Abstract
Aims. The aim of this study was to investigate the relationship of echocardiographic epicardial fat thickness (EFT) with carotid intima-media thickness (CIMT), in patients with type 2 diabetes mellitus (T2DM). Methods and Results. A total of 139 patients with T2DM (mean age 54.3 ± 9.2 and 49.6% male) and 40 age and sex-matched control subjects were evaluated. Echocardiographic EFT and ultrasonographic CIMT were measured in all subjects. Patients with T2DM had significantly increased EFT and CIMT than those of the controls (6.0 ± 1.5 mm versus 4.42 ± 1.0 mm, P < 0.001 and 0.76 ± 0.17 mm versus 0.57 ± 0.14 mm, P < 0.001, resp.). EFT was correlated with CIMT, waist circumference, BMI, age, duration of T2DM, HbA1c in the type 2 diabetic patients. Linear regression analysis showed that CIMT (β = 3.52, t = 3.72, P < 0.001) and waist circumference (β = 0.36, t = 2.26, P = 0.03) were found to be independent predictors of EFT. A cutoff high risk EFT value of 6.3 mm showed a sensitivity and specificity of 72.5% and 71.7%, respectively, for the prediction of subclinical atherosclerosis. Conclusion. We found that echocardiographic EFT was significantly higher in patients with T2DM. Our study also showed that EFT was strongly correlated with waist circumference and CIMT as being independent of sex.
1. Introduction
Type 2 diabetes mellitus (T2DM) is one of the most common chronic diseases in the worldwide, the incidence of which tends to grow steadily. It is associated with a high risk of cardiovascular disease (CVD) which is the leading cause of death in patients with type 2 diabetes mellitus [1].
Obesity, insulin resistance, and diabetes have identified a proinflammatory state associated with increased adiposity [2]. Epicardial adipose tissue (EAT) is a visceral fat depot of the heart located along the large coronary arteries and on the surface of the ventricles and apex [3]. The embryological origin of EAT is similar to intra-abdominal visceral adipose tissue [4]. Several studies have shown that EAT is not only an anatomic depot of fat but also may serve as a local source of proinflammatory cytokines related to coronary artery disease (CAD) [5]. Therefore, EAT thickness has been considered to be a possible cardiovascular risk indicator [6, 7]. Transthoracic echocardiography (TTE), magnetic resonance imaging (MRI), and multislice computed tomography (MSCT) scanning have been conventional methods for quantifying EAT [8]. Assessment of EAT by TTE could be a simple and practical tool for cardiovascular risk stratification in clinical practice [3].
Carotid intima-media thickness (CIMT) is a simple and inexpensive tool to assess the cumulative effect of atherosclerotic risk factors and is an independent predictor of future cardiovascular (CV) risk [9]. The ultrasound-based measurement of CIMT has become a standard for assessing arteriosclerosis and is recommended by the American Heart Association for the noninvasive assessment of cardiovascular risk [10, 11].
Previous studies have reported that increased EAT is associated with CAD, metabolic syndrome (MetS) and obesity [12–16]. In the present study, we evaluated type 2 diabetic patients to investigate epicardial fat thickness by TTE and investigate its relationship with CIMT.
2. Methods
2.1. Patient Population
In this observational, cross-sectional study, 139 type 2 diabetic patients, having this diagnosis for at least 1 year, were consecutively included in the study. The control group consisted of 40 sex and age-matched healthy people. T2DM was diagnosed according to the American Diabetes Association criteria [17]. The study protocol was approved by our local ethics committee, and all patients gave their written informed consent to participate in the study.
Exclusion criteria of the study were subjects with known ischemic heart disease, cerebrovascular disease, peripheral vascular disease, congestive heart failure, valvular heart disease, and chronic kidney disease.
