Abstract
Outcome evaluation is a cognitive process that plays an important role in our daily lives. In most paradigms utilized in the field of experimental psychology, outcome valence and outcome magnitude are the two major features investigated. The classical “independent coding model” suggest that outcome valence and outcome magnitude are evaluated by separate neural mechanisms that may be mapped onto discrete event-related potential (ERP) components: feedback-related negativity (FRN) and the P3, respectively. To examine this model, we presented outcome valence and magnitude sequentially rather than simultaneously. The results reveal that when only outcome valence or magnitude is known, both the FRN and the P3 encode that outcome feature; when both aspects of outcome are known, the cognitive functions of the two components dissociate: the FRN responds to the information available in the current context, while the P3 pattern depends on outcome presentation sequence. The current study indicates that the human evaluative system, indexed in part by the FRN and the P3, is more flexible than previous theories suggested.
Keywords: Decision-making, outcome evaluation, outcome valence, outcome magnitude, event-related potential (ERP), feedback-related negativity (FRN), P3
1. INTRODUCTION
The process of outcome evaluation constitutes a late stage of decision-making (Ernst & Paulus, 2005; Paulus, 2005; Platt, 2002). Its role in reinforcement learning, adaptation, and survival is crucial for human beings (Nieuwenhuis, Holroyd, Mol, & Coles, 2004). People adjust their behavior according to outcomes or consequences of their actions (Ernst & Paulus, 2005; Friedrich & Zentall, 2010; Holroyd & Coles, 2008). Our brain stores information about past outcomes so as to improve future decisions (Cohen & Ranganath, 2007; Frank, Seeberger, & O’Reilly, 2004; Platt, 2002). Abnormalities in outcome evaluation might lead to problematic behavior such as drug abuse or pathological gambling (Everitt et al., 2007; Fridberg et al., 2010; Shiv, Loewenstein, & Bechara, 2005). Thus far, most studies on outcome evaluation have focused on reward and punishment processing (Kim, Shimojo, & O’Doherty, 2006; Plassmann, Doherty, & Rangel, 2010; Rogers et al., 2004). This processing encodes outcome valence. Outcome magnitude, the size or degree of a reward or punishment, is also an important factor and has been investigated (Goyer, Woldorff, & Huettel, 2008; Sato et al., 2005; Shenhav & Greene, 2010; Wu & Zhou, 2009). We suggest the importance of outcome magnitude stems from larger magnitudes often being associated with stronger arousal and emotional feelings. In sum, outcome valence reveals whether or not the outcome is beneficial, while outcome magnitude modulates the level of arousal.
Event-related potentials (ERPs) of electroencephalogram (EEG), most notable for their exquisite temporal resolution (Liotti, Woldorff, Perez, & Mayberg, 2000), provide important real-time knowledge about the mechanism of outcome evaluation (e.g., Cohen, Elger, & Ranganath, 2007; Cohen & Ranganath, 2007). Among the ERP signals that could potentially be biomarkers of outcome evaluation, feedback-related negativity (FRN) and the P3 are the most often studied components (Polezzi, Sartori, Rumiati, Vidotto, & Daum, 2010; Wu & Zhou, 2009). The FRN is a negative-going component that is consistently larger after negative outcomes than positive outcomes (Gehring & Willoughby, 2002; Nieuwenhuis et al., 2004) while the P3 is a centro-parietal positivity that indicates the affective significance of stimuli (Polezzi et al., 2010; Wu & Zhou, 2009).
The reinforcement learning theory of error-related negativity (RL-ERN theory) provides a classical interpretation of the cognitive function associated with the FRN (Holroyd & Coles, 2002; Holroyd, Coles, & Nieuwenhuis, 2002). This theory suggests that the FRN is an index of the activities of the midbrain dopamine system, which evaluates the ongoing event along a binary “good-no good” dimension (Holroyd, Hajcak, & Larsen, 2006). Accordingly, the FRN responds to outcome valence but is insensitive to variations in magnitude (Hajcak, Moser, Holroyd, & Simons, 2006; Holroyd et al., 2006; Nieuwenhuis et al., 2004). To take a step further, Yeung & Sanfey (2004) suggest that both the FRN and the P3 are physiological reflections of the human evaluative function. Valence and magnitude, two fundamental features of outcome stimuli, are encoded by the FRN and the P3, respectively. The FRN is sensitive to outcome valence, regardless of outcome magnitude, and the P3 shows the opposite pattern. This idea, which indicates that our brain processes valence and magnitude separately, is called the “independent coding model” by its proposers. Yeung & Sanfey’s (2004) model remains controversial hitherto (Bellebaum & Daum, 2008; Hajcak, Holroyd, Moser, & Simons, 2005; Hajcak, Moser, Holroyd, & Simons, 2007; Pfabigan, Alexopoulos, Bauer, & Sailer, 2011; Wu & Zhou, 2009), and some researchers doubt whether valence and magnitude could be mapped onto the FRN and the P3, respectively (Goyer et al., 2008).
This issue remains unsolved partly because in previous studies the valence and the magnitude of outcomes are presented in an integrated way (i.e., simultaneously). Potential interactions between the processing of valence and magnitude might have been masked by the simultaneous presentation of both features. Hence, the ideal test of the model should separate the presentations of the valence and the magnitude during a task. We modified Yeung & Sanfey’s (2004) design such that outcome valence and outcome magnitude were presented sequentially rather than simultaneously. We expect that if the FRN and the P3 components are sensitive to outcome valence and outcome magnitude, respectively, in a separate presentation design, the reliability of the “independent coding model” would be confirmed.
