Abstract
Rift Valley fever virus (RVFV) causes severe disease in humans and livestock. There are currently no approved antivirals or vaccines for the treatment or prevention of RVF disease in humans. A major virulence factor of RVFV is the NSs protein, which inhibits host transcription including the interferon (IFN)-β gene and promotes the degradation of dsRNA-dependent protein kinase, PKR. We analyzed the efficacy of the live-attenuated MP-12 vaccine strain and MP-12 variants that lack the NSs protein as post-exposure vaccinations. Although parental MP-12 failed to elicit a protective effect in mice challenged with wild-type (wt) RVFV by the intranasal route, significant protection was demonstrated by vaccination with MP-12 strains lacking NSs when they were administered at 20 to 30 min post-exposure. Viremia and virus replication in liver, spleen and brain were also inhibited by post-exposure vaccination with MP-12 lacking NSs. The protective effect was mostly lost when vaccination was delayed 6 or 24 h after intranasal RVFV challenge. When mice were challenged subcutaneously, efficacy of MP-12 lacking NSs was diminished, most likely due to more rapid dissemination of wt RVFV. Our findings suggest that post-exposure vaccination with MP-12 lacking NSs may be developed as a novel post-exposure treatment to prevent RVF.
Keywords: Rift Valley fever virus, post-exposure vaccination, phlebovirus, viral hemorrhagic fever, treatment
1. INTRODUCTION
Rift Valley fever virus (RVFV) belongs to the genus Phlebovirus, family Bunyaviridae, and causes mosquito-borne zoonotic diseases involving humans and ruminants in endemic African countries (Schmaljohn and Nichol, 2007). The genome is comprised of three negative-stranded RNA segments. The S-segment encodes N and NSs proteins in an ambisense manner, the M-segment encodes Gn, Gc, 78kD and NSm proteins, and the L-segment encodes the RNA-dependent RNA Polymerase. NSs and NSm proteins are nonstructural, and the former is the major virulence factor for RVFV. RVFV infection in ruminants causes devastating abortion storms and newborn lambs or kids are highly susceptible to lethal disease (Pepin et al., 2010). Transmission to humans occurs through feeding of infected mosquitoes and handling or processing of infected animals (Wilson, 1994). RVFV infection typically causes a biphasic febrile illness characterized by fever, malaise, and headaches with most patients recovering without serious sequelae (Ikegami and Makino, 2011). However, in a small percentage of individuals, RVFV infection can progress to a lethal hemorrhagic fever, neurological disease or blindness, with an overall case fatality estimated to be 0.5–1% (McElroy and Nichol, 2012), although the rate has been reported to approach 30% in hospitalized cases (CDC, 2007). RVFV is a USDA and CDC overlap “Select Agent” and is classified as a Category A priority pathogen by the NIAID as it poses a significant risk to public health, agriculture, and national security (Bird et al., 2009; NIAID, 2006).
There are currently no approved antivirals for the treatment of RVF. Ribavirin has been shown to be effective against RVFV infection in nonhuman primates (Peters et al., 1986), but is only considered as an emergency provision due to associated toxicity (Borio et al., 2002; Rusnak et al., 2009; Russmann et al., 2006). While no vaccines are commercially available for RVF outside of endemic areas, formalin-inactivated RVFV (TSI-GSD-200) and live-attenuated MP-12 vaccines are at the status of Investigational New Drug for human clinical trials in the United States (Ikegami and Makino, 2009). Because North American mosquitoes are capable transmission vectors (Turell et al., 2010), RVFV could potentially cause a large outbreak involving humans and livestock. Importantly, there is no consensus therapeutic regimen or post-exposure prophylaxis to treat RVF patients or prevent the serious complications that can occur in some patients. The lack of approved interventions underscores the need for post-infection countermeasures and animal models to support early stage toxicity and efficacy studies to evaluate vaccine and drug candidates.
The MP-12 RVFV vaccine strain was developed by serial mutagenesis of ZH548 strain, which was isolated from a non-fatal human case during the Egyptian outbreak of 1977 (Caplen et al., 1985). The vaccine is highly efficacious in protecting of ruminants from disease caused by the virulent RVFV ZH501 strain, while demonstrating safety in the vaccination of ewes during late stages of pregnancy and in newborn lambs (Morrill et al., 1987; Morrill et al., 1997). MP-12 encodes attenuation mutations in the M- and L-segments, while the S-segment retains a virulent phenotype, and encodes a functional NSs gene (Billecocq et al., 2008). MP-12 NSs inhibits general host transcription including the IFN-β gene (Le May et al., 2004; Le May et al., 2008), and promotes post-translational degradation of double-stranded RNA-dependent protein kinase, PKR (Ikegami et al., 2009).
Because MP-12 lacks a marker for differentiation of infected from vaccinated animals (DIVA), the NSs gene was deleted from MP-12 to enable the virus to be used as a veterinary vaccine (Lihoradova et al., 2012). The MP-12 mutant rMP12-C13type encodes in-frame 69% truncation of NSs gene, and lacks all the NSs functions, while the rMP12-mPKRN167 mutant encodes a dominant-negative form of mouse PKR in place of NSs, thus additionally inhibiting the activation of PKR. Both candidate vaccines were shown to induce detectable type-I IFN upon vaccination in mice, while parental MP-12 did not (Lihoradova et al., 2012). Therefore, we hypothesized that post-exposure vaccination with MP-12 lacking NSs could inhibit wild-type (wt) RVFV replication at an early stage of infection resulting in protection against lethal disease. Here, we describe and compare RVFV infection in C57BL/6 mice by intranasal (i.n.) and subcutaneous (s.c.) challenge routes and employ these models to evaluate the efficacy of the MP-12 strain and the NSs deletion strains, rMP12-C13type and rMP12-mPKRN167, as post-exposure treatments.
2. MATERIALS AND METHODS
2.1. Ethics statement
All animal procedures complied with USDA guidelines and were conducted at the AAALAC-accredited Laboratory Animal Research Center at Utah State University under protocol 1502, approved by the Utah State University Institutional Animal Care and Use Committee.
