Abstract
This study examines intercorrelations among waist circumference (WC), intraperitoneal fat (IPF), and subcutaneous abdominal fat (SAF) in ethnically diverse Dallas Heart Study consisting of 1538 women and 1212 men (50% Black). Correlations between fat depots and triglyceride or HOMA2-IR, biomarkers of metabolic syndrome, are also reported. Total abdominal fat (TAF), ASF, and IPF masses were measured by magnetic resonance imaging. The highest correlations with WC according to ethnicity and gender were noted for TAF (R 2 = 0.81 − 0.88) with progressively lower correlations with ASF (0.65–0.82) and IPF (0.29–0.85). The percentage of IPF relative to TAF was not significantly correlated with WC. For all WC categories, higher IPF/ASF ratios were associated with higher triglyceride levels. In contrast, differences in ratios had little or no association with HOMA2-IR. However, when all data were pooled, IPF was positively correlated with both triglyceride (r = 0.358 (men) and 0.363 (women)) and HOMA2-IR (r = 0.480 (men) and 0.517 (women)); after adjustment for ASF, IPF was still correlated with triglyceride (r = 0.353 (men) and 0.348 (women)) and HOMA2-IR (r = 0.290 (men) and 0.221 (women)). WC measures TAF reliably, but its association with IPF depends on IPF/ASF ratios that vary by gender and ethnicity.
1. Introduction
Abdominal obesity is one component of the metabolic syndrome [1]. Clinically, abdominal obesity is identified by an increase in waist circumference (WC). Increased WC has repeatedly been linked to metabolic risk. It is unclear, however, whether this measure is a correlate of increased risk through its correlation with total abdominal fat (TAF) or a specific, metabolically unhealthy depot of adipose tissue. Many investigators postulate that the key component of body fat underlying the metabolic syndrome is intraperitoneal fat (IPF) or visceral fat [2–7]. Others nonetheless contend that abdominal subcutaneous fat (ASF) is a more important pathogenic factor [8–14]. Since previous studies have shown that IPF and ASF are intercorrelated [15], the more important adipose-tissue compartment underlying the metabolic syndrome is difficult to identify.
The primary aim of this study was to determine the strength of the correlations between WC and TAF, and ASF and IPF measured by magnetic resonance imaging (MRI). These analyses were made for gender in whites, blacks, and Hispanics of the Dallas Heart Study [16]. We additionally correlated SAF and IPF with plasma triglyceride (TG) and homeostatic model assessment of insulin resistance (HOMA2-IR) [17], both accompanying the metabolic syndrome.
2. Methods
Details of DHS study recruitment have been published previously [16]. The current cohort consisted of 1538 women (50% black, 29% white, and 21% Hispanic) and 1212 men (50% black, 36% white, and 16% Hispanic) that had measurement of ASF, IPF, and retroperitoneal fat (RPF). DHS study participants of other ethnicities were excluded from the study. All study volunteers gave written informed consent to participate in an Institutional Review-Board-approved study.
Body weight was measured with a portable scale (Ever Weigh, Lithium electronic scale no. 34067, Health O Meter, Bridgeview, IL, USA) to the nearest 0.1 kg. Height was measured with a stadiometer. Subjects were in a standing position with arms on side, legs straight, and knees together, with feet flat pointed outward. Waist circumference was measured at the midpoint between the lower margin of the last palpable rib and the top of the ileal crest using a stretch-resistant tape with a spring providing constant tension. Fasting plasma lipids, glucose, and insulin were measured as described previously [16]. Insulin resistance was estimated with the HOMA2 computer model (HOMA Calculator version 2.2) [17]. Three categories of WC were defined for this study: low, intermediate, and high. Low WC corresponded to <90 cm in men and <80 cm in women; intermediate WCs were 90–101 cm in men and 80–89 cm in women; and high WCs were ≥102 cm in men and ≥90 cm in women. These cut points corresponded to DHS body mass index categories of <25 kg/m2, 25–29.9 kg/m2, and ≥30 kg/m2. Zhu et al. [18] reported essentially the same ranges based on NHANES III data. Measurements of abdominal compartments of body fat were performed using 1.5 Tesla MRI scanners (Intera; Philips Medical Systems, Best, The Netherlands). The entire abdomen from the diaphragm to the pelvis was scanned using contiguous axial 10 mm slices, as previously described [19]. A single MRI slice at the L2-L3 level was used to quantify total abdominal fat (TAF), ASF, IPF, and retroperitoneal fat (RPF) as detailed by Abate et al. [19]. Briefly, the validation of this method to quantify total abdominal fat subregions involved MRI measurements from the 12th thoracic to 1st sacral vertebra calculated from contiguous 10 mm thick slices that covered the entire abdomen. Regression functions were derived that predicted total fat masses in the respective compartments and that correlated best with the single slice measurement at L2-L3 level. Similar analyses were done to validate the measurement in women [15].
