic acid (Becker, 2004b) | ||||||
| Linoleic acid (Gouveia and Oliveira, 2009) | ||||||
| quadricauda | D (Healey, 1970) | NP (Kim et al., 2007) | FS (Kim et al., 2007, Opuszynski, 1981) | Haemagglutinin (Chu et al., 2004) | ||
| (Desmodesmus) | α-linolenic acid (Ahlgren et al., 1992) | |||||
| Sulfonated polysaccharide (Mohamed, 2008) | ||||||
| Vitamin B (Becker, 2004b) | ||||||
| Vitamin C (Becker, 2004b) | ||||||
| Vitamin E (Becker, 2004b) | ||||||
| Extract w antimicrobial/ antifungal activity (Abedin and Taha , 2008) | ||||||
| spinosus | NP (Kim et al., 2007) | FS (Kim et al., 2007) | ||||
| Scotiella | chlorelloidea | Mycosporine-like amino acids (Xiong et al., 1999) | ||||
| nivalis | D (Boichenko and Hoffmann, 1994) | |||||
| Selenastrum | sp. | D (Boichenko and Hoffmann, 1994) | ||||
| capricornutum | Haemagglutinin (Chu et al., 2004) | |||||
| gracile | D (Kessler, 1974) | |||||
| Stichococcus | sp. | Mycosporine-like amino acids (Karsten et al., 2007) | ||||
| Tetraedron | sp. | FS (Turker et al., 2003) | ||||
| bitridens | D (Brand et al., 1989) | |||||
| caudatum | D (Boichenko and Hoffmann, 1994) | |||||
| Tetracystis | intermedium | Lutein (Del Campo et al., 2000) | ||||
| tetrasporum | Lutein (Del Campo et al., 2000) | |||||
| Tetraselmis | sp | PL, BL, BP, AL, BS, MR, SC (D'Souza and Loneragan, 1999, Lavens and Sorgeloos, 1996, Puello-Cruz et al., 2009, Raja et al., 2008, Zmora et al., 2005) | Eicosapentaenoic acid (Patil et al., 2007, Reitan et al., 1994, Tzovenis et al., 2009) | |||
| α-Linolenic acid (Patil et al., 2007, Reitan et al., 1994) | ||||||
| β-carotene (Egeland et al., 1995) | ||||||
| Lutein (Egeland et al., 1995) | ||||||
| Violoaxanthin (Egeland et al., 1995) | ||||||
| Neoxanthin (Egeland et al., 1995) | ||||||
| chui | FS, BS, SC, MR, PL (Cunha et al., 2008, Duerr et al., 1998) | |||||
| kochinensis | D (Bhosale et al., 2009) | |||||
| subcordiformis | D (Guan et al., 2004) | D (Guan et al., 2004) | BL, BP (Duerr et al., 1998) | |||
| suecica | BL, FS, PL, SC, MR, BS (Carballo-Cárdenas et al., 2003, D'Souza and Kelly,. 2000, Dahl et al., 2009, Puello-Cruz et al., 2009, Seixas et al., 2008, Souto et al., 2008) | Vitamin E (Carballo-Cárdenas et al., 2003) | ||||
| Sterols (Cardozo et al., 2007) | ||||||
| Linoleic acid (D'Souza and Loneragan, 1999) | ||||||
| α-Linolenic acid (D'Souza and Loneragan, 1999) | ||||||
| Stearidonic acid (D'Souza and Loneragan, 1999) | ||||||
| Eicosapentaenoic acid (D'Souza and Loneragan, 1999) | ||||||
| Hexadecatetraenoic acid (D'Souza and Loneragan, 1999) | ||||||
| tetrahele | FS, BS, SC, MR (Duerr et al., 1998) | |||||
| wettsteinii | β -carotene (Egeland et al., 1995) | |||||
| Lutein (Egeland et al., 1995) | ||||||
| Tetraspora | sp. | D (Maneeruttanarungroj et al., 2010) | D (Maneeruttanarungroj et al., 2010) | |||
| cylindrica | Hexadecaedienoic acid (Ghazala et al., 2004) | |||||
| Heptadecaedienoic acid (Ghazala et al., 2004) | ||||||
| Trimethyl dodecatrienoic acid (Ghazala et al., 2004) | ||||||
| Extracts w antifungal, antibacterial and phytotoxic activity (Ghazala et al., 2004) | ||||||
| gelatinosa | Trimethyl dodecatrienoic acid (Ghazala et al., 2004) | |||||
| Heptadecatrienoic acid (Ghazala et al., 2004) | ||||||
| Pentadecatetraenoate (Ghazala et al., 2004) | ||||||
| Extracts w phytotoxic activity (Ghazala et al., 2004) |
The metabolites are produced under different culturing conditions, see text.
D, detected; NP, human consumption for nutritional purposes; HF, hen feed; PL, penaeid shrimp larvae feed; BL, bivalve mollusc larvae feed; ML, freshwater prawn larvae feed; BP, bivalve mollusc postlarvae feed; AL, abalone larvae feed; MR, marine rotifers feed; BS, brine shrimp feed; SC, saltwater copepods feed; FZ, freshwater zooplankton feed; FS, fish feed; NS, organisms not specified.
Some of the species might have changed names since the cited work was published. This has not been taken into account in this study, although in some cases synonyms are presented in parentheses below species names.
Hydrogen production and hydrogen production during sulfur deprivation, refers to species where hydrogen production has been detected, or where expression of hydrogenase enzyme has been seen.
Polyunsaturated fatty acids (PUFA): Hexadecatetraenoic acid 16:4 (ω-3), α-linolenic acid (ALA) 18:3 (ω-3), Stearidonic acid (STD) 18:4 (ω-3), Eicosapentaenoic acid (EPA) 20:5 (ω-3), Eicosatrienoic acid (ETA) 20:3 (ω-3), Docosahexaenoic acid (DHA) 22:6 (ω-3), γ-linolenic acid (GLA) 18:3 (ω-6), Linoleic acid (LA) 18:2 (ω-6), Arachidonic acid (AA) 20:4(ω-6), Trimethyl-dodecatrienoate (15:3), Hexadecaedienoic acid (16:2), Heptadecaedienoic acid (17:2), Heptadecatrienoic acid (17:3), Pentadecatetraenoic acid (15:4).
Valuable metabolites listed in the table include components that are either produced in high amounts under stress conditions, or components that are produced under environmental conditions optimal for growth. In the case of carotenoids and fatty acids, the listed variants of these are in some cases produced in high amounts by the specific algae compared to the production by other algae species, in other cases represent dominant variants within the strain. See cited papers for details in each case.
Some basic reactions of Chlamydomonas to major nutrient limitation are reviewed by Grossman, (2000). Nutrient limitation, in combination with other forms of stress factors, often causes a synergistic effect where the cells need to apply more efficient stress reactions and adaptation strategies in order to survive the stressful conditions. Exposure to a combination of light stress and nutrient limitation has, for example, been studied extensively (Antal et al., 2007; Demeter et al., 1995; Domínguez-Bocanegra et al., 2004; Garcia-Malea et al., 2005; Nield et al., 2004; Solovchenko et al., 2008).
The effects of sulfur limitation in green algae have recently been studied more specifically. One of the reasons for this is that sulfur limitation also can lead to photoproduction of hydrogen in green algae, a mechanism that has been studied intensively (Ghirardi et al., 2009; Melis, 2007). This process is described in detail in the Section “Hydrogen production”. While some species have shown the ability to produce significant amounts of hydrogen during S-deprivation (see Table 1), other species have been explored and found not to have this ability, like for example Dunaliella salina (Cao et al., 2001).
Nutrient limitation can cause a number of different stress reactions, leading to production of for example high amounts of secondary carotenoids and PUFA. There are indications that also products with haemagglutinating activity are induced by nutrient deprivation. The oxidative stress caused by nutrient limitation is likely to induce production of antioxidants of different kinds, one example being induction of Vitamin E production during N-limitation (Durmaz, 2007). In general, production of carotenoids as antioxidants during nutrient deprivation is extensively studied in green algae (Del Campo et al., 2007; Jin et al., 2006).
Hydrogen production
A large number of algae species have shown the ability to produce hydrogen gas, some of which are listed in Table 1, although the majority of research on hydrogen production from green algae has been performed with the model organism, Chlamydomonas reinhardtii. The ability of algae to produce hydrogen from solar energy has been explored for many years, and different methods have been developed and evaluated.
A prerequisite for hydrogen production from algae has been to create an anaerobic environment. This can allow the highly oxygen sensitive hydrogen producing enzyme FeFe-hydrogenase to be induced and remain active throughout the anaerobic phase, as discussed below.
The perhaps simplest method for inducing hydrogen production in green algae is to create anaerobic conditions by physically or chemically removing oxygen from the culture medium. This can be done for example by adding a sodium dithionite solution, which will reduce the oxygen and thereby create a chemically induced anaerobic culture condition. Another approach is to physically remove the oxygen by aerating the culture with an inert gas like N2 or Ar. These methods can also be combined (Wünschiers and Lindblad, 2002), and are suitable for screening species of algae for the ability to produce hydrogen. A quick and simple method like this for inducing hydrogen production can be applied prior to more elaborate experiments, which are performed with species that are new or unexplored in respect to hydrogen production.
Alternatively, hydrogen production can be induced by creating an anaerobic environment during dark incubation. By incubating the culture in darkness, the photosynthesis and oxygen production will pause and the remaining oxygen in the culture will be used up by the respiration. By cycling the culture between light and dark intervals, algal biomass can be built up by photosynthesis in the light phase, and consumed by fermentative reactions in the dark phase. This method was found to be efficient in combination with fermentation of organic compounds during light by phototrophic bacteria (Kim et al., 2006b; Miura et al., 1997).
However, during the last decade the most well studied method for hydrogen production from green algae has been hydrogen production in the light during sulfur deprivation. This method, which was discovered in Chlamydomonas reinhardtii by Melis and co-workers (Ghirardi et al., 2000; Melis et al., 2000), takes advantage of stress reactions that are implemented by the algae in order to survive an environment without sulfur. When some green algae are deprived of sulfur, they will enter into a state where release of energy in the form of hydrogen is part of a survival mechanism in order to survive this form of environmental stress.
As described in the Section “Nutrient limitation”, algae that are deprived of a major nutrient will start several emergency reactions, including enhanced uptake systems of the nutrient from the environment, and breakdown of intracellular compounds which are no longer needed, but which contain the nutrient. When major nutrients are missing, the algae can no longer multiply, and production of structural components, like certain proteins, lipids and carbohydrates, will stop. During sulfur deprivation, there will be a specific degradation of RuBisCO, which contains a high amount of sulfur, resulting in an inactivation of the Calvin cycle (Zhang et al., 2002b). The RuBisCO pool of the Chlamydomonas chloroplast has been estimated to contain the equivalent of 50mM sulfur (Grossman, 2000). A few days after the algae have been deprived of sulfur, this enzyme is degraded more rapidly than the total protein content (Zhang et al., 2002b). However, during the first 1-3 days of sulfur deprivation, there may be a specific increase in the cellular contents of starch (Hemschemeier et al., 2008). At the same time, there will be a gradual inactivation of the PSII reaction center, caused by several factors. A sulfolipid in the thylakoid membrane associated with PSII is specifically degraded during the first hours of sulfur deprivation. This is believed to function as a source of sulfur to maintain the synthesis of essential enzymes, and contributes to a partial inactivation of the PSII complex (Sato et al., 1995; Sugimoto et al., 2007). A light inhibition of PSII occurs as described in the Section “Light intensity”. No similar inhibition is seen in PSI in this situation. The partial inactivation of PSII means that the oxygen production in PSII also is partly impaired. When the algae are deprived of sulfur, the oxygen production from PSII is sufficiently low to enable the respiratory oxygen consumption in mitochondria to turn the culture anaerobic (Antal et al., 2003). In this situation, when the Calvin cycle has stopped and can no longer function as a sink for carbon and energy, the system is in a reduced state and the cells will be dependent on releasing electrons from the system in order to prevent oxidative damage.
To release the reductive pressure in this anaerobic environment, some algae are able to produce hydrogenases that catalyze the production of hydrogen by receiving electrons from ferredoxin (Long et al., 2008). There are mainly two possible light dependent routes for electrons to reach hydrogen production: Electrons can either originate from the water splitting complex of PSII (PSII dependent pathway), or from fermentation products from the degradation of starch, which enter into the thylakoid electron transport chain through the PQ pool (PSII independent pathway) (Chochois et al., 2009). It has been shown that hydrogen production during sulfur deprivation is closely linked to starch contents of the cells. Inhibition of starch synthesis has led to a significant decrease in hydrogen production (Posewitz et al., 2004), and increased starch reserves in the cells have had influence on increased hydrogen production (Kruse et al., 2005). Some studies have indicated that the main source of electrons are the PSII water splitting and that starch contributes by increased metabolism (Antal et al., 2009; Chochois et al., 2009), while others indicate that the electrons are likely to originate from a combination of the two pathways (Hemschemeier et al., 2008). Some studies have shown that in the early stages of sulfur deprivation, most of the electrons originate from water splitting through the direct pathway, while this amount decreases significantly during the final stages of sulfur deprivation (Laurinavichene et al., 2004). However, the reactions linked to electron transport from original donor to hydrogen form a very complicated process where there still are many uncertainties. Figure 3 provides a simplified overview of the basic principles of hydrogen production during sulfur deprivation, as it has been explored in Chlamydomonas reinhardtii, see the above mentioned studies for thorough discussions on this issue. The correlation between starch content and hydrogen production efficiency seen in Chlamydomonas reinhardtii could indicate that species with the ability to accumulate large amounts of intracellular starch also might be suitable for an efficient hydrogen production process. Examples of species that can accumulate large amounts of starch during sulfur deprivation are, in addition to Chlamydomonas, Chlorella sp. such as Chlorella sorokiniana. This species is also able to produce significant amounts of hydrogen during the same conditions (Chader et al., 2009; Rigano et al., 2000).
Figure 3. .
Schematic overview of suggested mechanisms for hydrogen production during sulfur deprivation in light, as it has been described for Chlamydomonas reinhardtii. Deprivation from sulfur leads to a degradation of PSII components, which partly inhibits the oxidation of water, and less oxygen is thereby produced in the photosystem. The low level of oxygen that is still produced in PSII is continuously consumed by the respiration, and the culture becomes anaerobic. Sulfur deprivation also leads to degradation of the enzymes in the Calvin cycle, causing this CO2 fixation pathway and energy sink to come to a halt. When the Calvin cycle is no longer available for reducing CO2, the whole system of PSII and PSI is reduced, creating a potentially dangerous situation for the algae. To remove the reductive pressure, the algae dispose of the electrons by transferring electrons from ferredoxin to hydrogenase. This enzyme then uses the reductive energy to form hydrogen which can easily be released from the cell. Depending on culturing conditions and other factors, a certain amount of electrons released in the form of hydrogen may originate from degradation of starch. This reducing power enters the electron transport chain from the PQ pool.
For a practical implementation of this method for producing hydrogen from algae, several different solutions have been proposed and tested. The most common suggestion has been a two-stage process whereby batch cultures are grown aerobically in the first stage, followed by a second stage where the algae are deprived of sulfur and produce hydrogen in an anaerobic environment (Melis et al., 2000). The algae can be collected from the growth phase by for example centrifugation and transferred to a separate reactor for hydrogen production or, alternatively, sulfur can be removed by dilution techniques (Laurinavichene et al., 2002). In such systems, the algae may produce hydrogen for 1–2 weeks, leaving the excess biomass for recycling or use for other purposes. The first stage of the system can take place in an open or closed reactor. The second step must be performed in a strictly anaerobic environment, which not only must be optimized to supply the best physical conditions for the culture, but also must be optimized for collection of the produced hydrogen gas. Different setups for this method have been discussed previously (Fouchard et al., 2008; Hankamer et al., 2007; Melis, 2002).
Prolonged hydrogen production has been obtained in S-deprived cultures by re-addition of sulfur. By re-adding small amounts of sulfur, the algal cells were able to recover from the stress reaction and reactivate PSII temporarily without creating an aerobic environment (Kosourov et al., 2005). When small amounts of sulfur were re-added to sulfur-deprived cultures at regular intervals, the cells were allowed to reconstitute themselves. Re-addition of sulfur five times over a period of one month gave a three to four times increase in total hydrogen production compared to a culture with no re-addition (Kim et al., 2010). The possibilities for establishing continuous systems for hydrogen production have also been explored. By using a two-stage chemostat bioreactor, and continuous dilution of the hydrogen producing culture with fresh cells and small amounts of sulfur, hydrogen production was maintained for a total of five and a half months (Fedorov et al., 2005). Immobilization of algal cells on a solid phase made of glass has also been used to obtain a continuous hydrogen production during 90 days (Laurinavichene et al., 2006). Alginate has also been used as a matrix for immobilization of hydrogen producing cells, and this method has led to increased specific hydrogen production activity and better tolerance against oxygen compared to non-immobilized cultures (Kosourov and Seibert, 2009).
Initial experiments in this field all used cultures grown under photoheterotrophic or photomixotrophic conditions, using acetate as a carbon source for Chlamydomonas reinhardtii. Later studies have shown that algae grown under photoautotrophic conditions are also able to accumulate starch and produce significant amounts of hydrogen (Tolstygina et al., 2009; Tsygankov et al., 2006).
A significant amount of research has been performed to explore culture conditions for hydrogen production from green algae, mainly Chlamydomonas reinhardtii. It is clear that many environmental factors, such as light intensity, temperature, pH, nutrient composition, as well as cell density and cell age can have a significant effect on the efficiency of hydrogen production (Hahn et al., 2004; Jo et al., 2006; Kim et al., 2006a; Kim et al., 2005; Kosourov et al., 2003; Kosourov et al., 2002; Tsygankov et al., 2002). Light intensity was shown to have a considerable effect on the total hydrogen production under both autotrophic and heterotrophic sulfur deprived conditions. Light intensities below optimum gave, under heterotrophic conditions, a longer lag-phase before the culture became anaerobic and thereby a shorter hydrogen production period, in addition to a lower specific hydrogen production rate. Light intensities above optimum gave a shorter lag-time before anaerobiosis, but a lower specific hydrogen production rate (Kim et al., 2006a). During autotrophic conditions, hydrogen production was shown to be highly dependent on light intensities both during pre-growth of the cultures and during the oxygen consumption- and hydrogen production phases. The optimal hydrogen production occurred using low light intensities during pre-growth and higher light intensities during the oxygen consumption stage. This allowed for a temporal degradation of PSII activities and accumulation of a high amount of starch. A lower intensity during the hydrogen production stage allowed for a maintained production. Light intensities above optimal led to an irreversible inactivation of PSII (Tolstygina et al., 2009; Tsygankov et al., 2006). It has also been shown that small changes in pH can have a marked effect on hydrogen production, possibly caused by implications on the degradation of PSII or changes in starch accumulation (Kosourov et al., 2003; Tolstygina et al., 2009).
Possibilities for optimization of the hydrogen production process are explored by many researchers, and there is a significant attention towards gene modification. Gene modification issues are not addressed in this review, other than in general terms. However, some significant possibilities should be mentioned, for example, modifications of the hydrogenase enzyme itself. Studies have been performed to create a more oxygen tolerant system by screening activity of random mutations (Flynn et al., 2002), by using gene shuffling techniques (Nagy et al., 2007), and studies of possibilities for narrowing oxygen channels (Ghirardi et al., 2006). Other approaches include mutations for increased starch accumulation, and state transition block in state 1, leading to an inhibition of cyclic electron transport around PSI, an approach which has led to a five times increase in hydrogen production compared to the wild type of Chlamydomonas reinhardtii (Kruse et al., 2005). Additionally, worth mentioning is the possibility of creating a sulfur deprived environment inside the chloroplast by impairing the sulfate transport systems (Chen et al., 2005; Lindberg and Melis, 2008), and generation of strains with truncated light-harvesting chlorophyll antenna size (Melis, 2009). Further possibilities for an efficient hydrogen production process in the future, using molecular biology and gene modifications, might be within the field of synthetic biology (Picataggio, 2009), see also the Section “Summary and perspectives”.
Even if the majority of research on hydrogen production by sulfur deprivation has been performed on Chlamydomonas reinhardtii, other wild type species of green algae have also been explored in this respect and found to produce hydrogen under these conditions (Chader et al., 2009; Guan et al., 2004; Meuser et al., 2009; Skjånes et al., 2008; Timmins et al., 2009a). These species are listed in Table 1. In many of these cases, no attempts have yet been made to optimize the conditions for hydrogen production from each individual species and, at the moment, there are no strong indications that Chlamydomonas reinhardtii should be the most promising species if a wild type was to be used in such a process.
A total of about 70 species of green microalgae from more than 30 genera, which are reported to have the ability to produce hydrogen, are listed in Table 1. This study aims to explore the possibility of finding species of algae capable of producing hydrogen and other valuable products in the same process.
Analysis of current knowledge, potential products from algae
Table 1 presents, in addition to hydrogen producing species, a selection of species of green microalgae with the ability to produce metabolites of industrial/pharmaceutical interest. The major products from green microalgae with industrial use today include carotenoids and algal biomass for health food and aquaculture. These are at present obtained from a very limited number of species, as described in the Section “Present commercial uses of green microalgae”. However, the table gives an indication of the high number of species that are already known to be able to perform useful processes. The useful properties included in the table are hydrogen production during anaerobic induction, hydrogen production during sulfur deprivation, relatively high production of many different carotenoids, vitamins, other antioxidants, glycerol, PUFA, mycosporine-like amino acids (MAA), haemagglutinin, polysaccharides and other extracts with anticancer, antimicrobial and anti-inflammatory activity, in addition to several other medical activities. The species listed in Table 1 have shown production of the given metabolites during either normal conditions or under environmental stress. However, there are no indications that the ability to produce high amounts of these metabolites is restricted to the species listed in this table. Also included in Table 1 are species in use today for health food and aquaculture/animal feed purposes.
