Abstract
Pneumococcal meningitis is a life-threatening disease characterized by an acute purulent infection affecting the pia mater, the arachnoid, and the subarachnoid spaces. Streptococcus pneumoniae crosses the blood-brain barrier (BBB) by both transcellular traversal and disruption of the intraepithelial tight junctions to allow intercellular traversal. During multiplication, pneumococci release their bacterial products, which are highly immunogenic and may lead to an increased inflammatory response in the host. Thus, these compounds are recognized by antigen-presenting cells through the binding of toll-like receptors. These receptors induce the activation of myeloid differentiation factor 88 (MyD88), which interacts with various protein kinases, including IL-1 receptor-associated kinase-4 (IRAK4), which is phosphorylated and dissociated from MyD88. These products also interact with tumor necrosis factor receptor-associated factor 6 dependent signaling pathway (TRAF6). This cascade provides a link to NF-κB-inducing kinase, resulting in the nuclear translocation of NF-κB leading to the production of cytokines, chemokines, and other proinflammatory molecules in response to bacterial stimuli. Consequently, polymorphonuclear cells are attracted from the bloodstream and then activated, releasing large amounts of NO•, O2 •, and H2O2. This formation generates oxidative and nitrosative stress, subsequently, lipid peroxidation, mitochondrial damage, and BBB breakdown, which contributes to cell injury during pneumococcal meningitis.
1. Introduction
Pneumococcal meningitis is the most complex and serious infection of the central nervous system (CNS) that is associated with neurological sequelae [1]. The host immune response, through the production of cytokines and chemokines and the migration of leukocytes, is the first line of defense in response to bacterial infection [2]. In addition, polymorphonuclear leukocytes produce nitric oxide (NO•), superoxide anion radicals (O2 −•), and hydrogen peroxide (H2O2). O2 −• and NO• can lead to the formation of peroxynitrite (ONOO), which is a strong oxidant [3]. This oxidant exerts cytotoxic effects on endothelial cells [4], increases the permeability of the BBB, induces the peroxidation of lipids, and induces many other complex interactions that seem to be involved in the pathophysiology of pneumococcal meningitis [3].
The aim of this review is to summarize the current knowledge of the relevant pathophysiological steps of pneumococcal meningitis: (a) the crossing of the pneumococcus through the BBB; (b) the activation of innate immune system mechanisms; (c) the migration of leukocytes and (d) the induction of oxidative and nitrosative stress in the context of pneumococcal meningitis.
2. Microbial Traversal of the Blood-Brain Barrier
The CNS is protected by a bony skull, the leptomeninges, the blood-brain barrier (BBB), and the blood-cerebrospinal fluid barrier [5]. The BBB is formed by microvascular endothelial cells, astrocytes, and pericytes. This barrier acts by controlling the exchange of substances into and out of the brain [6] and thereby protects the brain from toxins and pathogens [5]. S. pneumoniae crosses the BBB through both transcellular traversal and paracellular traversal [6, 7]. In transcellular traversal, the pathogen interacts with cell-wall phosphorylcholine and the platelet-activating-factor (PAF) receptor. In addition, the protein C (PspC) pneumococcal surface binds to both the laminin receptor and the polymeric Ig receptor (pIgR), which are located on brain microvascular endothelial cells [8]. Later, the pathogen transmigrates through endothelial cells to the basolateral side without any evidence of disruption of intercellular tight junctions [5, 6].
Paracellular traversal involves the penetration of bacteria between barrier cells with or without evidence of tight-junction disruption [6]. Both the host immune response and bacterial virulence factors, such as pneumolysin, and the ability of pneumococci to bind to fibronectin [9], vitronectin, and collagen in the extracellular matrix, act together to increase the permeability of the BBB [10, 11]. This interaction facilitates the passage of the microorganism into the brain [1] Figure 1.
Figure 1.
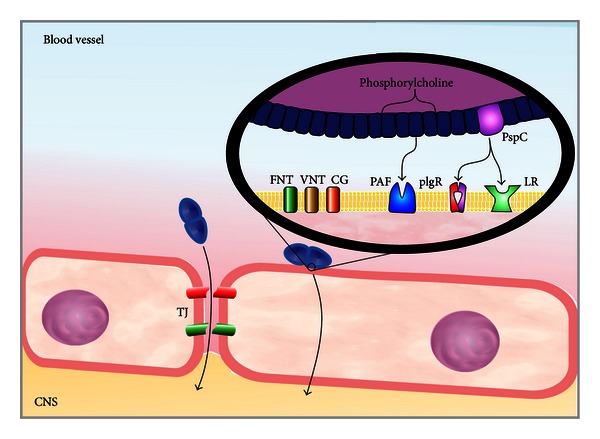
Mechanisms of microbial traversal of the BBB. S. pneumoniae crosses the BBB through transcellular traversal and paracellular traversal.