3. Statistics
Linear descriptive statistics were employed in data analyses. Data are summarized as means ±S.D. or S.E. for metabolic parameters. For data not normally distributed, results are given as medians (with interquartiles), and data were log-transformed prior to parametric statistical comparisons. Comparisons of means of metabolic risk factors among ethnic groups within each gender were done for metabolic parameters using ANOVA with Bonferroni adjustments for multiplicity of testing or in selected cases using a posthoc Fisher F test. Pearson's correlation coefficients were determined for analyses of linear associations of waist girth to abdominal fat parameters measured by MRI. Spearman's and partial correlations were also calculated for relating adipose tissue compartments to triglycerides and HOMA2-IR. A SAS version of StatView (version 5.1.26) was employed for the analyses.
4. Results
The clinical characteristics of subjects according to ethnicity and gender are shown in Table 1. Mean ages were in the 40's. Mean BMIs ranged from 28.6 to 32.9 km/m2 for all groups. Mean WCs ranged from 99 cm to 101 cm for the three groups of men and from 91.4 cm to 100.7 in women; WCs were higher in Black women. In both men and women, Blacks had the lowest TG and Hispanics had the highest. Black men had higher mean HDL-C levels compared to Whites and Hispanics; differences among the three groups of women were less. Black men had lower non-HDL-C levels than Whites and Hispanics, but they had higher systolic blood pressures.
Table 1.
Subject characteristics.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Black | White | Hispanic | Black | White | Hispanic | |
| Number of subjects | 579 | 434 | 199 | 767 | 449 | 272 |
| Age (years) | 46 (10)a | 45 (9)a | 41 (9)a | 45 (10) | 46 (10) | 41 (9)a |
| BMI (kg/m2) | 29.5 (6.8) | 28.9 (5.5) | 29.4 (4.6) | 32.9 (8.3)a | 28.6 (6.9)b | 30.9 (7.4) |
| Waist circumference (cm) | 101 (14) | 103 (14)b | 99 (11) | 100.7 (17.2)d | 91.4 (15.8) | 94.5 (16.2) |
| Hip circumference (cm) | 106 (14) | 106 (11) | 102 (8)a | 115.6 (16.4)d | 109.4 (14.9) | 109.5 (15.3) |
| Glucose (mg/dL) | 108 (52) | 98 (29)c | 104 (34) | 103.5 (48.3) | 95.4 (31.6)b | 107.6 (48.5) |
| Insulin (pmol/L) median (IQ) | 85 (103) | 75 (82)b | 91 (83) | 106 (94) | 68 (75)b | 103 (100) |
| HOMA2-IR (%) median (IQ) | 1.63 (1.93) | 1.42 (1.51)b | 1.72 (1.58) | 3.63 (3.77) | 2.23 (2.75)b | 3.51 (3.60) |
| Triglycerides (mg/dL) median (IQ) | 121 (120)d | 157 (121) | 170 (131) | 80 (52)d | 98 (75) | 111 (77) |
| HDL cholesterol (mg/dL) | 50 (15)d | 42 (10) | 42 (10) | 54 (15) | 55 (17) | 49 (12)a |
| Non-HDL cholesterol (mg/dL) | 128 (43)d | 142 (39) | 145 (41) | 125 (41) | 128 (38) | 129 (38) |
| Systolic blood pressure (Hg mm) | 132 (18)d | 127 (13)e | 124 (13) | 120 (15)d | 124 (16) | 129 (19) |
| Diastolic blood pressure (Hg mm) | 79 (10) | 79 (9) | 76 (9)a | 80 (10)d | 76 (9) | 78 (9) |
| % Metabolic syndrome | 30.0 | 33.9 | 35.0 | 42.4 | 31.5 | 39.3 |
| % Diabetes mellitus | 13.2 | 5.6 | 9.7 | 11.3 | 5.6 | 10.8 |
aSignificantly different from Blacks and Whites; P ≤ 0.0002; bsignificantly different from Blacks and Hispanics; P ≤ 0.03; csignificantly different from Blacks; P = 0.0001; dsignificantly different from Whites and Hispanic; P < 0.0001; esignificantly different from Hispanics; P < 0.0001.
Pearson's correlations (R 2) for linear regression analyses between WCs and different abdominal-fat compartments are shown in Figure 1. The highest coefficients of correlation were noted for TAF with progressively lower correlation coefficient with ASF and IPF. IPF was better correlated with WC in men than in women, but still, WC was not a good indicator of IPF. The strength of the correlations was similar within each ethnic group at a P < 0.0001.
Figure 1.
