Abstract
Background
Coronary endothelial function (endoFx) is abnormal in patients with established coronary artery disease (CAD) and was recently shown by MRI to relate to the severity of luminal stenosis. Recent advances in MRI now allow the non-invasive assessment of both anatomic and functional (endoFx) changes that previously required invasive studies. We tested the hypothesis that abnormal coronary endoFx is related to measures of early atherosclerosis such as increased coronary wall thickness (CWT).
Methods and Results
Seventeen arteries in fourteen healthy adults and seventeen arteries in fourteen patients with non-obstructive CAD were studied. To measure endoFx, coronary MRI was performed before and during isometric handgrip exercise, an endothelial-dependent stressor and changes in coronary cross-sectional area (CSA) and flow were measured. Black blood imaging was performed to quantify CWT and other indices of arterial remodeling. The mean stress-induced change in CSA was significantly higher in healthy adults (13.5%±12.8%, mean±SD, n=17) than in those with mildly diseased arteries (-2.2±6.8%, p<0.0001, n=17). Mean CWT was lower in healthy subjects (0.9±0.2mm) than in CAD patients (1.4±0.3mm, p<0.0001). In contrast to healthy subjects, stress-induced changes in CSA, a measure of coronary endoFx, correlated inversely with CWT in CAD patients (r= -0.73, p=0.0008).
Conclusions
There is an inverse relationship between coronary endothelial function and local CWT in CAD patients but not in healthy adults. These findings demonstrate that local endothelial-dependent functional changes are related to the extent of early anatomic atherosclerosis in mildly diseased arteries. This combined MRI approach enables the anatomic and functional investigation of early coronary disease.
Keywords: coronary disease, endothelium, magnetic resonance imaging
Coronary atherosclerosis results in anatomic changes involving increases in coronary wall thickness (CWT) and later luminal stenosis, as well as functional changes such as reduced endothelial-dependent coronary vasoreactivity.1, 2 Although both anatomic and functional changes are important indices of coronary atherosclerosis, their assessment has traditionally required invasive techniques and thus any relationship has not been delineated non-invasively in patients with early coronary atherosclerosclerotic disease, nor studied in healthy and low-risk populations.1, 3
Both anatomic and functional changes of the coronaries can now be measured non-invasively using magnetic resonance imaging (MRI). Early anatomic changes in the development of atherosclerosis include outward arterial remodeling with relative preservation of the lumen.4 Because thickening of the vessel wall precedes luminal narrowing, the degree of CAD may be underestimated with conventional, x-ray angiographic imaging of the coronary lumen.5 Using black blood MRI techniques, early increases in the coronary vessel wall thickness can be visualized6, 7 and both vessel wall thickness and area quantified,8 enabling the non-invasive detection of subclinical coronary atherosclerosis.
Abnormal coronary endothelial vasoreactivity is a functional vascular change, which predicts late cardiovascular events. 9-14 Our current understanding of coronary endothelial biology and its relationship to atherosclerotic development and progression in humans is limited, primarily because invasive imaging techniques were previously required. However, we recently developed high-field MRI methods capable of quantifying coronary endothelial vasoreactivity non-invasively with good reproducibility.15 This technique demonstrated abnormal coronary endothelial function in patients with coronary disease that, in a given patient, is heterogeneous and related to the extent of local stenotic disease.15 Luminal stenosis develops late in the atherosclerotic process and although abnormalities in endothelial function have been observed invasively in patients with mild stenotic disease,16 the extent to which both anatomic and functional changes of the coronary arteries are related in early atherosclerosis is unknown. Therefore, by means of previously described non-invasive MRI methods to assess endothelial-dependent coronary vasoreactivity15 and CWT,6,7 as one of the earliest indices of anatomic coronary atherosclerosis, we sought to test the hypothesis that local coronary endothelial vasoreactivity is inversely related to local coronary wall thickness and other indices of positive arterial remodeling in early, mildly diseased coronary arteries.
Methods
Participants
Subjects were outpatients with no contraindications to MRI. Healthy subjects were those under age 50 years without a history of coronary artery disease (CAD) and traditional CAD risk factors, and for those over age 50 with an Agatston coronary artery calcium score17 <10. CAD subjects were individuals with no unstable coronary syndromes and no history of myocardial infarction who had mild coronary artery disease (<30% maximum stenosis) in the vessel imaged (and <50% maximum stenosis in other coronaries) documented on coronary x-ray angiography performed within 8 months of the MRI exam. The subjects in the study were recruited consecutively de novo and were not a subset of subjects reported in prior published studies. The protocol was approved by the Institutional Review Board at Johns Hopkins University School of Medicine and complies with the Declaration of Helsinki. All participants provided written informed consent.