Medical history was obtained and physical examination was performed in all patients and controls. Blood pressure was measured three times—5 min apart—in a sitting position, on the right arm, and the mean value was calculated. Weight and height of the patients were measured without heavy outer garments and shoes, after a 12 h fasting period. Body-mass index (BMI) was calculated as body weight divided by the square of the height. Waist circumference was measured at the level of midway between the lower rib margin and iliac crest after removal of the clothes. Blood samples were withdrawn by venipuncture from all subjects following 12 h of fasting. Fasting blood glucose, serum creatinine, total cholesterol, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), and triglyceride levels were recorded. Glucose, creatinine, and lipid profile were determined using standard methods. Hemoglobin A1c (HbA1c) levels were measured by high pressure liquid chromatography with a thermo system. Serum CRP levels were evaluated using the nephelometric method.
2.2. Measurements of Epicardial Adipose Tissue Thickness
Each patient underwent a complete transthoracic echocardiography using the American Society of Echocardiography guidelines of measurement [18]. Echocardiogram was performed using a Vivid 7 (General Electronic, Wauke-sha, Wisconsin, USA) with a 2.5–3.5 MHz transducer, placed on the III–IV left intercostal space along the parasternal line, with patients being supine in left lateral decubitus and the head of the bed kept at 30°. All examinations were performed by an experienced cardiologist, blind to the patient's clinical information. Epicardial fat was identified as the space or layer anterior to the right ventricle with decreased echoreflectivity compared with the myocardium and pericardium. Epicardial fat thickness (EFT) was measured in end diastole on the free wall of the right ventricle from the parasternal long- and short-axis views, as previously described [19, 20]. The maximum values at any site were measured, and the average value was considered. Imaging constraints were used to ensure that the epicardial fat thickness was not measured obliquely. The intraobserver correlation coefficient was 0.96. Parasternal long- and short-axis views allow the most accurate measurement of EAT on the right ventricle, with optimal cursor beam orientation in each view.
2.3. Carotid Ultrasonography
Carotid arteries were evaluated using a Logiq 7 (General Electronic, Wauke-sha, Wisconsin, USA) with a 7.5 MHz transducer. All examinations were performed by an experienced radiologist, blind to the patient's clinical information. Measurements involved a primary transverse and longitudinal scanning of the common carotid artery, bifurcation, and internal carotid. The CIMT was measured on the far wall at 1 cm from bifurcation of the common carotid artery as the distance between the lumen-intima interface and the media-adventitia interface. At least three measurements were performed on both sides, and the average measurement was taken as the CIMT. All measurements were made at a plaque-free site. The intraobserver correlation coefficient was 0.97.
2.4. Statistical Analysis
SPSS statistical software (SPSS for Windows, version 17.0, Inc., Chicago, IL, USA) was used for all statistical calculations. Categorical variables were expressed as number and proportions, while continuous variables were expressed as mean ± standard deviation. Chi-square (χ 2) test was used to compare groups regarding categorical variables. Continuous variables were compared with Student t-test (while comparing parametric variables between diabetic patients and controls) or Mann-Whitney U test (while comparing nonparametric variables between diabetic patients and controls). Correlation analysis was performed using Pearson or Spearman tests. Linear regression analysis was used to explore the independent determinants of EFT. Receiver operating characteristic (ROC) curve analysis was performed to determine cutoff high risk value of EFT when patients are divided into two groups according to CIMT (< or ≥ 0.9 mm). Levels of statistical significance were set at a P value <0.05.