2. METHODS
2.1 Participants
24 right-handed students (13 females; mean age 22.25 ± 2.42 years) from Beijing Normal University participated in the experiment. All participants were free of regular use of medication or other nonmedical substances that might influence the central nervous system. All had normal vision (with or without correction); none had history of neurological disease. All participants gave their informed consent prior to the experiment. The experimental protocol was approved by the Local Ethics Committee (Beijing Normal University). None of the participants reported knowledge of the Hebrew alphabet.
2.2 Procedure
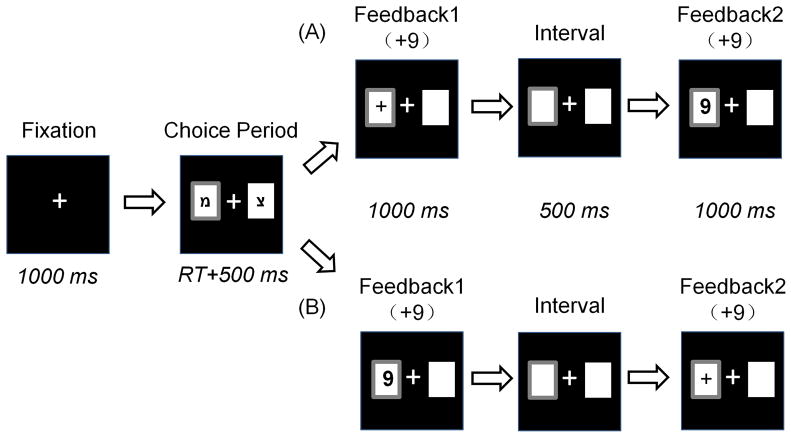
Stimulus display and behavioral data acquisition were conducted using E-Prime software (Version 1.1, Psychology Software Tools, Inc.). During the task, the participant sat comfortably in an electrically-shielded room approximately 100 cm from a computer screen. Each trial began with the presentation of two white rectangles (2.5 degree × 2.5 degree of visual angle) in which two Hebrew letters (“מ” and “צ”) were individually presented to indicate two alternative options on the left and right sides of a fixation point. The positions of the two letters were counterbalanced across trials. The participant was asked to make a selection by pressing the “F” or “J” key on the keyboard with the left or right index finger, respectively. The alternatives remained on the screen until the participant chose a rectangle, which was then highlighted by a thick red outline for 500 ms. Thereafter, the outcome of the participant’s choice was presented such that its valence and magnitude were displayed sequentially, with a 500 ms interval between presentations (see Figure 1).
Figure 1. Each participant was asked to select one of two alternatives and indicate his/her selection using the left or right index finger.
His/her choice was immediately highlighted with a thick red border for 500 ms. Afterward, the outcome was presented in two formats that were counterbalanced across trials: (A) outcome valence was presented antecedently, and outcome magnitude was presented subsequently; (B) outcome magnitude was presented antecedently, and outcome valence was presented subsequently. In this sample, both formats indicate the result “+9”.
There were four kinds of outcomes: “+9”, “+99”, “−9”, and “−99.” Each indicated the points the participant won (when the valence of outcome was “+”) or lost (when the valence of outcome was “−”) in the current trial. The formal task consisted of four blocks of 96 trials each. Unbeknownst to the participant, the outcomes were provided according to a pre-determined pseudorandom sequence, and each participant received exactly 96 of each kind of outcome.
There are two kinds of outcome sequences: (A) the valence of outcomes was presented antecedently, then the magnitude of outcomes subsequently; and (B) the reversal of (A). Prior to the experiment, each participant was told that the sequence of outcome presentation was irrelevant to its value. The two kinds of outcome sequences were counterbalanced within each kind of outcome.
Before the experiment, each participant was instructed about the rules and the meaning of the symbols in the task. In addition, he/she was encouraged to respond in a way that would maximize the total score amount. He/she was told that the higher the score he/she earned, the more bonus money he/she would receive at the end of the experiment. However, after the participant finished the task, he/she was briefed that there was no optimal strategy for the task. Each participant was paid 60 Chinese Yuan (approximately $10) for participation.
2.3 Electrophysiological Recording and Measures
Electroencephalogram (EEG) activity was recorded from 64 scalp sites using tin electrodes mounted in an elastic cap (NeuroScan Inc.) with an online reference to the left mastoid and off-line algebraic re-reference to the average of the left and right mastoids. Horizontal electrooculogram (HEOG) was recorded from electrodes placed at the outer canthi of both eyes. Vertical electrooculogram (VEOG) was recorded from electrodes placed above and below the left eye. All inter-electrode impedance was maintained at < 5 kΩ. EEG and EOG signals were amplified with a bandpass from 0.05 Hz to 100 Hz and continuously sampled at 500 Hz/channel.
During the offline analysis, ocular artifacts were removed from the EEG signal using a regression procedure implemented with Neuroscan software (Semlitsch, Anderer, Schuster, & Presslich, 1986). After digital filtering through a zero phase shift (see below), the EEG data were epoched time-locked to the onset of each kind of outcome stimulus. Separate EEG epochs of 1000 ms were baseline-corrected by subtracting from each sample the average activity of that channel during the − 200 − 0 ms baseline period. Any trials where EEG voltages exceeded a threshold of ±100 μV during the recording epoch were excluded from the analysis.