Study Protocol
A commercial human 3T MRI scanner (Achieva, Philips, Best, NL) with a 32-element cardiac coil for signal reception was used. MRI was performed in the morning after an overnight fast and before administration of prescribed vasoactive medications. Images were taken perpendicular to a proximal, linear segment of the coronary artery best identified on survey scans and without stenosis by invasive coronary angiography (Figure 1, A). When the image quality on the survey scan was equivalent for the RCA and LAD, both arteries were imaged and analyzed. To ensure slice orientation perpendicular to the coronary artery, double oblique scout scanning was performed as previously reported.18 The imaging plane for both endothelial function and wall thickness measurements was localized in a proximal or mid arterial segment that was straight over a distance of approximately 2 cm. All acquisitions were performed during a pre-specified period of least cardiac motion.19
Figure 1. Typical anatomical and functional coronary images using magnetic resonance imaging at rest and with isometric handgrip stress.
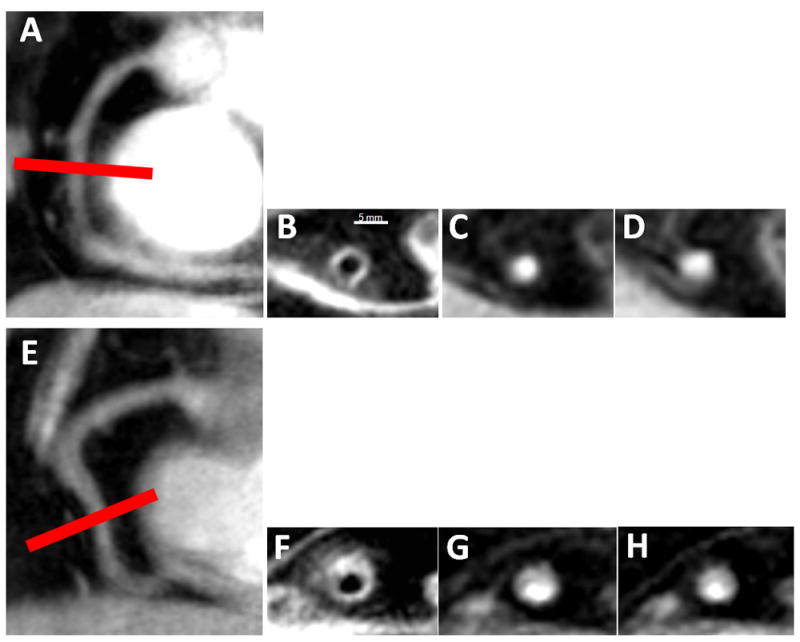
In a healthy subject, in image A, a scout scan obtained along the RCA (right coronary artery) in a healthy adult subject is shown together with the location for cross-sectional imaging (red line). In B, a view perpendicular to the RCA in a healthy adult subject is shown, illustrating a black blood vessel wall cross section. In C, a view perpendicular to the RCA in a healthy subject is shown at rest (C) and during stress (D). In a patient with CAD, shown in images E-H, a scout scan obtained parallel to the RCA is shown (E) together with the location for cross-sectional imaging (red line). F: RCA black blood vessel wall cross-sectional image in the same CAD patient. In G, a view perpendicular to the RCA in a CAD patient is shown at rest (G) and during stress (H).
Cross-sectional anatomical20 and flow velocity encoded spiral MRI21 were performed using single breath-hold cine sequences.22 Black blood coronary vessel wall imaging was performed at rest. For endothelial function imaging, alternating anatomical and velocity-encoded images were collected at baseline and during 4½ minutes of continuous isometric handgrip exercise as previously described.15 Each subject performed sustained isometric handgrip exercise using an MRI-compatible handgrip dynamometer (Stoelting, Wood Dale, IL, USA) at 30% of their maximum grip strength23 while under direct supervision. Heart rate and blood pressure were measured throughout using a non-invasive and MRI-compatible ECG and blood pressure monitor (Invivo, Precess, Orlando, FL, USA). The rate pressure product (RPP) was calculated as systolic blood pressure × heart rate.