3. Innate Immune Mechanisms of the Pneumococcal Meningitis
After S. pneumoniae reaches the subarachnoid space, it multiplies rapidly and releases compounds, such as cell wall fragments, lipoteichoic acid, teichoic acid, pneumolysin, and peptidoglycan [1]. These compounds are highly immunogenic and may elicit an inflammatory response in the host. These immunogenic molecular determinants are better known as pathogen-associated molecular patterns (PAMPs) [12, 13]. These PAMPs are recognized by different sensors of the innate immune system called pattern recognition receptors (PRRs) [14]. These PRRs comprise toll-like receptors (TLRs), NOD-like receptors (NLRs), and DNA sensors [14, 15]. At present, there are 13 members of the TLR family described in humans and 10 described in mice. These members are separated into two broad categories. One category is expressed at the cell surface for extracellular ligand recognition. The other category is localized in the endosomal compartment for the recognition of pathogen nucleic acids [16]. Microglia express all TLRs identified to date, whereas astrocytes only express TLR1, TLR2, TLR3 and TLR9. Neurons only express TLR3, TLR7, TLR8, and TLR9, and oligodendrocytes only express TLR2 and TLR3 [15, 17]. TLR2 is activated by pneumococcal cell wall compounds, lipoteichoic acid, and lipoproteins. TLR4 is activated by pneumolysin, and TLR9 is activated by pneumococcal DNA containing CpG motifs within endosomes [14, 15]. TLR2, TLR4, and TLR9 transduce their signals through a common intracellular adapter protein known as myeloid differentiation factor 88 (MyD88) [14, 18]. Of note, the deficiency of this intracellular adapter protein in children increases their susceptibility to invasive pneumococcal infections, including meningitis [19]. MyD88 interacts with a protein kinase, IL-1 receptor-associated kinase-4 (IRAK4) [1, 20]. The IRAK4 dependent, TLRs, and IL-1Rs are vital for childhood immunity to pyrogenic bacteria, which are mainly invasive pneumococcal infections [21]. After IRAK has been phosphorylated, it is dissociated from MyD88 and interacts with tumor necrosis factor receptor-associated factor 6 dependent signaling pathway (TRAF6) [22]. TRAF6 stimulates the transforming growth factor β-activated kinase (TAK1), which is a MAPKKK. Thus, TAK1 activates IKK (Inhibitor of IkB kinase), which results in the destruction of IkB and the subsequent activation and nuclear translocation of NF-κB [23, 24]. NF-κB comprises a closely related family of transcription factors, which play a key role in the expression of genes involved in the development of accessory cell and leukocyte populations, inducing the expression of many proteins implicated in inflammation and in the immune response [25]. NF-κB is also a transcriptional activator of various genes implicated in neuronal pathogenesis and in the production of cytokines and chemokines [20, 26]. The nucleotide-binding-oligomerization-domains-NOD-like receptors (NLRs) are also involved in the recognition of S. pneumoniae by the innate immune system. The family members consist of intracellular receptors, such as inflammasome-forming proteins (NLRPs), NLRP1, NLRP3, and NLRP6, which mediate the assembly of inflammasome complexes leading to the activation of procaspase-1. The second group of NLRs includes intracellular recognition receptors, such as NOD1/CARD4 and NOD2/CARD15. These receptors mediate the assembly of complexes that activate MAPK and NF-κB signaling pathways, and they are involved in the detection of cell wall peptidoglycan [27, 28]. NLRP3 (cryopyrin) and AIM2 (absent in melanoma 2) inflammasomes are activated by pneumolysin and bacterial DNA. These inflammasomes use an adapter molecule, known as apoptosis-associated speck-like protein (ASC), which is a key component of multimeric protein complexes that mediate inflammation and host defenses [29]. NLRP3 and AIM2 promote caspase-1 activation and the subsequent conversion of pro-IL-1β into mature IL-1β in pneumococcal meningitis [30]. Furthermore, pneumolysin activates the NLRP3 inflammasome and promotes the production of the proinflammatory cytokines independently of TLR4 [31], Figure 2.
Figure 2.