Following the methods of Yeung & Sanfey (2004), FRN amplitude was measured for each participant as the base-to-peak difference in voltage between the most negative peak within the 200–400 ms window and the average voltage of the immediately preceding and following positive peaks, after 0.05–30 Hz band-pass filtering. P3 amplitude was measured as the most positive peak within the 200–600 ms window after 2 Hz low-pass filtering (Yeung & Sanfey, 2004). The electrode at which the ERP components reached their maximum was detected along the midline (Fz, FCz, Cz, CPz, Pz, POz and Oz); thereafter, the mean amplitude of this electrode and 8 adjacent electrodes were calculated for further analysis.
3. RESULTS
For all the analyses listed below, the significance level was set at 0.05. Greenhouse–Geisser correction for ANOVA tests was used whenever appropriate. Post-hoc testing of significant main effects was conducted using LSD method. Significant interactions were analyzed using simple-effects models. Partial eta-squared was reported to demonstrate the effect size in ANOVA tests, where 0.05 represents a small effect, 0.10 equals a medium effect, and 0.20 represents a large effect (Pfabigan et al., 2011). Statistical analysis was performed using SPSS (17.0; SPSS, Inc., Chicago, IL).
3.1 Behavioral results
The percentage of trials where alternative “מ” was selected was 49.65 ± 3.11 %; this number was 50.36 ± 3.11 % for “צ”. Average decision-making time was 760.76 ± 447.26 ms. Since there was no optimal strategy for each participant during the task, behavioral data was not analyzed any further.
3.2 ERP Results
3.2.1 Sequence (A): Outcome Valence -> Outcome Magnitude
Outcome Valence (presented antecedently)
FRN
The analysis across the midline revealed that the FRN elicited by outcome valence (presented antecedently) was largest at electrode FCz (− 2.96 μV). Accordingly, this electrode and 8 adjacent electrodes (F1, Fz, F2, FC1, FC2, C1, Cz, C2) were chosen for further analysis. The mean amplitudes of these electrodes were entered into a paired-samples t test using outcome valence (positive/negative) as the within-subject factor.
The main effect of outcome valence was significant (t (23) = 5.889, p < 0.001). Consistent with classical studies (Gehring & Willoughby, 2002; Holroyd et al., 2006; Miltner, Braun, & Coles, 1997), this result indicated that the FRN was larger when outcome valence was negative (− 3.69 μV) and smaller when outcome valence was positive (− 1.80 μV) (see Figure 2a). Please note that the FRN is a negative-going component. Thereby, smaller amplitude indicates a larger FRN.
Figure 2.
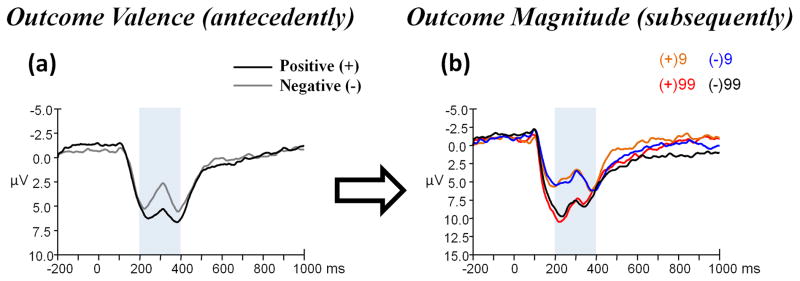
(a) Grand-average ERPs evoked by outcome valence (presented antecedently) at the FCz recording site, and (b) that evoked by outcome magnitude (presented subsequently) at the Fz recording site using a 0.05 – 30 Hz band-pass filter. The time point “0” indicates outcome presentation onset. The light gray shaded areas indicate the 200–400 ms time window for the detection of the most negative peak. The scalp topographies of each condition are presented beneath.
P3
The analysis across the midline revealed that the P3 elicited by outcome valence (presented antecedently) was largest at electrode CPz (8.11 μV). Accordingly, this electrode and 8 adjacent electrodes (C1, Cz, C2, CP1, CP2, P1, Pz, & P2) were chosen for further analysis. The mean amplitudes of these electrodes were entered into a paired-samples t test using outcome valence (positive/negative) as the within-subject factor.
The main effect of outcome valence was significant (t (23) = 3.705, p = 0.001); the P3 was larger when outcome valence was positive (8.50 μV) and smaller when outcome valence was negative (7.19 μV) (see Figure 3a; see also Hajcak et al., 2005; Polezzi et al., 2010).
Figure 3.
(a) Grand-average ERPs evoked by outcome valence (presented antecedently), and (b) that evoked by outcome magnitude (presented subsequently) using a 2 Hz low-pass filter. Both were observed at the CPz recording site. The time point “0” indicates the outcome presentation onset. The light gray shaded areas indicate the 200–600 ms analysis window for the P3. The scalp topographies of each condition are presented beneath.