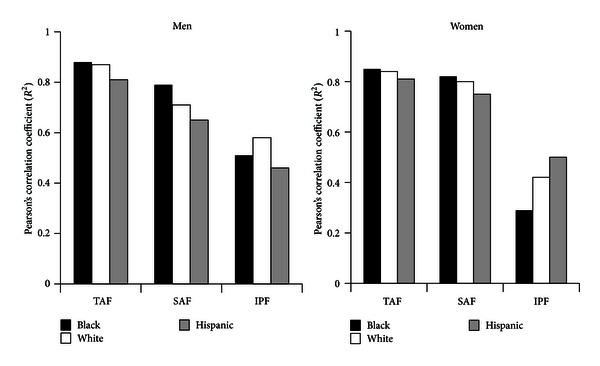
Pearson's correlation coefficients (r 2) by ethnicity and gender for linear regression analyses of waist circumference versus total abdominal fat (TAF), subcutaneous abdominal fat (SAF), and intraperitoneal fat (IPF). Highest correlations were found for TAF, intermediate for SAF, and lowest for IPF. All correlations were significant at P < 0.001.
Absolute fat masses in IPF and ASF for three categories of WC—low, intermediate, and high—according to ethnicity and gender are presented in Figures 2(a) and 2(b), respectively. Women of all ethnicities had much lower IPF masses than men. Further, in both men and women, blacks had lower IPF masses than whites and Hispanics at all levels of WC (P < 0.02); white men with low and intermediate WC had lower IPF than Hispanics (P < 0.02); and white women at intermediate WC had lower IPF than Hispanic women (P < 0.02). In contrast, men and women had similar patterns of ASF masses for each waist circumference category. Black men had significantly lower ASF than white and Hispanic for those with a low waist circumference, and they also had the highest ASF for those in the highest waist circumference category. The same pattern of ASF fat was noted in black women.
Figure 2.
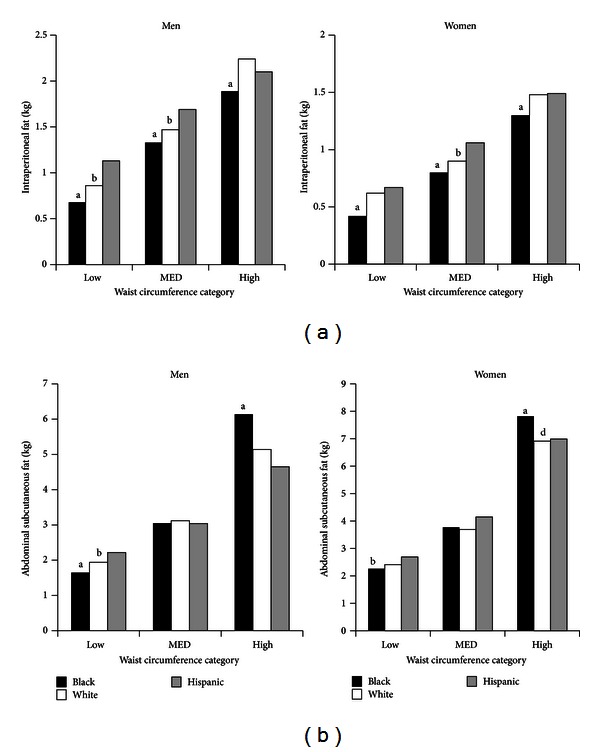
(a) Masses (kg) of intraperitoneal fat (IPF) for each waist circumference category for ethnicity and gender. Amounts of IPF increased for low, intermediate (MED), and high waist circumference categories. aSignificantly different from whites and Hispanics (P < 0.02); bSignificantly different from Hispanics (P < 0.02). (b) Masses (kg) of abdominal subcutaneous fat (ASF) for each waist circumference category for ethnicity and gender. Amounts of ASF increased for low, intermediate (MED), and high waist circumference categories. aSignificantly different from whites and Hispanics (P < 0.02); bsignificantly different from Hispanics (P < 0.02).
Figure 3 displays the IPF as a percentage of TAF. In all ethnicities, higher WCs matched with greater fat masses in each compartment (Figures 2(a) and 2(b)). Although fat masses rose with increasing WCs, the percentage of IPF relative to TAF did not rise for low, intermediate (MED), and high WCs for men or women (Figure 3). In general, blacks had slightly lower percentages of IPF than both other groups.
Figure 3.