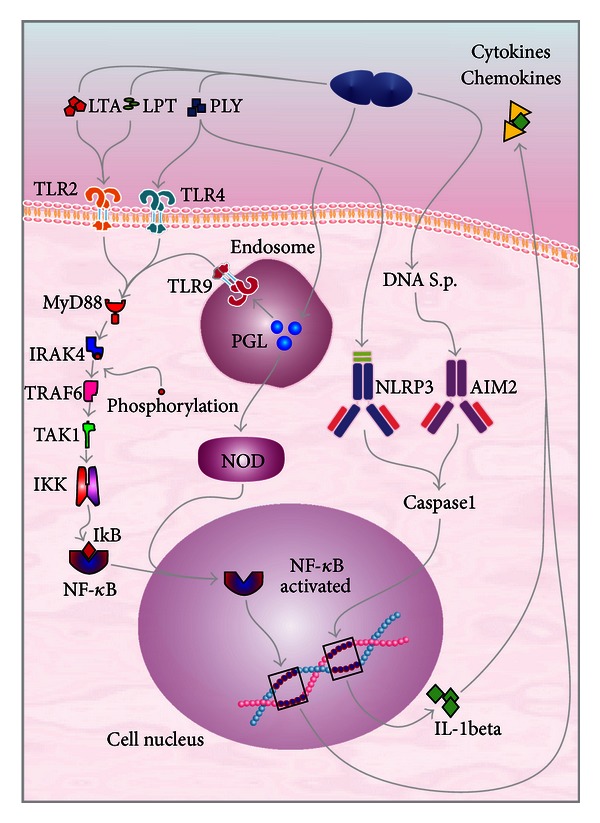
Innate immune system in pneumococcal meningitis infection. The majority of TLRs utilize a common intracellular adapter protein known as myeloid differentiation factor 88 (MyD88): it activates IRAK, which is phosphorylated and dissociated from MyD88. Thus, it interacts with the tumor necrosis factor receptor-associated factor 6 dependent signaling pathway (TRAF6). TRAF6 stimulates to the transforming growth factor β-activated kinase (TAK1). TAK1 activates the IKK (Inhibitor of IκB kinase), resulting in the destruction of IkB and subsequently, NF-κB activation resulting in the nuclear translocation of NF-κB. This cascade provides a link to NF-κB-inducing kinase, resulting in the nuclear translocation of NF-κB, which induces the production of cytokines, chemokines, and others proinflammatory molecules in response to bacterial stimuli.
4. Leukocyte Migration
Pneumococcal compounds are proinflammatory mediators that induce an innate immune response that activates NF-κB and subsequently triggers the production of proinflammatory cytokines and chemokines and the expression of co-stimulatory molecules [32]. In response, neutrophils leave the blood and migrate to sites of infection. Sialyl-LewisX on leukocytes binds to selectins P and E on endothelial cells. This binding becomes stronger when CXCL-8 binds to its specific receptor on neutrophils, which triggers the production of integrin LFA-1 and CX3 (mac-1). Inflammatory cytokines, such as TNF-α, are also necessary to induce expression of adhesion molecules ICAM-1 and ICAM-2. The link between endothelial cells and ICAM-1 allows the passage of neutrophils along a gradient of chemoattractants substances [33, 34], Figure 3. Consistent with the polymorphonuclear migration, as explained previously, TNF-α is produced mainly in the first 6 to 24 hours after pneumococcal meningitis induction [35]. Patients with bacterial meningitis also have increased the levels of TNF-α in the CSF early in the course of the disease [36]. In bacterial meningitis, approximately 90% of the migrating leukocytes are neutrophilic granulocytes [37]. However, blocking the accumulation of leukocytes in cerebrospinal fluid augments bacteremia and lethality in experimental pneumococcal meningitis [38].
Figure 3.
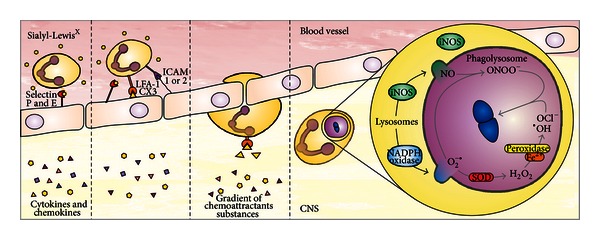
Leukocyte migration. Leukocytes leave the blood and migrate to sites of infection. Sialyl-LewisX on leukocytes binds to selectins P and E on endothelial cells. This binding becomes stronger when CXCL-8 binds to its specific receptor on neutrophils, triggering the production of integrin LFA-1 and CX3 (mac-1). Inflammatory cytokines, such as TNF-α, are also necessary to induce expression of the adhesion molecules ICAM-1 and ICAM-2. The interaction between endothelial cells and ICAM-1 allows the passage of neutrophils along a gradient of chemoattractants substances.