3. Results
There were 139 patients (mean age 54.3 ± 9.2 and 49.6% male) in the T2DM group and 40 patients (mean age 52.1 ± 7.3 and 50% male) in the control group. The demographic findings and laboratory values of the study groups are presented on Table 1. The mean duration of disease in patients with T2DM was 6.5 ± 3.9 years. The Age, frequencies of sex distribution, hypertension, hyperlipidaemia, smoking, family history of the CAD, and waist circumference were similar between patients with T2DM and the controls. BMI was higher in the control group compared with the value of the type 2 diabetic patients (29.1 ± 4.2 versus 27.6 ± 3.1, P = 0.03, resp.). Patients with T2DM had significantly increased EFT and CIMT than those of the controls (6.0 ± 1.5 mm versus 4.42 ± 1.0 mm, P < 0.001 and 0.76 ± 0.17 mm versus 0.57 ± 0.14 mm, P < 0.001, resp.). When laboratory findings were compared, type 2 diabetic patients had significantly higher fasting blood glucose, creatinine, C-reactive protein levels, and HbA1c, but total cholesterol, LDL, HDL and triglyceride levels did not differ between the two groups.
Table 1.
Clinical and biochemical characteristics of the patients with type 2 diabetic patients and healthy controls.
| Parameters | DM (n = 139) | Controls (n = 40) | P value |
|---|---|---|---|
| Age (years) | 54.3 ± 9.2 | 52.1 ± 7.3 | 0.109 |
| Gender (male, %) | 49.6% | 50% | 0.968 |
| Disease duration (yrs) | 6.5 ± 3.9 | — | — |
| Hypertension (%) | 41% | 35% | 0.494 |
| Smoking (%) | 20.9% | 15% | 0.410 |
| Hyperlipidaemia (%) | 35.3% | 32.5% | 0.747 |
| Family history of CAD (%) | 16.5% | 15% | 0.815 |
| Epicardial fat thickness (mm) | 6.0 ± 1.5 | 4.42 ± 1.0 | <0.001 |
| Carotid artery intima-media thickness (mm) | 0.76 ± 0.17 | 0.57 ± 0.14 | <0.001 |
| Body mass index (kg/m2) | 27.6 ± 3.1 | 29.1 ± 4.2 | 0.03 |
| Waist circumference (cm) | 90.8 ± 9.3 | 89.1 ± 11.2 | 0.392 |
| Systolic blood pressure (mm Hg) | 133.9 ± 17.6 | 129.4 ± 12.4 | 0.209 |
| Diastolic blood pressure (mm Hg)* | 78 (40–100) | 80 (60–90) | 0.440 |
| Fasting glucose (mg/dL) | 147 ± 38.8 | 94.1 ± 6.3 | <0.001 |
| Creatinine (mg/dL) | 0.88 ± 0.15 | 0.77 ± 0.17 | 0.03 |
| Total cholesterol (mg/dL) | 199.1 ± 28.1 | 198.4 ± 23.9 | 0.933 |
| LDL (mg/dL) | 116.1 ± 25.0 | 118.3 ± 18.0 | 0.734 |
| HDL (mg/dL) | 43.5 ± 9.4 | 46.6 ± 10.0 | 0.247 |
| Triglyceride (mg/dL) | 172 ± 72.7 | 163.2 ± 85.2 | 0.681 |
| C-reactive protein (mg/dL) | 3.0 (2–13.1) | 1.9 (0.93–7.6) | 0.002 |
| HbA1c (%) | 7.4 ± 1.2 | 5.1 ± 0.41 | <0.001 |
| Hemoglobin (g/dL) | 14.2 ± 1.6 | 14.3 ± 1.7 | 0.741 |
| Platelets (×109/L) | 276.2 ± 70.4 | 251.5 ± 42.9 | 0.167 |
Data are presented as mean ± s.d. or n (%). CAD: coronary artery disease; HDL: high-density lipoprotein; LDL: low-density lipoprotein. *Diastolic blood pressure is presented as the median (min–max).