Outcome Magnitude (presented subsequently)
FRN
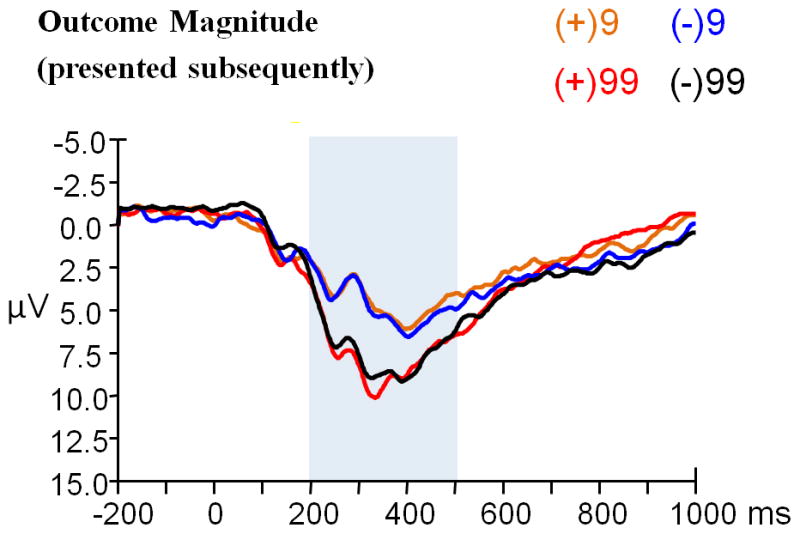
The analysis across the midline revealed that the FRN elicited by outcome magnitude (presented subsequently) was largest at electrode Fz (− 4.27 μV). Accordingly, this electrode and 8 adjacent electrodes (FP1, FPz, FP2, F1, F2, FC1, FCz, FC2) were chosen for further analysis. The mean amplitudes of these electrodes were entered into a 2 (magnitude: 9/99) × 2 (valence: positive/negative) ANOVA test.
The main effect of outcome magnitude was significant (F (1, 23) = 30.282, p < 0.001, partial η2 = 0.568); the FRN was larger when outcome magnitude was “9” (−4.76 μV) and smaller when outcome magnitude was “99” (− 3.34 μV) (see Figure 2b). The main effect of outcome valence was not significant (F (1, 23) = 0.138, p = 0.714). The magnitude x valence interaction was not significant (F (1, 23) = 0.021, p = 0.886).
P3
The analysis across the midline revealed that the P3 elicited by outcome magnitude (presented subsequently) was largest at electrode CPz (9.90 μV). Accordingly, this electrode and 8 adjacent electrodes (C1, Cz, C2, CP1, CP2, P1, Pz, & P2) were chosen for further analysis. The mean amplitudes of these electrodes were entered into a 2 (magnitude: 9/99) × 2 (valence: positive/negative) ANOVA test.
The main effect of outcome magnitude was significant (F (1, 23) = 44.784, p < 0.001, partial η2 = 0.661); the P3 was larger when outcome magnitude was “99” (11.24 μV) and smaller when outcome magnitude was “9” (7.72 μV) (see Figure 3b). The main effect of outcome valence was not significant (F (1, 23) = 0.077, p = 0.784). The magnitude x valence interaction was not significant (F (1, 23) = 0.470, p = 0.500).
3.2.2 Sequence (B): Outcome Magnitude -> Outcome Valence
Outcome Magnitude (presented antecedently)
FRN
The analysis across the midline revealed that the FRN elicited by outcome magnitude (presented antecedently) was largest at electrode Fz (− 3.92 μV). Accordingly, this electrode and 8 adjacent electrodes (FP1, FPz, FP2, F1, F2, FC1, FCz, FC2) were chosen for further analysis. The mean amplitudes of these electrodes were entered into a paired-samples t test using outcome magnitude (9/99) as the within-subject factor.
The main effect of outcome magnitude was significant (t (23) = − 4.714, p < 0.001); the FRN was larger when the magnitude was “9” (− 4.44 μV) and smaller when the magnitude was “99” (− 2.81 μV) (see Figure 4a).
Figure 4.
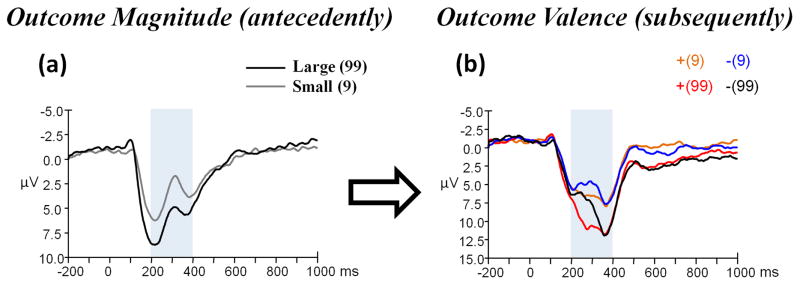
(a) Grand-average ERPs evoked by outcome magnitude (presented antecedently) at the Fz recording site, and (b) that evoked by outcome valence (presented subsequently) at the FCz recording site using a 0.05 – 30 Hz band-pass filter. The time point “0” indicates the outcome presentation onset. The light gray shaded areas indicate the 200–400 ms time window for the detection of the most negative peak. The scalp topographies of each condition are presented beneath.
P3
The analysis across the midline revealed that the P3 elicited by outcome magnitude (presented antecedently) was largest at electrode CPz (7.61 μV). Accordingly, this electrode and 8 adjacent electrodes (C1, Cz, C2, CP1, CP2, P1, Pz, & P2) were chosen for further analysis. The mean amplitudes of these electrodes were entered into a paired-samples t test using outcome magnitude (9/99) as the within-subject factor.