Initially, phagocytized pathogens are internalized in the phagosome. The phagosome is acidified by fusion with lysosomes, becoming a phagolysosome. In this period, high amounts of reactive oxygen species (ROS) and reactive nitrogen species (RNS) are formed [39]. The relevant antimicrobial systems of phagocytic cells are the NADPH phagocytic oxidase and inducible nitric oxide synthase (iNOS) pathways, which are expressed in neutrophil and macrophage cells [40]. Macrophages and neutrophils produce NO•, O2 −•, and H2O2. NO• is produced by iNOS2, and O2 −• is produced by NADPH oxidase. O2 −• and NO• can lead to the formation of ONOO− [3], which is a strong oxidant that exerts cytotoxic effects on endothelial and vascular smooth muscle cells [4]. Moreover, chemical and enzymatic reactions produce a variety of toxic chemical agents; O2 −• is converted by the enzyme superoxide dismutase (SOD) into H2O2 [38, 39]. H2O2 can kill the microorganisms and also be converted by the peroxidase enzyme in the presence of Fe2+ into hypochlorite (OCl−) and hydroxyl radicals (•OH), which are microbicides [3, 39, 41].
5. Oxidative Stress in the Context of Pneumococcal Meningitis
During pneumococcal meningitis, RNS and ROS are produced by resident immune cells of the brain as part of the host response to invasive bacterial infections [1, 42]. Furthermore, ROS are produced in greater quantities in neutrophils than in macrophages; however, macrophages produce more RNS than neutrophils [43]. S. pneumoniae also produces H2O2, which interacts with NO• forming ONOO− [44, 45]. ONOO− can damage neurons and glial cells by lipid peroxidation and cell membrane destabilization; it can also cause DNA disintegration and subsequent poly (ADP-ribose) polymerase (PARP) activation, which leads to cell energy reduction and cell death [2]. In pneumococcal meningitis, adjuvant therapy with an ONOO− scavenger reduces the number of CSF leukocytes concentrations and reduces the brain concentrations of IL-1β and MIP-2 [46]. This reduction is associated with a decrease of the number of leukocytes in the CSF, suggesting the involvement of ROS/RNS and proinflammatory cytokines and chemokines in the attraction of leukocytes from the blood into the subarachnoid space [3]. ONOO− can contribute to the development of meningeal inflammation and increase the production of IL-8. This chemokine is equivalent to rat MIP-2; it is a chemoattractant and is involved in the migrations of leukocytes in pneumococcal meningitis [47]. In addition, treatment with a monoclonal antibody that binds with IL-8 attenuates pleocytosis in experimental pneumococcal meningitis in rabbits [48].
In vitro, the production of cytokines by human mononuclear cells was regulated by ONOO−. This activation was mediated via the transcription factor NF-κB by a mechanism that may involve nitration or dephosphorylation of IκB-a which leads to NF-κB translocation and release of TNF-α [49].
One of the major and first pathologies during pneumococcal meningitis is the breakdown of the BBB. In an animal model, the BBB breakdown occurred at 12 hours after pneumococcal meningitis induction [50], subsequent to the cytokine production [35]. ROS and RNS have been implicated as mediators of the BBB breakdown [3], suggesting that the increase of the BBB permeability appears to be related to the presence of NO• and O2 −• [51]. Furthermore, treatment with antioxidant prevented BBB disruption [41, 46].
Neurological sequelae from pneumococcal meningitis are estimated to occur in 30 to 52% of surviving patients [1, 52]. This damage has been demonstrated in a bacterial meningitis animal model; in this model, the surviving animals showed memory and learning impairment, depressive-like-behaviors, and anxiety-like symptoms [53]. In addition, coadjuvant treatment with antioxidants prevented cognitive impairment and oxidative stress in the brain of the survivor rats of the bacterial meningitis animal model [54]. ROS and RNS are related to these cognitive sequelae because of the cellular damage that they cause. The nervous system is a unique network of diverse cell types, comprising multiple proteins, lipids, and carbohydrates, and has important interactions with all major organs in the body [55]. Thus, the brain becomes particularly vulnerable to oxidative damage due to its high oxygen consumption, the abundance of iron, relatively low expression of antioxidants levels [55], and high presence of the polyunsaturated fatty acids [3]. H2O2 and pneumolysin produced by pneumococcus can cause neuronal cell death through mitochondrial damage [45, 56], leading to the release of apoptosis-inducing factor (AIF) into the cytosol and subsequently inducing apoptosis by a caspase-independent pathway [56]. Furthermore, leukocytes activate the tumor suppressor protein (p53) and the ataxia telangiectasia-mutated (ATM) kinase, which induce mitochondria to release cytochrome-c. Cytochrome-c, Apaf-1, and dATP/ATP are needed to form the apoptosome which is a special protein complex. Subsequently, apoptosome activates the caspase-9, that results in the activation of caspase-3 and apoptosis [56, 57]. The formation of ROS can cause direct damage through lipid peroxidation and carbonylation. Lipid peroxidation can be increased in serum [58] and in the CSF of children with bacterial meningitis [59].