MRI Parameters
For the assessment of endothelial function, the temporal/spatial resolution for the anatomical images was 15ms/0.89×0.89×8.0mm3 and 34ms/0.8×0.8×8mm3 for the flow velocity images with velocity encoding of 35cm per second. Approximately 15-22 cardiac phases were acquired for the coronary flow scan, depending on heart rate. The radiofrequency (RF) excitation angle was 20°, 17 spiral interleaves were acquired and all scans were prospectively triggered. For coronary vessel wall imaging, black-blood dual-inversion spiral imaging was used with a heart rate-dependent inversion time.24 MRI parameters were: echo time =0.84 ms, spectrally selective fat suppression, breath-hold duration~15-23sec, acquisition window=21ms, spatial resolution (acquired/reconstructed) = 0.6×0.6×8.00mm3 /0.49×0.49×8.00 mm3 and an RF excitation angle=45°. A 45° spectral spatial RF excitation angle was used because it led to the highest visual vessel conspicuity when compared to higher flip angles and is preferred at higher heart rates. The total duration of the MRI was 35-40 minutes.
Image Analysis
Images were analyzed for cross-sectional area changes and coronary wall thickness using a semi-automated software tool (Cine version 3.15.17, General Electric, Milwaukee, WI, USA) after being magnified four-fold. A circular region-of-interest was manually traced around the coronary artery in diastole during the period of least coronary motion. The coronary cross-sectional area was then automatically calculated using a full width half maximum computer algorithm, which was shown to be highly reproducible with good inter- and intra- observer variability on blinded analysis.15,25 For the black blood coronary cross sectional images, semi-automated measurements were made of the inner and outer vessel borders (Cine, version 3.15.17) to determine total vessel wall area and mean vessel wall thickness using the full width half maximum algorithm. The normalized wall index (NWI), a measure of arterial remodeling which accounts for differences in vessel size, was calculated by dividing the wall area by the total vessel area.26
For flow measurements, images were analyzed using semi-automated commercial software (FLOW Version 3.0, Medis, NL). Peak diastolic coronary flow velocity was used for the velocity measurement to avoid adverse effects of motion blurring and because through-plane motion of the coronaries is minimized during diastole. Coronary artery blood-flow (in ml/minute) was calculated using the adapted equation (to convert units to mL/minute) as coronary artery cross-sectional area × coronary artery peak diastolic velocity × 0.3.27
Statistical Analysis
Data are expressed as mean values ± one standard deviation. Paired Student’s t-tests were used to compare baseline and stress coronary artery cross-sectional area, diastolic coronary flow velocity and blood-flow measurements. Student’s unpaired t-tests were used to compare the changes from rest to stress in coronary cross-sectional area, peak diastolic coronary flow velocity, blood-flow measurements and hemodynamic variables between the healthy and CAD subjects as well as to compare coronary wall thicknesses between the two groups. Linear regression analysis was performed to assess the relationship between continuous variables of coronary vasoreactivity, age, body mass index (BMI) and the anatomic atherosclerosis indices of coronary wall thickness and normalized wall index. We performed separate univariate regression analysis to assess the relationships of the independent variables coronary wall thickness and normalized wall index with the outcome variable, coronary vasoreactivity (% change in CSA with stress) in the two groups. We also performed separate, univariate linear regression models to assess the relationship between the independent variable of age with the outcome variable, coronary wall thickness in the healthy population. The data were tested for normality using the Shapiro-Wilk test and the results indicated that parametric testing was appropriate. Statistical Package for the Social Sciences (SPSS) 12.0 for Windows was used for statistical analyses. Statistical significance was defined as a two-tailed p-value <0.05.
Results
All subjects completed the study without complication and no subject was excluded prior to the MRI exam. One CAD patient and one healthy volunteer were excluded from the analysis of coronary flow velocity because of poor image quality related to patient motion. A total of 17 arterial segments in 14 CAD patients and 17 segments in 14 healthy volunteers were analyzed. Baseline characteristics of the study population are shown in the Table.
Table.