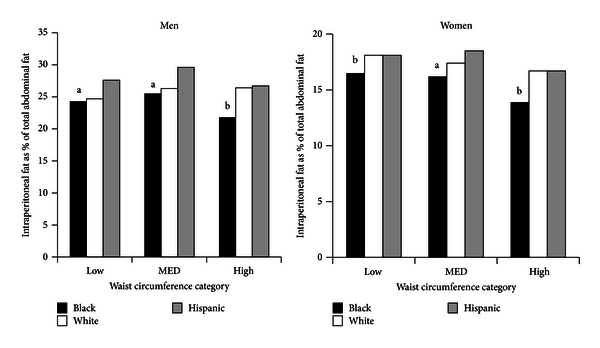
Percentage of intraperitoneal fat (IPF) or total abdominal fat (TAF) for each waist circumference category for ethnicity and gender. Blacks generally had a lower percentage IPF compared to whites and Hispanics. aSignificantly different from whites and Hispanics (P < 0.02); bsignificantly different from Hispanics (P < 0.02).
In Figures 4(a) and 4(b), ranges of IPF masses are given for quintiles of TAF in men and women, respectively. As TAF rose, so did IPF, showing that most of IPF mass was determined by TAF content. Within each TAF category, nonetheless, there was a range of IPF masses. This range broadened with higher TAF masses, suggesting heterogeneity of IPF response to obesity. In the most obese subjects, IPF masses varied over extremes of about 1.5 kg in men and 1.0 kg in women.
Figure 4.
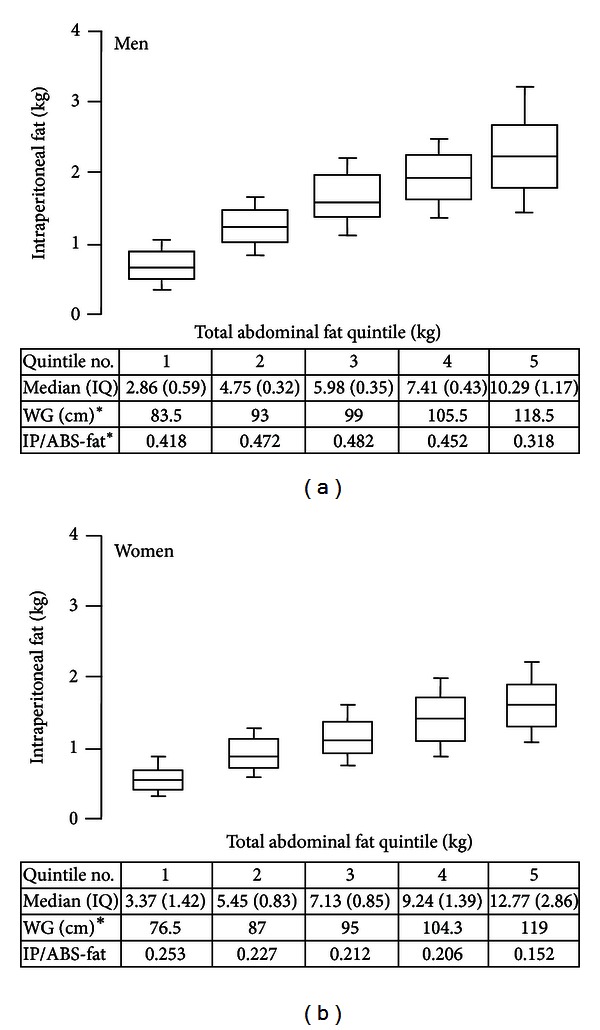
(a) Masses (kg) of intraperitoneal fat (IPF) plotted against masses of quintiles of total abdominal fat for all men. IPF masses increased progressively with TAF, and the distribution of IPF for each category widened. Boxes show mean and one standard deviation; whiskers show 2 standard deviations. The chart gives median values for each quintile, the mean waist girth, and ratio of IPF to abdominal subcutaneous fat. The latter ratio changed a little across ratios except in the highest quintile. (b) Masses (kg) of intraperitoneal fat (IPF) plotted against masses of quintiles of total abdominal fat for all women. IPF masses increased progressively with TAF, and the distribution of IPF for each category widened. Boxes show mean and one standard deviation; whiskers show 2 standard deviations. The chart gives median values for each quintile, the mean waist girth, and ratio of IPF to abdominal subcutaneous fat. The latter ratio changed a little across ratios except in the highest quintile.
The means and distributions of the IPF/ASF ratio are shown for men and women of the three ethnic groups (Table 2). The distributions were skewed so that mean and 50th percentiles (medians) are not identical. Although mean percentage IPF was relatively constant with increasing WCs, great individual variation was noted across the span of IPF/ASF ratios.
Table 2.