The variables correlated with EFT in the type 2 diabetic patients were CIMT (r = 0.479, P < 0.001) (Figure 1), waist circumference (r = 0.371, P < 0.001), BMI (r = 0.315, P < 0.001), age (r = 0.260, P = 0.002), duration of DM (r = 0.258, P = 0.003) (Figure 2), and HbA1c (r = 0.200, P = 0.032) (Table 2), and those in the controls were; CIMT (r = 0.690, P < 0.001), waist circumference (r = 0.420, P = 0.02), and age (r = 0.365, P = 0.02). When we performed subgroup analysis in the patients with T2DM according to sex, EFT was correlated with CIMT (r = 0.481, P < 0.001), waist circumference (r = 0.429, P < 0.001), age (0.348, P = 0.003), BMI (r = 0.391, P < 0.001), duration of DM (r = 0.293, P = 0.014), and weight (r = 0.285, P = 0.018) in female patients and CIMT (r = 0.481, P < 0.001) and waist circumference (r = 0.263, P < 0.03) in male patients. After multivariate stepwise linear regression analysis, CIMT (β = 3.52, t = 3.72, P < 0.001) and waist circumference (β = 0.36, t = 2.26, P = 0.03) were found to be independent relevant factors of EFT in patients with T2DM (Table 3).
Figure 1.
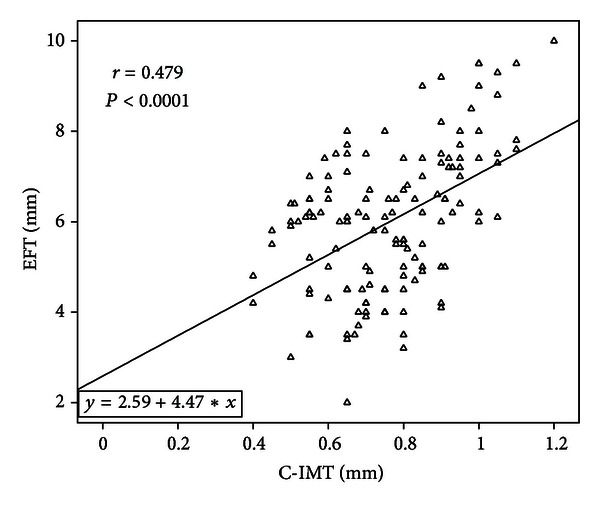
Epicardial fat thickness (EFT) is positively correlated with carotid intima-media thickness (CIMT) in diabetic patients.
Figure 2.
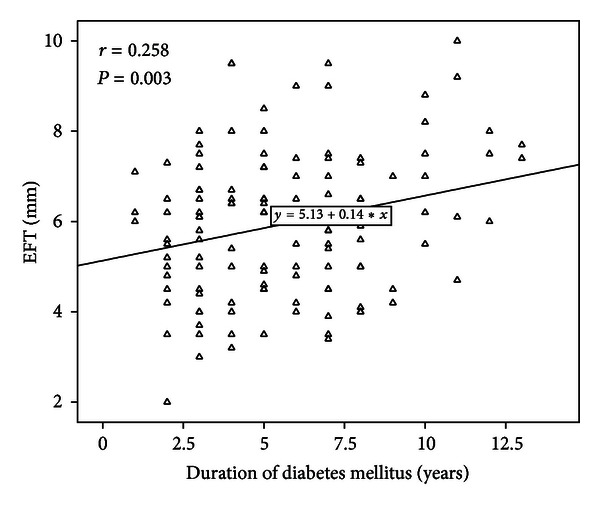
Duration of diabetes mellitus is linear and positively correlated with epicardial fat thickness (EFT).
Table 2.
The univariate correlations of the epicardial fat thickness.
| Variable | r | P |
|---|---|---|
| Carotid artery intima-media thickness | 0.479 | <0.001 |
| Waist circumference | 0.371 | <0.001 |
| Body mass index | 0.315 | <0.001 |
| Age | 0.260 | 0.002 |
| Disease duration | 0.258 | 0.003 |
| HbA1c | 0.200 | 0.032 |
| Weight | 0.165 | 0.054 |
The correlations with a P > 0.10 are not shown.
Table 3.