Antioxidants in general
Microalgae are often exposed to high oxygen levels and irradiance stress and, as a result, these organisms have developed defense systems in the form of antioxidants to prevent damage to the cells. Antioxidants are produced by algae in large amounts under certain stressful conditions in order to protect the photosynthetic cells from oxidative stress. Oxidative stress refers to a situation where ROS, such as hydrogen peroxide and oxygen derived free radicals, are produced and can start a chain reaction and cause damage to the cellular systems. ROS are produced when the photosystems absorb more energy than can either be transferred by the electron transport chain to an electron acceptor, or dissipated as heat.
ROS can damage DNA, proteins and lipids in all living organisms; oxidative stress leads to severe health problems in humans and animals. Oxidative damage is linked to aging, atherogenesis, cancer, neurodegenerative diseases, infant retinopathy, macular degeneration and renal failure, along with other problems (Granot and Kohen, 2004; Guerin et al., 2003; Pham-Huy et al., 2008). Dietary intake of antioxidants from algae has shown the ability to limit or prevent certain health problems, as described below. Studies in plants have showed that transgenic overproduction of antioxidants is in many cases a poor strategy for protection against stressful conditions (Logan et al., 2006), implying that additional mechanisms are required to handle the stress.
Many substances found in algae have antioxidant effects. The major group of antioxidants in algae is carotenoids, but there are also significant amounts of other antioxidants such as Vitamin C, Vitamin E, BHT and others. Antioxidants have been attributed many medical effects, as described in the Sections “Carotenoids” and “Other antioxidants”. Although there is a considerable number of studies concluding that antioxidants have positive health effects in humans and animals, some of these studies have been disputed, to the effect that dietary supplements of Vitamin A, Vitamin E and β-carotene in adults may in fact increase mortality when large doses are used (Bjelakovic et al., 2007; Pham-Huy et al., 2008).
Carotenoids
Carotenoids are lipophilic pigments with isoprenoid structure that occur widely in nature. Many carotenoids show a strong antioxidant effect that is used to protect the organisms against oxidative stress.
All carotenoids directly involved in photosynthesis are called primary carotenoids. Secondary carotenoids, however, are present in the cells as a response to different environmental factors, like exposure to high light intensity, nutrient deprivation, temperature changes, high or low pH, high salinity and oxidative stress. Examples of primary carotenoids are α-carotene, β-carotene, lutein, violaxanthin, zeaxanthin and neoxanthin, while typical secondary carotenoids include astaxanthin, canthaxanthin and echinenone (Leya et al., 2009). Primary carotenoids are typically localized in the thylakoid membrane, while secondary carotenoids are also located in lipid vesicles in the cytosol or the chloroplast stroma. Secondary carotenoids, although produced in the chloroplast, are often transported into the cytoplasm where they react with fatty acids to form carotenoid esters. These hydrophobic molecules accumulate, together with other secondary carotenoids, into extraplastidial lipid globules. For example, overproduction of astaxanthin in Haematococcus leads to accumulation of astaxanthin in oleic acid rich triacylglycerol (TAG) globules in the cytoplasm (Jin et al., 2003; Zhekisheva et al., 2002). However, when β-carotene is overproduced, like for example in Dunaliella bardawil during light stress or under nutrient limitation, this pigment also functions as a secondary carotenoid and is accumulated in TAG droplets inside the chloroplast (Rabbani et al., 1998). The production of β-carotene and TAG are interdependent, suggesting that TAG functions as a carotenoid sink under environmental stress conditions which helps to avoid end-product inhibition of the carotenoid pathway.
Carotenoids can also be divided into carotenes, which are non-polar molecules, and xanthophylls which are more polar. Many of the xanthophylls are primary carotenoids and are bound with chlorophyll to the proteins in the light harvesting complex (LHC) where they function as light harvesting pigments that transfer excitation energy to the chlorophylls. Xanthophylls also have an important role in protection against oxidative damage (Bhosale and Bernstein, 2005).
As mentioned above, carotenoids protect against oxidative stress, and are therefore induced under stressful conditions. Reactive oxygen species trigger up regulation of genes coding for production of carotenoids (Li et al., 2009b). In Chlorella zofingiensis hydroxyl radicals, as ROS formed during stress conditions, lead to increased production of astaxanthin (Ip and Chen, 2005). It has been suggested that not only the astaxanthin molecules themselves, but also their synthesis pathway, can serve as protection mechanisms against oxidative stress in Haematococcus (Hu et al., 2008c).
Production of carotenoids is regulated by interplay of the different environmental factors listed above and described in the Section “Analysis of current knowledge, stress and adaptation mechanisms”. In many cases several stress factors have to be present in order to induce an optimal production of these valuable metabolites. As an example, studies of β-carotene in Dunaliella have shown that nutrient limitation is essential for a high production, but when this condition is in place, the production can be increased by high light intensity and/or high salinity (Coesel et al., 2008). Another example is Chlorella zofingiensis, where the increased production of astaxanthin under nitrogen limitation is dependent on light stress, while the production of canthaxanthin can be increased by nitrogen limitation and salt stress, without high light exposure (Pelah et al., 2004).
As illustrated in Figure 4 and described above, carotenoid production in algae can be increased by stress factors such as high light intensity, nutrient limitation, high salt concentrations and temperature stress.
Figure 4. .
Graphic summary of the most common stress reactions having influence on the synthesis of some important valuable metabolites in green algae.
Applications
Humans and animals are incapable of synthesizing carotenoids, and therefore depend on obtaining them through their diet. Despite of this, carotenoids occur commonly in important roles of different animals, often giving red or orange color in for example salmon, flamingoes and shellfish. In humans, some carotenoids have Vitamin A activity, in addition to general antioxidant functions (Scott and Rodriquez-Amaya, 2000).
The carotenoids lutein and zeaxanthin are necessary for our vision to function normally, due to their roles in the yellow spot of the human retina. The amount of macular pigment correlates with the incidence of age-related macular degeneration, which is the major cause of blindness in the elderly (Krinsky et al., 2003). Lutein is responsible for pigmentation in fish and poultry, and is therefore sold as a feed additive. This pigment is also used as a colorant in cosmetics and food products (E161b) (Jin et al., 2003). High amounts of lutein can be produced by species like Muriellopsis sp. and Scenedesmus almeriensis; the production per cell has been observed to be induced by e.g. high temperature (Del Campo et al., 2000, 2007). However, the total production of lutein in the cultures was in these cases lower due to decreased growth rates. The same tendency has also been seen in respect to pH in Chlamydomonas zofingiensis and Dunaliella salina. The maximum level of lutein in the culture was observed when the measured pH was close to the optimum of growth, while the optimum content of lutein per cell was produced under low and high pH (Jin et al., 2003).
The secondary carotenoid canthaxanthin is used as a food dye (E161g), giving color to egg yolks and chicken skin; dietary supplements to poultry have been shown to be associated with increased vitamin E contents of the liver (Surai et al., 2003). Canthaxanthin is known to have antioxidative, anti-inflammatory and neuroprotective effects (Chan et al., 2009). It is produced in large amounts by algae such as Coelastrella striolata (Abe et al., 2007) and Chlorella zofingiensis (Pelah et al., 2004) under salt stress and N-deprivation.
Astaxanthin is a carotenoid that causes the red color in salmon and shellfish, and these organisms are dependent on obtaining this pigment through their diet. The most well-known algal producer of astaxanthin is Haematococcus pluvialis, which is often used as a dietary supplement in aquaculture. High irradiance stimulates production of astaxanthin (Harker et al., 1996), while the production of lutein can decrease under high light intensity (Del Campo et al., 2000). This difference can be explained by the fact that lutein is a primary carotenoid that is used directly in light harvesting, while astaxanthin, being a secondary carotenoid, is produced to protect the organism from oxidative stress. Major factors determining production of astaxanthin are high light intensity and nutrient deficiency (Del Campo et al., 2007; He et al., 2007).
The most important function of astaxanthin is its role as an antioxidant. Oxidative stress can cause many different health problems in humans and other organisms, but can be prevented by dietary intake of powerful antioxidants like astaxanthin (E161j). Intake of astaxanthin in humans or other mammals has been shown to reduce inflammation and help to fight ulcer caused by Helicobacter pylori (Kamath et al., 2008); affect cholesterol levels in the blood, which could benefit heart health (Olaizola, 2005); protect liver cells from oxidative damage (Kim et al., 2009b); improve immune response by enhancing the production of immunoglobulin and antibodies (Jyonouchi et al., 1995; Park et al., 2010). The effect of astaxanthin on cancer in mammals has been thoroughly studied, and examples of effects are protection against cancer in colon, urinary bladder, mouth, and direct reduction of tumor growth (Jyonouchi et al., 2000; Palozza et al., 2009). Astaxanthin has also been shown to prevent obesity (Ikeuchi et al., 2007), and effect on age-related cognitive function in humans has been indicated (Satoh et al., 2009). Subjective evaluation by patients with back pain and muscle pain concluded that astaxanthin relieved pain as good as, or better than over-the-counter drugs. Health benefits from oral intake of astaxanthin have been summarized in Guerin et al. (2003). Astaxanthin has no provitamin A activity after dietary intake, as opposed to β-carotene (Jyonouchi et al., 1995).
β-carotene is an important food coloring agent (E160), and is produced in large amounts by Dunaliella salina. It is used for human food, aquaculture feed and animal feed, and as addition to cosmetics. β-carotene is also a strong antioxidant, although not as powerful as astaxanthin (Jyonouchi et al., 1995). β-carotene is converted to retinol (Vitamin A) in the body, a vitamin which is essential for humans. Dietary intake of β-carotene has a high number of health effects. Vitamin A is important for sustaining vision and preventing eye diseases, preventing different skin conditions and helping the immune system. Oral intake of β-carotene from Dunaliella can prevent UV-induced erythema in humans (Heinrich et al., 2003). β-carotene has antioxidant activities and has been shown to prevent cancer, heart disease, degenerative disease and arthritis (Dufossé et al., 2005).
β-carotene and other carotenes are not absorbed by the body as efficiently as the oxygenated, more polar xanthophylls. However, β-carotene produced by algae is absorbed better by the body compared to artificial synthesized β-carotene. This is due to the fact that algae produce a combination of cis- and trans-isomers of β-carotene, while mainly the trans-forms are produced synthetically (Yeum and Russell, 2002). Like other antioxidants, many carotenoids are claimed to have anti-cancer activity (Cha et al., 2008; Mignone et al., 2009), as described in the Section “Other antioxidants”.
All wild types of green microalgae are likely to show an increase in secondary carotenoid content under certain stress conditions. Table 1 lists a selection of species that have been specifically studied in this respect and found to produce relatively high amounts of the given pigment.
Other antioxidants
Vitamin E
Many algae have high content of vitamins (Becker, 2004b; Brown et al., 1999; Running et al., 2002). α-/β-Tocopherol/α-Tocothrienol (Vitamin E) are fat soluble phenols with antioxidant activity. They are produced in high amounts by algae such as Dunaliella tertiolecta and Tetraselmis suecica (Carballo-Cárdenas et al., 2003), and the production of vitamin E can be increased by stressful conditions such as N-deprivation (Durmaz, 2007). These vitamin compounds are claimed to have activities against cancer, heart disease, eye disease, Alzheimer’s disease, Parkinson’s disease and other medical activity (Pham-Huy et al., 2008). They also have applications in the food industry, as a preservative and health improving additive (E306), and as photoprotection in skin cream (Alberts et al., 1996). Total phenol content has been measured in Chlamydomonas nivalis and found to be induced by UV-C exposure (Duval et al., 2000).
Vitamin C
Ascorbic acid (Vitamin C) is a water soluble vitamin with antioxidant activity. It is produced in high amounts in algae such as Chlorella sp. and Dunaliella sp. (Barbosa et al., 2005; Running et al., 2002), and its production can be increased by high light exposure (Barbosa et al., 2005). Vitamin C is essential for collagen, carnitine and neurotransmitters biosynthesis. It is used as a food additive (E300) and its health beneficiary effects include activity against cancer, atherosclerosis and as an immunomodulator. This vitamin has antioxidant activity that works synergistically with Vitamin E. However, both of these vitamins have been disputed in regards to safety, for example is a high intake of dietary supplements claimed to increase mortality and risk of cancer (Pham-Huy et al., 2008).
Butylated hydroxytoluene
BHT is a lipophilic antioxidant which can be produced in high amounts in Botryococcus braunii (Babu and Wu, 2008). BHT is used as a food additive (E321), or antioxidant additive in other products, but it is usually obtained synthetically (Capitani et al., 2009).
Glutathione
Glutathione is a non-protein thiol compound with activity as antioxidant, immunity booster and detoxifier; it is one of the most potent anti-virus agents known. Deficiency of glutathione is related to a long range of human diseases (Wu et al., 2004). It is used as a pharmaceutical compound, but has also potential for use in food production and cosmetic industry. The main industrial production today comes from yeast, but Dunaliella sp. has also been seen to produce a high amount of glutathione (Li et al., 2004; Sies, 1999). Although little has been done to determine which conditions one should apply to a culture in order to get an optimal production from algae, its function as an antioxidant indicates that glutathione can be induced by factors producing oxidative stress as explained in the Section “Analysis of current knowledge, stress and adaptation mechanisms”.
Fatty acids and their derivatives
Fatty acids can, for example, occur in the cells as glycolipids and phospholipids forming the cellular membranes, or as storage products in the form of TAG (Hu et al., 2008b). Chloroplast membranes are dominated by glycolipids, while phospholipids and other lipids dominate extra-chloroplast membranes (Sugimoto et al., 2005). Fatty acids are either saturated or unsaturated, defined by the presence of double bonds. TAG serves no structural function and is mostly present in the cell as a storage product for energy and carbon storage. In some cases, TAG may also have a role in protection against oxidative stress (Hu et al., 2008b). For practical use of the biomass and extraction of the lipids, it is important to know how the fatty acids are bound in the cell; this will not be addressed in this review.
Essential fatty acids (EFA) are PUFAs involved in biological processes, but not synthesized in animal cells. These fatty acids are a necessary part of the diet, and lack of this nutrient can cause severe damage to the organism. They are, however, produced by algae and, in some cases, in large amounts (Yongmanitchai and Ward, 1989).
The truly essential fatty acids are linoleic acid (LA) (18:2 omega-6) and α-linolenic acid (ALA) (18:3 omega-3). Both humans and animals are dependent on obtaining these from the diet, and they are used as starting points for building longer chains of fatty acids. Because they are used by many biological processes, the following fatty acids are often also referred to as essential: AA (20:4 omega-6), EPA (20:5 omega-3), DHA (22:6 omega-3) and γ-linolenic acid (GLA) (18:3 omega-6) (Russo, 2009).
Food supplements of omega-3 fatty acids are known to have many beneficiary health effects. They have for example anti-inflammatory, anti-thrombotic, anti-arrhythmic, hypolipidemic and vasodilatory properties. These beneficial effects of omega-3 fatty acids have been shown in the secondary prevention of coronary heart disease, hypertension, type 2 diabetes, and in some patients with renal disease, rheumatoid arthritis, ulcerative colitis, Crohn’s disease, and chronic obstructive pulmonary disease (Simopoulos, 1999). Omega-3 fatty acids have also shown positive effects on infant development, cancer and mental illness such as depression, ADHD and dementia (Riediger et al., 2009).
Many green algae are able to produce a high amount of lipids; an average of 23% (dry cell weight) of lipids has been detected in a selection of green algae, without stress-exposure. During nutrient deprivation, the lipid contents per cell increases in many species of green microalgae, while others react by producing starch (Griffiths and Harrison, 2009). Lipid production in algae is also affected by autotrophic versus mixotrophic growth, where autotrophic growth tends to give higher cellular content of lipids, but lower total production of lipids in the culture (Liang et al., 2009), and less relative amount of PUFA (Poerschmann et al., 2004). Many algae produce predominantly saturated or mono-unsaturated fatty acids (Becker, 2004b), although many will also produce a significant proportion of unsaturated fatty acids that are preferred for nutritional use of the algal biomass. For example will N-deprivation of Parietochloris incisa lead to an increase, not only in the total lipid content, but to a relative increase in the content of AA (Solovchenko et al., 2008).
Lower temperatures tend to promote increasing unsaturation of fatty acids (Hu et al., 2008a; Sushchik et al., 2003), as a way of maintaining fluidity of membranes and thereby maintaining cellular processes. Temperature tends to affect the composition of fatty acids rather than the total amount of lipids in the cell (Hu, 2004). Higher level of saturated acids have been seen at low pH in acidotolerant strains of Chlamydomonas, possibly as an adaptation to avoid too high membrane fluidity (Tatsuzawa et al., 1996). High salt concentration leads to higher ratio of unsaturated fatty acids in microsomes of halotolerant Dunaliella, this is suggested to be linked with adaptation to high intracellular glycerol levels (Azachi et al., 2002). Studies from other algae have shown that high salt concentrations may decrease the proportions of unsaturated fatty acids, as well as a total decrease in TAG content (Ben-Amotz et al., 1985; Chen et al., 2008). Light intensity can also have effect on the proportion of fatty acids. High light intensity can cause production of more saturated and mono-unsaturated fat, while low light intensity may induce formation of PUFA. Algae in stationary phase often produce more TAG, and a lower relative amount of PUFA (Hu et al., 2008b). UV exposure has shown to suppress PUFA synthesis (Goes et al., 1994; Hessen et al., 1997). Metabolism of important lipids has been reviewed by Guschina and Harwood (2006).
Certain PUFAs are of particular interest, and species of green algae known to produce high amounts of these are listed in Table 1. However, many species that are able to produce high amounts of lipids have not been analyzed in detail, and production of specific PUFAs have therefore not been identified in all algae that could turn out to be important producers (Hu et al., 2008b).
Arachidonic acid can, for example, be produced in large amounts by some green algae. The most efficient producer of this lipid is the green algae Parietochloris incisa, where the cell can contain AA up to 20% of the biomass under N-limiting conditions (Khozin-Goldberg et al., 2002). Parietochloris incisa is resistant to low temperatures. AA resides in TAG storage globules in the cell, and can be transported with the TAGs to cell membranes when temperature decreases (Bigogno et al., 2002). Incorporating AA into the cell membrane when temperature decreases is a mechanism for protection against cold stress, as described in the Section “Temperature”. AA-rich TAGs from the microalga Parietochloris incisa have shown medical effects on recovery from infection (Khozin-Goldberg et al., 2006). EPA is being produced in high amounts by for example the marine green alga Chlorella minutissima, and the production of this fatty acid can be increased by reduced temperature and increased salinity (Seto et al., 1984).
In some cases unsaturated fatty acids from algae can have an inhibitory effect on other aquatic microorganisms. For example, a mixture of the fatty acids α-linolenic, oleic and linoleic acid extracted from Botryococcus braunii showed a toxic effect on several aquatic organisms (Chiang et al., 2004). Extracts of the C18 fatty acids linolenic, linoleic and oleic acid from Chlamydomonas reinhardtii had a toxic effect on other organisms, including algae species, by reducing growth rate and increasing mortality (McCracken et al., 1980; Spruell, 1984). Linolenic acids from Chlorococcum and Dunaliella can act as antibiotics against microorganisms (Ohta et al., 1995). There are several mechanisms that may explain these inhibitory effects. Oxidation products from the breakdown of fatty acids like aldehydes, alcohols or others can be the inhibiting factor, or the fatty acids may have a direct regulatory role in the enzymatic activities (Ikawa, 2004). Production of inhibiting substances in general makes it possible for the species to dominate in their environment.
Other lipids include sterols, which are a group of lipids important as nutrition for aquaculture organisms (Gatenby et al., 2003). They are produced in significant amounts in green algae like for example Tetraselmis suecica (Cardozo et al., 2007), Pyramimonas sp. (Ponomarenko et al., 2004) and many other species. They are essential for cell membranes and act in signal transduction. Alkadienes represent a group of lipids produced by for example Botryococcus braunii that can be used for wax in the cosmetics industry (Mendes et al., 2003). Some algae can produce acetylenic lipids that can have antitumor, antibacterial, antimicrobial, antifouling, antifungal, pesticidal, phototoxic, HIV inhibitory, and immuno-suppressive properties (Dembitsky, 2006). However, this group of lipids is not extensively explored in green algae.
As illustrated in Figure 4 and described above, the production of PUFA may be increased by applying stress factors like low temperature, salt stress and nutrient deprivation.
Metabolites with other properties or medical effects
Polysaccharides
Certain polysaccharides from algae have been shown to have activities with medical effects; most of these studies have been performed with Chlorella spp. Chlorella vulgaris and Scenedesmus quadricauda are able to produce polysaccharides that function as protection against oxidative stress and, in one case, exposure to microcystin (Mohamed, 2008). A mixture of polysaccharides extracted from Chlorella stigmatophora showed anti-inflammatory and immunosuppressant effects, however, the structure of the active polysaccharide was not identified (Guzman et al., 2003). A polysaccharide with high molecular weight identified from Chlorella pyrenoidosa showed a very high immunostimulatory and antitumor effect with potential for cancer therapy (Pugh et al., 2001; Yang et al., 2006). Other attempts to identify the active constituents in extracts of polysaccharides in this species have been made, but the exact structure is still unknown (Sheng et al., 2007). The exact function of most of these polysaccharides in the algal cells is not known. The polysaccharide β-1,3-glucan from Chlorella has been found to be an active immunostimulator, a free radical scavenger and a reducer of blood lipids. Other health-promoting effects can be efficacy on gastric ulcers, wounds, and constipation; preventive action against atherosclerosis and hypercholesterolemia; and antitumor action (Iwamoto, 2004; Spolaore et al., 2006). In these studies no attempts were made to expose the algae to sub-optimal growth conditions, i.e. to find out if stress conditions would induce a higher production of the mentioned active metabolites.