Distribution of intraperitoneal/abdominal subcutaneous fat ratio.
| Mean (SD) | 10th | 25th | 50th | 75th | 90th | |
|---|---|---|---|---|---|---|
| All men | 0.462 (0.196) | 0.247 | 0.323 | 0.426 | 0.568 | 0.731 |
| Black | 0.416 (0.184)a | 0.222 | 0.286 | 0.387 | 0.505 | 0.662 |
| White | 0.488 (0.195) | 0.262 | 0.353 | 0.462 | 0.592 | 0.759 |
| Hispanic | 0.541 (0.200) | 0.318 | 0.405 | 0.498 | 0.655 | 0.837 |
|
| ||||||
| All women | 0.222 (0.091) | 0.118 | 0.157 | 0.209 | 0.273 | 0.334 |
| Black | 0.198 (0.085)a | 0.101 | 0.138 | 0.185 | 0.247 | 0.309 |
| White | 0.248 (0.090) | 0.154 | 0.186 | 0.234 | 0.29 | 0.358 |
| Hispanic | 0.247 (0.092) | 0.143 | 0.183 | 0.226 | 0.301 | 0.375 |
aSignificantly different from White and Hispanic (P < 0.0001).
To examine whether differences in IPF/ASF ratios affect plasma TG or HOMA2-IR, ratios were split into upper and lower halves and were related to these metabolic measures. The results for men are given in Figure 5(a). On the whole, for the three WC categories, greater IPF/ASF ratios associated with higher TG levels. In contrast, differences in ratios had little or no influence on HOMA2-IR. In women, a similar but less pronounced trend was noted for differences in IPF/ASF ratios on TG levels (Figure 5(b)). Differences in ratios again had little or no effect on HOMA2-IR.
Figure 5.
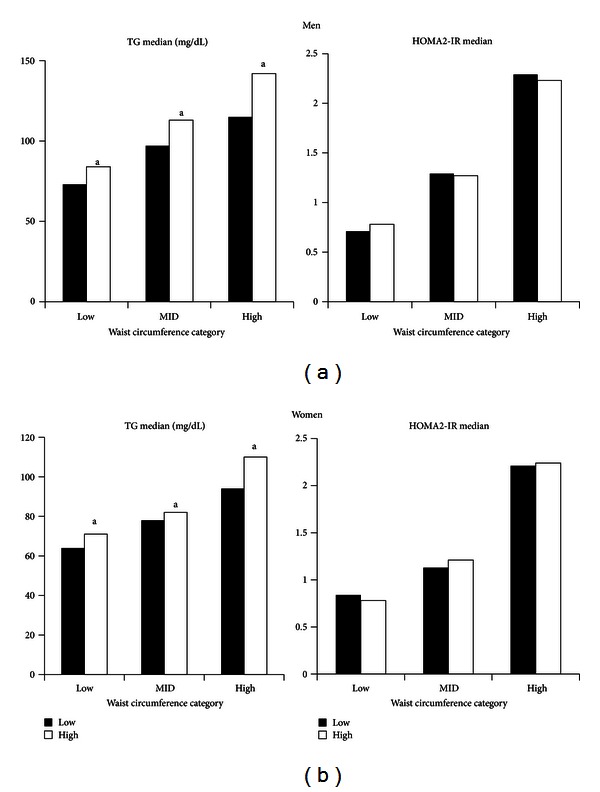
(a) Plasma triglyceride (TG) and HOMA2-IR for upper and lower halves of the intraperitoneal fat/abdominal subcutaneous fat ratios for all men. For TG, those with the high ratios had significantly higher triglyceride in each waist circumference category (a P < 0.05). For HOMA2-IR, there were no differences between higher and lower ratios. (b) Plasma triglyceride (TG) and HOMA2-IR for upper and lower halves of the intraperitoneal fat/abdominal subcutaneous fat ratios for all women. For TG, those with the high ratios had significantly higher triglyceride in each waist circumference category (a P < 0.05). For HOMA2-IR, there were no differences between higher and lower ratios.
To determine whether a more sensitive analysis might identify an effect of IPF on HOMA2-IR, Spearman's correlation coefficients were determined on all men and all women, regardless of ethnicity or WC (Figure 6). In both men and women, ASF was less strongly correlated with TG than was IPF. After cross-adjustment, ASF lost its association with TG levels, whereas IPF did not. In both men and women, ASF and IPF were similarly correlated with HOMA2-IR. After cross-adjustment, the strength of the correlation for each compartment diminished, but IPF remained significantly correlated.
Figure 6.