Characteristics of the Subjects
| Healthy subjects (N=14) | CAD patients (N=14) | p-value | |
|---|---|---|---|
| Age -- years | 39 ± 19 | 59 ± 7 | <0.001 |
| Mean ± one SD Range | 18-66 | 49-70 | |
| Male --no. (%) | 6 (43) | 4 (28) | NS |
| Left Ventricular Ejection Fraction -- % | N/A | 58 ± 11.7 | |
| Coronary artery imaged | |||
| RCA alone --no. (%) | 8 (57) | 6 (43) | NS |
| LAD alone--no. (%) | 3 (21) | 5 (36) | NS |
| Both RCA and LAD --no. (%) | 3 (21) | 3 (21) | NS |
| CAD risk factors * Mean ± one SD | 0 | 2.3 ± 0.8 | <0.001 |
| Hypertension | 0 | 11 (79) | |
| High cholesterol | 0 | 10 (71) | |
| Smoking | 0 | 7 (50) | |
| Diabetes | 0 | 3 (21) | |
| Family history of premature CAD | 0 | 3 (21) | |
| Body Mass Index (BMI) | 23.0± 3.0 | 25.3± 6.6 | 0.05 |
| ACE-inhibitor use --no. (%) | 0 | 9 (64) | <0.001 |
| Beta-blocker use --no. (%) | 0 | 10 (71) | <0.001 |
| Statin use --no. (%) | 0 | 11 (79) | <0.001 |
Abbreviations; N/A=not available, SD=standard deviation, CAD=coronary artery disease, RCA=right coronary artery, LAD=left anterior descending artery, ACE-angiotensin converting enzyme inhibitor, NS=non-significant.
CAD risk factors excluding age and gender. One point is assigned for each risk factor: hypertension, high cholesterol, diabetes, family history of premature CAD and smoking.
Hemodynamic Effect of Isometric Handgrip Stress
Isometric handgrip exercise induced significant hemodynamic effects in both healthy subjects and those with CAD. In the healthy group, we observed a 9.6% increase in mean systolic blood pressure (p<0.0001 vs. baseline, N=14 subjects) and a 15.7% increase in mean heart rate with stress (p=0.001) while in CAD patients (N=14), the increases were 9.2% and 17.0%, respectively (both p<0.001 vs. baseline). The RPP increased by 28% with handgrip stress (p<0.001) in healthy subjects to 10784±2358mmHg*beats/minute, and by 28% (p<0.0001) in CAD patients, to 11335±1046mmHg*beats/minute. Absolute RPP during stress and the percent increase in RPP from baseline did not significantly differ between healthy subjects and CAD patients.
Coronary Vasoreactivity
In the healthy group, coronary arteries dilated significantly with stress (baseline coronary CSA, 11.1±3.4 mm2 vs. stress, 12.6±4.2 mm2, p=0.002, N=17 arteries in 14 subjects) whereas in the CAD group, the coronary CSA did not significantly change with stress (baseline area, 13.8±3.5 mm2 vs. 13.5±3.7 mm2, p=0.80, N=17 arteries in 14 subjects). The percent change in stress-induced CSA was significantly higher in healthy subjects (13.5±12.8%) than in those with CAD (-2.2±6.8%, p<0.0001, Figure 2A). The baseline coronary CSA was higher (although not significantly) in patients with CAD (13.8±3.5 mm2) than in healthy subjects (11.1±3.4 mm2, p=0.07). Coronary area measurements (% CSA change with stress) showed excellent intraobserver agreement (r =0.99, P<0.001) and interobserver agreement (r = 0.98, P<0.001) similar to prior published observations.15
Figure 2. Relative changes in coronary artery area, peak diastolic coronary flow velocity and blood-flow during isometric handgrip stress and mean coronary wall thickness.
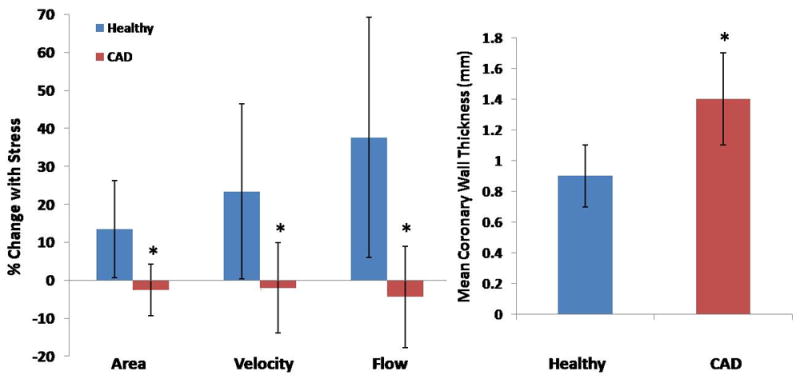
(A) Relative changes in coronary vasoreactive parameters with stress for healthy subjects (blue bars, n=17 arteries studied in 14 subjects) and patients with CAD (red bars, n=17 arteries in 14 subjects). Bars indicate standard deviations. (B) Average coronary wall thickness (mm) for healthy individuals and CAD patients (* p<0.0001 vs. healthy).