Glycerol
Glycerol is an organic osmolyte that is highly soluble and non-inhibitory of metabolic processes produced as a response to extracellular osmotic pressure. It can function as both osmoregulator and osmoprotector of enzymes. It can be accumulated in large amounts in halotolerant species during salt stress; an increase in intracellular glycerol content linear with salt concentration in the medium has been seen in Chlamydomonas pulsatilla (Ahmad and Hellebust, 1986). Halotolerant Dunaliella species can accumulate up to 17% w/w intracellular glycerol (Kaçka and Dönmez, 2008). Freshwater species like Chlamydomonas reinhardtii can also produce high amounts of glycerol as a response to osmotic stress, but in this case the glycerol is excreted into the medium rather than being accumulated (León and Galván, 1994). The production of glycerol in algae is regulated by external water activity, but high light intensities may inhibit the production (León and Galván, 1999). In some cases, the algae can also excrete glycerol as a response to high CO2 rather than salt stress, as has been observed in a marine Chlamydomonas sp. strain (Miyasaka et al., 1998). Glycerol is widely used in industries such as cosmetics, pharmaceuticals, paint, automotive, food (E422), tobacco, pulp and paper, leather, and textile, or as a feedstock for the production of various chemicals (Wang et al., 2001). In addition to being produced in high amounts during osmotic stress, an increase in glycerol content has also been showed in Chlamydomonas reinhardtii during sulfur deprivation (Bölling and Fiehn, 2005), although this was only measured after 24 h of deprivation.
Lectins
Lectins are carbohydrate binding proteins located within protein bodies in the cells. Lectins from algae have high specificity for complex oligosaccharides, glycoprotein or glycolipids. They are useful in medical science for a variety of applications, like detection of disease-related alterations of glycan synthesis, blood group typing and definition of secretor status, quantification of aberrations of cell surface glycan presentation and cell markers for diagnostic purposes including infectious agents (viruses, bacteria, fungi, parasites). Moreover, lectins are useful as bioadhesives that bind to mucosal surfaces and to deliver vaccines across mucosal surfaces (Cardozo et al., 2007). Studies of lectins in green microalgae often focus on haemagglutinating activity, and a high number of Chlamydomonas and Chlorella species have been identified with ability to agglutinate erythrocytes. Many species of Chlorella produce metabolites with antimicrobial activity, and this activity has been hypothesized to be caused by lectins (Table 1) (Chu et al., 2004). Studies in other algae have indicated that the production of these metabolites can be induced by growth limiting conditions like nutrient deprivation and light stress (Liao et al., 2003).
Mycosporine-like amino acids
Mycosporine-like amino acids (MAA) are a group of molecules consisting of an amino acid bound to a chromophore that absorbs low wavelength light. A database listing different MAAs identified in various organisms is available (Sinha et al., 2007), out of which ten are green microalgae (Table 1). These amino acids are involved in protecting the organism against UV radiation and are produced in significant amounts by for example the highly UV-tolerant snow algae Chlamydomonas nivalis and other green algal species. Production of MAA is induced by exposure to UV-light and the resulting irradiance stress reactions, but there are indications that N-limitation leads to a decreased production (Karsten et al., 2007; Xiong et al., 1999). MAA from other algae phyla have been shown to protect skin of higher organisms against UV damage (de la Coba et al., 2009). MAAs from algae have been explored for commercial purposes, which have resulted in commercial skin-care products for UV protection (Schmid et al., 2006).
Glycoprotein
A glycoprotein from Chlorella vulgaris shows anticancer activity through antimetastatic immunopotentiation (Hasegawa et al., 2002; Tanaka et al., 1998). Little has been done neither to identify similar compounds with activity from other algal species, nor to consider possibilities for optimization of the production of these glycoproteins by manipulating growth conditions.
Antifreeze proteins
Cold adapted strains of green algae are often producers of antifreeze proteins that prevent damage occurring as a result of very low temperatures. These proteins are able to bind to ice crystals, prevent recrystallization and protect other proteins from damage. Antifreeze proteins extracted from algae or other microorganisms can be used for agricultural, biomedical and industrial applications (Christner, 2010; Fernandes et al., 2010; Kang and Raymond, 2004).
Antibiotics
Some strains of algae are able to produce metabolites with antibiotic activity by killing or inhibiting growth of bacteria. In some cases, this activity has only been identified in general extracts from the algal culture without determining the identity of the active substrate (Chu et al., 2004; Ördög et al., 2004). In other cases, the antibiotic agents have been identified, as described above. There are indications that antibiotics are more likely to occur in strains isolated from environments polluted by bacteria, than in strains isolated from cleaner environments (Lustigman, 1988).
General extracts containing unidentified metabolites with activity
A large number of studies have been performed screening for algal extracts with medical effects. The path from a certain effect is detected in an algal extract, to a pharmaceutical product is sold commercially, is very long and expensive. Even if these screenings have resulted in a high number of extracts with potential pharmaceutical interest, many studies have not concluded with identification of the specific metabolites leading to these effects.
Pharmacological activities measured in Dunaliella tertiolecta include antihypertensive, bronchodilator, antiseritonin, polysynaptic block, analgesic, muscle relaxant and antioedema (Borowitzka, 1995). Dunaliella tertiolecta showed activity as a central nervous system depressant (Villar et al., 1992) and Chlorella stigmatophora extracts showed anti-dopaminergic activity on the central nervous system (Laguna et al., 1993). Out of 174 strains from 24 genera of green microalgae screened, 10 strains from the species Desmococcus olivaeceus, Chlorella minutissima and other Chlorella species, and Scenedesmus sp. showed antimicrobial and anticancer activity (Ördög et al., 2004). Extracts from several species of Chlorella, Chlamydomonas and Scenedesmus showed haemagglutinating effects, and five Chlorella species had antimicrobial activity (Chu et al., 2004). The active metabolites were all produced without exposing the cells to suboptimal conditions, which in some cases may have increased their production.
Whole cells of Chlorella sorokiniana have activity as a sulfoxidation biocatalyst, and can therefore have significance in industrial processes involving flavor and aroma precursors, antibiotics, enzyme inhibitors, metabolites and pharmaceuticals (Daligault et al., 2006). Extracts from a high number of algae have shown inhibitory effects on other algae, and this is often seen in connection with unsaturated fatty acids (Ikawa, 2004).
Present commercial uses of green microalgae
Commercial production of algae is often quite secretive and the identities of the species used are not always revealed. In most cases, the identities of the algae are only known at the genus level. Table 2 shows a list of companies with commercial production of green microalgae for many purposes; health food and aquaculture feed constituting the major parts of the market. The number of species in use today is very limited, and the algae are usually selected for their ability to grow fast and produce high amounts of specific metabolites.
Table 2. .
Companies producing green microalgae for health food/ aquaculture/ animal feed/ pharmaceutical and industrial purposes.
| Algae | Common products | Company | Country |
|---|---|---|---|
| Dunaliella | Biomass for health food, cosmetics or aquaculture feed, produced in the form of powder, paste, capsules or tablets. Extracts of β-carotene as color and antioxidant in food and cosmetics,produced in the form of powder or oil extract. | Aqua Carotene Ltd | Australia |
| Betatene Ltd. | Australia | ||
| Cognis Nutrition and Health | Australia | ||
| Western Biotechnology Ltd. | Australia | ||
| Inner Mongolia Biological Eng. | P.R.China | ||
| Tianjin Lantai Biotechnology | P.R.China | ||
| Cyanotech Corp. | Hawaii, USA | ||
| ABL Biotechnologies Ltd. | India | ||
| Parry Nutraceuticals | India | ||
| Proalgen Biotech | India | ||
| Nature Beta Technologies Ltd. | Israel | ||
| Seambiotic | Israel | ||
| Nikken Sohonsha Corporation | Japan | ||
| Dutch State Mines | The Netherlands | ||
| Easy Algae | Spain | ||
| Haematococcus | Biomass for health food, cosmetics or aquaculture feed, produced in the form of powder, paste, capsules or tablets. Extracts of astaxanthin for color and antioxidants in food and cosmetics,produced in the form of powder or oil extract. | WefirstBiotechnologyCo.,Ltd | P.R. China |
| Blue BioTech Int. | Germany | ||
| Cyanotech Corp. | Hawaii, USA | ||
| Mera Pharmaceuticals | Hawaii, USA | ||
| Parry Nutraceuticals | India | ||
| AlgaTechnologies | Israel | ||
| Dutch State Mines | The Netherlands | ||
| Bioreal Inc. | Sweden, USA, Japan | ||
| Chlorella | Biomass for health food, cosmetics or animal feed, produced in the form of powder, paste, capsules or tablets. | Ocean Nutrition | Canada |
| Bioprodukte Prof. Steinberg Produktions- undVertriebsGmbH& Co KG | Germany | ||
| Blue BioTech Int. | Germany | ||
| Nikken Sohonsha Corporation | Japan | ||
| Sun Chlorella Corp. | Japan | ||
| YaeyamaShokusanCO.Ltd. | Japan | ||
| Taiwan Chlorella Manufacturing and Co. | Taiwan | ||
| Tetraselmis | Biomass for aquaculture feed, produced in the form of powder, paste or tablets. | Astaxa GmbH | Germany |
| Seambiotic | Israel | ||
| EasyAlgae | Spain | ||
| Reed Mariculture | USA | ||
| Nannochloris | Biomass for aquaculture feed. | Seambiotic | Israel |
| Chlorococcum | Biomass for aquaculture feed. | Seambiotic | Israel |
See text for details regarding use of algae products.
Production efficiency of biomass is the key-factor for financial success in most commercial systems today. Moreover, to be used in aquaculture, microalgal species must also be selected on the basis of their mass-culture potential, nutritive constituents, non-toxicity, sanitary state as a diet, size and shape of the cells and digestibility (Becker, 2004a; Brown et al., 1999; de la Noue and de Pauw, 1988; Tzovenis et al., 2003). It should be noted that production of high amounts of secondary metabolites under stress conditions, is associated with decreased growth rates and thereby decreased production of total biomass. Although the selected stress conditions may induce a high production of the targeted metabolite on a per cell basis, the success of a commercial process depends on the total productivity of the system. Growth rates will vary greatly between species, and are highly dependent on cultivation methods, especially bioreactor design, and physical and chemical parameters. The simplest way of growing microalgae commercially is considered to be cultivation in open ponds, and this system is commonly applied in commercial algal production. In such systems, species of algae that are tolerant to extreme conditions have been preferred, due to the decreased risk of contamination from competing organisms. The application of closed bioreactors in microalgal cultivation leads to reduced risk of contamination, which has opened up for using a wider choice of species. Extensive research is ongoing within the field of bioreactors, culturing systems and production efficiencies, and has previously been reviewed (Barbosa et al., 2003; Carvalho et al., 2006; Dasgupta et al., 2010; Eriksen, 2008; Posten, 2009; Pulz, 2001; Richmond, 2003; Tsygankov, 2001; Xu et al., 2009). The commercial production of green microalgae today is mainly focused on species from four genera: Chlorella, Dunaliella, Haematococcus and Tetraselmis, which all are cultivated essentially for health food industry and aquaculture production.
Health food
Dunaliella spp., dominated by Dunaliella salina, are widely used for production of the antioxidant and coloring agent β-carotene. These algae are known to produce about 10% β-carotene of algal dry weight under stress conditions (Lamers et al., 2008). A high number of companies produce Dunaliella for commercial products (Table 2). The most common products include extracted β-carotene, dried algal powder for human use and dried algal powder for animal feed. Dunaliella powder for human use can be sold directly as a health supplement due to its high content of proteins and antioxidants (Becker, 2007), and can also be sold as a protein source for food production (Finney et al., 1984). Most extracted β-carotene is sold in vegetable oil for coloring various food products and for cosmetics (Dufossé et al., 2005). β-carotene is added to cosmetics and food products like margarine, cheese, fruit juices, baked goods, dairy products, canned goods, confectionary and health condiments as a colorant and antioxidant, and as a precursor to Vitamin A (Dufossé et al., 2005). Extracts from Dunaliella salina is claimed to stimulate skin cell growth and proliferation (Kim et al., 2008). β-carotene extracted from Dunaliella is also added to pet food for the same reasons, and the addition of this pigment to animal fodder can improve the color of chicken skin, egg yolks, salmon flesh and shellfish. β-carotene produced from algae is considered to be more valuable than synthetic versions since the combination of cis- and trans-isomers leads to higher bioavailability and bioefficiency (Yeum and Russell, 2002).
As with Dunaliella, Haematococcus spp., dominated by Haematococcus pluvialis, are considered to be a rich source of proteins, vitamins and other nutrients, in addition to high amounts of the antioxidant and coloring agent astaxanthin (Dufossé et al., 2005). Haematococcus biomass can be more expensive to produce than Dunaliella because growth requirements cannot exclude contaminating organisms; closed bioreactor systems are therefore advantageous. However, the high value of Haematococcus biomass makes this production highly successful commercially (Table 2). These algae can grow and produce high contents of astaxanthin under both autotrophic and heterotrophic conditions. The astaxanthin production is usually a two-stage process where biomass is firstly produced under optimal growth conditions. In the second stage, the algae are exposed to sub-optimal conditions that lead to a cell resting stage and formation of haematocysts where the cells produce high amounts of astaxanthin. These sub-optimal conditions include nitrate and phosphate deprivations, increased temperature and light intensity, or osmotic stress caused by addition of NaCl (Lorenz and Cysewski, 2000). A concentration of 7.7% astaxanthin has been reached at lab scale (Kang et al., 2005). Products from Haematococcus include extracted astaxanthin, dried algal powder for human use and dried algal powder for animal feed, these products have been available to consumers for about 10 years (Guerin et al., 2003). Astaxanthin has many medical effects in both human and animals, as described in the Section “Carotenoids”, and its antioxidant activity is 10 times higher than β-carotene and 500 times higher than α-tocopherol (Dufossé et al., 2005).
Chlorella spp., dominated by Chlorella vulgaris, is widely used as human health food due to its high nutritional value (Pulz and Gross, 2004), and the majority of Chlorella biomass for human consumption has been sold as tablets or capsules. Although whole cells of Chlorella and Haematococcus have been added to food as a coloring agent and antioxidant (Gouveia et al., 2006), the market for direct addition of microalgal biomass to food for human consumption has been limited due to its distinct flavor. In order to maximize the nutritional value of the product, the Chlorella cell wall is mechanically broken in the production process (Janczyk et al., 2007). In addition to having a very high protein content (Becker, 2007), different Chlorella species are able to accumulate high concentrations of carotenoids like lutein, astaxanthin and canthaxanthin. They are also able to produce PUFA, polysaccharides and extracts with different medical activities (Table 1). Oral intake of Chlorella biomass as a food supplement can lead to medical effects such as reduction of maternal transfer of dioxins (Nakano et al., 2005). Extracts from Chlorella have been used for commercial cosmetic products, and skin cream containing Chlorella extracts have been claimed to stimulate collagen synthesis and thus prevent wrinkles (Kim et al., 2008). Regarding safety, some concerns have been raised regarding intake of nucleic acids, which are present in most food sources. A high intake of purine may in some cases lead to increased plasma uric acid, which again may lead to gout. Studies have shown that for endangered hyper-uremic persons, a daily intake of 20 g algae is considered safe (Becker, 2004b). Despite of this, safety evaluations of green algal biomass as a food additive, cultured under normal conditions without exposure to heavy metals, have concluded that this product is safe for oral intake (Day et al., 2009; Mokady et al., 1989).
Aquaculture
The main applications of microalgae in aquaculture are, directly or indirectly, related to nutrition of various species of aquatic farmed animals. They are frequently used as food source for marine herbivores and in the first feeding process of some carnivorous larvae (Lavens and Sorgeloos, 1996; Reitan et al., 1997). Microalgae are used as food source for producing live preys such as rotifers, copepods and brine shrimp, which serve in turn as food for early stages of carnivorous cephalopods, crustaceans and fish (Dahl et al., 2009; Seixas et al., 2008; Souto et al., 2008; Watanabe et al., 1983). Microalgae are consumed mostly as whole cells, as a basic diet component or as a food additive to supply basic nutrients (Albentosa et al., 1997). They can be valuable fresh or dried in the green water technique or as color additive. “Green water” refers to the feeding technique whereby algae are added to the organisms’ environment as a suspension (Neori, 2011). Use of algae in the first-feeding process of aquatic animals may enhance the rearing success, including survival, growth and larvae quality (Conceicão et al., 2010; Lavens and Sorgeloos,, 1996; Reitan et al., 1997). The most important algal species used as nutrient source in aquaculture production worldwide belong to the following classes: Bacillariophyceae, Chlorophyceae, Cyanophyceae, Eustigmatphyceae, Prasinophyceae and Prymnesiophyceae. Out of the algal species that are most commonly used as feed in commercial aquaculture today, only Chlorella spp. and Tetraselmis spp. belong to the green algae group.
In fish, a lack of PUFAs at various stages affects negatively the eye development, pigmentation, vertebrae development, fecundity, spawning, egg quality and larval hatching rates (Bell et al., 2003; Lall and Lewis-McCrea, 2007; Rainuzzo et al., 1997). PUFAs, such as AA, EPA and DHA as described in the Section “Fatty acids and their derivatives”, are essential for the growth and survival of marine fish larvae (Sargent et al., 1999). Since they have a very limited ability to biosynthesize PUFA de novo, their PUFAs are derived from zooplankton that consume algae (Yongmanitchai and Ward, 1989). In rotifers production, Tetraselmis sp. increases the DHA and EPA contents of the rotifers even with a short term enrichment period. However, to observe positive effects on growth and survival of fish larvae using rotifers with short term enrichment in microalgae, microalgae need to also be added as green water (Reitan et al., 1997).
Several species of green microalgae have been reported to accumulate canthaxanthin, asthaxanthin, lutein, β-carotene and other carotenoids (Table 1). The aquaculture fodder industry (especially pigmentation of salmonids) is the largest market for use of extracts from Dunaliella and Haematococcus. Other species of green algae can also be potential sources of carotenoids, such as lutein from Muriellopsis (Del Campo et al., 2000). Carotenoid pigments fulfill very important roles in the growth of fish larvae. An astaxanthin- canthaxanthin- and β-carotene supplemented diet enhances pigmentation of organisms such as domesticated shrimp, red sea bream, salmon, trout, sea urchin, lobster and ornamental fish (Baker et al., 2002; Barclay et al., 2006; Choubert, 2010; Gouveia et al., 2002; Gouveia and Empis, 2003; Shpigel et al., 2006; Tejera et al., 2007). Astaxanthin has also been shown to be essential for growth and survival during the initial feeding period of shrimp, salmon and trout (Lakeh et al., 2010; Lorenz and Cysewski, 2000; Niu et al., 2009).
In aquaculture, vaccines are often delivered orally and the algae have an important potential for producing high quality recombined proteins (León-Bañares et al., 2004; Maliga, 2003). Chlamydomonas reinhardtii is known to have chloroplast features that are suited for developing an oral vaccine delivery system (Surzycki et al., 2009), which can lead to further development of the potential for a microalgal vaccine strategy for immunization of aquatic animals.
Algal biomass produced in large scale represents a potential for high quality substitute for fish-based ingredients in aquaculture feeds. Specifically, algal preparations could be superior alternative sources for EPA, DHA and AA enrichment products in a variety of aquaculture feeds, and there may also be the additional benefit that microalgae may act as immuno-stimulants (Spolaore et al., 2006).
Summary and perspectives
The multidisciplinary process for use of algal technology described in Skjånes et al. (2007), can either be used as a complete process, or parts of the process can be used independently. Based on the current efficiency rates which are obtained with hydrogen production from green algae (Kruse et al., 2005; Timmins et al., 2009b), and recent economic feasibility studies (Stephens et al., 2010), it is likely that hydrogen production from green algae must be combined with subsequent use of the biomass, to achieve a viable, sustainable system.
There are many studies available which report for example efficiencies of algal biomass production, the nutrient composition of algae, algal production of chemicals, and algal tolerance to separate stress factors, as discussed above. What most of these studies have in common is that they either report comparisons of a species under different conditions, or compare several species under fixed conditions. The values reported by these studies are the result of physical conditions used in the lab for those specific experiments, like for example size and form of culture vessel, bubble size of CO2 mixture, and stirring speed. Because of this, comparing any type of efficiency numbers between different studies is very difficult, in many cases impossible, even if basic measurable conditions such as light, temperature and pH are identical. In this review, the only references to efficiencies have been “high” or “low”, referring to comparisons within each paper, or a general overall picture from several papers. Because of this fact, this review does no attempt to suggest which algae are most efficient for the different uses. The purpose here is to discuss which species are likely to be able to perform at several stages in a combined process, based on the currently available information.
A substantial amount of research is currently ongoing within genomic and metabolic engineering of algae for efficient production of different potential algal products, including hydrogen (Beer et al., 2009; Griesbeck et al., 2005; Li and Tsai, 2009a). Considerable progress has been achieved in this field during the last decade, and there is a clear potential for increased exploitation of modified algae, compared to wild types. However, in many countries there is, and may continue to be, a severe resistance against gene modified organisms. In some cases there will be regulations preventing the use of these organisms either in general, or for specific uses, and in other cases there will simply be disapprovement and fear among the potential customers. This will severely limit the market potential, as might be the case in examples like health food for human consumption. It is our opinion that there is, and will continue to be, a need for “natural” products from organisms isolated directly from nature. It is therefore important to find out more about which naturally occurring species are able to perform useful processes, something this study has attempted to clarify.
In many cases, screening for specific metabolites or general extracts having activities with medical application is performed with algae grown under optimal-, or close to optimal conditions. Examples are studies where species of algae are screened for metabolites with antimicrobial, anticancer and haemagglutinating activity (Chou et al., 2008; Chu et al., 2004; Hasegawa et al., 2002; Kim et al., 2009a; Ördög et al., 2004). As described in the Section “Analysis of current knowledge, potential products from algae”, many of these substances may be secondary metabolites that are induced under stress or sub-optimal conditions. There is reason to believe that many species may have the ability to produce a high amount of valuable molecules given the right conditions, and that there still is a great undetected potential in that respect. Many secondary metabolites are produced by applying several stress factors to the algal culture. The synergistic effect of several stress factors can be necessary to get an optimal production of these chemicals, indicating that this could also be the case for other algal products.