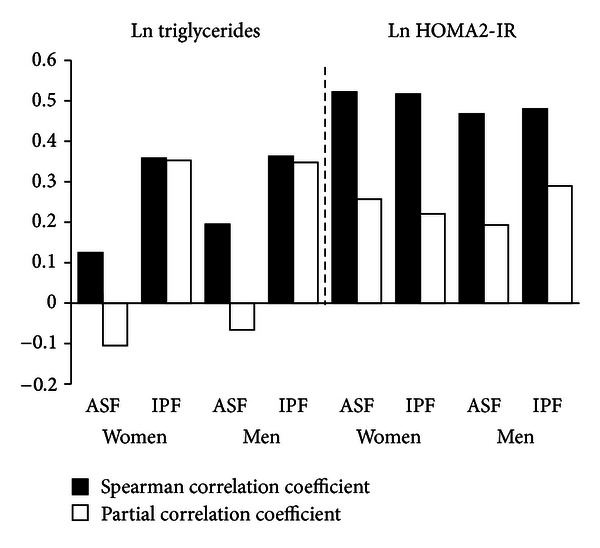
Black bars show Spearman correlation coefficients (r) for regression analyses of ln triglyceride and ln HOMA2-IR versus abdominal subcutaneous fat (ASF) and intraperitoneal fat (IPF) in women and men. White bars show partial correlation coefficients after adjustment for the opposite fat compartment. For this analysis, all men and all women were combined. For triglycerides, ASF was modestly correlated but became negatively correlated after adjustment for IPF. IPF was more strongly correlated with triglycerides and remained equally correlated after adjustment for ASF. Both ASF and IPF were strongly correlated with ln HOMA2-IR, but after adjustment for the opposing compartment, partial correlation coefficient was diminished. All correlation coefficients were significant at a level of P < 0.001.
5. Discussion
The major findings of this study were the following. First, WC correlated strongly with TAF and ASF, whereas WC less strongly predicted IPF (Figure 1). Second, IPF constituted only about one-fourth of TAF in men and one-fifth in women (Figure 4). Third, for all groups, the distributions of IPF/ASF ratios showed considerable variability among individuals; this explains the relatively low correlations between WC and IPF (Table 2). Fourth, IPF rose progressively with increasing TAF, but at each step of increase, IPF masses varied considerably (Figures 4(a) and 4(b)). Fifth, for all WC categories, persons with higher IPF/ASF ratios had higher plasma TG levels than did those with lower ratios; this relationship was not observed for HOMA2-IR (Figures 5(a) and 5(b)). Even so when all ethnic groups were combined, a positive correlation was uncovered between IPF and HOMA2-IR, which persisted after adjustment for ASF (Figure 6).
IPF, ASF, and the distribution of their ratios were compared to blacks, whites, and Hispanic men and women. Both black men and women had lower median IPF/ASF ratios compared to whites and Hispanics (Table 2). In the three ethnic groups, TAF, ASF, and IPF were similarly correlated with waist girths (Figure 1). In low, intermediate, and high WC categories, both black men and women had lower masses of IPF, compared to whites and Hispanics (Figure 2(a)). In low and intermediate WC categories of men, Hispanics had greater masses of IPF than whites as well as black; at high WG, only black men were different from the other ethnicities. In women, only blacks were consistently different from whites and Hispanics in IFP masses (Figure 2(a)). Black men and women had relatively low ASF mass compared to whites and Hispanics; but in the high WG categories, blacks of both genders had higher ASF mass than whites and Hispanics (Figure 2(b)).
A high WC clearly associates with all metabolic risk factors [1]; and it is commonly believed that WC is a surrogate measurement for visceral adipose tissue [20–22]. The current study revealed that amounts of IPF increased progressively through each category of increasing WC. In this sense, therefore, it can be said that WC is a surrogate for IPF. The current data, nonetheless, indicate that WC is much more strongly correlated with TAF and ASF than with IPF. This being the case, it cannot be assumed that the relation between increased WC and metabolic syndrome is mediated predominantly through a higher IPF.
Abdominal obesity is well recognized to predispose to hypertriglyceridemia [23]. Our study found clear evidence that IPF correlates with plasma TG. For all WC categories and in men and women, those with higher IPF/ASF ratios had higher plasma TG levels. In addition, partial correlation analysis indicated that IPF independently associates with TG levels. The mechanism for this relationship can be readily visualized. Since IPF drains its fatty acids directly into the splanchnic circulation, these fatty acids should add an excess load of lipid on the liver beyond what would be derived from subcutaneous adipose tissue beds. This extra load should translate into higher TG levels.
Several reports suggest that IPF is related to insulin resistance. For instance, Carr et al. [2] reported that intra-abdominal fat is independently associated with insulin resistance, and others found a similar relationship [12, 24, 25]. It might be expected that if IPF causes insulin resistance, a high level of IPF should be a risk factor for type 2 diabetes. Such has been reported [6, 25, 26]. In the current study, IPF appeared to be correlated with HOMA2-IR, albeit weakly. This relationship could not be found when people with high and low IPF/ASF ratios were compared. But partial correlations suggest that higher levels of IPF associate with increased HOMA2-IR independently of ASF. In the light of previous reports, there seems to be little doubt that a positive correlation between IPF and insulin resistance exists.