The main effect of outcome magnitude was significant (t (23) = − 6.472, p < 0.001); the P3 was larger when outcome magnitude was “99” (8.46 μV) and smaller when outcome magnitude was “9” (6.25 μV) (see Figure 5a).
Figure 5.
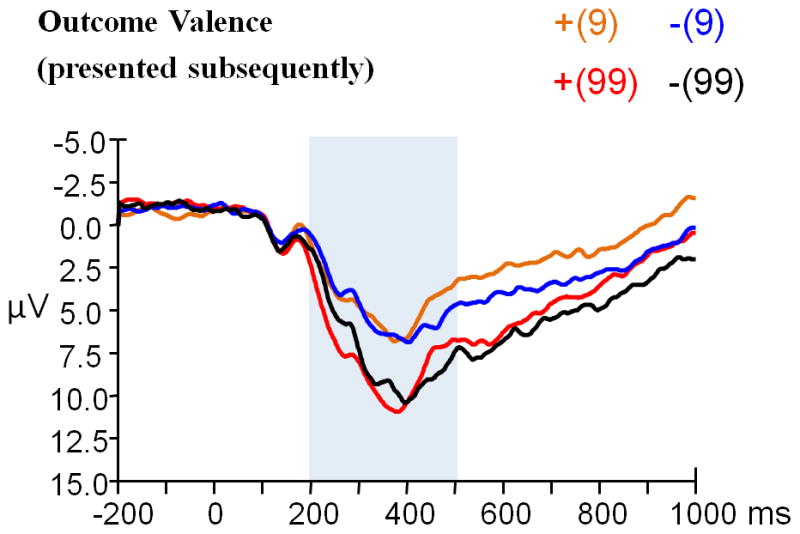
(a) Grand-average ERPs evoked by outcome magnitude (presented antecedently), and (b) that evoked by outcome valence (presented subsequently) using a 2 Hz low-pass filter. Both were observed at the CPz recording site. The time point “0” indicates the outcome presentation onset. The light gray shaded areas indicate the 200–600 ms analysis window for the P3. The scalp topographies of each condition are presented beneath.
Outcome Valence (presented subsequently)
FRN
The analysis across the midline revealed that the FRN elicited by outcome valence (presented subsequently) was largest at electrode FCz (− 4.21 μV). Accordingly, this electrode and 8 adjacent electrodes (F1, Fz, F2, FC1, FC2, C1, Cz, C2) were chosen for further analysis. The mean amplitudes of these electrodes were entered into a 2 (valence: positive/negative) × 2 (magnitude: 9/99) ANOVA test.
The main effect of outcome valence was significant (F (1, 23) = 16.886, p < 0.001, partial η2 = 0.423); the FRN was larger when outcome valence was negative (− 4.70 μV) and smaller when outcome valence was positive (− 3.15 μV) (see Figure 4b). The main effect of outcome magnitude was not significant (F (1, 23) = 2.085, p = 0.162). The valence x magnitude interaction was significant (F (1, 23) = 17.018, p < 0.001, partial η2 = 0.425). Simple effect analysis indicated that the effect of outcome valence was significant when outcome magnitude was “99” (F (1, 23) = 23.85, p < 0.001), but not significant when outcome magnitude was “9” (F (1, 23) = 0.65, p = 0.427).
P3
The analysis across the midline revealed that the P3 elicited by outcome valence (presented subsequently) was largest at electrode CPz (11.34 μV). Accordingly, this electrode and 8 adjacent electrodes (C1, Cz, C2, CP1, CP2, P1, Pz, & P2) were chosen for further analysis. The mean amplitudes of these electrodes were entered into a 2 (valence: positive/negative) × 2 (magnitude: 9/99) ANOVA test.
The main effect of outcome valence was significant (F (1, 23) = 5.138, p = 0.033, partial η2 = 0.183); the P3 was larger when outcome valence was positive (11.18 μV) and smaller when outcome valence was negative (10.51 μV). The main effect of outcome magnitude was also significant (F (1, 23) = 46.791, p < 0.001, partial η2 = 0.670); the P3 was larger when outcome magnitude was “99” (12.92 μV) and smaller when outcome magnitude was “9” (8.78 μV) (see Figure 5b). The valence x magnitude interaction was not significant (F (1, 23) = 1.322, p = 0.262).
4. DISCUSSION
According to the classical independent coding model, the FRN and the P3 are associated with the coding of outcome valence and outcome magnitude, respectively. This model would predict that when outcome valence and outcome magnitude were presented separately, the FRN would be selectively sensitive to outcome valence presentation, and the P3 would show the opposite pattern. Contrary to this prediction, the present results reveal that both the FRN and the P3 were sensitive to valence and magnitude. We therefore suggest the processing of valence and magnitude are not indexed by separate ERP components.
4.1 The Cognitive Function of the FRN
Many studies suggest that the FRN, which appears to be larger after unfavorable outcomes or feedback that indicates incorrect performance, may reflect a binary evaluation of “good” versus “no good” outcomes (Hajcak et al., 2005; Polezzi et al., 2010; Toyomaki & Murohashi, 2005). Given that binary evaluation interpretation, we proposed that the relationship between outcome valence and the FRN should not be influenced by outcome presentation style. Nevertheless, in the current study, the FRN could respond to either valence or magnitude, depending upon which of the two was being presented. It remains unknown, however, why the magnitude “9” consistently elicited a larger FRN than “99.” One possibility is that the participants, always seeking higher scores, evaluated “9” more unfavorably than “99.” Our results indicate that the cognitive function associated with the FRN goes beyond encoding outcome valence.