Hydrogen production represents a reaction to stress factors like for example light and nutrient deprivation, where the cell needs to dispose of excess energy absorbed by the photosystems. A practical system for producing significant amounts of metabolites from algae usually consists of a two stage process, where the algae firstly are grown under optimal conditions to produce a dense culture. In the second stage, stress conditions are applied that induce production of the interesting metabolites (Hu, 2004). As pointed out above a practical hydrogen production process from green algae is often described as a two stage process as well (Melis and Happe, 2001). However, it should be noted that hydrogen production by algae during sulfur deprivation is at present a very sensitive process where small changes in physical factors such as light intensity, pH and nutrient composition can cause major effects on the hydrogen production efficiency (Kim et al., 2006a; Kosourov et al., 2003). Careful studies must be performed in order to obtain a production of valuable metabolites that does not compromise hydrogen production rates. The current knowledge of hydrogen production from green algae by sulfur deprivation suggests that the hydrogen production is strongly dependent on the algae’s ability to accumulate starch (Kruse et al., 2005; Posewitz et al., 2004). The studies of a combined process may therefore explore a potential combination of biosynthesis of starch and valuable metabolites during the initial step of sulfur deprivation. Alternatively, the targeted algae may produce the metabolites simultaneously with hydrogen or after the hydrogen production has stopped as a protection mechanism. These options are illustrated in Figure 1. All these three possibilities must be explored experimentally.
Figure 4 presents a selection of secondary metabolites produced by green algae, and the main stress factors known to induce their production. See Table 1 for information on which species have been shown to produce large amounts of the different products, and text above for more details.
Nevertheless, the most important secondary metabolites currently available commercially are carotenoids, mainly astaxanthin from Haematococcus sp. and β-carotene from Dunaliella sp. These pigments are induced by several stress factors like high light intensity, nutrient deprivation and high salt concentration. Their main purpose is to protect the cell from oxidative stress, which is also the main purpose of hydrogen production during sulfur starvation. Carotenoids dissipate energy, but have been shown to be co-produced with TAG containing lipid bodies (Rabbani et al., 1998).
Another important product from algae is PUFA. Algae will accumulate increased amounts of unsaturated fat during stress conditions like low temperature, nutrient deprivation and high salt concentration. These unsaturated fatty acids can accumulate in the membranes in order to maintain their biological functions or, in some cases, in storage globules in the cytoplasm functioning as energy reserves.
Glycerol production in green algae is induced by osmotic stress and, in some cases, also by exposure to high CO2 concentrations or nutrient deprivation. Under osmotic stress, glycerol functions as an osmolyte to maintain a normal salt balance inside the cell. In some cases, there can be a correlation between glycerol production and degradation of starch, indicating that starch is used as an energy source and possibly also carbon source for glycerol synthesis. Assuming this is the case and, considering the fact that hydrogen production during sulfur deprivation also partly uses starch as an energy source, a combination of these two processes may not be optimal. Moreover, the market value of glycerol has dropped significantly the most recent years, due to the fact that this compound is a by-product from biodiesel production (Johnson and Taconi, 2007). Products from breakdown of starch in species of green algae have been previously presented, and the degradation pathways leading to hydrogen and other products were shown to vary greatly between species (Meuser et al., 2009).
As for hydrogen production, nutrient deprivation and high light intensities are stress reactions that have been shown to induce lectins. Production of lectins is highly dependent on their functions in the cells and, as they are a highly diverse group of proteins, it is therefore difficult to make general assumptions for a combined production with hydrogen. Vitamins, other antioxidants and MAA are induced by high light intensity or UV-light as part of a photoprotective response. Several compounds with medical effects have been identified, like for example glycoproteins, glutathione, different polysaccharides and unidentified antibiotics, but in these cases little information is available regarding the conditions leading to their induction.
In cases where certain secondary metabolites are produced in large amounts as a response to oxidative stress, the metabolites may function as energy sinks. In a combined process where the goal is to produce an optimal proportion of hydrogen and valuable products, a very large content of a metabolite (%w/w) may lead to decreased hydrogen production efficiency. Examples of metabolites produced as a reaction to oxidative stress that reach a high concentration in the cells are β-carotene and astaxanthin (7–10%), as described in the Section “Carotenoids”. This may indicate that in a practical process where hydrogen and a valuable metabolite are to be produced simultaneously, the best option may be to target production of a metabolite that is produced in smaller amounts, but which have a correspondingly higher market value. The identification and selection of the most suitable metabolite for this process is a topic for further studies. An alternative can be, as described above and illustrated in Figure 1C, a second step after the hydrogen production where other conditions are applied, to induce production of large amounts of a valuable metabolite.
From this study, it is clear that with the current status in the field it is difficult to conclude on which combination of stress reactions and which species are preferable to explore for potential combined production of optimal amounts of hydrogen and valuable side products, either simultaneously or in sequence. The majority of research on a practical use of algae is focusing on the study of each process separately, and often on just one species at the time. Algae’s reactions to different stress factors represent a complicated interaction by many metabolic processes, and the production of metabolites and hydrogen will depend on more than just single stress factors applied to different species. A significant amount of research is still necessary, and some is currently ongoing to identify optimal conditions for a combined system that would provide a practically sustainable process.
The model organism, Chlamydomonas reinhardtii, has been thoroughly studied in many respects, including hydrogen production. However, regarding production of metabolites with industrial/ pharmaceutical interest from wild types, the research has focused more on the species mentioned in the Section “Present commercial uses of green microalgae”, which have already shown a clear commercial potential. Although Chlamydomonas spp. are also able to produce interesting metabolites, little research has focused on how to promote and optimize their production, except in the case of gene modification. This could be due to well-known superior growth rates by other species, making a practical production process of the metabolites more likely to succeed. As reported in Table 1, a higher number of Chlamydomonas species have been found to produce hydrogen, than other genera. This can be explained by the fact that many more Chlamydomonas species have been identified and can be found in culture collections and databases. As one example, there are currently 1163 Chlamydomonas species names in the Algaebase database (Guiry and Guiry, 2012), out of which 417 have been flagged as currently accepted taxonomically. For comparison, the same database has registered 76 Chlorella species, 28 species of Dunaliella, 15 species of Haematococcus, and 15 species of Botryococcus.
Although some species have a better productivity than others, it is possible that some main characteristics to some extent can be transferred among species of the same genus. Algal genera used commercially today that are also known to produce hydrogenases, include Chlorella, Chlorococcum, Haematococcus and Tetraselmis.
The halotolerant/ halophilic Dunaliella spp. are green algae able to produce large amounts of β-carotene and glycerol under high light intensity and salt stress (Ben-Amotz, 2004). Even if Dunaliella species are already produced commercially, hydrogenase has never been observed in any species of this genus, and Dunaliella is therefore less likely to be a candidate for our proposed combined process.
One example of an algal species that has several qualities sought after in a potentially commercial context is Chlorella sorokiniana. Like many other Chlorella species, it is considered easy to culture with high growth rates (Morita et al., 2000; Qiao and Wang, 2009; Ugwu and Aoyagi, 2008), is able to produce high amounts of carotenoids and other antioxidants (Matsukawa et al., 2000), fatty acids with activities of medical application (Chou et al., 2008), and significant amounts of hydrogen (Chader et al., 2009). It is highly tolerant to heat stress and irradiant stress (Cuaresma et al., 2009; de-Bashan et al., 2008). It also shows high lipid productivity, although the lipid content of the cells does not necessarily increase during N-limitation, as is the case with many other species (Griffiths and Harrison, 2009).
In many cases, algae’s reaction to stress will vary within each genus, and in some cases even within each species. In order to obtain strains with a good ability for stress adaptation reactions it could be useful to isolate algae from stress exposed environments. When choosing algae for a practical process of hydrogen production, we suggest that the algae fulfill the following conditions:
Presence of hydrogenase and ability for hydrogen production,
fast growth during optimal environmental conditions,
adaptation mechanisms for nutrient deprivation and other environmental stress factors,
ability to produce metabolites of pharmaceutical/ industrial interest,
high production of these metabolites before or after hydrogen production.
In conclusion, this review is an initial attempt to create a conceptual overview of possibilities for innovative use of different species of green microalgae. One of the intentions has been to create a basis for selecting algae to screen for the above mentioned characteristics, with the goal to perform an innovative combined process where hydrogen and other valuable products can be produced either simultaneously or in sequence. The review also presents an overview of stress mechanisms and how these can be applied in order to induce production of valuable chemicals. The results show that green algae from at least 12 genera have shown ability to produce both hydrogen and several interesting metabolites, and these representatives could form a starting point for a systematic screening for candidates suited for a combined energy and high value compound production.
Acknowledgements
We acknowledge Stig A. Borgvang at Bioforsk for comments to the manuscript.
Declaration of interest
We gratefully acknowledge the Norwegian Ministry of Foreign Affairs via the Royal Norwegian Embassy in New Delhi, India, for their financial contributions through the BioCO2 project, and Nordic Energy Research for financial contributions through the AquaFEED project.
References
- Abe K, Hattori H, Hirano M. Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga Coelastrella striolata var. multistriata . Food Chem. 2007;100:656–661. [Google Scholar]
- Abedin RMA, Taha HM. Antibacterial and antifungal activity of cyanobacteria and green microalgae. Evaluation of medium components by Plackett-Burman design for antimicrobial activity of Spirulina platensis . Glob J Biotech Biochem. 2008;3:22–31. [Google Scholar]
- Ahlgren G, Gustafsson IB, Boberg M. Fatty-acid content and chemical-composition of fresh-water microalgae. J Phycol. 1992;28:37–50. [Google Scholar]
- Ahmad I, Hellebust JA. The role of glycerol and inorganic ions in osmoregulatory responses of the euryhaline flagellate Chlamydomonas pulsatilla Wollenweber. Plant Physiol. 1986;82:406–410. doi: 10.1104/pp.82.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albentosa M, Pérez-Camacho A, Labarta U, Fernández-Reiriz MJ. Evaluation of freeze-dried microalgal diets for the seed culture of Ruditapes decussatus using physiological and biochemical parameters. Aquaculture. 1997;154:305–321. [Google Scholar]
- Alberts DS, Goldman R, Xu MJ, Dorr RT, Quinn J, Welch K, Guillen-Rodriguez J, Aickin M, Peng YM, Loescher L, Gensler H. Disposition and metabolism of topically administered alpha-tocopherol acetate: a common ingredient of commercially available sunscreens and cosmetics. Nutr Cancer. 1996;26:193–201. doi: 10.1080/01635589609514475. [DOI] [PubMed] [Google Scholar]
- Allen EJ, Nelson EW. On the artificial culture of marine plankton organisms. J Mar Biol Assoc UK. 1910;8:421–474. [Google Scholar]
- Alyabyev AJ, Loseva NL, Gordon LK, Andreyeva IN, Rachimova GG, Tribunskih VI, Ponomareva AA, Kemp RB. The effect of changes in salinity on the energy yielding processes of Chlorella vulgaris and Dunaliella maritima cells. Thermochim Acta. 2007;458:65–70. [Google Scholar]
- Antal TK, Krendeleva TE, Laurinavichene TV, Makarova VV, Ghirardi ML, Rubin AB, Tsygankov AA, Seibert M. The dependence of algal H2 production on Photosystem II and O2 consumption activities in sulfur-deprived Chlamydomonas reinhardtii cells. Biochim Biophys Acta. 2003;1607:153–160. doi: 10.1016/j.bbabio.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Antal TK, Krendeleva TE, Rubin AB. Study of photosystem 2 heterogeneity in the sulfur-deficient green alga Chlamydomonas reinhardtii . Photosyn Res. 2007;94:13–22. doi: 10.1007/s11120-007-9202-0. [DOI] [PubMed] [Google Scholar]
- Antal TK, Volgusheva AA, Kukarskih GP, Krendeleva TE, Rubin AB. Relationships between H2 photoproduction and different electron transport pathways in sulfur-deprived Chlamydomonas reinhardtii . Int J Hydrogen Energy. 2009;34:9087–9094. [Google Scholar]
- Apt KE, Behrens PW. Commercial developments in microalgal biotechnology. J Phycol. 1999;35:215–226. [Google Scholar]
- Arisz SA, van Himbergen JA, Musgrave A, van den Ende H, Munnik T. Polar glycerolipids of Chlamydomonas moewusii . Phytochemistry. 2000;53:265–270. doi: 10.1016/s0031-9422(99)00505-1. [DOI] [PubMed] [Google Scholar]
- Azachi M, Sadka A, Fisher M, Goldshlag P, Gokhman I, Zamir A. Salt induction of fatty acid elongase and membrane lipid modifications in the extreme halotolerant alga Dunaliella salina . Plant Physiol. 2002;129:1320–1329. doi: 10.1104/pp.001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu B, Wu JT. Production of natural butylated hydroxytoluene as an antioxidant by freshwater phytoplankton. J Phycol. 2008;44:1447–1454. doi: 10.1111/j.1529-8817.2008.00596.x. [DOI] [PubMed] [Google Scholar]
- Baker RTM, Pfeiffer AM, Schöner FJ, Smith-Lemmon L. Pigmenting efficacy of astaxanthin and canthaxanthin in fresh-water reared Atlantic salmon, Salmo salar . Anim Feed Sci Technol. 2002;99:97–106. [Google Scholar]
- Barbosa MJ, Janssen M, Ham N, Tramper J, Wijffels RH. Microalgae cultivation in air-lift reactors: modeling biomass yield and growth rate as a function of mixing frequency. Biotechnol Bioeng. 2003;82:170–179. doi: 10.1002/bit.10563. [DOI] [PubMed] [Google Scholar]
- Barbosa MJ, Zijffers JW, Nisworo A, Vaes W, van Schoonhoven J, Wijffels RH. Optimization of biomass, vitamins, and carotenoid yield on light energy in a flat-panel reactor using the A-stat technique. Biotechnol Bioeng. 2005;89:233–242. doi: 10.1002/bit.20346. [DOI] [PubMed] [Google Scholar]
- Barclay MC, Irvin SJ, Williams KC, Smith DM. Comparison of diets for the tropical spiny lobster Panulirus ornatus: astaxanthin-supplemented feeds and mussel flesh. Aquac Nutr. 2006;12:117–125. [Google Scholar]
- Barnhart MC. Buckets of muckets: A compact system for rearing juvenile freshwater mussels. Aquaculture. 2006;254:227–233. [Google Scholar]
- Baroli I, Melis A. Photoinhibitory damage is modulated by the rate of photosynthesis and by the photosystem II light-harvesting chlorophyll antenna size. Planta. 1998;205:288–296. doi: 10.1007/s004250050323. [DOI] [PubMed] [Google Scholar]
- Barsanti L, Coltelli P, Evangelista V, Frassanito AM, Passarelli V, Vesentini N, Gualtieri P. Oddities and curiosities in the algal world. In: Evangelista V, Barsanti L, Frassanito AM, Passarelli V, Gualtieri P, editors. Algal toxins: nature, occurrence, effect and detection. Springer; Dordrecht: 2008. pp. 353–391. (Eds.) [Google Scholar]
- Becker EW. Micro-algae as a source of protein. Biotechnol Adv. 2007;25:207–210. doi: 10.1016/j.biotechadv.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Becker W. Microalgae for aquaculture - The nutritional value of microalgae for aquaculture. In: Richmond A, editor. Handbook of microalgal culture: Biotechnology and applied phycology. Blackwell Science Ltd; Oxford: 2004a. pp. 380–391. (Ed.) [Google Scholar]
- Becker W. Microalgae in human and animal nutrition. In: Richmond A, editor. Handbook of microalgal culture: Biotechnology and applied phycology. Blackwell Science Ltd; Oxford: 2004b. pp. 312–351. (Ed.) [Google Scholar]
- Beer LL, Boyd ES, Peters JW, Posewitz MC. Engineering algae for biohydrogen and biofuel production. Curr Opin Biotechnol. 2009;20:264–271. doi: 10.1016/j.copbio.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Belgis C, Guido P. Cyst-based toxicity tests. XI. Influence of the type of food on the intrinsic growth rate of the rotifer Brachionus calyciflorus in short-chronic toxicity tests. Chemosphere. 2003;50:365–372. doi: 10.1016/s0045-6535(02)00496-4. [DOI] [PubMed] [Google Scholar]
- Bell JG, McEvoy LA, Estevez A, Shields RJ, Sargent JR. Optimising lipid nutrition in first-feeding flatfish larvae. Aquaculture. 2003;227:211–220. [Google Scholar]
- Ben-Amotz A. . Industrial production of microalgal cell-mass and secondary products - Major industrial species - Dunaliella . In: Richmond A, editor. Handbook of microalgal culture: Biotechnology and applied phycology. Blackwell Science Ltd; Oxford: 2004. pp. 273–280. (Ed.) [Google Scholar]
- Ben-Amotz A, Avron M. On the factors which determine massive beta-carotene accumulation in the halotolerant alga Dunaliella bardawil . Plant Physiol. 1983;72:593–597. doi: 10.1104/pp.72.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amotz A, Tornabene TG, Thomas WH. Chemical profile of selected species of microalgae with emphasis on lipids. J Phycol. 1985;21:72–81. [Google Scholar]
- Bethmann B, Schönknecht G. pH regulation in an acidophilic green alga - a quantitative analysis. New Phytol. 2009;183:327–339. doi: 10.1111/j.1469-8137.2009.02862.x. [DOI] [PubMed] [Google Scholar]
- Bhosale P, Bernstein PS. Microbial xanthophylls. Appl Microbiol Biotechnol. 2005;68:445–455. doi: 10.1007/s00253-005-0032-8. [DOI] [PubMed] [Google Scholar]
- Bhosale SH, Pant A, Khan MI. Purification and characterization of putative alkaline [Ni-Fe] hydrogenase from unicellular marine green alga, Tetraselmis kochinensis NCIM 1605. Microbiol Res. 2009;164:131–137. doi: 10.1016/j.micres.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Bigogno C, Khozin-Goldberg I, Cohen Z. Accumulation of arachidonic acid-rich triacylglycerols in the microalga Parietochloris incisa (Trebuxiophyceae, Chlorophyta) Phytochemistry. 2002;60:135–143. doi: 10.1016/s0031-9422(02)00037-7. [DOI] [PubMed] [Google Scholar]
- Bishop NI, Frick M, Jones LW. Academic Press Inc; New York - San Fransisco - London: 1977. Photohydrogen production in green algae: Water serves as the primary substrate for hydrogen and oxygen production. [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- Blackwell JR, Gilmour DJ. Determination of intracellular volume and internal solute concentrations of the green alga Chlorococcum submarinum . Arch Microbiol. 1991;157:80–85. [Google Scholar]