IPF could be related to either insulin resistance in skeletal muscle or liver. The mechanisms whereby IPF per se could cause skeletal muscle insulin resistance are not readily apparent. An increased release of fatty acids from IPF is one possibility; but amounts must be relatively small compared to the total adipose tissue output of fatty acids. It is thus unlikely that a relatively small increment in release of fatty acids from IPF could substantially worsen insulin resistance in skeletal muscle [27–30].
If IPF increases insulin resistance, it is more likely to be hepatic insulin resistance; this condition is characterized by increased hepatic glucose output. Presumably an increased fatty acid influx into the liver suppresses insulin action, stimulates gluconeogenesis, and raises hepatic glucose output [31, 32]. A report suggests that HOMA2-IR reflects hepatic glucose output more than skeletal muscle insulin resistance [33].
The positive correlation between IPF and HOMA2-IR thus could be mediated through fatty acid stimulation of hepatic glucose output. An interesting question is what are the sources of fatty acids reaching the liver? This question has been examined by Nielsen et al. [28]. They found that the contribution of IPF to hepatic fatty acid delivery ranged from <10% to approximately 50% depending on amounts of IPF. The remainder of fatty acid flux to liver derived from subcutaneous adipose tissue. Thus their findings suggest that excess fatty acids from IPF could drive gluconeogenesis and raise HOMA2-IR.
In reference to the association between visceral obesity and metabolic risk factors, it seems important to distinguish between the rise in IPF with total body obesity and the occurrence of excessive amounts of IPF with obesity. The findings of Nielsen et al. [28] suggest that with increasing obesity, a higher percentage of splanchnic flux of fatty acids is derived from IPF. In addition, for any given level of obesity, there is heterogeneity in IPF content; therefore, obese persons with the greatest IPF masses could have the highest splanchnic flux of fatty acids, worsening liver-associated risk factors.
In summary, this study shows that WC correlates with IPF but not strongly. For any given WC, IPF can vary greatly. The two factors affecting this variation are ethnicity and gender. Black men and women have lower median IPF masses than whites and Hispanics. In the high WG category, blacks have both lower IPF and greater ASF, which contributes to the overall variability between WC and IPF. Both IPF and ASF contribute to metabolic risk factors. IPF was found to correlate with both serum triglyceride levels and HOMA2-IR, whereas ASF correlated only with HOMA2-IR. The mechanisms responsible for these latter correlations require further study.
Acknowledgments
Grant support for the Dallas Heart Study was provided by the Donald W. Reynolds Foundation and by US Public Health Service General Clinical Research Center Grant M01-RR00633. This work was supported by award no. T32HL007360 from the National Heart, Lung, and Blood Institute to Dr. Neeland, by Grants UL1DE019584 and PL1DK081182 from the National Institutes of Health, and by a Clinical and Translational Science Award, UL1TR000451, from the National Center for Advancing Translational Sciences of the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International atherosclerosis society; And international association for the study of obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Carr DB, Utzschneider KM, Hull RL, et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53(8):2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 3.von Eyben FE, Mouritsen E, Holm J, et al. Intra-abdominal obesity and metabolic risk factors: a study of young adults. International Journal of Obesity. 2003;27(8):941–949. doi: 10.1038/sj.ijo.0802309. [DOI] [PubMed] [Google Scholar]
- 4.Nakao YM, Miyawaki T, Yasuno S, et al. Intra-abdominal fat area is a predictor for new onset of individual components of metabolic syndrome: metabolic syndrome and abdominal obesity (MERLOT study) Proceedings of the Japan Academy Series B. 2012;88(8):454–461. doi: 10.2183/pjab.88.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JD, Borel A-L, Nazare J-A, et al. Visceral adipose tissue indicates the severity of cardiometabolic risk in Patients with and without type 2 diabetes: results from the INSPIRE me IAA Study. Journal of Clinical Endocrinology and Metabolism. 2012;97(5):1517–1525. doi: 10.1210/jc.2011-2550. [DOI] [PubMed] [Google Scholar]
- 6.Neeland IJ, Turer AT, Ayers CR, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. Journal of the American Medical Association. 2012;308(11):1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumner AE, Micklesfield LK, Ricks M, et al. Waist circumference, BMI, and visceral adipose tissue in white women and women of African descent. Obesity. 2011;19(3):671–674. doi: 10.1038/oby.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men. Journal of Clinical Investigation. 1995;96(1):88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg A. Regional adiposity and insulin resistance. Journal of Clinical Endocrinology and Metabolism. 2004;89(9):4206–4210. doi: 10.1210/jc.2004-0631. [DOI] [PubMed] [Google Scholar]