6. Conclusion
Understanding the interactions between the complex immune network, composed of cytokines, chemokine, leukocytes, and oxidative stress, and bacterial virulence factors may help to establish more effective therapeutic strategies for CNS infections and, therefore, a better outcome for affected subjects.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgments
This research was supported by grants from CNPq, FAPESC, UNESC, NENASC project (PRONEX program CNPq/FAPESC), INCT-TM, and L'Oréal-UNESCO Brazil Fellowship for Women in Science 2011.
Abbreviations
- AIF:
Apoptosis-inducing factor
- AIM2:
Absent in melanoma 2
- Apaf:
Apoptosis protease activating factor
- ASC:
apoptosis speck-like protein
- ATM:
Ataxia telangiectasiamutated
- BBB:
Blood-brain barrier
- CNS:
Central nervous system
- CSF:
Cerebrospinal fluid
- CXCL-8:
Interleukin 8
- DNA:
Desoxyribonucleic acid
- Fe2:
Iron ion
- H2O2:
Hydrogen peroxide
- ICAM:
Intercellular adhesion molecule
- IkB:
Immunome knowledge base
- IKK:
Immunome knowledge base kinase
- IL:
Interleukin
- IL-1Rs:
interleukin receptors
- iNOS:
Inducible nitric oxide synthase
- IRAK4:
IL-1 receptor-associated kinase-4
- LFA:
Lymphocyte-function-associated antigen
- MAPKKK:
Mitogen-activated protein kinase kinase kinase
- MIP-2:
Macrophage inflammatory protein
- MyD88:
Myeloid differentiation factor 88
- NADPH:
Nicotinamide adenine dinucleotide phosphate
- NF-κB:
Nuclear factor kappa B
- NLR:
NOD-Like receptor
- NO:
Nitric oxide
- NOD/CARD:
Nucleotide oligomerization domain/caspase recruitment domain
- O2−•:
Superoxide anion radicals
- OCl:
Hypochlorite
- OH:
Hydroxyl radicals
- ONOO‒:
Peroxynitrite
- PAF:
Platelet-activating-factor
- PAMP:
Pathogen-associated molecular patterns
- PARP:
Poly-ADP-ribose polymerase
- pIgR:
Polymeric Ig receptor
- PRRs:
Pattern recognition receptors
- PspC:
Protein C pneumococcal surface
- RNS:
Reactive nitrogen species
- ROS:
Reactive oxygen species
- S. pneumoniae:
Streptococcus pneumoniae
- SOD:
Superoxide dismutase
- TAK1:
TGF-β-activated kinase 1
- TLR:
Toll-like receptor
- TRAF6:
Tumor necrosis factor receptor associated factor 6.