- Blanco AM, Moreno J, Del Campo JA, Rivas J, Guerrero MG. Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl Microbiol Biotechnol. 2007;73:1259–1266. doi: 10.1007/s00253-006-0598-9. [DOI] [PubMed] [Google Scholar]
- Blunden G. Marine algae as sources of biologically active compounds. Interdiscip Sci Rev. 1993;18:73–80. [Google Scholar]
- Blunt JW, Copp BR, Hu WP, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2007;24:31–86. doi: 10.1039/b603047p. [DOI] [PubMed] [Google Scholar]
- Blunt JW, Copp BR, Hu WP, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2008;25:35–94. doi: 10.1039/b701534h. [DOI] [PubMed] [Google Scholar]
- Blunt JW, Copp BR, Hu WP, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2009;26:170–244. doi: 10.1039/b805113p. [DOI] [PubMed] [Google Scholar]
- Blunt JW, Copp BR, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2003;20:1–48. doi: 10.1039/b207130b. [DOI] [PubMed] [Google Scholar]
- Blunt JW, Copp BR, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2004;21:1–49. doi: 10.1039/b305250h. [DOI] [PubMed] [Google Scholar]
- Blunt JW, Copp BR, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2005;22:15–61. doi: 10.1039/b415080p. [DOI] [PubMed] [Google Scholar]
- Blunt JW, Copp BR, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2006;23:26–78. doi: 10.1039/b502792f. [DOI] [PubMed] [Google Scholar]
- Boichenko VA, Hoffmann P. Photosynthetic hydrogen-production in prokaryotes and eukaryotes - occurrence, mechanism, and functions. Photosynth. 1994;30:527–552. [Google Scholar]
- Boichenko VA, Wiessner W, Klimov VV, Mende D, Demeter S. Hydrogen photoevolution indicates an increase in the antenna size of Photosystem I in Chlamydobotrys stellata during transition from autotrophic to photoheterotrophic nutrition. Plant Physiol. 1992;100:518–524. doi: 10.1104/pp.100.1.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölling C, Fiehn O. Metabolite profiling of Chlamydomonas reinhardtii under nutrient deprivation. Plant Physiol. 2005;139:1995–2005. doi: 10.1104/pp.105.071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitzka MA. Vitamins and fine chemicals from micro-algae. In: Borowitzka MA, Borowitzka LJ, editors. Micro-algal Biotechnology. Cambridge University Press; Cambridge: 1988. pp. 153–196. (Eds.) [Google Scholar]
- Borowitzka MA. Microalgae as sources of pharmaceuticals and other biologically-active compounds. J Appl Phycol. 1995;7:3–15. [Google Scholar]
- Brand JJ, Wright JN, Lien S. Hydrogen production by eukaryotic algae. Biotechnol Bioeng. 1989;33:1482–1488. doi: 10.1002/bit.260331116. [DOI] [PubMed] [Google Scholar]
- Brown MR, Jeffrey SW, Volkman JK, Dunstan GA. Nutritional properties of microalgae for mariculture. Aquaculture. 1997;151:315–331. [Google Scholar]
- Brown MR, Mular M, Miller I, Farmer C, Trenerry C. The vitamin content of microalgae used in aquaculture. J Appl Phycol. 1999;11:247–255. [Google Scholar]
- Cao HM, Zhang LP, Melis A. Bioenergetic and metabolic processes for the survival of sulfur-deprived Dunaliella salina (Chlorophyta) J Appl Phycol. 2001;13:25–34. [Google Scholar]
- Capitani CD, Carvalho ACL, Rivelli DP, Barros SBM, Castro IA. Evaluation of natural and synthetic compounds according to their antioxidant activity using a multivariate approach. Eur J Lipid Sci Technol. 2009;111:1090–1099. [Google Scholar]
- Capon JF, Gloaguen F, Pétillon FY, Schollhammer P, Talarmin J. Electron and proton transfers at diiron dithiolate sites relevant to the catalysis of proton reduction by the [FeFe]-hydrogenases. Coord Chem Rev. 2009;253:1476–1494. [Google Scholar]
- Carballo-Cárdenas EC, Tuan PM, Janssen M, Wijffels RH. Vitamin E (alpha-tocopherol) production by the marine microalgae Dunaliella tertiolecta and Tetraselmis suecica in batch cultivation. Biomol Eng. 2003;20:139–147. doi: 10.1016/s1389-0344(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Cardozo KH, Guaratini T, Barros MP, Falcão VR, Tonon AP, Lopes NP, Campos S, Torres MA, Souza AO, Colepicolo P, Pinto E. Metabolites from algae with economical impact. Comp Biochem Physiol C Toxicol Pharmacol. 2007;146:60–78. doi: 10.1016/j.cbpc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Carvalho AP, Meireles LA, Malcata FX. Microalgal reactors: a review of enclosed system designs and performances. Biotechnol Prog. 2006;22:1490–1506. doi: 10.1021/bp060065r. [DOI] [PubMed] [Google Scholar]
- Cerón MC, Campos I, Sánchez JF, Acién FG, Molina E, Fernández-Sevilla JM. Recovery of lutein from microalgae biomass: development of a process for Scenedesmus almeriensis biomass. J Agric Food Chem. 2008;56:11761–11766. doi: 10.1021/jf8025875. [DOI] [PubMed] [Google Scholar]
- Cerón MC, García-Malea MC, Rivas J, Acien FG, Fernandez JM, Del Río E, Guerrero MG, Molina E. Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Appl Microbiol Biotechnol. 2007;74:1112–1119. doi: 10.1007/s00253-006-0743-5. [DOI] [PubMed] [Google Scholar]
- Cha KH, Koo SY, Lee DU. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J Agric Food Chem. 2008;56:10521–10526. doi: 10.1021/jf802111x. [DOI] [PubMed] [Google Scholar]
- Chader S, Hacene H, Agathos SN. Study of hydrogen production by three strains of Chlorella isolated from the soil in the Algerian Sahara. Int J Hydrogen Energy. 2009;34:4941–4946. [Google Scholar]
- Chan KC, Mong MC, Yin MC. Antioxidative and anti-inflammatory neuroprotective effects of astaxanthin and canthaxanthin in nerve growth factor differentiated PC12 cells. J Food Sci. 2009;74 doi: 10.1111/j.1750-3841.2009.01274.x. H225. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay P, Chatterjee S, Sen SK. Biotechnological potential of natural food grade biocolorants. Afr J Biotechnol. 2008;7:2972–2985. [Google Scholar]
- Chen F. Chin J . Oceanol Limnol 16 Suppl.: 1998. Bioreactor technology for production of valuable algal products. [Google Scholar]
- Chen GQ, Jiang Y, Chen F. Salt-induced alterations in lipid composition of diatom Nitzschia laevis (Bacillariophyceae) under heterotrophic culture condition. J Phycol. 2008;44:1309–1314. doi: 10.1111/j.1529-8817.2008.00565.x. [DOI] [PubMed] [Google Scholar]
- Chen H, Jiang JG. Osmotic responses of Dunaliella to the changes of salinity. J Cell Physiol. 2009;219:251–258. doi: 10.1002/jcp.21715. [DOI] [PubMed] [Google Scholar]
- Chen HC, Newton AJ, Melis A. Role of SulP, a nuclear-encoded chloroplast sulfate permease, in sulfate transport and H2 evolution in Chlamydomonas reinhardtii . Photosyn Res. 2005;84:289–296. doi: 10.1007/s11120-004-7157-y. [DOI] [PubMed] [Google Scholar]
- Chiang IZ, Huang WY, Wu JT. Allelochemicals of Botryococcus braunii (Chlorophyceae) J Phycol. 2004;40:474–480. [Google Scholar]
- Cho SH, Ji SC, Hur SB, Bae J, Park IS, Song YC. Optimum temperature and salinity conditions for growth of green algae Chlorella ellipsoidea and Nannochloris oculata . Fish Sci. 2007;73:1050–1056. [Google Scholar]
- Chochois V, Dauvillée D, Beyly A, Tolleter D, Cuiné S, Timpano H, Ball S, Cournac L, Peltier G. Hydrogen production in Chlamydomonas: photosystem II-dependent and -independent pathways differ in their requirement for starch metabolism. Plant Physiol. 2009;151:631–640. doi: 10.1104/pp.109.144576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YC, Prakash E, Huang CF, Lien TW, Chen X, Su IJ, Chao YS, Hsieh HP, Hsu JT. Bioassay-guided purification and identification of PPARalpha/gamma agonists from Chlorella sorokiniana . Phytother Res. 2008;22:605–613. doi: 10.1002/ptr.2280. [DOI] [PubMed] [Google Scholar]
- Choubert G. Response of rainbow trout (Oncorhynchus mykiss) to varying dietary astaxanthin/canthaxanthin ratio: colour and carotenoid retention of the muscle. Aquac Nutr. 2010;16:528–535. [Google Scholar]
- Choubert G, Mendes-Pinto MM, Morais R. Pigmenting efficacy of astaxanthin fed to rainbow trout Oncorhynchus mykiss: Effect of dietary astaxanthin and lipid sources. Aquaculture. 2006;257:429–436. [Google Scholar]
- Chow WS, Lee HY, Park YI, Park YM, Hong YN, Anderson JM. The role of inactive photosystem-II-mediated quenching in a last-ditch community defence against high light stress in vivo. Philos Trans R Soc Lond, B, Biol Sci. 2002;357:1441–49; discussion 1449. doi: 10.1098/rstb.2002.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christner BC. Bioprospecting for microbial products that affect ice crystal formation and growth. Appl Microbiol Biotechnol. 2010;85:481–489. doi: 10.1007/s00253-009-2291-2. [DOI] [PubMed] [Google Scholar]
- Chu CY, Liao WR, Huang R, Lin LP. Haemagglutinating and antibiotic activities of freshwater microalgae. World J Microbiol Biotechnol. 2004;20:817–825. [Google Scholar]
- Coesel SN, Baumgartner AC, Teles LM, Ramos AA, Henriques NM, Cancela L, Varela JC. Nutrient limitation is the main regulatory factor for carotenoid accumulation and for Psy and Pds steady state transcript levels in Dunaliella salina (Chlorophyta) exposed to high light and salt stress. Mar Biotechnol. 2008;10:602–611. doi: 10.1007/s10126-008-9100-2. [DOI] [PubMed] [Google Scholar]
- Conceicão LEC, Yufera M, Makridis P, Morais S, Dinis MT. Live feeds for early stages of fish rearing. Aquac Res. 2010;41:613–640. [Google Scholar]
- Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature. 2005;438:90–93. doi: 10.1038/nature04056. [DOI] [PubMed] [Google Scholar]
- Cuaresma M, Janssen M, Vílchez C, Wijffels RH. Productivity of Chlorella sorokiniana in a short light-path (SLP) panel photobioreactor under high irradiance. Biotechnol Bioeng. 2009;104:352–359. doi: 10.1002/bit.22394. [DOI] [PubMed] [Google Scholar]
- Cunha L, Mascaro M, Chiapa X, Costa A, Simoes N. Experimental studies on the effect of food in early larvae of the cleaner shrimp Lysmata amboinensis (De Mann, 1888) (Decapoda: Caridea: Hippolytidae) Aquaculture. 2008;277:117–123. [Google Scholar]
- D’Souza FML, Kelly GJ. Effects of a diet of a nitrogen-limited alga (Tetraselmis suecica) on growth, survival and biochemical composition of tiger prawn (Penaeus semisulcatus) larvae. Aquaculture. 2000;181:311–329. [Google Scholar]
- D’Souza FML, Loneragan NR. Effects of monospecific and mixed-algae diets on survival, development and fatty acid composition of penaeid prawn (Penaeus spp.) larvae. Mar Biol. 1999;133:621–633. [Google Scholar]
- Dahl U, Lind CR, Gorokhova E, Eklund B, Breitholtz M. Food quality effects on copepod growth and development: implications for bioassays in ecotoxicological testing. Ecotoxicol Environ Saf. 2009;72:351–357. doi: 10.1016/j.ecoenv.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Daligault F, Nugier-Chauvin C, Patin H. Microalga Chlorella sorokiniana: a new sulfoxidation biocatalyst. Org Biomol Chem. 2006;4:1474–1477. doi: 10.1039/b516813a. [DOI] [PubMed] [Google Scholar]
- Dasgupta CN, Gilbert J, Lindblad P, Heidorn T, Borgvang SA, Skjånes K, Das D. Recent trends on the development of photo-biological processes and photobioreactors for the improvement of hydrogen production. Int J Hydrogen Energy. 2010;35:10218–10238. [Google Scholar]
- Day AG, Brinkmann D, Franklin S, Espina K, Rudenko G, Roberts A, Howse KS. Safety evaluation of a high-lipid algal biomass from Chlorella protothecoides . Regul Toxicol Pharmacol. 2009;55:166–180. doi: 10.1016/j.yrtph.2009.06.014. [DOI] [PubMed] [Google Scholar]
- de-Bashan LE, Trejo A, Huss VA, Hernandez JP, Bashan Y. Chlorella sorokiniana UTEX 2805, a heat and intense, sunlight-tolerant microalga with potential for removing ammonium from wastewater. Bioresour Technol. 2008;99:4980–4989. doi: 10.1016/j.biortech.2007.09.065. [DOI] [PubMed] [Google Scholar]
- de la Coba F, Aguilera J, de Gálvez MV, Álvarez M, Gallego E, Figueroa FL, Herrera E. Prevention of the ultraviolet effects on clinical and histopathological changes, as well as the heat shock protein-70 expression in mouse skin by topical application of algal UV-absorbing compounds. J Dermatol Sci. 2009;55:161–169. doi: 10.1016/j.jdermsci.2009.06.004. [DOI] [PubMed] [Google Scholar]
- de la Noue J, de Pauw N. The potential of microalgal biotechnology – A review of production and uses of microalgae. Biotechnol Adv. 1988;6:725–770. doi: 10.1016/0734-9750(88)91921-0. [DOI] [PubMed] [Google Scholar]
- de Pauw N, Persoone G. Micro-algae for aquaculture. In: Borowitzka MA, Borowitzka LJ, editors. Micro-algal Biotechnology. Cambridge University Press; Cambridge: 1988. pp. 197–221. (Eds.) [Google Scholar]
- Del Campo JA, García-González M, Guerrero MG. Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl Microbiol Biotechnol. 2007;74:1163–1174. doi: 10.1007/s00253-007-0844-9. [DOI] [PubMed] [Google Scholar]
- Del Campo JA, Moreno J, Rodríguez H, Vargas MA, Rivas J, Guerrero MG. Carotenoid content of chlorophycean microalgae: factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta) J Biotechnol. 2000;76:51–59. doi: 10.1016/s0168-1656(99)00178-9. [DOI] [PubMed] [Google Scholar]
- Del Campo JA, Rodríguez H, Moreno J, Vargas MA, Rivas J, Guerrero MG. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta) Appl Microbiol Biotechnol. 2004;64:848–854. doi: 10.1007/s00253-003-1510-5. [DOI] [PubMed] [Google Scholar]
- Dembitsky VM. Anticancer activity of natural and synthetic acetylenic lipids. Lipids. 2006;41:883–924. doi: 10.1007/s11745-006-5044-3. [DOI] [PubMed] [Google Scholar]
- Demeter S, Janda T, Kovacs L, Mende D, Wiessner W. Effects of in-vivo CO2 depletion on electron-transport and photoinhibition in the green algae, Chlamydobotrys stellata and Chlamydomonas reinhardtii . Biochim Biophys Acta - Bioenergetics. 1995;1229:166–174. [Google Scholar]
- Devos N, Ingouff M, Loppes R, Matagne RF. Rubisco adaptation to low temperatures: A comparative study in psychrophilic and mesophilic unicellular algae. J Phyco. 1998;34:655–660. [Google Scholar]
- Domínguez-Bocanegra AR, Guerrero Legarreta I, Martinez Jeronimo F, Tomasini Campocosio A. Influence of environmental and nutritional factors in the production of astaxanthin from Haematococcus pluvialis . Bioresour Technol. 2004;92:209–214. doi: 10.1016/j.biortech.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Duerr EO, Molnar A, Sato V. Cultured microalgae as aquaculture feeds. J Mar Biotechnol. 1998;6:65–70. [Google Scholar]
- Dufossé L, Galaup P, Yaron A, Arad SM. Microorganisms and microalgae as sources of pigments for food use: a scientific oddity or an industrial reality? Trends Food Sci Technol. 2005;16:389–406. [Google Scholar]
- Durmaz Y. Vitamin E (alpha-tocopherol) production by the marine microalgae Nannochloropsis oculata (Eustigmatophyceae) in nitrogen limitation. Aquaculture. 2007;272:717–722. [Google Scholar]
- Duval B, Shetty K, Thomas WH. Phenolic compounds and antioxidant properties in the snow alga Chlamydomonas nivalis after exposure to UV light. J Appl Phycol. 2000;11:559–566. [Google Scholar]
- Egeland ES, Eikrem W, Throndsen J, Wilhelm C, Zapata M, Liaaen-Jensen S. Carotenoids from further prasinophytes. Biochem Syst Ecol. 1995;23:747–755. [Google Scholar]
- Egeland ES, Guillard RRL, Liaaen-Jensen S. Additional carotenoid prototype representatives and a general chemosystematic evaluation of carotenoids in Prasinophyceae (Chlorophyta) Phytochemistry. 1997;44:1087–1097. [Google Scholar]
- Elliott P, Aldridge DC, Moggridge GD. Zebra mussel filtration and its potential uses in industrial water treatment. Water Res. 2008;42:1664–1674. doi: 10.1016/j.watres.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Eriksen NT. The technology of microalgal culturing. Biotechnol Lett. 2008;30:1525–1536. doi: 10.1007/s10529-008-9740-3. [DOI] [PubMed] [Google Scholar]
- Fedorov AS, Kosourov S, Ghirardi ML, Seibert M. Continuous hydrogen photoproduction by Chlamydomonas reinhardtii: using a novel two-stage, sulfate-limited chemostat system. Appl Biochem Biotechnol. 2005;121124:403–412. doi: 10.1385/abab:121:1-3:0403. [DOI] [PubMed] [Google Scholar]
- Fernandes BD, Dragone GM, Teixeira JA, Vicente AA. Light regime characterization in an airlift photobioreactor for production of microalgae with high starch content. Appl Biochem Biotechnol. 2010;161:218–226. doi: 10.1007/s12010-009-8783-9. [DOI] [PubMed] [Google Scholar]
- Finney KF, Pomeranz Y, Bruinsma BL. Use of algae Dunaliella as a protein supplement in bread. Cereal Chem. 1984;61:402–406. [Google Scholar]
- Flynn T, Ghirardi ML, Seibert M. Accumulation of O2 tolerant phenotypes in H2 producing strains of Chlamydomonas reinhardtii by sequential applications of chemical mutagenesis and selection. Int J Hydrogen Energy. 2002;27:1421–1430. [Google Scholar]
- Fouchard S, Pruvost J, Degrenne B, Legrand J. Investigation of H2 production using the green microalga Chlamydomonas reinhardtii in a fully controlled photobioreactor fitted with on-line gas analysis. Int J Hydrogen Energy. 2008;33:3302–3310. [Google Scholar]
- Fried A, Tietz A, Benamotz A, Eichenberger W. Lipid-composition of the halotolerant alga, Dunaliella bardawil . Biochim Biophys Acta. 1982;713:419–426. [Google Scholar]
- Fujii K, Nakajima H, Anno Y. Potential of Monoraphidium sp GK12 for energy-saving astaxanthin production. J Chem Technol Biotechnol. 2008;83:1578–1584. [Google Scholar]
- Fujii Y, Sakamoto S, Ben-Amotz A, Nagasawa H. Effects of beta-carotene-rich algae Dunaliella bardawil on the dynamic changes of normal and neoplastic mammary cells and general metabolism in mice. Anticancer Res. 1993;13:389–393. [PubMed] [Google Scholar]
- Gerloff-Elias A, Barua D, Mölich A, Spijkerman E. Temperature- and pH-dependent accumulation of heat-shock proteins in the acidophilic green alga Chlamydomonas acidophila . FEMS Microbiol Ecol. 2006;56:345–354. doi: 10.1111/j.1574-6941.2006.00078.x. [DOI] [PubMed] [Google Scholar]
- Garbayo I, Cuaresma M, Vilchez C, Vega JM. Effect of abiotic stress on the production of lutein and beta-carotene by Chlamydomonas acidophila . Process Biochem. 2008;43:1158–1161. [Google Scholar]
- Garcia-Malea MC, Brindley C, Del Rio E, Acien FG, Fernandez JM, Molina E. Modelling of growth and accumulation of carotenoids in Haematococcus pluvialis as a function of irradiance and nutrients supply. Biochem Eng J. 2005;26:107–114. [Google Scholar]
- Gatenby CM, Orcutt DM, Kreeger DA, Parker BC, Jones VA, Neves RJ. Biochemical composition of three algal species proposed as food for captive freshwater mussels. J Appl Phycol. 2003;15:1–11. [Google Scholar]
- Gerloff-Elias A, Barua D, Mölich A, Spijkerman E. Temperature- and pH-dependent accumulation of heat-shock proteins in the acidophilic green alga Chlamydomonas acidophila . FEMS Microbiol Ecol. 2006;56:345–354. doi: 10.1111/j.1574-6941.2006.00078.x. [DOI] [PubMed] [Google Scholar]
- Gerloff-Elias A, Spijkerman E, Proschold T. Effect of external pH on the growth, photosynthesis and photosynthetic electron transport of Chlamydomonas acidophila Negoro, isolated from an extremely acidic lake (pH 2.6) Plant Cell Environ. 2005;28:1218–1229. [Google Scholar]
- Ghazala B, Shameel M, Choudhary MI, Shahzad S, Leghari SM. Phycochemistry and bioactivity of Tetraspora(Volvocophyta) from Sindh. Pak J Bot. 2004;36:531–547. [Google Scholar]
- Ghirardi ML, Dubini A, Yu J, Maness PC. Photobiological hydrogen-producing systems. Chem Soc Rev. 2009;38:52–61. doi: 10.1039/b718939g. [DOI] [PubMed] [Google Scholar]
- Ghirardi ML, King P, Kosourov S, Forestier M, Zhang L, Seibert M. Development of algal systems for hydrogen photoproduction: Addressing the hydrogenase oxygen-sensitivity problem. In: Collings AF, Critchley C, editors. Artificial photosynthesis: From basic biology to industrial application. WILEY-VCH Verlag GmbH & Co; Weinheim: 2006. pp. 213–227. (Eds.) [Google Scholar]
- Ghirardi ML, Zhang L, Lee JW, Flynn T, Seibert M, Greenbaum E, Melis A. Microalgae: a green source of renewable H(2) Trends Biotechnol. 2000;18:506–511. doi: 10.1016/s0167-7799(00)01511-0. [DOI] [PubMed] [Google Scholar]
- Glaser KB, Mayer AM. A renaissance in marine pharmacology: from preclinical curiosity to clinical reality. Biochem Pharmacol. 2009;78:440–448. doi: 10.1016/j.bcp.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Goes JI, Handa N, Taguchi S, Hama T. Effect of UV-B radiation on the fatty-acid composition of the marine-phytoplankton Tetraselmis sp – relationship to cellular pigments. Mar Ecol-Prog Ser. 1994;114:259–274. [Google Scholar]
- Gouveia L, Choubert G, Pereira N, Santinha J, Empis J, Gomes E. Pigmentation of gilthead seabream, Sparus aurata (L. 1875), using Chlorella vulgaris (Chlorophyta, Volvocales) microalga. Aquac Res. 2002;33:987–993. [Google Scholar]