In spite of our results, most studies have reported that the FRN is selectively sensitive to valence (but see Bellebaum & Daum, 2008; Goyer et al., 2008). A possible explanation is that the rapid evaluative system indexed by the FRN (Gehring & Willoughby, 2002; Yeung & Sanfey, 2004) encodes whichever information is contextually most salient (Goyer et al., 2008; Potts, Martin, Burton, & Montague, 2006). Bradley (2000) claimed “for adult humans, object meaning tends to outweigh stimulus magnitude as the eliciting cue,” implying that valence is considered more important than magnitude. Accordingly, when the valence and the magnitude are presented simultaneously, the evaluative system indexed by the FRN focuses on valence rather than magnitude.
However, if valence is unknown, we suggest that magnitude alone could be motivationally significant in many scenarios. A night watchman who observes hundreds of people approaching may need to respond to the crowd’s magnitude in spite of their “valence” (i.e. their positive or negative intentions) being unknown. Consistent with our viewpoint, Schneirla (1959) proposed that the intensity of stimulus is an important factor that determines approach and withdrawal behaviors, with low-intensity stimuli associated with approach and high-intensity stimuli associated with withdrawal (cited from Bradley, 2000). This viewpoint demonstrates how our revised interpretation of the cognitive function indexed by the FRN harmonizes with existing theory suggesting the FRN’s role in motivationally-significant evaluation (Gehring & Willoughby, 2002; Holroyd, Baker, Kerns, & Muller, 2008; Yeung, Holroyd, & Cohen, 2005).
In our opinion, it is evolutionarily advantageous for the rapid evaluative system to respond to the most salient environmental features rather than immutably processing valence and magnitude separately. Researchers have pointed out that it is often necessary to make a quick, imperfect decision, so as to meet the challenges of the environment (Rahman, Sahakian, Cardinal, Rogers, & Robbins, 2001; Slovic, Peters, Finucane, & MacGregor, 2005). For this purpose, focusing attention on the contextually most salient information may quicken decision-making (Rahman et al., 2001). When outcome features are not fully known, encoding the most salient information regardless of its category, may be crucial for adaptation and survival (see also Anokhin et al., 2006).
4.2 The Cognitive Function of the P3
Like the FRN, the P3 elicited by outcome information (presented antecedently) could be sensitive to either valence or magnitude, depending upon which feature was being presented. Moreover, the pattern of the P3 elicited by outcome information (presented subsequently) was significantly influenced by outcome presentation sequence.
Using laboratory gambling tasks, some previous studies have reported that the time-course of the FRN and P3 temporally overlap (Foti, Weinberg, Dien, & Hajcak, 2011; Wu & Zhou, 2009; Yeung et al., 2005). These two components are even referred to as the “FRN-P3 complex” by some researchers (Dywan, Mathewson, Snyder, Tays, & Segalowitz, 2008; see also Folstein & Van Petten, 2008; Holroyd, 2004). We suggest that when outcome information is incomplete (e.g. either valence or magnitude is unknown), the P3 encodes the contextually most salient information in tandem with the FRN, so as to guide adaptive behavior more effectively (Nieuwenhuis et al., 2004; Prinzel, Freeman, Scerbo, Mikulka, & Pope, 2003).
The P3 elicited by outcome valence (presented subsequently) responded to both magnitude (presented antecedently) and valence. Regarding the valence, positive outcomes evoked a larger P3 than negative outcomes, indicating stronger affective significance (van Lankveld & Smulders, 2008; Wu & Zhou, 2009). These results fit well with the idea that the P3 encodes both valence and magnitude (Hajcak et al., 2005, 2007; Martin & Potts, 2004; Polezzi et al., 2010; Wu & Zhou, 2009). One possible explanation for this phenomenon is that when outcome features are fully known, the P3 reflects the process of overall outcome evaluation; all the major properties of the outcome are taken into account (Wu & Zhou, 2009). However, this interpretation does not account for the P3 elicited by outcome magnitude (presented subsequently) being insensitive to valence (presented antecedently). As such, it may be that the cognitive process indexed by the P3 is significantly affected by outcome presentation style. Different outcome presentation sequences may produce distinct neurological responses to the outcome. Further investigation into this issue may be necessary.
4.3 Conclusions
Using a new experimental design, the current study reveals that the human evaluative system, indexed in part by the FRN and the P3, encodes outcome information in a flexible way. When the outcome is partly unknown, both the FRN and the P3 encode the most salient feature (either valence or magnitude) of the outcome; when the outcome is fully known, the cognitive functions of the two components dissociate: the FRN still responds to the contextually most salient information, but the P3 could be sensitive to both valence and magnitude in specific outcome presentation sequence. More research is needed to test these findings.