2.2. Animals
Female 7–8-week-old C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) were used. The mice were quarantined for 72 h prior to challenge and fed Harlan Lab Block and tap water ad libitum.
2.3. Viruses
RVFV, strain ZH501, was obtained from Dr. Stuart Nichol (CDC, Atlanta, GA). The virus stock (1 passage in BSRT7 cells, 3 passages in Vero E6 cells) used was from a clarified cell culture lysate preparation and was diluted in sterile medium and inoculated by either intranasal i.n. instillation or s.c. injection (ventral, right side of abdomen). MP-12 was obtained from Dr. Robert Tesh (World Reference Center for Emerging Viruses and Arboviruses (WRCEVA), University of Texas Medical Branch (UTMB), Galveston, TX). The rMP12-C13type and rMP12-mPKRN167 recombinant vaccine virus strains have been previously described (Ikegami et al., 2006; Lihoradova et al., 2012). MP-12 and the NSs deletion variants were diluted in sterile PBS and administered s.c. (ventral, left side of abdomen).
2.4. Characterization of virulent RVFV infection in C57BL/6J mice
To determine the most appropriate RVFV dose for challenge efficacy studies, groups of mice (n=5) were challenged by i.n. or s.c. exposure routes with 0.1 ml of inoculum containing varying log10 dilutions of virus. The animals were observed daily for 14 days for morbidity and mortality and weighed every 3 days. Based on the virus titrations, challenge doses of 103.9 and 103.3 PFU were used for a temporal analysis studies to evaluate the development of serum and tissue virus titers, as well as aminotransferase serum concentrations during i.n. and s.c. infections, respectively. In these studies, groups of mice (n=3/group) were designated for sacrificed on days 1 through 6 and liver, lung, spleen, kidney, and brain tissues were collected for infectious virus titer determination, as described below. Serum was assayed for viral load and alanine aminotransferase (ALT) levels.
2.5. Post-exposure RVFV vaccination with NSs deletion vaccines
In the first experiment, mice (n=10–15/group) were vaccinated by s.c. injection with 105 plaque-forming units (PFU) of MP-12, rMP12-C13type, or rMP12-mPKRN167 at 20 min, 6 h, or 24 h post-infection with 103.3 PFU of RVFV by the i.n. route. Included as a negative control, one group of mice was vaccinated s.c. with the vehicle placebo at 20 min post-challenge. Cohorts of 3–5 animals from each group were sacrificed on day 3 of infection for determination of liver, spleen, and serum virus titers. For the second experiment, mice (n=22 for treatment groups, n=32 for the placebo group) were vaccinated with the vehicle placebo or 105 PFU of MP-12, rMP12-C13type, or rMP12-mPKRN167 by s.c. injection 30 minutes after contralateral s.c. challenge with 102.5 PFU of RVFV. Four animals from each group were sacrificed on days 1, 2 and 3 of infection for analysis of liver disease, viral titers, histopathology, and systemic IFN-β levels. Sham-infected normal animals were included in both studies as controls for the virus inoculation medium and to establish baselines for all parameters and the assay detection limits for the viral titers.
2.6. Tissue and serum virus titers
Virus titers were assayed using an infectious cell culture assay as previously described (Gowen et al., 2007). Briefly, tissue samples were homogenized in a fixed volume of Minimal Essential Medium (MEM) and the homogenates and serum were serially diluted and added to quadruplicate wells of Vero 76 cell monolayers in 96-well microplates. The viral cytopathic effect (CPE) was determined 4 days post-infection and the 50% endpoints were calculated as described (Reed and Muench, 1938). The detection limit for serum virus was 1.75 log10 50% cell culture infectious dose (CCID50)/ml. The lower limit of detection for tissues varied from 2.25 – 4.8 log10 CCID50/g of tissue.
2.7. Measurement of serum ALT
Detection of ALT in serum is an indirect method for evaluating liver disease. Serum ALT levels were measured using the ALT (SGPT) Reagent Set purchased from Pointe Scientific, Inc. (Lincoln Park, MI) per the manufacturer’s recommendations. The reagent volumes were adjusted for analysis on 96-well microplates.
2.8. Interferon-beta (IFN-β) enzyme linked immunosorbant assay (ELISA)
Levels of IFN-β in serum were determined using the VeriKine Mouse Interferon-beta ELISA kit (PBL Interferon Source, Piscataway, NJ) following the manufacturer’s recommendations. The kit uses a sandwich enzyme immunoassay format with recombinant mouse IFN-β expressed in mammalian cells provided as the standard. Based on dilution of serum samples, the assay lower limit of detection was 50 pg/ml.
2.9. Histopathology
For the second post-exposure vaccine efficacy study in the s.c. RVFV challenge mouse model, liver and brain samples were sent to the Utah Veterinary Diagnostic Laboratory (Logan, UT) for histopathologic examination. Sections were analyzed by a blinded board certified pathologist and lesions scored on a scale of 0–3 based on disease severity: 0 = normal, 1 = mild, 2 = moderate, 3 = severe.
2.10. Statistical analysis
The Mantel-Cox log-rank test was used for analysis of Kaplan-Meier survival curves. A one-way analysis of variance (ANOVA) with a Newman-Keuls posttest was performed to compare differences in viral titers, ALT and IFN-β concentrations, and histopathology scores. All statistical evaluations were done using Prism (GraphPad Software, CA).
3. RESULTS
3.1 Characterization of RVFV infection in C57BL/6 mice
We first titrated the ZH501 strain of RVFV in C57BL/6 mice by i.n. instillation to establish a respiratory route infection model to evaluate post-exposure vaccination interventions with MP-12 and NSs deletion viruses. The virus was uniformly lethal in 4–6 days by the i.n. route at doses of 1000 PFU or greater (Fig. 1A). At lower challenge doses (10–100 PFU), significant delay in time of death out to 6–9 days post-infection was observed. LD50 and LD90 were estimated to be approximately 30 and 260 PFU, respectively. Next, we challenged C57BL/6 mice by s.c. injection to more closely resemble mosquito-borne transmission. At a challenge dose of 103.3 PFU of RVFV, complete lethality was observed within 3–6 days (Fig. 1B). There was some variability with the intermediate doses as reflected by 60% lethality at the dose of 101.3 PFU and 20% mortality with the 102.3 PFU challenge dose. If we assume that the latter was an aberrant result and remove it from the analysis, the LD50 and LD90 were 70 and 620 PFU, respectively. Notably, 2 of the animals challenged with 101.3 PFU of RVFV succumbed 11 and 13 days after challenge (Fig. 1B), with encephalitic disease being the presumptive cause of death.