- Gouveia L, Empis J. Relative stabilities of microalgal carotenoids in microalgal extracts, biomass and fish feed: Effect of storage conditions. Innov Food Sci Emerg Technol. 2003;4:227–233. [Google Scholar]
- Gouveia L, Oliveira AC. Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol. 2009;36:269–274. doi: 10.1007/s10295-008-0495-6. [DOI] [PubMed] [Google Scholar]
- Gouveia L, Raymundo A, Batista AP, Sousa I, Empis J. Chlorella vulgaris and Haematococcus pluvialis biomass as colouring and antioxidant in food emulsions. Eur Food Res Technol. 2006;222: 362–367. [Google Scholar]
- Gouveia L, Veloso V, Reis A, Fernandes H, Novais J, Empis J. Evolution of pigment composition in Chlorella vulgaris . Bioresour Technol. 1996;57:157. [Google Scholar]
- Goyal A. Osmoregulation in Dunaliella, Part II: Photosynthesis and starch contribute carbon for glycerol synthesis during a salt stress in Dunaliella tertiolecta . Plant Physiol Biochem. 2007;45:705–710. doi: 10.1016/j.plaphy.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Granot E, Kohen R. Oxidative stress in childhood–in health and disease states. Clin Nutr. 2004;23:3–11. doi: 10.1016/s0261-5614(03)00097-9. [DOI] [PubMed] [Google Scholar]
- Greenbaum E, Guillard RRL, Sunda WG. Hydrogen and oxygen photoproduction by marine algae. Photochem Photobiol. 1983;37:649–655. [Google Scholar]
- Griesbeck C, Kobl I, Heitzer M. Chlamydomonas reinhardtii: a protein expression system for pharmaceutical and biotechnological proteins. Mol Biotechnol. 2006;34:213–223. doi: 10.1385/MB:34:2:213. [DOI] [PubMed] [Google Scholar]
- Griffiths MJ, Harrison STL. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol. 2009;21:493–507. [Google Scholar]
- Grossman A. Acclimation of Chlamydomonas reinhardtii to its nutrient environment. Protist. 2000;151:201–224. doi: 10.1078/1434-4610-00020. [DOI] [PubMed] [Google Scholar]
- Guan YF, Deng MC, Yu XJ, Zhang W. Two-stage photo-biological production of hydrogen by marine green alga Platymonas subcordiformis . Biochem Eng J. 2004;19:69–73. [Google Scholar]
- Guerin M, Huntley ME, Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. 2003;21:210–216. doi: 10.1016/S0167-7799(03)00078-7. [DOI] [PubMed] [Google Scholar]
- Guiry MD, Guiry GM. AlgaeBase. 2012 http://wwwalgaebaseorg World-wide electronic publication, National University of Ireland, Galway. Available at:
- Guschina IA, Harwood JL. Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res. 2006;45:160–186. doi: 10.1016/j.plipres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Guzmán S, Gato A, Lamela M, Freire-Garabal M, Calleja JM. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum . Phytother Res. 2003;17:665–670. doi: 10.1002/ptr.1227. [DOI] [PubMed] [Google Scholar]
- Hadi MR, Shariati M, Afsharzadeh S. Microalgal biotechnology: carotenoid and glycerol production by the green algae Dunaliella isolated from the Gave-Khooni salt marsh, Iran, Biotechnol Bioprocess Eng. 2008;13:540–544. [Google Scholar]
- Hahn JJ, Ghirardi ML, Jacoby WA. Effect of process variables on photosynthetic algal hydrogen production. Biotechnol Prog. 2004;20:989–991. doi: 10.1021/bp0341287. [DOI] [PubMed] [Google Scholar]
- Hankamer B, Lehr F, Rupprecht J, Mussgnug JH, Posten C, Kruse O. Photosynthetic biomass and H2 production by green algae: from bioengineering to bioreactor scale-up. Physiol Plant. 2007;131:10–21. doi: 10.1111/j.1399-3054.2007.00924.x. [DOI] [PubMed] [Google Scholar]
- Harker M, Tsavalos AJ, Young AJ. Factors responsible for astaxanthin formation in the chlorophyte Haematococcus pluvialis . Bioresour Technol. 1996;55:207–214. [Google Scholar]
- Harrigan GG, Goetz G. Symbiotic and dietary marine microalgae as a source of bioactive molecules-experience from natural products research. J Appl Phycol. 2002;14:103–108. [Google Scholar]
- Hasegawa T, Matsuguchi T, Noda K, Tanaka K, Kumamoto S, Shoyama Y, Yoshikai Y. Toll-like receptor 2 is at least partly involved in the antitumor activity of glycoprotein from Chlorella vulgaris . Int Immunopharmacol. 2002;2:579–589. doi: 10.1016/s1567-5769(02)00002-4. [DOI] [PubMed] [Google Scholar]
- Havaux M, Dall’osto L, Bassi R. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol. 2007;145:1506–1520. doi: 10.1104/pp.107.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Duncan J, Barber J. Astaxanthin accumulation in the green alga Haematococcus pluvialis: Effects of cultivation parameters. J Integr Plant Biol. 2007;49:447–451. [Google Scholar]
- Healey FP. Hydrogen evolution by several algae. Planta. 1970;91:220–226. doi: 10.1007/BF00385481. [DOI] [PubMed] [Google Scholar]
- Heinrich U, Gärtner C, Wiebusch M, Eichler O, Sies H, Tronnier H, Stahl W. Supplementation with beta-carotene or a similar amount of mixed carotenoids protects humans from UV-induced erythema. J Nutr. 2003;133:98–101. doi: 10.1093/jn/133.1.98. [DOI] [PubMed] [Google Scholar]
- Hejazi MA, Wijffels RH. Effect of light intensity on beta-carotene production and extraction by Dunaliella salina in two-phase bioreactors. Biomol Eng. 2003;20:171–175. doi: 10.1016/s1389-0344(03)00046-7. [DOI] [PubMed] [Google Scholar]
- Hellebust JA, Lin YH. Regulation of glycerol and starch metabolism in Chlamydomonas pulsatilla in response to changes in salinity. Plant Cell Environ. 1989;12:621–627. [Google Scholar]
- Hemschemeier A, Fouchard S, Cournac L, Peltier G, Happe T. Hydrogen production by Chlamydomonas reinhardtii: an elaborate interplay of electron sources and sinks. Planta. 2008;227:397–407. doi: 10.1007/s00425-007-0626-8. [DOI] [PubMed] [Google Scholar]
- Herrero M, Ibáñez E, Cifuentes A, Reglero G, Santoyo S. Dunaliella salina microalga pressurized liquid extracts as potential antimicrobials. J Food Prot. 2006;69:2471–2477. doi: 10.4315/0362-028x-69.10.2471. [DOI] [PubMed] [Google Scholar]
- Hessen DO, De Lange HJ, Van Donk E. UV-induced changes in phytoplankton cells and its effects on grazers. Freshw Biol. 1997;38:513–524. [Google Scholar]
- Hu HH, Ll HY, Xu XD. Alternative cold response modes in Chlorella (Chlorophyta, Trebouxiophyceae) from Antarctica. Phycologia. 2008a;47:28–34. [Google Scholar]
- Hu Q. Environmental effects on cell composition. In: Richmond A, editor. Handbook of microalgal culture: Biotechnology and applied phycology. Blackwell Science Ltd; Oxford: 2004. pp. 83–93. (Ed.) [Google Scholar]
- Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J. 2008b;54:621–639. doi: 10.1111/j.1365-313X.2008.03492.x. [DOI] [PubMed] [Google Scholar]
- Hu ZY, Li YT, Sommerfeld M, Chen F, Hu Q. Enhanced protection against oxidative stress in an astaxanthin-overproduction Haematococcus mutant (Chlorophyceae) Eur J Phycol. 2008c;43:365–376. [Google Scholar]
- Huner NPA, Öquist G, Sarhan F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998;3:224–230. [Google Scholar]
- Hunt B, Vincent AC. Scale and sustainability of marine bioprospecting for pharmaceuticals. Ambio. 2006;35:57–64. doi: 10.1579/0044-7447(2006)35[57:sasomb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ikawa M. Algal polyunsaturated fatty acids and effects on plankton ecology and other organisms. UNH Center Freshwat Biol Res. 2004;6:17–44. [Google Scholar]
- Ikeuchi M, Koyama T, Takahashi J, Yazawa K. Effects of astaxanthin in obese mice fed a high-fat diet. Biosci Biotechnol Biochem. 2007;71:893–899. doi: 10.1271/bbb.60521. [DOI] [PubMed] [Google Scholar]
- Illman AM, Scragg AH, Shales SW. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb Technol. 2000;27:631–635. doi: 10.1016/s0141-0229(00)00266-0. [DOI] [PubMed] [Google Scholar]
- Imamoglu E, Dalay MC, Sukan FV. Influences of different stress media and high light intensities on accumulation of astaxanthin in the green alga Haematococcus pluvialis . N Biotechnol. 2009;26:199–204. doi: 10.1016/j.nbt.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Ip PF, Chen F. Employment of reactive oxygen species to enhance astaxanthin formation in Chlorella zofingiensis in heterotrophic culture. Process Biochem. 2005;40:3491–3496. [Google Scholar]
- Iwamoto H. Industrial production of microalgal cell-mass and secondary products - Major industrial species - Chlorella . In: Richmond A, editor. Handbook of microalgal culture: Biotechnology and applied phycology. Blackwell Science Ltd; Oxford: 2004. pp. 255–263. (Ed.) [Google Scholar]
- Jahns P, Latowski D, Strzalka K. Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids. Biochim Biophys Acta. 2009;1787:3–14. doi: 10.1016/j.bbabio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Janczyk P, Franke H, Souffrant WB. Nutritional value of Chlorella vulgaris: Effects of ultrasonication and electroporation on digestibility in rats. Anim Feed Sci Technol. 2007;132:163–169. [Google Scholar]
- Janczyk P, Halle B, Souffrant WB. Microbial community composition of the crop and ceca contents of laying hens fed diets supplemented with Chlorella vulgaris . Poult Sci. 2009;88:2324–2332. doi: 10.3382/ps.2009-00250. [DOI] [PubMed] [Google Scholar]
- Jin E, Lee CG, Polle JEW. Secondary carotenoid accumulation in Haematococcus (Chlorophyceae): Biosynthesis, regulation, and biotechnology. J Microbiol Biotechnol. 2006;16:821–831. [Google Scholar]
- Jin E, Polle JEW, Lee HK, Hyun SM, Chang M. Xanthophylls in microalgae: From biosynthesis to biotechnological mass production and application. J Microbiol Biotechnol. 2003;13:165–174. [Google Scholar]
- Jo JH, Lee DS, Park JM. Modeling and optimization of photosynthetic hydrogen gas production by green alga Chlamydomonas reinhardtii in sulfur-deprived circumstance. Biotechnol Prog. 2006;22:431–437. doi: 10.1021/bp050258z. [DOI] [PubMed] [Google Scholar]
- Johnson DT, Taconi KA. The glycerin glut: Options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ Prog. 2007;26:338–348. [Google Scholar]
- Jyonouchi H, Sun S, Iijima K, Gross MD. Antitumor activity of astaxanthin and its mode of action. Nutr Cancer. 2000;36:59–65. doi: 10.1207/S15327914NC3601_9. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Sun S, Gross M. Effect of carotenoids on in vitro immunoglobulin production by human peripheral blood mononuclear cells: astaxanthin, a carotenoid without vitamin A activity, enhances in vitro immunoglobulin production in response to a T-dependent stimulant and antigen. Nutr Cancer. 1995;23:171–183. doi: 10.1080/01635589509514373. [DOI] [PubMed] [Google Scholar]
- Kaçka A, Dönmez G. Isolation of Dunaliella spp. from a hypersaline lake and their ability to accumulate glycerol. Bioresour Technol. 2008;99:8348–8352. doi: 10.1016/j.biortech.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Kamath BS, Srikanta BM, Dharmesh SM, Sarada R, Ravishankar GA. Ulcer preventive and antioxidative properties of astaxanthin from Haematococcus pluvialis . Eur J Pharmacol. 2008;590:387–395. doi: 10.1016/j.ejphar.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Kamp C, Silakov A, Winkler M, Reijerse EJ, Lubitz W, Happe T. Isolation and first EPR characterization of the [FeFe]-hydrogenases from green algae. Biochim Biophys Acta. 2008;1777:410–416. doi: 10.1016/j.bbabio.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Kang CD, Lee JS, Park TH, Sim SJ. Comparison of heterotrophic and photoautotrophic induction on astaxanthin production by Haematococcus pluvialis . Appl Microbiol Biotechnol. 2005;68:237–241. doi: 10.1007/s00253-005-1889-2. [DOI] [PubMed] [Google Scholar]
- Kang JS, Raymond JA. Reduction of freeze-thaw-induced hemolysis of red blood cells by an algal ice-binding protein. Cryo Letters. 2004;25:307–310. [PubMed] [Google Scholar]
- Kaplan A, Schreiber U, Avron M. Salt-induced metabolic changes in Dunaliella salina . Plant Physiol. 1980;65:810–813. doi: 10.1104/pp.65.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten U, Lembcke S, Schumann R. The effects of ultraviolet radiation on photosynthetic performance, growth and sunscreen compounds in aeroterrestrial biofilm algae isolated from building facades. Planta. 2007;225:991–1000. doi: 10.1007/s00425-006-0406-x. [DOI] [PubMed] [Google Scholar]
- Kessler E. Effect of anaerobiosis on photosynthetic reactions and nitrogen metabolism of algae with and without hydrogenase. Arch Mikrobiol. 1973;93:91–100. doi: 10.1007/BF00424940. [DOI] [PubMed] [Google Scholar]
- Kessler E. Hydrogenase, photoreduction and anaerobic growth. In: Stewart WDP, editor. Algal physiology and biochemistry. Blackwell Sci.; Oxford: 1974. pp. 456–473. (Ed.) [Google Scholar]
- Khan SA, Rashmi, Hussain MZ, Prasad S, Banerjee UC. Prospects of biodiesel production from microalgae in India. Renew Sust Energy Rev. 2009;13:2361–2372. [Google Scholar]
- Khozin-Goldberg I, Bigogno C, Shrestha P, Cohen Z. Nitrogen starvation induces the accumulation of arachidonic acid in the freshwater green alga Parietochloris incisa (Trebuxiophyceae) J Phycol. 2002;38:991–994. [Google Scholar]
- Khozin-Goldberg I, Cohen Z, Pimenta-Leibowitz M, Nechev J, Zilberg D. Feeding with arachidonic acid-rich triacylglycerols from the microalga Parietochloris incisa improved recovery of guppies from infection with Tetrahymena sp. Aquaculture. 2006;255:142–150. [Google Scholar]
- Kim HK, Li L, Lee HS, Park MO, Bilehal D, Li W, Kim YH. Protective effects of Chlorella vulgaris extract on carbon tetrachloride induced acute liver injury in mice. Food Sci Biotechnol. 2009a;18:1186–1192. [Google Scholar]
- Kim JP, Kang CD, Park TH, Kim MS, Sim SJ. Enhanced hydrogen production by controlling light intensity in sulfur-deprived Chlamydomonas reinhardtii culture. Int J Hydrogen Energy. 2006a;31:1585–1590. [Google Scholar]
- Kim JP, Kang CD, Sim SJ, Kim MS, Park TH, Lee D, Kim D, Kim JH, Lee YK, Pak D. Cell age optimization for hydrogen production induced by sulfur deprivation using a green alga Chlamydomonas reinhardtii UTEX 90. J Microbiol Biotechnol. 2005;15:131–135. [Google Scholar]
- Kim JP, Kim KR, Choi SP, Han SJ, Kim MS, Sim SJ. Repeated production of hydrogen by sulfate re-addition in sulfur deprived culture of Chlamydomonas reinhardtii . Int J Hydrogen Energy 2010 [Google Scholar]
- Kim MK, Park JW, Park CS, Kim SJ, Jeune KH, Chang MU, Acreman J. Enhanced production of Scenedesmus spp. (green microalgae) using a new medium containing fermented swine wastewater. Bioresour Technol. 2007;98:2220–2228. doi: 10.1016/j.biortech.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Kim MS, Baek JS, Yun YS, Sim SJ, Park S, Kim SC. Hydrogen production from Chlamydomonas reinhardtii biomass using a two-step conversion process: Anaerobic conversion and photosynthetic fermentation. Int J Hydrogen Energy. 2006b;31:812–816. [Google Scholar]
- Kim SH, Jean DI, Lim YP, Lee C, An G. Weight gain limitation and liver protection by long-term feeding of astaxanthin in murines. J Korean Soc Appl Biol Chem. 2009b;52:180–185. [Google Scholar]
- Kim SK, Ravichandran YD, Khan SB, Kim YT. Prospective of the cosmeceuticals derived from marine organisms. Biotechnol Bioprocess Eng. 2008;13:511–523. [Google Scholar]
- Kirst GO. Salinity tolerance of eukaryotic marine algae. Annu Rev Plant Physiol Plant Molec Biol. 1989;41:21–53. [Google Scholar]
- Kosourov S, Makarova V, Fedorov AS, Tsygankov A, Seibert M, Ghirardi ML. The effect of sulfur re-addition on H(2) photoproduction by sulfur-deprived green algae. Photosyn Res. 2005;85:295–305. doi: 10.1007/s11120-005-5105-0. [DOI] [PubMed] [Google Scholar]
- Kosourov S, Seibert M, Ghirardi ML. Effects of extracellular pH on the metabolic pathways in sulfur-deprived, H2-producing Chlamydomonas reinhardtii cultures. Plant Cell Physiol. 2003;44:146–155. doi: 10.1093/pcp/pcg020. [DOI] [PubMed] [Google Scholar]
- Kosourov S, Tsygankov A, Seibert M, Ghirardi ML. Sustained hydrogen photoproduction by Chlamydomonas reinhardtii: Effects of culture parameters. Biotechnol Bioeng. 2002;78:731–740. doi: 10.1002/bit.10254. [DOI] [PubMed] [Google Scholar]
- Kosourov SN, Seibert M. Hydrogen photoproduction by nutrient-deprived Chlamydomonas reinhardtii cells immobilized within thin alginate films under aerobic and anaerobic conditions. Biotechnol Bioeng. 2009;102:50–58. doi: 10.1002/bit.22050. [DOI] [PubMed] [Google Scholar]
- Kreuzberg K. Starch fermentation via a formate producing pathway in Chlamydomonas reinhardii, Chlorogonium elongatum and Chlorella fusca . Physiol Plant. 1984;61:87–94. [Google Scholar]
- Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- Kruse O, Rupprecht J, Bader KP, Thomas-Hall S, Schenk PM, Finazzi G, Hankamer B. Improved photobiological H2 production in engineered green algal cells. J Biol Chem. 2005;280:34170–34177. doi: 10.1074/jbc.M503840200. [DOI] [PubMed] [Google Scholar]
- Laguna MR, Villar R, Calleja JM, Cadavid I. Effects of Chlorella stigmatophora extract on the central nervous system. Planta Med. 1993;59:125–130. doi: 10.1055/s-2006-959626. [DOI] [PubMed] [Google Scholar]
- Lakeh AAB, Ahmadi MR, Safi S, Ytrestøyl T, Bjerkeng B. Growth performance, mortality and carotenoid pigmentation of fry offspring as affected by dietary supplementation of astaxanthin to female rainbow trout (Oncorhynchus mykiss) broodstock. J Appl Ichthyol. 2010;26:35–39. [Google Scholar]
- Lall SP, Lewis-McCrea LM. Role of nutrients in skeletal metabolism and pathology in fish - An overview. Aquaculture. 2007:3–19. [Google Scholar]
- Lamers PP, Janssen M, De Vos RC, Bino RJ, Wijffels RH. Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends Biotechnol. 2008;26:631–638. doi: 10.1016/j.tibtech.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Langner U, Jakob T, Stehfest K, Wilhelm C. An energy balance from absorbed photons to new biomass for Chlamydomonas reinhardtii and Chlamydomonas acidophila under neutral and extremely acidic growth conditions. Plant Cell Environ. 2009;32:250–258. doi: 10.1111/j.1365-3040.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- Latasa M, Scharek R, Le Gall F, Guillou L. Pigment suites and taxonomic groups in Prasinophyceae. J Phycol. 2004;40:1149–1155. [Google Scholar]
- Laurinavichene T, Tolstygina I, Tsygankov A. The effect of light intensity on hydrogen production by sulfur-deprived Chlamydomonas reinhardtii . J Biotechnol. 2004;114:143–151. doi: 10.1016/j.jbiotec.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Laurinavichene TV, Fedorov AS, Ghirardi ML, Seibert M, Tsygankov AA. Demonstration of sustained hydrogen photoproduction by immobilized, sulfur-deprived Chlamydomonas reinhardtii cells. Int J Hydrogen Energy. 2006;31:659–667. [Google Scholar]
- Laurinavichene TV, Tolstygina IV, Galiulina RR, Ghirardi ML, Seibert M, Tsygankov AA. Dilution methods to deprive Chlamydomonas reinhardtii cultures of sulfur for subsequent hydrogen photoproduction. Int J Hydrogen Energy. 2002;27:1245–1249. [Google Scholar]
- Lavens P, Sorgeloos P. Manual on the production and use of live food for aquaculture. Food and Agriculture Organization of the United Nations; Rome: 1996. [Google Scholar]
- León-Bañares R, González-Ballester D, Galván A, Fernández E. Transgenic microalgae as green cell-factories. Trends Biotechnol. 2004;22:45–52. doi: 10.1016/j.tibtech.2003.11.003. [DOI] [PubMed] [Google Scholar]
- León R, Galván F. Halotolerance studies on Chlamydomonas reinhardtii – glycerol excretion by free and immobilized cells. J Appl Phycol. 1994;6:13–20. [Google Scholar]
- León R, Galván F. Interaction between saline stress and photoinhibition of photosynthesis in the freshwater green algae Chlamydomonas reinhardtii. Implications for glycerol photoproduction. Plant Physiol Biochem. 1999;37:623–628. [Google Scholar]
- Lewis LA, McCourt RM. Green algae and the origin of land plants. Am J Bot. 2004;91:1535–1556. doi: 10.3732/ajb.91.10.1535. [DOI] [PubMed] [Google Scholar]
- Leya T, Rahn A, Lütz C, Remias D. Response of arctic snow and permafrost algae to high light and nitrogen stress by changes in pigment composition and applied aspects for biotechnology. FEMS Microbiol Ecol. 2009;67:432–443. doi: 10.1111/j.1574-6941.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- Li SS, Tsai HJ. Transgenic microalgae as a non-antibiotic bactericide producer to defend against bacterial pathogen infection in the fish digestive tract. Fish Shellfish Immunol. 2009a;26:316–325. doi: 10.1016/j.fsi.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Li Y, Wei G, Chen J. Glutathione: a review on biotechnological production. Appl Microbiol Biotechnol. 2004;66:233–242. doi: 10.1007/s00253-004-1751-y. [DOI] [PubMed] [Google Scholar]