Independent predictors for epicardial fat thickness by multivariate stepwise linear regression analysis.
| Model | Unstandardized coefficients | Standardized coefficients | t | P value | 95.0% confidence interval for B | ||
|---|---|---|---|---|---|---|---|
| B | Std. error | Beta | Lower bound | Upper bound | |||
| Carotid artery intima-media thickness | 3.52 | 0.946 | 0.362 | 3.719 | <0.001 | 1.64 | 5.40 |
| Waist circumference | 0.36 | 0.016 | 0.220 | 2.263 | 0.026 | 0.004 | 0.068 |
The model including CIMT, age, weight, HbA1c, waist circumference, duration of DM and BMI. B: Coefficient of regression.
The patients with T2DM are divided into two groups according to the values of CIMT (< or ≥0.9 mm), which is an indicator level of subclinical organ damage as proposed by ESC hypertension guideline [21], to determine cutoff high risk value of EFT in patients T2DM by receiver operating characteristic (ROC) curve analysis. In the ROC curve analysis, the area under the curve (AUC, Figure 3) was found statistically significant (AUC = 0.797, 95% CI: 0.709–0.884, P < 0.001). As an optimal cutoff point, high risk EFT value of 6.3 mm was determined with a 72.5% sensitivity and 71.7% specificity.
Figure 3.
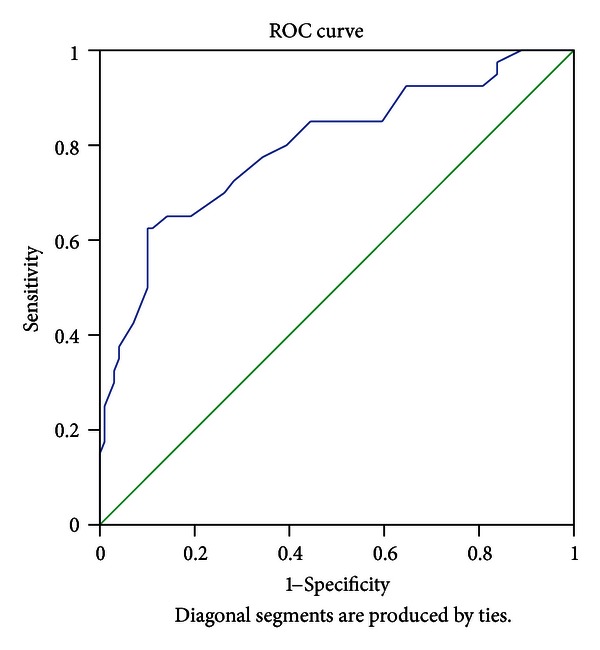
Receiver operating characteristics (ROC) curve (showing sensitivity and specify of the epicardial fat thickness to determine cutoff high risk value). The area under the ROC curve of 0.797 indicates a very good accuracy of the test.
4. Discussion
The major findings of the present study were first, patients with T2DM had increased EFT and CIMT compared with age- and sex-matched controls. Second, EFT was correlated with CIMT, waist circumference, BMI, age, duration of DM, and HbA1c in patients with T2DM. Third, CIMT and waist circumference were found to be independent predictors of EFT. Finally, EFT of 6.3 mm was determined as a high risk value for subclinical atherosclerosis with a 72.5% sensitivity and 71.7% specificity in ROC curve analysis.
Epicardial, mesenteric, and omental fats share the same origin from the splanchnopleuric mesoderm [4]. It has been shown that EAT produces inflammatory mediators such as interleukin (IL)-6, IL-1b, tumor necrosis factor (TNF)-a, and monocyte chemotactic protein (MCP-1) in patients with significant coronary artery disease [22] and expresses mRNAs of adiponectin, resistin, leptin, IL-6, TNF-a, and CD-45 [23]. Several studies have shown that EAT can play a role in the development and aggravation of CAD [8, 22–24]. In addition, EFT has been shown to be related to MetS, abdominal visceral adiposity, subclinical atherosclerosis, nonalcoholic fatty liver disease, type 1 DM, impaired fasting glucose, and hypertension [19, 20, 25–29].