In our opinion, brain imaging experiments that use our task paradigm would provide important knowledge about the neural underpinning of outcome evaluation. In addition, since the FRN and the P3 are both strongly affected by individual differences in personality (Foti & Hajcak, 2009; Gu, Ge, Jiang, & Luo, 2010; Gu, Huang, & Luo, 2010; Iacono & Mcgue, 2006; Martin & Potts, 2009; Onoda, Abe, & Yamaguchi, 2010), further studies utilizing personalities as grouping factors would be worthwhile.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (30930031), Ministry of Sci & Tech (973 Program, 2011CB711000), National Key Technologies R & D Program (2009BAI77B01), Global Research Initiative Program, United States National Institute of Health grants (1R01TW007897, P50DA005312), and Funds for Outstanding Doctoral Dissertation of Beijing Normal University. The authors thank Suyong Yang for help with data acquisition, Chunliang Feng for help with manuscript preparation, and three anonymous reviewers for their constructive comments.
References
- Anokhin AP, Golosheykin S, Sirevaag E, Kristjansson S, Rohrbaugh JW, Heath AC. Rapid discrimination of visual scene content in the human brain. Brain Research. 2006;1093:167–177. doi: 10.1016/j.brainres.2006.03.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellebaum C, Daum I. Learning-related changes in reward expectancy are reflected in the feedback-related negativity. European Journal of Neuroscience. 2008;27(7):1823–1835. doi: 10.1111/j.1460-9568.2008.06138.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM. Emotion and Motivation. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 2. Cambridge: Cambridge University Press; 2000. pp. 602–642. [Google Scholar]
- Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. Neuroimage. 2007;35(2):968–978. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Ranganath C. Reinforcement learning signals predict future decisions. Journal of Neuroscience. 2007;27(2):371–378. doi: 10.1523/JNEUROSCI.4421-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dywan J, Mathewson KJ, Snyder PJ, Tays WJ, Segalowitz SJ. Aging and electrocortical response to error feedback during a spatial learning task. Psychophysiology. 2008;45(6):936–948. doi: 10.1111/j.1469-8986.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: A selective review from a neurocognitive and clinical perspective. Biological Psychiatry. 2005;58(8):597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Linking Affect to Action: Critical Contributions of the Orbitofrontal Cortex. 2007;1121:576–597. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology. 2008;45(1):152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological Psychology. 2009;81(1):1–8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: Temporospatial principal components analysis and source localization of the feedback negativity. Human Brain Mapping. 2011 doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O’Reilly RC. By carrot or by stick: Cognitive reinforcement learning in Parkinsonism. Science. 2004;306(5703):1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Fridberg DJ, Queller S, Ahn WY, Kim W, Bishara AJ, Busemeyer JR, et al. Cognitive mechanisms underlying risky decision-making in chronic cannabis users. Journal of Mathematical Psychology. 2010;54(1):28–38. doi: 10.1016/j.jmp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich AM, Zentall TR. A relational differential outcomes effect: pigeons can classify outcomes as “good” and “better”. Animal Cognition. 2010;13(2):359–365. doi: 10.1007/s10071-009-0286-0. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Goyer JP, Woldorff MG, Huettel SA. Rapid Electrophysiological Brain Responses are Influenced by Both Valence and Magnitude of Monetary Rewards. Journal of Cognitive Neuroscience. 2008;20(11):2058–2069. doi: 10.1162/jocn.2008.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu R, Ge Y, Jiang Y, Luo Y-j. Anxiety and Outcome Evaluation: The Good, the Bad and the Ambiguous. Biological Psychology. 2010;85(2):200–206. doi: 10.1016/j.biopsycho.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu R, Huang Y-x, Luo Y-j. Anxiety and feedback negativity. Psychophysiology. 2010;47(5):961–967. doi: 10.1111/j.1469-8986.2010.00997.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Holroyd CB, Moser JS, Simons RF. Brain potentials associated with expected and unexpected good and bad outcomes. Psychophysiology. 2005;42(2):161–170. doi: 10.1111/j.1469-8986.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biological Psychology. 2006;71(2):148–154. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. It’s worse than you thought: The feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology. 2007;44(6):905–912. doi: 10.1111/j.1469-8986.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- Holroyd CB. A note on the oddball N200 and feedback ERN. Biological Psychology. 2004;56:191–198. [Google Scholar]
- Holroyd CB, Baker TE, Kerns KA, Muller U. Electrophysiological evidence of atypical motivation and reward processing in children with attention-deficit hyperactivity disorder. Neuropsychologia. 2008;46(8):2234–2242. doi: 10.1016/j.neuropsychologia.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. Dorsal anterior cingulate cortex integrates reinforcement history to guide voluntary behaviour. Cortex. 2008;44(5):548–559. doi: 10.1016/j.cortex.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH, Nieuwenhuis S. Medial prefrontal cortex and error potentials. Science. 2002;296(5573):1610–1611. doi: 10.1126/science.296.5573.1610. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Hajcak G, Larsen JT. The good, the bad and the neutral: Electrophysiological responses to feedback stimuli. Brain Research. 2006;1105:93–101. doi: 10.1016/j.brainres.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Mcgue M. Association between P3 event-related brain potential amplitude and adolescent problem behavior. Psychophysiology. 2006;43(5):465–469. doi: 10.1111/j.1469-8986.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O’Doherty JP. Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. Plos Biology. 2006;4(8):1453–1461. doi: 10.1371/journal.pbio.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, Woldorff MG, Perez R, Mayberg HS. An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia. 2000;38(5):701–711. doi: 10.1016/s0028-3932(99)00106-2. [DOI] [PubMed] [Google Scholar]