References
- 1.Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clinical Microbiology Reviews. 2011;24(3):557–591. doi: 10.1128/CMR.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheld WM, Koedel U, Nathan B, Pfister HW. Pathophysiology of bacterial meningitis: mechanism(s) of neuronal injury. Journal of Infectious Diseases. 2002;186(supplement 2):S225–S233. doi: 10.1086/344939. [DOI] [PubMed] [Google Scholar]
- 3.Klein M, Koedel U, Pfister HW. Oxidative stress in pneumococcal meningitis: a future target for adjunctive therapy? Progress in Neurobiology. 2006;80(6):269–280. doi: 10.1016/j.pneurobio.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Szabó C. Multiple pathways of peroxynitrite cytotoxicity. Toxicology Letters. 2003;140-141:105–112. doi: 10.1016/s0378-4274(02)00507-6. [DOI] [PubMed] [Google Scholar]
- 5.Kim KS. Mechanisms of microbial traversal of the blood-brain barrier. Nature Reviews Microbiology. 2008;6(8):625–634. doi: 10.1038/nrmicro1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim KS. Microbial translocation of the blood-brain barrier. International Journal for Parasitology. 2006;36(5):607–614. doi: 10.1016/j.ijpara.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Barichello T, Generoso JS, Milioli G, Elias SG, Teixeira AL. Pathophysiology of bacterial infection of the central nervous system and its putative role in the pathogenesis of behavioral changes. Revista Brasileira de Psiquiatria. 2013;35(1):81–87. doi: 10.1016/j.rbp.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Zhang JR, Mostov KE, Lamm ME, et al. The polymeric immunoglobulin receptor translocates pneumococci across nasopharyngeal human epithelial cells. Cell. 2000;102(6):827–837. doi: 10.1016/s0092-8674(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 9.van der Flier M, Chhun N, Wizemann TM, Min J, McCarthy JB, Tuomanen EI. Adherence of Streptococcus pneumoniae to immobilized fibronectin. Infection and Immunity. 1995;63(11):4317–4322. doi: 10.1128/iai.63.11.4317-4322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergmann S, Lang A, Rohde M, et al. Integrin-linked kinase is required for vitronectin-mediated internalization of Streptococcus pneumoniae by host cells. Journal of Cell Science. 2009;122(2):256–257. doi: 10.1242/jcs.035600. [DOI] [PubMed] [Google Scholar]
- 11.Kostrzynska M, Wadström T. Binding of laminin, type IV collagen, and vitronectin by Streptococcus pneumoniae . Zentralblatt fur Bakteriologie. 1992;277(1):80–83. doi: 10.1016/s0934-8840(11)80874-1. [DOI] [PubMed] [Google Scholar]
- 12.Barichello T, Generoso JS, Collodel A, Moreira AP, Almeida SM. Pathophysiology of acute meningitis caused by Streptococcus pneumoniae and adjunctive therapy approaches. Arquivos de Neuro-Psiquiatria. 2012;70(5):366–372. doi: 10.1590/s0004-282x2012000500011. [DOI] [PubMed] [Google Scholar]
- 13.Sellner J, Täuber MG, Leib SL. Pathogenesis and pathophysiology of bacterial CNS infections. In: Karen LR, Allan RT, editors. Handbook of Clinical Neurology. chapter 1. New York, NY, USA: Elsevier; 2010. pp. 1–16. [DOI] [PubMed] [Google Scholar]
- 14.Koppe U, Suttorp N, Opitz B. Recognition of Streptococcus pneumoniae by the innate immune system. Cellular Microbiology. 2012;14(4):460–466. doi: 10.1111/j.1462-5822.2011.01746.x. [DOI] [PubMed] [Google Scholar]
- 15.Hanke ML, Kielian T. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clinical Science. 2011;121(9):367–387. doi: 10.1042/CS20110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanamsagar R, Hanke ML, Kielian T. Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends in Immunology. 2012;33(7):333–342. doi: 10.1016/j.it.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanke ML, Angle A, Kielian T. MyD88-dependent signaling influences fibrosis and alternative macrophage activation during Staphylococcus aureus biofilm infection. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0042476.e42476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kronfol Z, Remick DG. Cytokines and the brain: implications for clinical psychiatry. The American Journal of Psychiatry. 2000;157(5):683–694. doi: 10.1176/appi.ajp.157.5.683. [DOI] [PubMed] [Google Scholar]