To our knowledge, as a main difference when compared to previously published data in patients with MetS, this is the first study in the literature focusing on the relationship between EFT and CIMT in patients with T2DM. It is also important that a positive linear and significant relationship between EFT and duration of diabetes mellitus was found in our study. According to these results, EFT may be used as a marker of subclinical atherosclerosis and disease progression in patients with T2DM. Further studies are required to support this hypothesis.
There is very limited study investigating the relationship between T2DM and EFT. Recently, in a study performed by Kim et al. [30], increased EAT thickness assessed by cardiovascular magnetic resonance (CMR) was an independent risk factor for significant coronary artery stenosis in asymptomatic type 2 diabetes. There were 100 patients and no control group in that trial. In another study reported in a series of 49 type 2 diabetic patients by Wang et al. [31], EAT volume assessed by cardiac multislice computed tomography was shown to be increased and was associated with unfavourable components of MetS and coronary atherosclerosis. In our study, similar to these trials, we found that EFT was increased in patients with T2DM. In contrast to these trials, we evaluated EFT by TTE in a larger population with a control group and sought relation between EFT and CIMT, which is increasingly used as a surrogate marker for atherosclerosis. Although epicardial fat is readily visualized on high-speed CT and MRI, widespread use of these methods for its assessment is not practical. Echocardiographic EFT measurement in the current practice appears to be feasible, as well as reliable due to good reproducibility which has been shown both in previous studies [19, 20, 32] and in this study.
In our study, patients with T2DM had lower BMI. However, EFT and CIMT were higher in the diabetic patients compared to the controls. Additionally, BMI correlated with EFT in female diabetic patients, but it did not correlate with EFT in male diabetic patients and did not find independent predictor for EFT. Waist circumference not only was correlated with EFT but also was independent predictor for EFT in patients with T2DM and also in both sex. It has been reported that BMI is not a good measure of body adiposity [33]. Similarly, we also found that waist circumference was more reliable parameter than BMI to predict EFT in this study.
Iacobellis et al. [34] have reported that threshold values of high risk EFT to predict MetS are median values of 9.5 mm (85% sensitivity and 63% specificity) and 7.5 mm (82% sensitivity and 62% specificity) in white men and women, respectively. In another study performed by Natale et al. [35] in a large population of hypertensives, Patients with EFT > 7 mm showed a significantly increase in CIMT (0.84 ± 0.2 mm). In a large study performed on patients presenting for cardiovascular preventive care, Nelson et al. [36] have reported that EAT thickness ≥5.0 mm may identify an individual with a higher likelihood of having detectable carotid atherosclerosis. In our study, we found that EFT of 6.3 mm was determined as a high risk value for subclinical atherosclerosis with a 72.5% sensitivity and 71.7% specificity. Different race and patient population in those studies may have created these different threshold values of EFT. Even if different threshold values of EFT have been found in all these studies, increased EFT seems to be related to atherosclerotic process.
Our study had some limitations. This is a case control study, and prospective studies are necessary to show relation between EFT with CIMT and waist circumference. All data were based on a single measurement and may not reflect the association of EFT and CIMT regarding changes with time. In order to establish EFT as a high risk criteria of atherosclerosis in type 2 diabetic patients, further studies are necessary on larger series.