- Martin LE, Potts GF. Impulsivity and decision-making: An event related potential investigation. Psychophysiology. 2004;41:S66–S66. doi: 10.1016/j.paid.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LE, Potts GF. Impulsivity in decision-making: An event-related potential investigation. Personality and Individual Differences. 2009;46(3):303–308. doi: 10.1016/j.paid.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-Related Brain Potentials Following Incorrect Feedback in a Time-Estimation Task: Evidence for a “Generic” Neural System for Error Detection. Journal of Cognitive Neuroscience. 1997;9(6):788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Holroyd CB, Mol N, Coles MGH. Reinforcement-related brain potentials from medial frontal cortex: origins and functional significance. Neuroscience and Biobehavioral Reviews. 2004;28(4):441–448. doi: 10.1016/j.neubiorev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Onoda K, Abe S, Yamaguchi S. Feedback-related negativity is correlated with unplanned impulsivity. Neuroreport. 2010;21(10):736–739. doi: 10.1097/WNR.0b013e32833bfd36. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Neurobiology of decision-making: Quo vadis? Cognitive Brain Research. 2005;23(1):2–10. doi: 10.1016/j.cogbrainres.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Pfabigan DM, Alexopoulos J, Bauer H, Sailer U. Manipulation of feedback expectancy and valence induces negative and positive reward prediction error signals manifest in event-related brain potentials. Psychophysiology. 2011;48(5):656–664. doi: 10.1111/j.1469-8986.2010.01136.x. [DOI] [PubMed] [Google Scholar]
- Plassmann H, Doherty JPO, Rangel A. Appetitive and Aversive Goal Values Are Encoded in the Medial Orbitofrontal Cortex at the Time of Decision Making. The Journal of Neuroscience. 2010;30(32):10799–10808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML. Neural correlates of decisions. Current Opinion in Neurobiology. 2002;12(2):141–148. doi: 10.1016/s0959-4388(02)00302-1. [DOI] [PubMed] [Google Scholar]
- Polezzi D, Sartori G, Rumiati R, Vidotto G, Daum I. Brain correlates of risky decision-making. Neuroimage. 2010;49(2):1886–1894. doi: 10.1016/j.neuroimage.2009.08.068. [DOI] [PubMed] [Google Scholar]
- Potts GF, Martin LE, Burton P, Montague PR. When things are better or worse than expected: The medial frontal cortex and the allocation of processing resources. Journal of Cognitive Neuroscience. 2006;18(7):1112–1119. doi: 10.1162/jocn.2006.18.7.1112. [DOI] [PubMed] [Google Scholar]
- Prinzel LJ, Freeman FG, Scerbo MW, Mikulka PJ, Pope AT. Effects of a psychophysiological system for adaptive automation on performance, workload, and the event-related potential P300 component. Human Factors. 2003;45(4):601–613. doi: 10.1518/hfes.45.4.601.27092. [DOI] [PubMed] [Google Scholar]
- Rahman S, Sahakian BJ, Cardinal RN, Rogers RD, Robbins TW. Decision making and neuropsychiatry. Trends in Cognitive Sciences. 2001;5(6):271–277. doi: 10.1016/s1364-6613(00)01650-8. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biological Psychiatry. 2004;55(6):594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Sato A, Yasuda A, Ohira H, Miyawaki K, Nishikawa M, Kumano H, et al. Effects of value and reward magnitude on feedback negativity and P300. Neuroreport. 2005;16(4):407–411. doi: 10.1097/00001756-200503150-00020. [DOI] [PubMed] [Google Scholar]
- Schneirla T. An evolutionary and development theory of biphasic processes underlying approach and withdrawal. In: Jones M, editor. Nebraska Symposimu on Motivation. Lincoln: University of Nebraska Press; 1959. pp. 1–42. [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A Solution for Reliable and Valid Reduction of Ocular Artifacts, Applied to the P300 Erp. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Greene JD. Moral Judgments Recruit Domain-General Valuation Mechanisms to Integrate Representations of Probability and Magnitude. Neuron. 2010;67(4):667–677. doi: 10.1016/j.neuron.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Shiv B, Loewenstein G, Bechara A. The dark side of emotion in decision-making: When individuals with decreased emotional reactions make more advantageous decisions. Cognitive Brain Research. 2005;23(1):85–92. doi: 10.1016/j.cogbrainres.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Slovic P, Peters E, Finucane ML, MacGregor DG. Affect, risk, and decision making. Health Psychology. 2005;24(4):S35–S40. doi: 10.1037/0278-6133.24.4.S35. [DOI] [PubMed] [Google Scholar]
- Toyomaki A, Murohashi H. The ERPs to feedback indicating monetary loss and gain on the game of modified “rock–paper–scissors”. International Congress Series. 2005;1278:381–384. [Google Scholar]
- van Lankveld JJDM, Smulders FTY. The effect of visual sexual content on the event-related potential. Biological Psychology. 2008;79(2):200–208. doi: 10.1016/j.biopsycho.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhou XL. The P300 and reward valence, magnitude, and expectancy in outcome evaluation. Brain Research. 2009;1286:114–122. doi: 10.1016/j.brainres.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Yeung N, Holroyd CB, Cohen JD. ERP correlates of feedback and reward processing in the presence and absence of response choice. Cerebral Cortex. 2005;15(5):535–544. doi: 10.1093/cercor/bhh153. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. Journal of Neuroscience. 2004;24(28):6258–6264. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]