- Li YT, Huang JC, Sandmann G, Chen F. High light and sodium chloride stress differentially regulate the biosynthesis of astaxanthin in Chlorella zofingiensis (chlorophyceae) J Phycol. 2009b;45:635–641. doi: 10.1111/j.1529-8817.2009.00689.x. [DOI] [PubMed] [Google Scholar]
- Liang Y, Sarkany N, Cui Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett. 2009;31:1043–1049. doi: 10.1007/s10529-009-9975-7. [DOI] [PubMed] [Google Scholar]
- Liao WR, Lin JY, Shieh WY, Jeng WL, Huang R. Antibiotic activity of lectins from marine algae against marine vibrios. J Ind Microbiol Biotechnol. 2003;30:433–439. doi: 10.1007/s10295-003-0068-7. [DOI] [PubMed] [Google Scholar]
- Libessart N, Maddelein ML, Koornhuyse N, Decq A, Delrue B, Mouille G, D’Hulst C, Ball S. Storage, photosynthesis, and growth: The conditional nature of mutations affecting starch synthesis and structure in Chlamydomonas . Plant Cell. 1995;7:1117–1127. doi: 10.1105/tpc.7.8.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg P, Melis A. The chloroplast sulfate transport system in the green alga Chlamydomonas reinhardtii . Planta. 2008;228:951–961. doi: 10.1007/s00425-008-0795-0. [DOI] [PubMed] [Google Scholar]
- Liu BH, Lee YK. Secondary carotenoids formation by the green alga Chlorococcum sp. J Appl Phycol. 2000;12:301–307. [Google Scholar]
- Logan BA, Kornyeyev D, Hardison J, Holaday AS. The role of antioxidant enzymes in photoprotection. Photosyn Res. 2006;88:119–132. doi: 10.1007/s11120-006-9043-2. [DOI] [PubMed] [Google Scholar]
- Long H, Chang CH, King PW, Ghirardi ML, Kim K. Brownian dynamics and molecular dynamics study of the association between hydrogenase and ferredoxin from Chlamydomonas reinhardtii . Biophys J. 2008;95:3753–3766. doi: 10.1529/biophysj.107.127548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz RT, Cysewski GR. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000;18:160–167. doi: 10.1016/s0167-7799(00)01433-5. [DOI] [PubMed] [Google Scholar]
- Luiten EE, Akkerman I, Koulman A, Kamermans P, Reith H, Barbosa MJ, Sipkema D, Wijffels RH. Realizing the promises of marine biotechnology. Biomol Eng. 2003;20:429–439. doi: 10.1016/s1389-0344(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Lustigman B. Comparison of antibiotic production from four ecotypes of the marine alga, Dunaliella . Bull Environ Contam Toxicol. 1988;40:18–22. doi: 10.1007/BF01689380. [DOI] [PubMed] [Google Scholar]
- Maliga P. Progress towards commercialization of plastid transformation technology. Trends Biotechnol. 2003;21:20–28. doi: 10.1016/s0167-7799(02)00007-0. [DOI] [PubMed] [Google Scholar]
- Maneeruttanarungroj C, Lindblad P, Incharoensakdi A. A newly isolated green alga, Tetraspora sp. CU2551, from Thailand with efficient hydrogen production. Int J Hydrogen Energy. 2010;35:13193–13199. [Google Scholar]
- Martinez-Fernandez E, Acosta-Salmon H, Southgate PC. The nutritional value of seven species of tropical microalgae for black-lip pearl oyster (Pinctada margaritifera, L.) larvae. Aquaculture. 2006;257:491–503. [Google Scholar]
- Martinez-Fernandez E, Southgate PC. Use of tropical microalgae as food for larvae of the black-lip pearl oyster Pinctada margaritifera . Aquaculture. 2007;263:220–226. [Google Scholar]
- Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: A review. Renew Sust Energy Rev. 2010;14:217–232. [Google Scholar]
- Matagne RF, Loppes R, Deltour R. Phosphatase of Chlamydomonas reinhardtii: biochemical and cytochemical approach with specific mutants. J Bacteriol. 1976;126:937–950. doi: 10.1128/jb.126.2.937-950.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa R, Hotta M, Masuda Y, Chihara M, Karube I. Antioxidants from carbon dioxide fixing Chlorella sorokiniana . J Appl Phycol. 2000;12:263–267. [Google Scholar]
- Mayer AM, Gustafson KR. Marine pharmacology in 2000: antitumor and cytotoxic compounds. Int J Cancer. 2003;105:291–299. doi: 10.1002/ijc.11080. [DOI] [PubMed] [Google Scholar]
- Mayer AM, Gustafson KR. Marine pharmacology in 2005–2006: antitumour and cytotoxic compounds. Eur J Cancer. 2008;44:2357–2387. doi: 10.1016/j.ejca.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM. The evolution of free radicals and oxidative stress. Am J Med. 2000;108:652–659. doi: 10.1016/s0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- McCracken MD, Middaugh RE, Middaugh RS. A chemical characterization of an algal inhibitor obtained from Chlamydomonas . Hydrobiologia. 1980;70:271–276. [Google Scholar]
- Melis A. Photosystem II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends Plant Sci. 1999;4:130–135. doi: 10.1016/s1360-1385(99)01387-4. [DOI] [PubMed] [Google Scholar]
- Melis A. Green alga hydrogen production: progress, challenges and prospects. Int J Hydrogen Energy. 2002;27:1217–1228. [Google Scholar]
- Melis A. Photosynthetic H2 metabolism in Chlamydomonas reinhardtii (unicellular green algae) Planta. 2007;226:1075–1086. doi: 10.1007/s00425-007-0609-9. [DOI] [PubMed] [Google Scholar]
- Melis A. Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 2009;177:272–280. [Google Scholar]
- Melis A, Happe T. Hydrogen production. Green algae as a source of energy. Plant Physiol. 2001;127:740–748. [PMC free article] [PubMed] [Google Scholar]
- Melis A, Zhang L, Forestier M, Ghirardi ML, Seibert M. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii . Plant Physiol. 2000;122:127–136. doi: 10.1104/pp.122.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes RL, Nobre BP, Cardoso MT, Pereira AP, Palavra AF. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorg Chim Acta. 2003;356:328–334. [Google Scholar]
- Meuser JE, Ananyev G, Wittig LE, Kosourov S, Ghirardi ML, Seibert M, Dismukes GC, Posewitz MC. Phenotypic diversity of hydrogen production in chlorophycean algae reflects distinct anaerobic metabolisms. J Biotechnol. 2009;142:21–30. doi: 10.1016/j.jbiotec.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Mignone LI, Giovannucci E, Newcomb PA, Titus-Ernstoff L, Trentham-Dietz A, Hampton JM, Willett WC, Egan KM. Dietary carotenoids and the risk of invasive breast cancer. Int J Cancer. 2009;124:2929–2937. doi: 10.1002/ijc.24334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y, Akano T, Fukatsu K, Miyasaka H, Mizoguchi T, Yagi K, Maeda I, Ikuta Y, Matsumoto H. Stably sustained hydrogen production by biophotolysis in natural day/night cycle. Energy Conv Manag. 1997;38:S533–S537. [Google Scholar]
- Miyasaka H, Ohnishi Y, Akano T, Fukatsu K, Mizoguchi T, Yagi K, Maeda I, Ikuta Y, Matsumoto H, Shioji N, Miura Y. Excretion of glycerol by the marine Chlamydomonas sp. strain W-80 in high CO2 cultures. J Ferment Bioeng. 1998;85:122–124. [Google Scholar]
- Mohamed ZA. Polysaccharides as a protective response against microcystin-induced oxidative stress in Chlorella vulgaris and Scenedesmus quadricauda and their possible significance in the aquatic ecosystem. Ecotoxicology. 2008;17:504–516. doi: 10.1007/s10646-008-0204-2. [DOI] [PubMed] [Google Scholar]
- Mokady S, Abramovici A, Cogan U. The safety evaluation of Dunaliella bardawil as a potential food supplement. Food Chem Toxicol. 1989;27:221–226. doi: 10.1016/0278-6915(89)90159-2. [DOI] [PubMed] [Google Scholar]
- Momirlan M, Veziroglu TN. Current status of hydrogen energy. Renew Sust Energy Rev. 2002;6:141–179. [Google Scholar]
- Morgan-Kiss RM, Priscu JC, Pocock T, Gudynaite-Savitch L, Huner NP. Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol Mol Biol Rev. 2006;70:222–252. doi: 10.1128/MMBR.70.1.222-252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, Watanabe Y, Saiki H. High photosynthetic productivity of green microalga Chlorella sorokiniana . Appl Biochem Biotechnol. 2000;87:203–218. doi: 10.1385/abab:87:3:203. [DOI] [PubMed] [Google Scholar]
- Myrand B, de la Noüe J. Ingestion and assimilation rates of Oocystis sp. by Daphnia magna in treated wastewaters. Environ Poll (Series A) – Ecol Biol. 1983;31:77–95. [Google Scholar]
- Nagy LE, Meuser JE, Plummer S, Seibert M, Ghirardi ML, King PW, Ahmann D, Posewitz MC. Application of gene-shuffling for the rapid generation of novel [FeFe]-hydrogenase libraries. Biotechnol Lett. 2007;29:421–430. doi: 10.1007/s10529-006-9254-9. [DOI] [PubMed] [Google Scholar]
- Nakano S, Noguchi T, Takekoshi H, Suzuki G, Nakano M. Maternal–fetal distribution and transfer of dioxins in pregnant women in Japan, and attempts to reduce maternal transfer with Chlorella (Chlorella pyrenoidosa) supplements. Chemosphere. 2005;61:1244–1255. doi: 10.1016/j.chemosphere.2005.03.080. [DOI] [PubMed] [Google Scholar]
- Napolitano MJ, Shain DH. Distinctions in adenylate metabolism among organisms inhabiting temperature extremes. Extremophiles. 2005;9:93–98. doi: 10.1007/s00792-004-0424-1. [DOI] [PubMed] [Google Scholar]
- Neale PJ, Melis A. Salinity stress enhances photoinhibition of photosynthesis in Chlamydomonas reinhardtii . J Plant Physiol. 1989;134:619–622. [Google Scholar]
- Neori A. “Green water” microalgae: the leading sector in world aquaculture. J Appl Phycol. 2011;23:143–149. [Google Scholar]
- Nield J, Redding K, Hippler M. Remodeling of light-harvesting protein complexes in Chlamydomonas in response to environmental changes. Eukaryotic Cell. 2004;3:1370–1380. doi: 10.1128/EC.3.6.1370-1380.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y, Allakhverdiev SI, Murata N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta. 2006;1757:742–749. doi: 10.1016/j.bbabio.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Allakhverdiev SI, Murata N. Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiol Plant. 2011;142:35–46. doi: 10.1111/j.1399-3054.2011.01457.x. [DOI] [PubMed] [Google Scholar]
- Niu J, Tian LX, Liu YJ, Yang HJ, Ye CX, Gao W, Mai KS. Effect of dietary astaxanthin on growth, survival, and stress tolerance of postlarval shrimp, Litopenaeus vannamei . J World Aquacult Soc. 2009;40:795–802. [Google Scholar]
- Niyogi KK. Safety valves for photosynthesis. Curr Opin Plant Biol. 2000;3:455–460. doi: 10.1016/s1369-5266(00)00113-8. [DOI] [PubMed] [Google Scholar]
- Oguchi R, Terashima I, Kou J, Chow WS. Operation of dual mechanisms that both lead to photoinactivation of Photosystem II in leaves by visible light. Physiol Plant. 2011;142:47–55. doi: 10.1111/j.1399-3054.2011.01452.x. [DOI] [PubMed] [Google Scholar]
- Ohta S, Shiomi Y, Kawashima A, Aozasa O, Nakao T, Nagate T, Kitamura K, Miyata H. Antibiotic effect of linolenic acid from Chlorococcum strain HS-101 and Dunaliella primolecta on methicillin resistant Staphylococcus aureus . J Appl Phycol. 1995;7:121–127. [Google Scholar]
- Olaizola M. The health benefits of Haematococcus astaxanthin: cardiovascular health. Agro Food Ind Hi-Tech. 2005;16:35–37. [Google Scholar]
- Olsen Y, Knutsen G, Lien T. Characteristics of phosphorus limitation in Chlamydomonas reinhardtii (Chlorophyceae) and its palmelloids. J Phycol. 1983;19:313–319. [Google Scholar]
- Opuszynski K. Comparison of the usefulness of the Silver Carp and the Bighead Carp as additional fish in carp ponds. Aquaculture. 1981;25:223–233. [Google Scholar]
- Ördög V, Stirk WA, Lenobel R, Bancírová M, Strnad M, van Staden J, Szigeti J, Néřmeth L. Screening microalgae for some potentially useful agricultural and pharmaceutical secondary metabolites. J Appl Phycol. 2004;16:309–314. [Google Scholar]
- Oren A. Diversity of organic osmotic compounds and osmotic adaptation in cyanobacteria and algae. In: Seckbach J, editor. Algae and cyanobacteria in extreme environments. Springer: Dordrecht, Netherlands; 2007. pp. 641–660. (Ed.) [Google Scholar]
- Oren A, Ionescu D, Hindiyeh M, Malkawi H. Microalgae and cyanobacteria of the Dead Sea and its surrounding springs. Isr J Plant Sci. 2008;56:1–13. [Google Scholar]
- Orosa M, Valero JF, Herrero C, Abalde J. Comparison of the accumulation of astaxanthin in Haematococcus pluvialis and other green microalgae under N-starvation and high light conditions. Biotechnol Lett. 2001;23:1079–1085. [Google Scholar]
- Palozza P, Torelli C, Boninsegna A, Simone R, Catalano A, Mele MC, Picci N. Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Lett. 2009;283:108–117. doi: 10.1016/j.canlet.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Park JS, Chyun JH, Kim YK, Line LL, Chew BP. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab (Lond) 2010;7:18. doi: 10.1186/1743-7075-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil V, Källqvist T, Olsen E, Vogt G, Gislerød HR. Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquac Int. 2007;15:1–9. [Google Scholar]
- Pelah D, Sintov A, Cohen E. The effect of salt stress on the production of canthaxanthin and astaxanthin by Chlorella zofingiensis grown under limited light intensity. World J Microbiol Biotechnol. 2004;20:483–486. [Google Scholar]
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- Picataggio S. Potential impact of synthetic biology on the development of microbial systems for the production of renewable fuels and chemicals. Curr Opin Biotechnol. 2009;20:325–329. doi: 10.1016/j.copbio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Piorreck M, Baasch KH, Pohl P. Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue-green algae under different nitrogen regimes. Phytochemistry. 1984;23:207–216. [Google Scholar]
- Plaza M, Herrero M, Cifuentes A, Ibáñez E. Innovative natural functional ingredients from microalgae. J Agric Food Chem. 2009;57:7159–7170. doi: 10.1021/jf901070g. [DOI] [PubMed] [Google Scholar]
- Poerschmann J, Spijkerman E, Langer U. Fatty acid patterns in Chlamydomonas sp. as a marker for nutritional regimes and temperature under extremely acidic conditions. Microb Ecol. 2004;48:78–89. doi: 10.1007/s00248-003-0144-6. [DOI] [PubMed] [Google Scholar]
- Pollock SV, Pootakham W, Shibagaki N, Moseley JL, Grossman AR. Insights into the acclimation of Chlamydomonas reinhardtii to sulfur deprivation. Photosyn Res. 2005;86:475–489. doi: 10.1007/s11120-005-4048-9. [DOI] [PubMed] [Google Scholar]
- Ponomarenko LP, Stonik IV, Aizdaicher NA, Orlova TY, Popovskaya GI, Pomazkina GV, Stonik VA. Sterols of marine microalgae Pyramimonas cf. cordata (Prasinophyta), Attheya ussurensis sp. nov. (Bacillariophyta) and a spring diatom bloom from Lake Baikal. Comp Biochem Physiol B, Biochem Mol Biol. 2004;138:65–70. doi: 10.1016/j.cbpc.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Posewitz MC, Smolinski SL, Kanakagiri S, Melis A, Seibert M, Ghirardi ML. Hydrogen photoproduction is attenuated by disruption of an isoamylase gene in Chlamydomonas reinhardtii . Plant Cell. 2004;16:2151–2163. doi: 10.1105/tpc.104.021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posten C. Design principles of photo-bioreactors for cultivation of microalgae. Eng Life Sci. 2009;9:165–177. [Google Scholar]
- Posten C, Schaub G. Microalgae and terrestrial biomass as source for fuels–a process view. J Biotechnol. 2009;142:64–69. doi: 10.1016/j.jbiotec.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Proksch P, Edrada RA, Ebel R. Drugs from the seas – current status and microbiological implications. Appl Microbiol Biotechnol. 2002;59:125–134. doi: 10.1007/s00253-002-1006-8. [DOI] [PubMed] [Google Scholar]
- Puello-Cruz AC, Mezo-Villalobos S, Gonzalez-Rodriguez B, Voltolina D. Culture of the calanoid copepod Pseudodiaptomus euryhalinus (Johnson 1939) with different microalgal diets. Aquaculture. 2009;290:317–319. [Google Scholar]
- Pugh N, Ross SA, ElSohly HN, ElSohly MA, Pasco DS. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, aphanizomenon flos-aquae and Chlorella pyrenoidosa . Planta Med. 2001;67:737–742. doi: 10.1055/s-2001-18358. [DOI] [PubMed] [Google Scholar]
- Pulz O. Photobioreactors: production systems for phototrophic microorganisms. Appl Microbiol Biotechnol. 2001;57:287–293. doi: 10.1007/s002530100702. [DOI] [PubMed] [Google Scholar]
- Pulz O, Gross W. Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol. 2004;65:635–648. doi: 10.1007/s00253-004-1647-x. [DOI] [PubMed] [Google Scholar]
- Qiao HJ, Wang G. Effect of carbon source on growth and lipid accumulation in Chlorella sorokiniana GXNN01. Chin J Oceanol Limnol. 2009;27:762–768. [Google Scholar]
- Qin S, Liu GX, Hu ZY. The accumulation and metabolism of astaxanthin in Scenedesmus obliquus (Chlorophyceae) Process Biochem. 2008;43:795–802. [Google Scholar]
- Rabbani S, Beyer P, Lintig J, Hugueney P, Kleinig H. Induced beta-carotene synthesis driven by triacylglycerol deposition in the unicellular alga Dunaliella bardawil . Plant Physiol. 1998;116:1239–1248. doi: 10.1104/pp.116.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainuzzo JR, Reitan KI, Olsen Y. The significance of lipids at early stages of marine fish: a review. Aquaculture. 1997;155:103–115. [Google Scholar]
- Raja R, Hemaiswarya S, Balasubramanyam D, Rengasamy R. Protective effect of Dunaliella salina (Volvocales, Chlorophyta) against experimentally induced fibrosarcoma on wistar rats. Microbiol Res. 2007;162:177–184. doi: 10.1016/j.micres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Raja R, Hemaiswarya S, Kumar NA, Sridhar S, Rengasamy R. A perspective on the biotechnological potential of microalgae. Crit Rev Microbiol. 2008;34:77–88. doi: 10.1080/10408410802086783. [DOI] [PubMed] [Google Scholar]
- Rao AR, Dayananda C, Sarada R, Shamala TR, Ravishankar GA. Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour Technol. 2007;98:560–564. doi: 10.1016/j.biortech.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Rao AR, Sarada R, Baskaran V, Ravishankar GA. Antioxidant activity of Botryococcus braunii extract elucidated in vitro models. J Agric Food Chem. 2006;54:4593–4599. doi: 10.1021/jf060799j. [DOI] [PubMed] [Google Scholar]
- Reitan KI, Rainuzzo JR, Olsen Y. Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. J Phycol. 1994;30:972–979. [Google Scholar]
- Reitan KI, Rainuzzo JR, Øie G, Olsen Y. A review of the nutritional effects of algae in marine fish larvae. Aquaculture. 1997;155:207–221. [Google Scholar]
- Remias D, Holzinger A, Lutz C. Physiology, ultrastructure and habitat of the ice alga Mesotaenium berggrenii (Zygnemaphyceae, Chlorophyta) from glaciers in the European Alps. Phycologia. 2009;48:302–312. [Google Scholar]
- Remias D, Lütz-Meindl U, Lütz C. Photosynthesis, pigments and ultrastructure of the alpine snow alga Chlamydomonas nivalis . Eur J Phycol. 2005;40:259–268. [Google Scholar]
- Richmond A. Growth characteristics of ultrahigh-density microalgal cultures. Biotechnol Bioprocess Eng. 2003;8:349–353. [Google Scholar]
- Richmond A, Soeder CJ. Microalgaculture. Crit Rev Biotechnol. 1986;4:369–438. [Google Scholar]
- Riediger ND, Othman RA, Suh M, Moghadasian MH. A systemic review of the roles of n-3 fatty acids in health and disease. J Am Diet Assoc. 2009;109:668–679. doi: 10.1016/j.jada.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Rigano VD, Vona V, Carfagna S, Esposito S, Carillo P, Rigano C. Effects of sulfate-starvation and re-supply on growth, NH4+ uptake and starch metabolism in Chlorella sorokiniana . Aust J Plant Physiol. 2000;27:335–342. [Google Scholar]
- Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR. Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng. 2009;102:100–112. doi: 10.1002/bit.22033. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Garcia I, Guil-Guerrero JL. Evaluation of the antioxidant activity of three microalgal species for use as dietary supplements and in the preservation of foods. Food Chem. 2008;108:1023–1026. doi: 10.1016/j.foodchem.2007.11.059. [DOI] [PubMed] [Google Scholar]
- Running JA, Severson DK, Schneider KJ. Extracellular production of L-ascorbic acid by Chlorella protothecoides, Prototheca species, and mutants of P. moriformis during aerobic culturing at low pH. J Ind Microbiol Biotechnol. 2002;29:93–98. doi: 10.1038/sj.jim.7000275. [DOI] [PubMed] [Google Scholar]
- Russo GL. Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol. 2009;77:937–946. doi: 10.1016/j.bcp.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Sakai N, Sakamoto Y, Kishimoto N, Chihara M, Karube I. Chlorella strains from hot springs tolerant to high temperature and high CO2 . Energy Conv Manag. 1995;36:693. [Google Scholar]
- Salguero A, de la Morena B, Vigara J, Vega JM, Vilchez C, León R. Carotenoids as protective response against oxidative damage in Dunaliella bardawil . Biomol Eng. 2003;20:249–253. doi: 10.1016/s1389-0344(03)00065-0. [DOI] [PubMed] [Google Scholar]