Figure 1. Survival of C57BL/6 mice challenged i.n. or s.c. with wt RVFV.
Groups of 5 mice were infected with the indicated PFU of RVFV. Mortality was monitored over a 14-day period. Survival of A) i.n. and B) s.c. challenged mice.
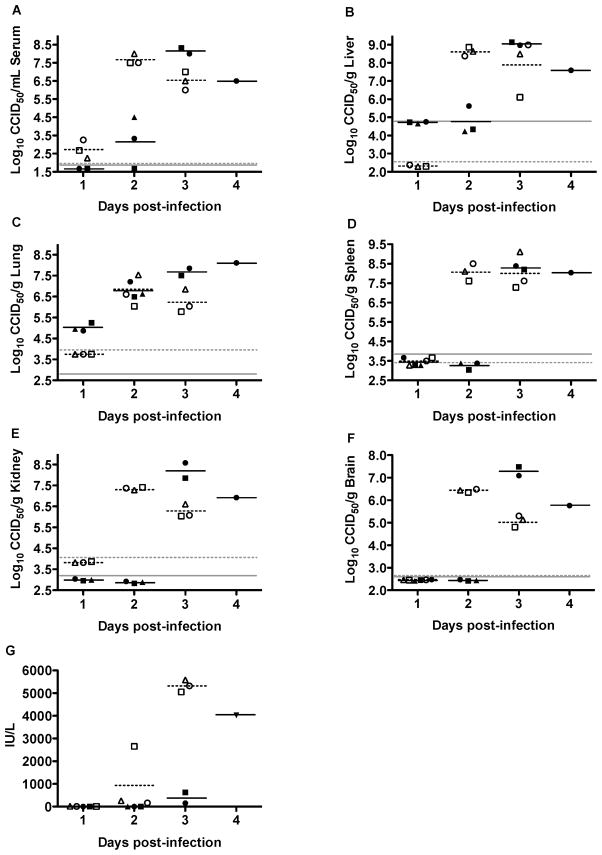
We next examined the temporal development of viremia and viral titers in tissues following i.n. and s.c. wt RVFV challenge. Mice infected s.c. with RVFV reached maximum serum virus titers (>7.5 log10 CCID50/ml) at 2 days post-infection (dpi), whereas it took 3 days for mice challenged by i.n. route to reach peak titers of 8 log10 CCID50/ml (Fig. 2A). A low level of viremia was detected as early as 1 dpi in mice challenged s.c., while viremia began on 2 dpi following i.n. challenge. Maximum viral titers were observed at 2 dpi in the liver, lung, spleen, kidney and brain of mice challenged by the s.c. route, whereas peak titers generally required 3 days to develop in mice infected i.n. (Fig. 2B–F). Notably, however, viral loads of >5 log10 CCID50/g were detected in lung tissue in mice challenged i.n. as early as 1 dpi, with titers equivalent to the s.c. infection observed on day 2 (Fig. 2C). Thus, with the exception of the lungs, dissemination of RVFV to the viscera progressed more rapidly following s.c. inoculation at the challenge doses evaluated.
Figure 2. Temporal analysis of tissue and serum virus titers and ALT levels during wt RVFV infection in C57BL/6 mice.
Animals were challenged i.n. or s.c. with 103.9 or 103.3 PFU of RVFV, respectively. Groups of 3 mice were designated for sacrifice on the specified days of infection for analysis of A) serum, B) liver, C) lung, D) spleen, E) kidney, and F) brain virus titers, and G) serum ALT concentrations. Unique symbols on each day post-infection represent values for the same animal across all parameters. The closed and open symbols represent mice challenged i.n. or s.c., respectively. The gray, solid and hashed lines indicate the limits of detection for the i.n. and s.c. challenged animals, respectively. Serum from one of the animals on day 1 was compromised and not included in the analysis. Due to death prior to time of sacrifice, data for several animals in the day 3 and 4 groups could not be obtained.
Serum ALT was used as a marker for liver damage in mice. Only 1 out of 3 animals that were challenged with wt RVFV via the s.c. route showed an elevation of ALT on day 2, but all 3 animals in the day 3 sacrifice group had >5000 IU/L (Fig. 2G). In contrast, only a slight elevation in ALT was observed on day 3 in 1 of 2 animals challenged i.n., with the only surviving mouse in the day 4 sacrifice group having a dramatically elevated ALT. Thus, the time course data suggest that day 3 is the optimal sampling time to measure the effects of candidate treatments for both challenge routes, although liver dysfunction in the i.n. challenge model may not be well resolved until day 4.