In conclusion, we found that EFT measure by TTE was increased in patients with T2DM. Our study also demonstrated that EFT was strongly correlated with waist circumference and CIMT as being independent of sex. Threshold value of high risk EFT was determined to be 6.3 mm. These results from our study population may suggest that the echocardiographic assessment of EFT is the reliable marker of atherosclerosis and increased CV risk in patients with T2DM. Further studies are needed to confirm these findings.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- 1.Morrish NJ, Wang SL, Stevens LK, et al. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia. 2001;44(2, supplement):S14–S21. doi: 10.1007/pl00002934. [DOI] [PubMed] [Google Scholar]
- 2.Vela D, Buja LM, Madjid M, et al. The role of periadventitial fat in atherosclerosis. Archives of Pathology & Laboratory Medicine. 2007;131:481–487. doi: 10.5858/2007-131-481-TROPFI. [DOI] [PubMed] [Google Scholar]
- 3.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nature Clinical Practice Cardiovascular Medicine. 2005;2(10):536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 4.Marchington JM, Mattacks CA, Pond CM. Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comparative Biochemistry and Physiology Part B. 1989;94(2):225–232. doi: 10.1016/0305-0491(89)90337-4. [DOI] [PubMed] [Google Scholar]
- 5.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. American Heart Journal. 2007;153(6):907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Iacobellis G, Sharma AM. Epicardial adipose tissue as new cardio-metabolic risk marker and potential therapeutic target in the metabolic syndrome. Current Pharmaceutical Design. 2007;13(21):2180–2184. doi: 10.2174/138161207781039670. [DOI] [PubMed] [Google Scholar]
- 7.De Vos AM, Prokop M, Roos CJ, et al. Peri-coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post-menopausal women. European Heart Journal. 2008;29(6):777–783. doi: 10.1093/eurheartj/ehm564. [DOI] [PubMed] [Google Scholar]
- 8.Verhagen SN, Visseren FLJ. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214(1):3–10. doi: 10.1016/j.atherosclerosis.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 9.O’Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults: cardiovascular health study collaborative research group. New England Journal of Medicine. 1999;340(1):14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 10.Pignoli P, Tremoli E, Poli A. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74(6):1399–1406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 11.Rajaram V, Pandhya S, Patel S, et al. Role of surrogate markers in assessing patients with diabetes mellitus and the metabolic syndrome and in evaluating lipid-lowering therapy. American Journal of Cardiology. 2004;93(11):32C–48C. doi: 10.1016/j.amjcard.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Taguchi R, Takasu J, Itani Y, et al. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis. 2001;157(1):203–209. doi: 10.1016/s0021-9150(00)00709-7. [DOI] [PubMed] [Google Scholar]
- 13.Gorter PM, de Vos AM, van der Graaf Y, et al. Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronaryartery calcium in patients undergoing coronary angiography. American Journal of Cardiology. 2008;102(4):380–385. doi: 10.1016/j.amjcard.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample the framingham heart study. Circulation. 2008;117(5):605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 15.Okyay K, Balcioglu AS, Tavil Y, Tacoy G, Turkoglu S, Abaci A. A relationship between echocardiographic subepicardial adipose tissue and metabolic syndrome. International Journal of Cardiovascular Imaging. 2008;24(6):577–583. doi: 10.1007/s10554-008-9295-3. [DOI] [PubMed] [Google Scholar]
- 16.Gorter PM, van Lindert ASR, de Vos AM, et al. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197(2):896–903. doi: 10.1016/j.atherosclerosis.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Garber AJ, Moghissi ES, Buonocore D, et al. American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control. Diabetes Care. 2006;29:1955–1962. doi: 10.2337/dc06-9913. [DOI] [PubMed] [Google Scholar]