- Sánchez JF, Fernández-Sevilla JM, Acién FG, Cerón MC, Pérez-Parra J, Molina-Grima E. Biomass and lutein productivity of Scenedesmus almeriensis: influence of irradiance, dilution rate and temperature. Appl Microbiol Biotechnol. 2008;79:719–729. doi: 10.1007/s00253-008-1494-2. [DOI] [PubMed] [Google Scholar]
- Sargent J, McEvoy L, Estevez A, Bell G, Bell M, Henderson J, Tocher D. Lipid nutrition of marine fish during early development: current status and future directions. Aquaculture. 1999;179:217–229. [Google Scholar]
- Sato N, Sonoike K, Kawaguchi A, Tsuzuki M. Contribution of lowered unsaturation levels of chloroplast lipids to high temperature tolerance of photosynthesis in Chlamydomonas reinhardtii . J Photochem Photobiol B-Biol. 1996;36:333–337. [Google Scholar]
- Sato N, Sonoike K, Tsuzuki M, Kawaguchi A. Impaired photosystem II in a mutant of Chlamydomonas reinhardtii defective in sulfoquinovosyl diacylglycerol. Eur J Biochem. 1995;234:16–23. doi: 10.1111/j.1432-1033.1995.016_c.x. [DOI] [PubMed] [Google Scholar]
- Satoh A, Tsuji S, Okada Y, Murakami N, Urami M, Nakagawa K, Ishikura M, Katagiri M, Koga Y, Shirasawa T. Preliminary clinical evaluation of toxicity and efficacy of a new astaxanthin-rich Haematococcus pluvialis extract. J Clin Biochem Nutr. 2009;44:280–284. doi: 10.3164/jcbn.08-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, Smith CM, Fork DC. Effects of salinity on primary processes of photosynthesis in the red alga Porphyra perforata . Plant Physiol. 1983;73:643–647. doi: 10.1104/pp.73.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B. Second generation biofuels: High-efficiency microalgae for biodiesel production. Bioenerg Res. 2008;1:20–43. [Google Scholar]
- Schmid D, Schürch C, Zülli F. Mycosporine-like amino acids from red algae protect against premature skin-aging. Euro Cosmetics. 2006;9:1–4. [Google Scholar]
- Schreiner O, Lien T, Knutsen G. The capacity for arylsulfatase synthesis in synchronous and synchronized cultures of Chlamydomonas reinhardtii . Biochim Biophys . 1975;384:Acta. 180–193. doi: 10.1016/0005-2744(75)90107-2. [DOI] [PubMed] [Google Scholar]
- Scott KJ, Rodriquez-Amaya D. Pro-vitamin A carotenoid conversion factors: retinol equivalents – fact or fiction? 2000;69:Food Chem. 125–127. [Google Scholar]
- Seckbach J. Algae and cyanobacteria in extreme environments. Springer; Dordrecht, Netherlands: 2007. [Google Scholar]
- Seixas P, Rey-Méndez M, Valente LMP, Otero A. Producing juvenile Artemia as prey for Octopus vulgaris paralarvae with different microalgal species of controlled biochemical composition. 2008;283:Aquaculture. 83–91. [Google Scholar]
- Seto A, Wang HL, Hesseltine CW. Culture conditions affect eicosapentaenoic acid content of Chlorella minutissima . J Am Oil Chem Soc. 1984;61:892–894. [Google Scholar]
- Seymour EH, Murray L, Fernandes R. Key challenges to the introduction of hydrogen – European stakeholder views. Int J Hydrogen Energy. 2008;33:3015–3020. [Google Scholar]
- Sheng JC, Yu F, Xin ZH, Zhao LY, Zhu XJ, Hu QH. Preparation, identification and their antitumor activities in vitro of polysaccharides from Chlorella pyrenoidosa . Food Chem. 2007;105:533–539. [Google Scholar]
- Shi X, Wu Z, Chen F. Kinetic modeling of lutein production by heterotrophic Chlorella at various pH and temperatures. Mol Nutr Food Res. 2006;50:763–768. doi: 10.1002/mnfr.200600037. [DOI] [PubMed] [Google Scholar]
- Shon YH, Nam KS, Kim MK. Cancer chemopreventive potential of Scenedesmus spp. cultured in medium containing bioreacted swine urine. J Microbiol Biotechnol. 2004;14:158–161. [Google Scholar]
- Shpigel M, Schlosser SC, Ben-Amotz A, Lawrence AL, Lawrence JM. Effects of dietary carotenoid on the gut and the gonad of the sea urchin Paracentrotus lividus . Aquaculture. 2006;261:1269–1280. [Google Scholar]
- Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr. 1999;70:560S–569S. doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- Sinha RP, Singh SP, Häder DP. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J Photochem Photobiol B, Biol. 2007;89:29–35. doi: 10.1016/j.jphotobiol.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Skjånes K, Knutsen G, Källqvist T, Lindblad P. H2 production from marine and freshwater species of green algae during sulfur starvation and considerations for bioreactor design. Int J Hydrogen Energy. 2008;33:511–521. [Google Scholar]
- Skjånes K, Lindblad P, Muller J. BioCO2 – a multidisciplinary, biological approach using solar energy to capture CO2 while producing H2 and high value products. Biomol Eng. 2007;24:405–413. doi: 10.1016/j.bioeng.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Soeder CJ, Hegewald E. Scenedesmus. In: Borowitzka MA, Borowitzka LJ, editors. Micro-algal biotechnology. Cambridge University Press; Cambridge: 1988. pp. 59–84. (Eds.) [Google Scholar]
- Solovchenko AE, Khozin-Goldberg I, Cohen Z, Merzlyak MN. Carotenoid-to-chlorophyll ratio as a proxy for assay of total fatty acids and arachidonic acid content in the green microalga Parietochloris incisa . J Appl Phycol. 2009;21:361–366. [Google Scholar]
- Solovchenko AE, Khozin-Goldberg I, Didi-Cohen S, Cohen Z, Merzlyak MN. Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa . J Appl Phycol. 2008;20:245–251. [Google Scholar]
- Souto M, Saavedra M, Pousão-Ferreira P, Herrero C. Riboflavin enrichment throughout the food chain from the marine microalga Tetraselmis suecica to the rotifer Brachionus plicatilis and to White Sea Bream (Diplodus sargus) and Gilthead Sea bream (Sparus aurata) larvae. Aquaculture. 2008;283:128–133. [Google Scholar]
- Spoehr HA, Milner HW. The chemical composition of Chlorella; effect of environmental conditions. Plant Physiol. 1949;24:120–149. doi: 10.1104/pp.24.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolaore P, Joannis-Cassan C, Duran E, Isambert A. Commercial applications of microalgae. J Biosci Bioeng. 2006;101:87–96. doi: 10.1263/jbb.101.87. [DOI] [PubMed] [Google Scholar]
- Spruell JA. Response of algae and zooplankton to C18 fatty acids of Chlamydomonas reinhardtii . Hydrobiologia. 1984;114:9–12. [Google Scholar]
- Stephens E, Ross IL, King Z, Mussgnug JH, Kruse O, Posten C, Borowitzka MA, Hankamer B. An economic and technical evaluation of microalgal biofuels. Nat Biotechnol. 2010;28:126–128. doi: 10.1038/nbt0210-126. [DOI] [PubMed] [Google Scholar]
- Stiller C, Bünger U, Møller-Holst S, Svensson AM, Espegren KA, Nowak M. Pathways to a hydrogen fuel infrastructure in Norway. Int J Hydrogen Energy. 2010;35:2597–2601. sp. iss. [Google Scholar]
- Stoynova-Bakalova E, Toncheva-Panova T. Subcellular adaptation to salinity and irradiance in Dunaliella salina . Biol Plantarum. 2003;47:233–236. [Google Scholar]
- Strizh IG, Popova LG, Balnokin YV. Physiological aspects of adaptation of the marine microalga Tetraselmis (Platymonas) viridis to various medium salinity. Russ J Plant Physiol. 2004;51:176–182. [Google Scholar]
- Subhadra BG. Sustainability of algal biofuel production using integrated renewable energy park (IREP) and algal biorefinery approach. Energy Policy. 2010;38:5892–5901. [Google Scholar]
- Sugimoto K, Sato N, Tsuzuki M. Utilization of a chloroplast membrane sulfolipid as a major internal sulfur source for protein synthesis in the early phase of sulfur starvation in Chlamydomonas reinhardtii . FEBS Lett. 2007;581:4519–4522. doi: 10.1016/j.febslet.2007.08.035. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Sato N, Watanabe A, Tsuzuki M. Effects of sulfur deprivation on sulfolipid metabolism in Chlamydomonas reinhardtii . In: Saito K., editor. Sulfur transport and assimilation in plants in the postgenomic era. Backhuys Publishers; Leiden, The Netherlands: 2005. pp. 133–135. (Ed.) [Google Scholar]
- Surai AP, Surai PF, Steinberg W, Wakeman WG, Speake BK, Sparks NH. Effect of canthaxanthin content of the maternal diet on the antioxidant system of the developing chick. Br Poult Sci. 2003;44:612–619. doi: 10.1080/00071660310001616200. [DOI] [PubMed] [Google Scholar]
- Surzycki R, Greenham K, Kitayama K, Dibal F, Wagner R, Rochaix JD, Ajam T, Surzycki S. Factors effecting expression of vaccines in microalgae. Biologicals. 2009;37:133–138. doi: 10.1016/j.biologicals.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Sushchik NN, Kalacheva GS, Zhila NO, Gladyshev MI, Volova TG. A temperature dependence of the intra- and extracellular fatty acid composition of green algae and cyanobacterium. Russ J Plant Physiol. 2003;50:374–380. [Google Scholar]
- Szyszka B, Ivanov AG, Hüner NP. Psychrophily is associated with differential energy partitioning, photosystem stoichiometry and polypeptide phosphorylation in Chlamydomonas raudensis. . Biochim Biophys Acta. 2007;1767:789–800. doi: 10.1016/j.bbabio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Badger MR. Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci. 2011;16:53–60. doi: 10.1016/j.tplants.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Milward SE, Yamori W, Evans JR, Hillier W, Badger MR. The solar action spectrum of photosystem II damage. Plant Physiol. 2010;153:988–993. doi: 10.1104/pp.110.155747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Yamada A, Noda K, Hasegawa T, Okuda M, Shoyama Y, Nomoto K. A novel glycoprotein obtained from Chlorella vulgaris strain CK22 shows antimetastatic immunopotentiation. Cancer Immunol Immunother. 1998;45:313–320. doi: 10.1007/s002620050448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BJ, Liu BZ, Wang GD, Zhang T, Xiang JH. Effects of various algal diets and starvation on larval growth and survival of Meretrix meretrix . Aquaculture. 2006;254:526–533. [Google Scholar]
- Tatsuzawa H, Takizawa E, Wada M, Yamamoto Y. Fatty acid and lipid composition of the acidophilic green alga Chlamydomonas sp. J Phycol. 1996;32:598–601. [Google Scholar]
- Tejera N, Cejas JR, Rodriguez C, Bjerkeng B, Jerez S, Bolanos A, Lorenzo A. Pigmentation, carotenoids, lipid peroxides and lipid composition of skin of red porgy (Pagrus pagrus) fed diets supplemented with different astaxanthin sources. Aquaculture. 2007;270:218–230. [Google Scholar]
- Thaipratum R, Melis A, Svasti J, Yokthongwattana K. Analysis of non-photochemical energy dissipating processes in wild type Dunaliella salina (green algae) and in zea1, a mutant constitutively accumulating zeaxanthin. J Plant Res. 2009;122:465–476. doi: 10.1007/s10265-009-0229-5. [DOI] [PubMed] [Google Scholar]
- Timmins M, Thomas-Hall SR, Darling A, Zhang E, Hankamer B, Marx UC, Schenk PM. Phylogenetic and molecular analysis of hydrogen-producing green algae. J Exp Bot. 2009;60:1691–1702. doi: 10.1093/jxb/erp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmins M, Zhou WX, Rupprecht J, Lim L, Thomas-Hall SR, Doebbe A, Kruse O, Hankamer B, Marx UC, Smith SM, Schenk PM. The metabolome of Chlamydomonas reinhardtii following induction of anaerobic H2 production by sulfur depletion. J Biol Chem. 2009b;284:23415–23425. doi: 10.1074/jbc.M109.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjahjono AE, Hayama Y, Kakizono T, Terada Y, Nishio N, Nagai S. Hyper accumulation of astaxanthin in a green alga Haematococcus pluvialis at elevated temperatures. Biotechnol Lett. 1994;16:133–138. [Google Scholar]
- Tolstygina IV, Antal TK, Kosourov SN, Krendeleva TE, Rubin AB, Tsygankov AA. Hydrogen production by photoautotrophic sulfur-deprived Chlamydomonas reinhardtii pre-grown and incubated under high light. Biotechnol Bioeng. 2009;102:1055–1061. doi: 10.1002/bit.22148. [DOI] [PubMed] [Google Scholar]
- Tsygankov A, Kosourov S, Seibert M, Ghirardi ML. Hydrogen photoproduction under continuous illumination by sulfur-deprived, synchronous Chlamydomonas reinhardtii cultures. Int J Hydrogen Energy. 2002;27:1239–1244. [Google Scholar]
- Tsygankov AA. Laboratory scale photobioreactors. Appl Biochem Microbiol. 2001;37:333–341. [Google Scholar]
- Tsygankov AA, Kosourov SN, Tolstygina IV, Ghirardi ML, Seibert M. Hydrogen production by sulfur-deprived Chlamydomonas reinhardtii under photoautotrophic conditions. Int J Hydrogen Energy . 2006;31:1574–1584. [Google Scholar]
- Tukaj Z, Matusiak-Mikulin K, Lewandowska J, Szurkowski J. Changes in the pigment patterns and the photosynthetic activity during a light-induced cell cycle of the green alga Scenedesmus armatus . Plant Physiol Biochem. 2003;41:337–344. [Google Scholar]
- Turker H, Eversole AG, Brune DE. Filtration of green algae and cyanobacteria by Nile tilapia, Oreochromis niloticus, in the Partitioned Aquaculture System. Aquaculture. 2003;215:93–101. [Google Scholar]
- Tyystjärvi E. Photoinhibition of Photosystem II and photodamage of the oxygen evolving manganese cluster. Coord Chem Rev. 2008;252:361–376. [Google Scholar]
- Tzovenis I, De Pauw N, Sorgeloos P. Optimisation of T-ISO biomass production rich in essential fatty acids: I. Effect of different light regimes on growth and biomass production. Aquaculture. 2003;216:203–222. [Google Scholar]
- Tzovenis I, Fountoulaki E, Dolapsakis N, Kotzamanis I, Nengas I, Bitis I, Cladas Y, Economou-Amilli A. Screening for marine nanoplanktic microalgae from Greek coastal lagoons (Ionian Sea) for use in mariculture. J Appl Phycol. 2009;21:457–469. [Google Scholar]
- Ueno Y, Kurano N, Miyachi S. Ethanol production by dark fermentation in the marine green alga, Chlorococcum littorale . J Ferment Bioeng. 1998;86:38–43. [Google Scholar]
- Ueno Y, Kurano N, Miyachi S. Purification and characterization of hydrogenase from the marine green alga, Chlorococcum littorale . FEBS Lett. 1999;443:144–148. doi: 10.1016/s0014-5793(98)01699-8. [DOI] [PubMed] [Google Scholar]
- Ugwu CU, Aoyagi H. Influence of shading inclined tubular photobioreactor surfaces on biomass productivity of C. sorokiniana . Photosynthetica. 2008;46:283–285. [Google Scholar]
- Uhlik DJ, Gowans CS. Synthesis of nicotinic acid in Chlamydomonas eugametos . Int J Biochem. 1974;5:79–84. [Google Scholar]
- Ukeles R. The effect of temperature on the growth and survival of several marine algal species. Biol Bull. 1961;120:255–264. [Google Scholar]
- van Eykelenburg C. Spirulina platensis – Morphology & Ultrastructure, PhD Thesis, Technische Hogeschool Delft. Delft: 1980. pp. 118. [Google Scholar]
- Vasilikiotis C, Melis A. Photosystem II reaction center damage and repair cycle: chloroplast acclimation strategy to irradiance stress. Proc Natl Acad Sci USA. 1994;91:7222–7226. doi: 10.1073/pnas.91.15.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan PT, Briggs M. Biodiesel production–current state of the art and challenges. J Ind Microbiol Biotechnol. 2008;35:421–430. doi: 10.1007/s10295-008-0312-2. [DOI] [PubMed] [Google Scholar]
- Vilchez C, Garbayo I, Lobato MV, Vega JM. Microalgae-mediated chemicals production and wastes removal. Enz Microb Technol. 1997;20:562–572. [Google Scholar]
- Villar R, Laguna MR, Calleja JM, Cadavid I. Effects of Phaeodactylum tricornutum and Dunaliella tertiolecta extracts on the central nervous system. Planta Med. 1992;58:405–409. doi: 10.1055/s-2006-961501. [DOI] [PubMed] [Google Scholar]
- Visviki I, Santikul D. The pH tolerance of Chlamydomonas applanata (Volvocales, Chlorophyta) Arch Environ Contam Toxicol. 2000;38:147–151. doi: 10.1007/s002449910018. [DOI] [PubMed] [Google Scholar]
- Wang ZT, Ullrich N, Joo S, Waffenschmidt S, Goodenough U. Algal lipid bodies: stress induction, purification, and biochemical characterization in wild-type and starchless Chlamydomonas reinhardtii . Eukaryotic Cell. 2009;8:1856–1868. doi: 10.1128/EC.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX, Zhuge J, Fang H, Prior BA. Glycerol production by microbial fermentation: a review. Biotechnol Adv. 2001;19:201–223. doi: 10.1016/s0734-9750(01)00060-x. [DOI] [PubMed] [Google Scholar]
- Warburg O. Über die Geschwindigkeit der photochemischen Kohlensaurezersetzung in lebenden Zellen. Biochem Z. 1919;100:230. [Google Scholar]
- Watanabe T, Kitajima C, Fujita S. Nutritional values of live organisms used in Japan for mass propagation of fish – A review. Aquaculture. 1983;34:115–143. [Google Scholar]
- Wei Z, Cady CW, Brudvig GW, Hou HJ. Photodamage of a Mn(III/IV)-oxo mixed-valence compound and photosystem II: evidence that a high-valent manganese species is responsible for UV-induced photodamage of the oxygen-evolving complex in photosystem II. J Photochem Photobiol B, Biol. 2011;104:118–125. doi: 10.1016/j.jphotobiol.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Winkler M, Hemschemeier A, Gotor C, Melis A, Happe T. [Fe]-hydrogenases in green algae: photo-fermentation and hydrogen evolution under sulfur deprivation. Int J Hydrogen Energy. 2002;27:1431–1439. [Google Scholar]
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Wu ZY, Wu SM, Shi XM. Supercritical fluid extraction and determination of lutein in heterotrophically cultivated Chlorella pyrenoidosa . J Food Process Eng. 2007;30:174–185. [Google Scholar]
- Wünschiers R, Lindblad P. Hydrogen in education – a biological approach. Int J Hydrogen Energy. 2002;27:1131–1140. [Google Scholar]
- Wykoff DD, Davies JP, Melis A, Grossman AR. The regulation of photosynthetic electron transport during nutrient deprivation in Chlamydomonas reinhardtii . Plant Physiol. 1998;117:129–139. doi: 10.1104/pp.117.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong FS, Kopecky J, Nedbal L. The occurrence of UV-B absorbing mycosporine-like amino acids in freshwater and terrestrial microalgae (Chlorophyta) Aquat Bot. 1999;63:37–49. [Google Scholar]
- Xu L, Weathers PJ, Xiong XR, Liu CZ. Microalgal bioreactors: Challenges and opportunities. Eng Life Sci. 2009;9:178–189. [Google Scholar]
- Yamaguchi K. Recent advances in microalgal bioscience in Japan, with special reference to utilization of biomass and metabolites: A review. J Appl Phycol. 1997;8:487–502. [Google Scholar]
- Yang FM, Shi Y, Sheng JC, Hu QH. In vivo immunomodulatory activity of polysaccharides derived from Chlorella pyrenoidosa . Eur Food Res Technol. 2006;224:225–228. [Google Scholar]
- Yeum KJ, Russell RM. Carotenoid bioavailability and bioconversion. Annu Rev Nutr. 2002;22:483–504. doi: 10.1146/annurev.nutr.22.010402.102834. [DOI] [PubMed] [Google Scholar]
- Yildiz FH, Davies JP, Grossman AR. Characterization of sulfate transport in Chlamydomonas reinhardtii during sulfur-limited and sulfur-sufficient growth. Plant Physiol. 1994;104:981–987. doi: 10.1104/pp.104.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yongmanitchai WC, Ward OP. Omega-3 fatty acids – Alternative sources of production. Process Biochem. 1989;24:117–125. [Google Scholar]
- Yuan JP, Chen F, Liu X, Li XZ. Carotenoid composition in the green microalga Chlorococcum . Food Chem. 2002;76:319–325. [Google Scholar]
- Zhang CW, Cohen Z, Khozin-Goldberg I, Richmond A. Characterization of growth and arachidonic acid production of Parietochloris incisa comb. nov (Trebouxiophyceae, Chlorophyta) J Appl Phycol. 2002a;14:453–460. [Google Scholar]
- Zhang L, Happe T, Melis A. Biochemical and morphological characterization of sulfur-deprived and H2-producing Chlamydomonas reinhardtii (green alga) Planta. 2002;214:552–561. doi: 10.1007/s004250100660. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Shrager J, Jain M, Chang CW, Vallon O, Grossman AR. Insights into the survival of Chlamydomonas reinhardtii during sulfur starvation based on microarray analysis of gene expression. Eukaryotic Cell. 2004;3:1331–1348. doi: 10.1128/EC.3.5.1331-1348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhekisheva M, Boussiba S, Khozin-Goldberg I, Zarka A, Cohen Z. Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters. J Phycol. 2002;38:325–331. [Google Scholar]
- Zhekisheva M, Zarka A, Khozin-Goldberg I, Cohen Z, Boussiba S. Inhibition of astaxanthin synthesis under high irradiance does not abolish triacylglycerol accumulation in the green alga Haematococcus pluvialis (Chlorophyceae) J Phycol. 2005;41:819–826. [Google Scholar]
- Zmora O, Findiesen A, Stubblefield J, Frenkel V, Zohar Y. Large-scale juvenile production of the blue crab Callinectes sapidus . Aquaculture. 2005;244:129–139. [Google Scholar]
- Zuppini A, Gerotto C, Moscatiello R, Bergantino E, Baldan B. Chlorella saccharophila cytochrome f and its involvement in the heat shock response. J Exp Bot. 2009;60:4189–4200. doi: 10.1093/jxb/erp264. [DOI] [PMC free article] [PubMed] [Google Scholar]