3.2. Post-exposure vaccination of mice with MP-12 lacking NSs protects mice from lethal i.n. RVFV challenge
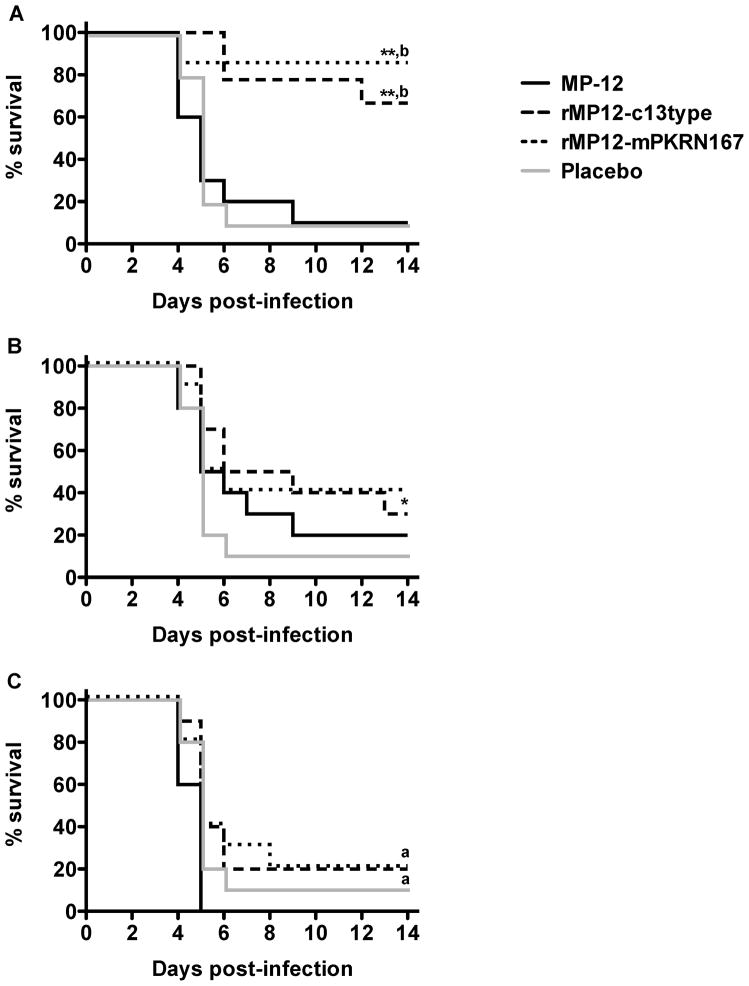
We next tested the efficacy of MP-12, rMP12-C13type and rMP12-mPKRN167 when administered s.c. at 20 min, 6 or 24 h after i.n. challenge with wt RVFV. Regardless of the time of post-exposure vaccination, most of the mice placebo-vaccinated or vaccinated with MP-12 succumbed within 4–9 days post challenge (Fig. 3). On the other hand, 70–80% of mice vaccinated with rMP12-C13type or rMP12-mPKRN167 were significantly protected from the i.n. wt RVFV infection when vaccinated 20 min post challenge (Fig 3A). Evidence of a weak protective effect compared to placebo or MP-12 vaccinations was also observed in groups that received rMP12-C13type or rMP12-mPKRN167 at 6 and 24 h post-infection (Fig. 3B, C). In parallel, sham-infected animals were also vaccinated with placebo, MP-12, rMP12-C13type or rMP12-mPKRN167. All groups appeared healthy and had similar weight increase throughout the experiment (data not shown), demonstrating that MP-12, rMP12-C13type, and rMP12-mPKRN167 vaccinations were well tolerated by the mice.
Figure 3. Effect of post-exposure vaccination with MP-12 or MP-12 lacking NSs on survival outcome in mice challenged by i.n route with wt RVFV.
Mice (n=7–10) in each group were challenged with 103.3 PFU of RVFV then treated s.c. with placebo (20 min post-exposure), or 105 PFU of MP-12, rMP12-C13type, or rMP12-mPKRN167 at A) 20 min, B) 6 h, or C) 24 h post-exposure. *P < 0.05, **P < 0.01 compared to placebo-treated animals; aP < 0.05, bP < 0.01 compared to MP-12-treated animals.
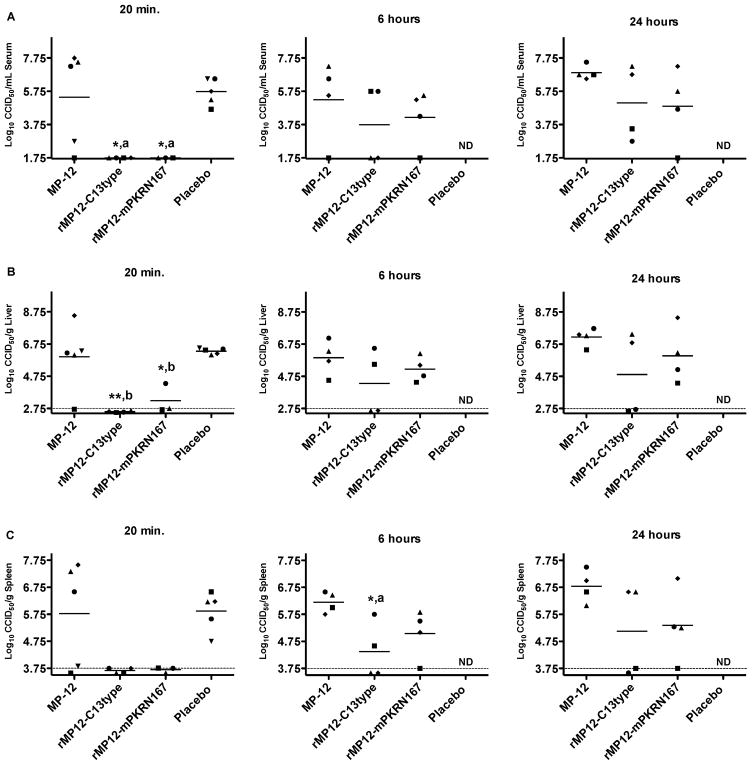
The impact of post-exposure vaccination on tissue and serum virus titers was investigated in a subset of mice sacrificed at 3 days post i.n. wt RVFV infection. In mice vaccinated with placebo 20 min after infection, all 5 animals developed high serum, liver, and spleen virus titers at 3 dpi (Fig. 4A–C, left panels). Most of the mice vaccinated with MP-12 at 20 min, 6 h or 24 h post- infection also showed abundant serum and tissue virus titers, with the exception of a few animals that were vaccinated within 20 min of wt i.n. RVFV exposure (Fig. 4). Consistent with the survival data, no virus was detected in serum, liver and spleen of mice vaccinated at 20 min post-infection with MP-12 viruses lacking NSs, with the exception of a single animal vaccinated rMP12-mPKRN167 having a low liver virus titer (Fig. 4A–C, left panels). The dramatic reductions in viral loads were still observed when vaccination was delayed until 6 and 24 h after wt RVFV challenge in 2 of 4 mice vaccinated with rMP12-C13type, and to a lesser extent with animals vaccinated with rMP12-mPKRN167 (Fig. 4A–C middle and right panels). Although not statistically significant, there was a trend that the 24 h samples collected from mice vaccinated with the parental MP-12 had slightly increased virus titers in serum, liver and spleen compared to the mice that were placebo-vaccinated at 20 min post-infection (Fig. 4A–C, right panels). This may be due to the contribution of MP-12 to the total infectious virus pool in tissues and serum. Taken together, the viral titer data suggests that the inhibition of wt RVFV replication resulting from vaccination within 20 min post-exposure is associated with a lack of NSs gene functions and the induction of type-I IFN during the early stages of infection.