- 19.von Bernuth H, Picard C, Jin Z, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321(5889):691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai T, Akira S. Signaling to NF-κB by toll-like receptors. Trends in Molecular Medicine. 2007;13(11):460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Ku CL, von Bernuth H, Picard C, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. Journal of Experimental Medicine. 2007;204(10):2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26(22):3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- 23.Malley R, Henneke P, Morse SC, et al. Recognition of pneumolysin by toll-like receptor 4 confers resistance to pneumococcal infection. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenneth NS, Mudie S, Naron S, Rocha S. TFR1 interacts with the IKK complex and is involved in IKK-NF-κB signalling. Biochemical Journal. 2013;449(1):275–284. doi: 10.1042/BJ20120625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tato CM, Hunter CA. Host-pathogen interactions: subversion and utilization of the NF-κB pathway during infection. Infection and Immunity. 2002;70(7):3311–3317. doi: 10.1128/IAI.70.7.3311-3317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koedel U, Bayerlein I, Paul R, Sporer B, Pfister HW. Pharmacologic interference with NF-κB activation attenuates central nervous system complications in experimental pneumococcal meningitis. Journal of Infectious Diseases. 2000;182(5):1437–1445. doi: 10.1086/315877. [DOI] [PubMed] [Google Scholar]
- 27.Opitz B, Püschel A, Schmeck B, et al. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae . The Journal of Biological Chemistry. 2004;279(35):36426–36432. doi: 10.1074/jbc.M403861200. [DOI] [PubMed] [Google Scholar]
- 28.Kersse K, Bertrand MJ, Lamkanfi M, Vandenabeele P. NOD-like receptors and the innate immune system: coping with danger, damage and death. Cytokine and Growth Factor Reviews. 2011;22(5):257–276. doi: 10.1016/j.cytogfr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Proell M, Gerlic M, Mace PD, Reed JC, Riedl SJ. The CARD plays a critical role in ASC foci formation and inflammasome signaling. Biochemical Journal. 2013;449(3):613–621. doi: 10.1042/BJ20121198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shoma S, Tsuchiya K, Kawamura I, et al. Critical involvement of pneumolysin in production of interleukin-1α and caspase-1-dependent cytokines in infection with Streptococcus pneumoniae in vitro: a novel function of pneumolysin in caspase-1 activation. Infection and Immunity. 2008;76(4):1547–1557. doi: 10.1128/IAI.01269-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNeela EA, Burke A, Neill DR, et al. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathogens. 2010;6(11) doi: 10.1371/journal.ppat.1001191.e1001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirst RA, Kadioglu A, O’Callaghan C, Andrew PW. The role of pneumolysin in pneumococcal pneumonia and meningitis. Clinical and Experimental Immunology. 2004;138(2):195–201. doi: 10.1111/j.1365-2249.2004.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84(7):2068–2101. [PubMed] [Google Scholar]
- 34.Hanna S, Etzioni A. Leukocyte adhesion deficiencies. Annals of the New York Academy of Sciences. 2012;1250:50–55. doi: 10.1111/j.1749-6632.2011.06389.x. [DOI] [PubMed] [Google Scholar]
- 35.Barichello T, dos Santos I, Savi GD, et al. TNF-α, IL-1β, IL-6, and cinc-1 levels in rat brain after meningitis induced by Streptococcus pneumoniae . Journal of Neuroimmunology. 2010;221(1-2):42–45. doi: 10.1016/j.jneuroim.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Brivet FG, Jacobs FM, Mégarbane B. Cerebral output of cytokines in patients with pneumococcal meningitis. Critical Care Medicine. 2005;33(11):2721–2723. doi: 10.1097/01.ccm.0000187092.73841.82. [DOI] [PubMed] [Google Scholar]
- 37.Polfliet MMJ, Zwijnenburg PJG, van Furth AM, et al. Meningeal and perivascular macrophages of the central nervous system play a protective role during bacterial meningitis. Journal of Immunology. 2001;167(8):4644–4650. doi: 10.4049/jimmunol.167.8.4644. [DOI] [PubMed] [Google Scholar]
- 38.Brandt CT, Lundgren JD, Frimodt-Møller N, et al. Blocking of leukocyte accumulation in the cerebrospinal fluid augments bacteremia and increases lethality in experimental pneumococcal meningitis. Journal of Neuroimmunology. 2005;166(1-2):126–131. doi: 10.1016/j.jneuroim.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Kastenbauer S, Koedel U, Becker BF, Pfister HW. Oxidative stress in bacterial meningitis in humans. Neurology. 2002;58(2):186–191. doi: 10.1212/wnl.58.2.186. [DOI] [PubMed] [Google Scholar]
- 40.Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nature Reviews Microbiology. 2004;2(10):820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 41.Kastenbauer S, Koedel U, Pfister HW. Role of peroxynitrite as a mediator of pathophysiological alterations in experimental pneumococcal meningitis. Journal of Infectious Diseases. 1999;180(4):1164–1170. doi: 10.1086/315048. [DOI] [PubMed] [Google Scholar]