- 18.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. Journal of the American Society of Echocardiography. 1989;2(5):358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 19.Iacobellis G, Ribaudo MC, Assael F, et al. Echocardiographic epicardial adipose tissue is related to anthropometricand clinical parameters of metabolic syndrome: a new indicator ofcardiovascular risk. The Journal of Clinical Endocrinology & Metabolism. 2003;88(11):5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 20.Iacobellis G, Assael F, Ribaudo MC, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obesity Research. 2003;11(2):304–310. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- 21.Rajaram V, Pandhya S, Patel S, et al. The task force for the management of arterial hypertension of the European Society of Hypertension, The task force for the management of arterial hypertension of the European Society of Cardiology. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH)and of the European Society of Cardiology (ESC) European Heart Journal. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 22.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 23.Baker AR, da Silva NF, Quinn DW, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovascular Diabetology. 2006;5:1–7. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Feyter PJ. Epicardial adipose tissue: an emerging role for thedevelopment of coronary atherosclerosis. Clinical Cardiology. 2011;34:143–144. doi: 10.1002/clc.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iacobellis G, Pellicelli AM, Sharma AM, Grisorio B, Barbarini G, Barbaro G. Relation of subepicardial adipose tissue to carotid intima-media thickness in patients with human immunodeficiency virus. American Journal of Cardiology. 2007;99(10):1470–1472. doi: 10.1016/j.amjcard.2006.12.082. [DOI] [PubMed] [Google Scholar]
- 26.Yazıcı D, Özben B, Yavuz D, et al. Epicardialadipose tissue thickness in type 1 diabetic patients. Endocrine. 2011;40:250–255. doi: 10.1007/s12020-011-9478-x. [DOI] [PubMed] [Google Scholar]
- 27.Colak Y, Karabay CY, Tuncer I, et al. Relation of epicardial adipose tissueand carotid intima-media thickness in patients with nonalcoholic fatty liverdisease. European Journal of Gastroenterology & Hepatology. 2012;24(6):613–618. doi: 10.1097/MEG.0b013e3283513f19. [DOI] [PubMed] [Google Scholar]
- 28.Iacobellis G, Barbaro G, Gerstein HC. Relationship of epicardial fat thickness and fasting glucose. International Journal of Cardiology. 2008;128(3):424–426. doi: 10.1016/j.ijcard.2007.12.072. [DOI] [PubMed] [Google Scholar]
- 29.Eroğlu S, Sade L, Yıldırır A, Demir O, Müderrisoğlu H. Association of epicardial adipose tissue thickness by echocardiography and hypertension. Archives of the Turkish Society of Cardiology. 2013;41(2):115–122. doi: 10.5543/tkda.2013.83479. [DOI] [PubMed] [Google Scholar]
- 30.Kim HM, Kim KJ, Lee HJ, et al. Epicardial adipose tissue thickness is an indicator for coronary artery stenosis in asymptomatic type 2 diabetic patients: its assessment by cardiacmagnetic resonance. Cardiovascular Diabetology. 2012;11:p. 83. doi: 10.1186/1475-2840-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang CP, Hsu HL, Hung WC, et al. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clinical Endocrinology. 2009;70(6):876–882. doi: 10.1111/j.1365-2265.2008.03411.x. [DOI] [PubMed] [Google Scholar]
- 32.Chaowalit N, Somers VK, Pellikka PA, Rihal CS, Lopez-Jimenez F. Subepicardial adipose tissue and the presence and severity of coronary artery disease. Atherosclerosis. 2006;186(2):354–359. doi: 10.1016/j.atherosclerosis.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Okorodudu DO, Jumean MF, Montori VM, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. International Journal of Obesity. 2010;34(5):791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 34.Iacobellis G, Willens HJ, Barbaro G, Sharma AM. Threshold values of high-risk echocardiographic epicardial fat thickness. Obesity. 2008;16(4):887–892. doi: 10.1038/oby.2008.6. [DOI] [PubMed] [Google Scholar]
- 35.Natale F, Tedesco MA, Mocerino R, et al. Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. European Journal of Echocardiography. 2009;10(4):549–555. doi: 10.1093/ejechocard/jep002. [DOI] [PubMed] [Google Scholar]
- 36.Nelson MR, Mookadam F, Thota V, et al. Epicardial fat: an additional measurement for subclinical atherosclerosis and cardiovascular risk stratification? Journal of the American Society of Echocardiography. 2011;24(3):339–345. doi: 10.1016/j.echo.2010.11.008. [DOI] [PubMed] [Google Scholar]