Figure 4. Effect of post-exposure vaccination with MP-12 or MP-12 lacking NSs on i.n. wt RVFV replication at 3 dpi.
Mice (n=3–5 per group) were treated as described in Figure 3 and sacrificed on day 3 of infection for evaluation of A) serum, B) liver, and C) spleen viral titers. The gray hashed lines indicate the lower limits of detection. Unique symbols in each treatment group represent values for the same animal across all parameters. *P < 0.05, **P < 0.01 compared to placebo-treated animals; aP < 0.05, bP < 0.01 compared to MP-12-treated animals. ND, not determined.
3.3. Efficacy of post-exposure vaccination with NSs deletion variants against lethal s.c. RVFV challenge
We next investigated the efficacy of MP-12, rMP12-C13type, and rMP12-mPKRN167 in mice challenged with wt RVFV via the s.c. route. Vaccines were administered at 30 min post-exposure, and not at later times, because we expected more rapid dissemination and replication of wt RVFV in the liver and other organs compared to the i.n. infection (Fig. 2). We used a contralateral s.c. vaccination strategy to evaluate the systemic effect of post-exposure vaccination, rather than local reactions occurring within a specific draining lymph node. As shown in Fig. 5, vaccination with rMP12-C13type or rMP12-mPKRN167 viruses significantly improved survival outcome in mice infected with wt RVFV compared to the placebo-vaccinated animals (P < 0.001) and the MP-12-vaccinated animals (P < 0.01). All of the MP-12-vaccinated animals succumbed to the infection by day 12 and the animals receiving the placebo vaccine by day 7 (Fig 5). Comparatively, 30% of the rMP12-C13type treatment group and 20% of the rMP12-mPKRN167 treatment group survived the lethal challenge.
Figure 5. Effect of post-exposure vaccination with MP-12 or MP-12 lacking NSs on survival outcome in mice challenged s.c. with wt RVFV.
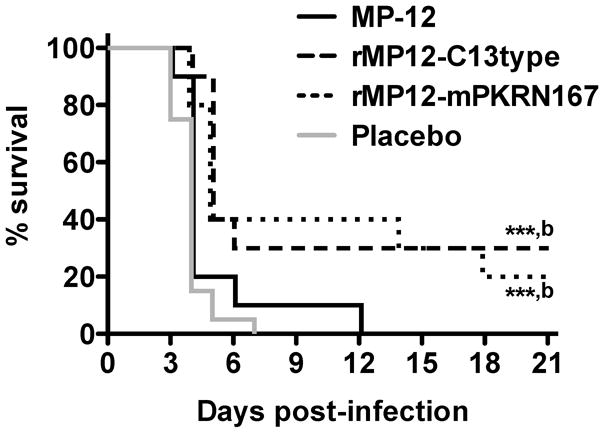
Mice in each group (n=10) were challenged with 102.5 PFU of RVFV, then treated s.c. with 105 PFU of MP-12, rMP12-C13type or rMP12-mPKRN167 at 30 minutes post-infection. ***P < 0.001 compared to animals receiving placebo (n=20), bP < 0.01 compared to animals receiving MP-12.
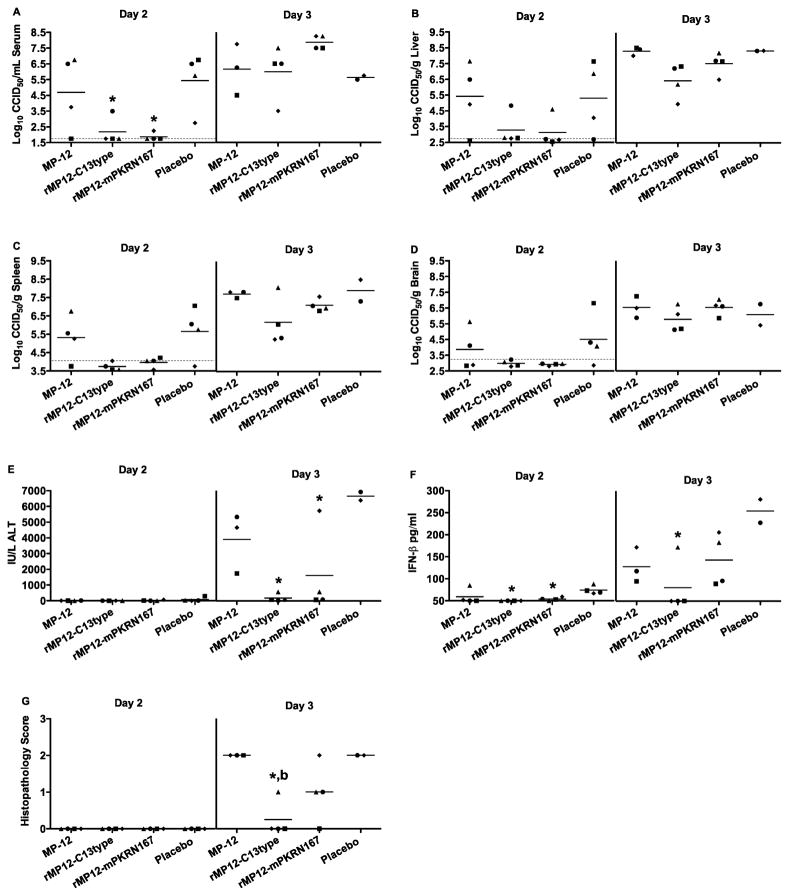
The efficacy of the 30 min post-exposure vaccinations with the NSs deletion virus were also evaluated by measurement of reductions in virus titers and several disease parameters over the first 3 days of infection. Spleen virus titers in was slightly increased in one mouse vaccinated with rMP12-C13type at 1 dpi, while all other measured parameters were at or below the limit of detection (data not shown). Virus loads were increased in most of the mice vaccinated with MP-12 or placebo at 2 dpi (Fig. 6A–D). In contrast, viral titers were at or below the limit of detection in most of the animals vaccinated with rMP12-C13type or rMP12-mPKRN167, with the exception of a couple of mice that had low level serum and/or liver virus titers. By day 3 post-infection, all mice had high levels of serum and tissue virus titers (Fig 6A–D).