- 42.Aycicek A, Iscan A, Erel O, Akcali M, Ocak AR. Oxidant and antioxidant parameters in the treatment of meningitis. Pediatric Neurology. 2007;37(2):117–120. doi: 10.1016/j.pediatrneurol.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(16):8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell TJ. Virulence factors and the pathogenesis of disease caused by Streptococcus pneumoniae . Research in Microbiology. 2000;151(6):413–419. doi: 10.1016/s0923-2508(00)00175-3. [DOI] [PubMed] [Google Scholar]
- 45.Braun JS, Sublett JE, Freyer D, et al. Pneumococcal pneumolysin and H2O2 mediate brain cell apoptosis during meningitis. Journal of Clinical Investigation. 2002;109(1):19–27. doi: 10.1172/JCI12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kastenbauer S, Koedel U, Becker BF, Pfister HW. Pneumococcal meningitis in the rat: evaluation of peroxynitrite scavengers for adjunctive therapy. European Journal of Pharmacology. 2002;449(1-2):177–181. doi: 10.1016/s0014-2999(02)01980-5. [DOI] [PubMed] [Google Scholar]
- 47.Spanaus KS, Nadal D, Pfister HW, et al. C-X-C and C-C chemokines are expressed in the cerebrospinal fluid in bacterial meningitis and mediate chemotactic activity on peripheral blood-derived polymorphonuclear and mononuclear cells in vitro . Journal of Immunology. 1997;158(4):1956–1964. [PubMed] [Google Scholar]
- 48.Stergaard C, Yieng-Kow RV, Larsen CG, et al. Treatment with a monoclonal antibody to IL-8 attenuates the pleocytosis in experimental pneumococcal meningitis in rabbits when given intravenously, but not intracisternally. Clinical and Experimental Immunology. 2000;122(2):207–211. doi: 10.1046/j.1365-2249.2000.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matata BM, Galiñanes M. Peroxynitrite is an essential component of cytokines production mechanism in human monocytes through modulation of nuclear factor-κB DNA binding activity. The Journal of Biological Chemistry. 2002;277(3):2330–2335. doi: 10.1074/jbc.M106393200. [DOI] [PubMed] [Google Scholar]
- 50.Barichello T, Generoso JS, Silvestre C, et al. Circulating concentrations, cerebral output of the CINC-1 and blood-brain barrier disruption in Wistar rats after pneumococcal meningitis induction. European Journal of Clinical Microbiology and Infectious Diseases. 2012;31(8):2005–2009. doi: 10.1007/s10096-011-1533-2. [DOI] [PubMed] [Google Scholar]
- 51.Mayhan WG. Nitric oxide donor-induced increase in permeability of the blood-brain barrier. Brain Research. 2000;866(1-2):101–108. doi: 10.1016/s0006-8993(00)02254-x. [DOI] [PubMed] [Google Scholar]
- 52.Kastenbauer S, Pfister HW. Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain. 2003;126(5):1015–1025. doi: 10.1093/brain/awg113. [DOI] [PubMed] [Google Scholar]
- 53.Barichello T, Silva GZ, Generoso JS, et al. Time-dependent behavioral recovery after pneumococcal meningitis in rats. Journal of Neural Transmission. 2010;117(7):819–826. doi: 10.1007/s00702-010-0435-2. [DOI] [PubMed] [Google Scholar]
- 54.Barichello T, Santos AL, Savi GD, et al. Antioxidant treatment prevents cognitive impairment and oxidative damage in pneumococcal meningitis survivor rats. Metabolic Brain Disease. 2012;27(4):587–593. doi: 10.1007/s11011-012-9315-9. [DOI] [PubMed] [Google Scholar]
- 55.Harris RA, Amor S. Sweet and sour—oxidative and carbonyl stress in neurological disorders. CNS and Neurological Disorders Drug Targets. 2011;10(1):82–107. doi: 10.2174/187152711794488656. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell L, Smith SH, Braun JS, Herzog KH, Weber JR, Tuomanen EI. Dual phases of apoptosis in pneumococcal meningitis. Journal of Infectious Diseases. 2004;190(11):2039–2046. doi: 10.1086/425520. [DOI] [PubMed] [Google Scholar]
- 57.Marek L. The role of the apoptosome in the activation of procaspase-9. Postepy Higieny i Medycyny Doswiadczalnej. 2013;67:54–64. doi: 10.5604/17322693.1032333. [DOI] [PubMed] [Google Scholar]
- 58.Çaksen H, Cemek M, Dede S, Dulger H, Cemek F. Brief clinical study: lipid peroxidation and antioxidant status in children with acute purulent meningitis and encephalitis. International Journal of Neuroscience. 2004;114(1):105–111. doi: 10.1080/00207450490249383. [DOI] [PubMed] [Google Scholar]
- 59.Miric D, Katanic R, Kisic B, et al. Oxidative stress and myeloperoxidase activity during bacterial meningitis: effects of febrile episodes and the BBB permeability. Clinical Biochemistry. 2010;43(3):246–252. doi: 10.1016/j.clinbiochem.2009.09.023. [DOI] [PubMed] [Google Scholar]