- 10.Chandalla M, Lin P, Seenivasan T, et al. Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS ONE. 2007;2(8, article e812) doi: 10.1371/journal.pone.0000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goedecke JH, Levitt NS, Lambert EV, et al. Differential effects of abdominal adipose tissue distribution on insulin sensitivity in black and white South African women. Obesity. 2009;17(8):1506–1512. doi: 10.1038/oby.2009.73. [DOI] [PubMed] [Google Scholar]
- 12.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. American Journal of Physiology. 2000;278(5):E941–E948. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 13.Tulloch-Reid MK, Hanson RL, Sebring NG, et al. Both subcutaneous and visceral adipose tissue correlate highly with insulin resistance in african americans. Obesity Research. 2004;12(8):1352–1359. doi: 10.1038/oby.2004.170. [DOI] [PubMed] [Google Scholar]
- 14.Frederiksen L, Nielsen TL, Wraae K, et al. Subcutaneous rather than visceral adipose tissue is associated with adiponectin levels and insulin resistance in young men. Journal of Clinical Endocrinology and Metabolism. 2009;94(10):4010–4015. doi: 10.1210/jc.2009-0980. [DOI] [PubMed] [Google Scholar]
- 15.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. Journal of Clinical Endocrinology and Metabolism. 2006;91(11):4459–4466. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 16.Victor RG, Haley RW, Willett DL, et al. The Dallas heart study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. American Journal of Cardiology. 2004;93(12):1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Zhu S, Heymsfield SB, Toyoshima H, Wang Z, Pietrobelli A, Heshka S. Race-ethnicity-specific waist circumference cutoffs for identifying cardiovascular disease risk factors. American Journal of Clinical Nutrition. 2005;81(2):409–415. doi: 10.1093/ajcn.81.2.409. [DOI] [PubMed] [Google Scholar]
- 19.Abate N, Garg A, Coleman R, Grundy SM, Peshock RM. Prediction of total subcutaneous abdominal, intraperitoneal, and retroperitoneal adipose tissue masses in men by a single axial magnetic resonance imaging slice. American Journal of Clinical Nutrition. 1997;65(2):403–408. doi: 10.1093/ajcn/65.2.403. [DOI] [PubMed] [Google Scholar]
- 20.Mateo-Gallego R, Bea AM, Jarauta E, Perez-Ruiz MR, Civeira F. Age and sex influence the relationship between waist circumference and abdominal fat distribution measured by bioelectrical impedance. Nutrition Research. 2012;32(6):466–469. doi: 10.1016/j.nutres.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Katashima M, Yasumasu T, Li KJ. Visceral fat area, waist circumference and metabolic risk factors in abdominally obese Chinese adults. Biomedical and Environmental Sciences. 2012;25(2):141–148. doi: 10.3967/0895-3988.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Onat A, Avci GŞ, Barlan MM, Uyarel H, Uzunlar B, Sansoy V. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. International Journal of Obesity. 2004;28(8):1018–1025. doi: 10.1038/sj.ijo.0802695. [DOI] [PubMed] [Google Scholar]
- 23.Arsenault BJ, Lemieux I, Després JP, et al. The hypertriglyceridemic-waist phenotype and the risk of coronary artery disease: results from the EPIC-Norfolk Prospective Population Study. Canadian Medical Association Journal. 2010;182(13):1427–1432. doi: 10.1503/cmaj.091276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preis SR, Massaro JM, Robins SJ, et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the framingham heart study. Obesity. 2010;18(11):2191–2198. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the framingham heart study. Circulation. 2007;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 26.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26(2):372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 27.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23(4):465–471. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. Journal of Clinical Investigation. 2004;113(11):1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miles JM, Jensen MD. Counterpoint: visceral adiposity is not causally related to insulin resistance. Diabetes Care. 2005;28(9):2326–2328. doi: 10.2337/diacare.28.9.2326. [DOI] [PubMed] [Google Scholar]
- 30.Jensen MD. Is visceral fat involved in the pathogenesis of the metabolic syndrome? Human model. Obesity. 2006;14(supplement 1):20S–24S. doi: 10.1038/oby.2006.278. [DOI] [PubMed] [Google Scholar]
- 31.Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cε and hepatic insulin resistance. Cell Metabolism. 2012;15(5):574–584. doi: 10.1016/j.cmet.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Z, Lazar MA. Dissociating fatty liver and diabetes. Trends in Endocrinology and Metabolism. 2013;24(1):4–12. doi: 10.1016/j.tem.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman RP. Indices of insulin action calculated from fasting glucose and insulin reflect hepatic, not peripheral, insulin sensitivity in African-American and Caucasian adolescents. Pediatric Diabetes. 2008;9(3):57–61. doi: 10.1111/j.1399-5448.2007.00350.x. [DOI] [PubMed] [Google Scholar]