Figure 6. Viral titers, serum ALT, IFN-β, and histopathology in mice challenged s.c. with wt RVFV and vaccinated with MP-12 or MP-12 lacking NSs at 30 minutes post-exposure.
Animals were treated as described in Figure 5 and sacrificed on day 1 (not shown), 2 or 3 post-infection for analysis of A) serum, B) liver, C) spleen, and D) brain virus titers, E) serum ALT, F) serum IFN-β levels, and G) histopathology of the liver. The gray hashed lines indicate the limits of detection. Unique symbols on each day of sacrifice represent values for the same animal across all parameters. Due to death prior to time of sacrifice, data for several animals in the MP-12 and placebo groups could not be obtained. *P < 0.05 compared to animals receiving placebo, bP < 0.01 compared to animals receiving MP-12.
In regards to mitigating liver damage, significantly less ALT was detected in the serum of mice vaccinated with rMP12-C13type or rMP12-mPKRN167 at 3 dpi, compared to the placebo-vaccinated animals (P < 0.05) (Fig. 6E). These results were consistent with the day 2 liver virus titer data (Fig. 6B), and are indicative of reduced liver damage in most of the mice vaccinated with the NSs deletion viruses. We also evaluated systemic IFN-β levels on days 1–3 since it is known that concentrations are increased in mice infected with wt RVFV at 3 dpi and linked to disease progression (Jansen van Vuren et al., 2011), and we had previously shown that both rMP12-C13type and rMP12-mPKRN167, but not MP-12, could induce detectable serum IFN-α at 1 day post s.c. vaccination in CD-1 mice (Lihoradova et al., 2012). Serum IFN-β levels were detectable in all mice in the placebo vaccination group at 2 dpi, with significantly reduced and nearly undetectable levels present in the animals vaccinated with the NSs deletion viruses (P < 0.05) (Fig. 6F). On day 3 post-infection, INF-β could be readily detected in mice vaccinated with MP-12, rMP12-mPKRN167, and placebo, whereas 3 of 4 mice vaccinated with rMP12-C13type did not have detectable levels (Fig. 6F). Therefore, the data suggests that the increase in IFN-β is primarily induced by the host response to wt RVFV, and not the vaccine viruses. The liver histopathology scores were also consistent with the serum ALT and day 2 liver virus titer data (Fig. 6B, E). Mice vaccinated with rMP12-C13type had significantly less hepatocellular damage than the placebo (P < 0.05) or MP-12-vaccinated animals (P < 0.01) at 3 dpi, while those vaccinated with rMP12-mPKRN167 did not have a significant reduction in liver lesions (Fig. 6G). The results suggest that vaccination with rMP12-C13type induces a liver-protective effect, which is not observed following vaccination with rMP12-mPKRN167.
At the completion of the 21-day observation period, virus titers in spleen and brain were determined in mice vaccinated with rMP12-C13type or rMP12-mPKRN167 that survived wt RVFV challenge. At 21 dpi, all the survivors had baseline levels of serum IFN-β or ALT, with no detectable virus present in liver (data not shown). Further, virus was not found in any spleen or brain tissue samples with the exception of one animal vaccinated with rMP12-mPKRN167, which had a low level of virus in the spleen (<5 log10 CCID50/g) (Fig. 7A and B). In addition to the above survivors, a mouse treated with rMP12-mPKRN167 was found moribund at 17 dpi (Fig. 5), and sacrificed for inclusion in the analysis. Similar to the survivors, the moribund mouse had baseline concentrations of IFN-β and ALT, and there was no evidence of infectious RVFV in the liver (data not shown). The moribund animal did have a low level of virus in the spleen (Fig. 7A), but most remarkable was the 1010 log10 CCID50 of virus per g of brain tissue (Fig. 7B), suggesting that it was deteriorating from a severe brain infection and associated encephalitis. Consistent with this assessment, the histopathology score reflected the severe encephalopathy observed (Fig. 7C). The brain in this animal showed multifocal, perivascular lymphocytic and neutrophilic encephalitis with necrotic debris in the neurophil surrounding some affected vessels and meninges multifocally thickened by moderate numbers of lymphocytes and neutrophils (not shown). The result suggests that rMP12-C13type may be more effective than rMP12-mPKRN167 in preventing wt RVFV replication after the acute infection.
Figure 7. Analysis of tissue viral titers and histopathology in survivors and moribund mice treated with rMP12-C13type or rMP12-mPKRN167 RVFV vaccine viruses at 30 minutes post-exposure.
Five mice survived the 21-day observation period. One mouse vaccinated with rMP12-mPKRN167 that became moribund on day 17 was also included. Shown are data for A) spleen, and B) brain virus titers, and C) histopathology of the brain. The gray hashed lines indicate the limits of detection. Unique symbols in each treatment group represent values for the same animal across all parameters.
4. DISCUSSION
The present study describes a series of experiments designed to investigate the post-exposure efficacy of the MP-12 vaccine strain and MP-12 variants lacking a functional NSs gene in C57BL/6 mice challenged by i.n. and s.c. routes with wt RVFV. A recent comprehensive study of s.c. RVFV infection in BALB/c mice described a lethal disease with acute hepatitis and encephalitis being the most prominent features (Smith et al., 2010). Survival and viral replication data obtained using our C57BL/6 model were consistent with those reported in BALB/c mice and other studies using C57BL/6 mice (Gray et al., 2012). We also observed that disease in C57BL/6 mice challenged s.c. progresses more rapidly compared to i.n. infection, as reflected by earlier time to death, and faster development of viral titers and liver disease as measured by elevation in ALT concentration. Consequently, the window of opportunity for effective post-exposure vaccination to suppress wt RVFV replication and disease likely depends on the speed of virus spread. Indeed, challenge by the i.n. route followed by vaccination with rMP12-C13type or rMP12-mPKRN167 within 20 min protected 70–80% of mice from mortality, whereas similar post-exposure vaccination against wt RVFV inoculated s.c. was less effective. However, it is important to note that the uniform lethality in the s.c. challenge study (i.n. challenge was 90% lethal), combined with the delay in post-exposure vaccination from 20 min to 30 min, likely influenced the decreased efficacy.
Previous studies employing rMP12-C13type and rMP12-mPKRN167 NSs deletion viruses have demonstrated improved immunogenicity and increased efficacy with vaccination prior to wild-type RVFV challenge (Lihoradova et al., 2012). In the present study, both the NSs deletion virus strains provided the significant protection in mice challenged i.n. with RVFV when administered 20 min post-exposure, with only a weak protective effect seen at 6 h or beyond. To a lesser extent, both NSs deletion strains also provided significant protection from mortality in the s.c. RVFV-infected mice when given as a 30-minute post-exposure vaccination. As discussed above, the reduced efficacy was consistent with a more rapid disease progression in mice infected s.c. Importantly, parental MP-12 failed to protect the mice from wt RVFV challenges when administered post-exposure. Because MP-12 NSs is functional and inhibits host innate immune responses (Billecocq et al., 2008), we interpreted the efficacy of rMP12-C13type and rMP12-mPKRN167 to be due to induction of host innate immune responses by those viruses at an early stage of wt RVFV infection. We previously showed that mice infected with Punta Toro virus could be treated post-exposure with a replication-incompetent adenovirus encoding consensus human IFN-α (Gowen et al., 2012). Studies to elucidate the precise mechanism of protection elicited by post-exposure vaccination with rMP12-C13type or rMP12-mPKRN167 will be forthcoming.
The large amount of infectious virus present in the brain on day 17 post-infection in the moribund mouse vaccinated with rMP12-mPKRN167 suggests that mice surviving beyond the acute hepatic phase of the disease succumb to encephalitis. This was also supported by the lack of viral burden in the serum and other extraneural tissues, with the exception of a low spleen virus titer, in this animal and was consistent with previous work in BALB/c mice wherein neuroinvasion and encephalitis was reported in animals succumbing later during the course of infection (Smith et al., 2010). It was possible that rMP12-C13type may be more effective than rMP12-mPKRN167 as a post-exposure vaccine. Both viruses lack NSs functions, but rMP12-mPKRN167 also encodes a dominant-negative form of mouse PKR, and therefore is designed to increase protein synthesis in infected cells (Lihoradova et al., 2012). Thus, differences in host cytokine profiles in mice vaccinated with rMP12-C13type and rMP12-mPKRN167 may result in more a favorable response with the former when administered post-exposure. Understanding the effect of these vaccines on host proteins may be useful to determine markers for successful treatment of RVF.
In summary, post-exposure vaccination of mice with MP-12 lacking NSs within 20–30 min of wt RVFV challenge significantly improved survival outcome when compared to MP-12- and placebo-vaccinated mice. In humans, there is a 4–6 day incubation period following exposure to RVFV, and in severe cases of the hemorrhagic disease, patients generally die within 3–6 days after the onset of signs of clinical illness (Ikegami and Makino, 2011). Because mice challenged with RVFV succumb to the acute phase of the infection within a day of showing disease signs such as lethargy and ruffling of fur, it is difficult to translate our results to the human condition. Nevertheless, considering the rapid progression of the disease in mice, the ability to significantly protect animals against wild-type RVFV infection with vaccination is encouraging. Our findings suggest that accidental laboratory infection or other scenarios wherein post-exposure prophylaxis can be administered within a few hours of exposure may be a safe and effective alternative to prophylaxis with ribavirin, the currently indicated emergency provision (Borio et al., 2002).
Highlights.
A model of RVFV infection in C57BL/6 mice was used for post-exposure vaccination studies.
Early post-exposure vaccination with NSs deletion viruses improves survival outcome.
Reduced viral loads and disease severity are associated with post-exposure vaccination efficacy.
Acknowledgments
We thank Luci Wandersee and Yohichi Kumaki for technical support.
BG was supported by funding from the National Institutes of Health (HHSN272201000039I) and TI was supported by R01 AI08764301-A1 (NIAID), and funding from the Sealy Center for Vaccine Development at UTMB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Billecocq A, Gauliard N, Le May N, Elliott RM, Flick R, Bouloy M. RNA polymerase I-mediated expression of viral RNA for the rescue of infectious virulent and avirulent Rift Valley fever viruses. Virology. 2008;378:377–384. doi: 10.1016/j.virol.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird BH, Ksiazek TG, Nichol ST, Maclachlan NJ. Rift Valley fever virus. J Am Vet Med Assoc. 2009;234:883–893. doi: 10.2460/javma.234.7.883. [DOI] [PubMed] [Google Scholar]
- Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, Jahrling PB, Ksiazek T, Johnson KM, Meyerhoff A, O’Toole T, Ascher MS, Bartlett J, Breman JG, Eitzen EM, Jr, Hamburg M, Hauer J, Henderson DA, Johnson RT, Kwik G, Layton M, Lillibridge S, Nabel GJ, Osterholm MT, Perl TM, Russell P, Tonat K. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA. 2002;287:2391–2405. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- Caplen H, Peters CJ, Bishop DH. Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. The Journal of General Virology. 1985;66:2271–2277. doi: 10.1099/0022-1317-66-10-2271. [DOI] [PubMed] [Google Scholar]
- CDC. Rift Valley fever outbreak--Kenya, November 2006-January 2007. MMWR Morb Mortal Wkly Rep. 2007;56:73–76. [PubMed] [Google Scholar]
- Gowen BB, Ennis J, Sefing EJ, Wong MH, Jung KH, Turner JD. Extended protection against phlebovirus infection conferred by recombinant adenovirus expressing consensus interferon (DEF201) Antimicrob Agents Chemother. 2012;56:4168–4174. doi: 10.1128/AAC.00376-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Wong MH, Jung KH, Sanders AB, Mendenhall M, Bailey KW, Furuta Y, Sidwell RW. In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother. 2007;51:3168–3176. doi: 10.1128/AAC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KK, Worthy MN, Juelich TL, Agar SL, Poussard A, Ragland D, Freiberg AN, Holbrook MR. Chemotactic and inflammatory responses in the liver and brain are associated with pathogenesis of Rift Valley fever virus infection in the mouse. PLoS Negl Trop Dis. 2012;6:e1529. doi: 10.1371/journal.pntd.0001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T, Makino S. Rift valley fever vaccines. Vaccine. 2009;27(Suppl 4):D69–72. doi: 10.1016/j.vaccine.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T, Makino S. The Pathogenesis of Rift Valley Fever. Viruses. 2011;3:493–519. doi: 10.3390/v3050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T, Narayanan K, Won S, Kamitani W, Peters CJ, Makino S. Rift Valley fever virus NSs protein promotes post-transcriptional downregulation of protein kinase PKR and inhibits eIF2alpha phosphorylation. PLoS Pathog. 2009;5:e1000287. doi: 10.1371/journal.ppat.1000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T, Won S, Peters CJ, Makino S. Rescue of infectious rift valley fever virus entirely from cDNA, analysis of virus lacking the NSs gene, and expression of a foreign gene. J Virol. 2006;80:2933–2940. doi: 10.1128/JVI.80.6.2933-2940.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen van Vuren P, Tiemessen CT, Paweska JT. Anti-nucleocapsid protein immune responses counteract pathogenic effects of Rift Valley fever virus infection in mice. PLoS One. 2011;6:e25027. doi: 10.1371/journal.pone.0025027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le May N, Dubaele S, Proietti De Santis L, Billecocq A, Bouloy M, Egly JM. TFIIH transcription factor, a target for the Rift Valley hemorrhagic fever virus. Cell. 2004;116:541–550. doi: 10.1016/s0092-8674(04)00132-1. [DOI] [PubMed] [Google Scholar]
- Le May N, Mansuroglu Z, Leger P, Josse T, Blot G, Billecocq A, Flick R, Jacob Y, Bonnefoy E, Bouloy M. A SAP30 complex inhibits IFN-beta expression in Rift Valley fever virus infected cells. PLoS Pathog. 2008;4:e13. doi: 10.1371/journal.ppat.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lihoradova O, Kalveram B, Indran SV, Lokugamage N, Juelich TL, Hill TE, Tseng CT, Gong B, Fukushi S, Morikawa S, Freiberg AN, Ikegami T. The Dominant-Negative Inhibition of Double-Stranded RNA-Dependent Protein Kinase PKR Increases the Efficacy of Rift Valley Fever Virus MP-12 Vaccine. J Virol. 2012;86:7650–7661. doi: 10.1128/JVI.00778-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy AK, Nichol ST. Rift Valley fever virus inhibits a pro-inflammatory response in experimentally infected human monocyte derived macrophages and a pro-inflammatory cytokine response may be associated with patient survival during natural infection. Virology. 2012;422:6–12. doi: 10.1016/j.virol.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill JC, Jennings GB, Caplen H, Turell MJ, Johnson AJ, Peters CJ. Pathogenicity and immunogenicity of a mutagen-attenuated Rift Valley fever virus immunogen in pregnant ewes. Am J Vet Res. 1987;48:1042–1047. [PubMed] [Google Scholar]
- Morrill JC, Mebus CA, Peters CJ. Safety and efficacy of a mutagen-attenuated Rift Valley fever virus vaccine in cattle. Am J Vet Res. 1997;58:1104–1109. [PubMed] [Google Scholar]
- NIAID; NIAID, editor. National Institute of Allergy and Infectious Diseases Biodefense Research Agenda for CDC Category A Agents. NIAID; Bethesda, MD: 2006. [Google Scholar]
- Pepin M, Bouloy M, Bird BH, Kemp A, Paweska J. Rift Valley fever virus(Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res. 2010;41:61. doi: 10.1051/vetres/2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters CJ, Reynolds JA, Slone TW, Jones DE, Stephen EL. Prophylaxis of Rift Valley fever with antiviral drugs, immune serum, an interferon inducer, and a macrophage activator. Antiviral Res. 1986;6:285–297. doi: 10.1016/0166-3542(86)90024-0. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- Rusnak JM, Byrne WR, Chung KN, Gibbs PH, Kim TT, Boudreau EF, Cosgriff T, Pittman P, Kim KY, Erlichman MS, Rezvani DF, Huggins JW. Experience with intravenous ribavirin in the treatment of hemorrhagic fever with renal syndrome in Korea. Antiviral Res. 2009;81:68–76. doi: 10.1016/j.antiviral.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russmann S, Grattagliano I, Portincasa P, Palmieri VO, Palasciano G. Ribavirin-induced anemia: mechanisms, risk factors and related targets for future research. Curr Med Chem. 2006;13:3351–3357. doi: 10.2174/092986706778773059. [DOI] [PubMed] [Google Scholar]
- Schmaljohn C, Nichol ST. Bunyaviridae. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields Virology. 5. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1741–1789. [Google Scholar]
- Smith DR, Steele KE, Shamblin J, Honko A, Johnson J, Reed C, Kennedy M, Chapman JL, Hensley LE. The pathogenesis of Rift Valley fever virus in the mouse model. Virology. 2010;407:256–267. doi: 10.1016/j.virol.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Wilson WC, Bennett KE. Potential for North American mosquitoes (Diptera: Culicidae) to transmit rift valley fever virus. J Med Entomol. 2010;47:884–889. doi: 10.1603/me10007. [DOI] [PubMed] [Google Scholar]
- Wilson ML. Rift Valley fever virus ecology and the epidemiology of disease emergence. Ann N Y Acad Sci. 1994;750:169–180. doi: 10.1111/j.1749-6632.1994.tb19867.x. [DOI] [PubMed] [Google Scholar]