Coronary Flow Velocity and Blood-flow Measures
Peak diastolic coronary flow velocity increased in healthy subjects with stress (baseline velocity, 20.9±5.2 cm/s vs. stress 25.8±7.3 cm/s, p=0.0009, N=17) whereas there was no significant change in peak diastolic velocity in CAD subjects (baseline velocity 21.7±5.0 cm/s vs. stress 21.3±6.1 cm/s, p=0.87, N=17). The relative exercise-induced change in peak diastolic coronary flow velocity was also greater in healthy subjects (+23.4±23.0%) than in CAD patients (-1.8±11.0%, p<0.001).
In healthy subjects, coronary blood-flow increased significantly with isometric handgrip (71.5±34.5 ml/minute vs. 98.4±46.3 ml/minute, p=0.0002, N=17), while blood-flow was not significantly changed with stress in CAD patients (91.5±38.1ml/minute vs. 88.5±41.5ml/minute, p=0.83, N=17). The percentage change in coronary blood-flow changes with stress was significantly greater in the healthy group (+37.6±31.7%) than in the CAD group (-4.3±13.0%, p<0.0001, Figure 2A).
Coronary Wall Thickness
Mean coronary wall thickness was significantly greater (1.4±0.3mm) in patients with mild CAD than in healthy subjects (0.9±0.2mm, p<0.0001 healthy vs. CAD, Figure 2B, N=17 each group) as was the mean coronary vessel wall area (19.8±5.6 mm2 vs. 11.5±3.0 mm2, p<0.0001). The normalized wall index (NWI), a marker of positive arterial remodeling, was also significantly higher in CAD patients (0.72±0.08, N=17) than in the healthy group (0.59±0.08, p=0.0009, N=17). Consistent with prior observations,28,29 wall thickness measurements showed excellent intraobserver (r =0.99, P<0.001) and interobserver agreement (r = 0.98, P<0.001).
Relationship between Arterial Remodeling and Endothelial-dependent Coronary Vasoreactivity
In the entire population of healthy adults and CAD patients combined (N=34 arterial segments), there was a significant relationship between CWT and % coronary artery area change with stress (r=-0.64, p<0.0001, Figure 3). However, in the healthy group alone we observed no significant relationship between CWT and % CSA with handgrip stress (r= -0.19, p=0.47, Figure 4A). In contrast, in the CAD patient group, there was a strong inverse relationship between CWT and % area change with stress (r= -0.73, p=0.0008, Figure 4B). The significant relationship between the two parameters persisted even after excluding those CAD patients whose endothelial function was in the top and bottom 10% of the group (r = -0.67, p=0.001). Similarly, there was no significant relationship between NWI and stress-induced % CSA change in healthy subjects (r= -0.11, p=0.67), while we observed a strong inverse relationship between NWI and % CSA change in the CAD patients (r= -0.67, p=0.003, Figure 5).
Figure 3. MRI measures of coronary wall thickness (mm) versus % coronary cross sectional area changes with isometric handgrip stress in healthy subjects and patients with coronary artery disease (combined).
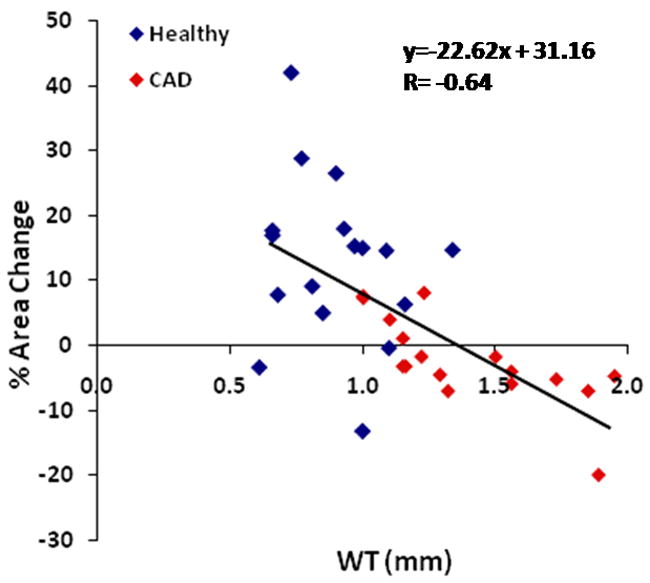
Individual data points shown for healthy subjects (blue diamonds) and patients with coronary artery disease (red diamonds). The standard error of the estimate (SEE)=0.27.
Figure 4. MRI measures of coronary wall thickness (CWT) versus % coronary cross sectional area changes and % velocity changes with isometric handgrip stress.
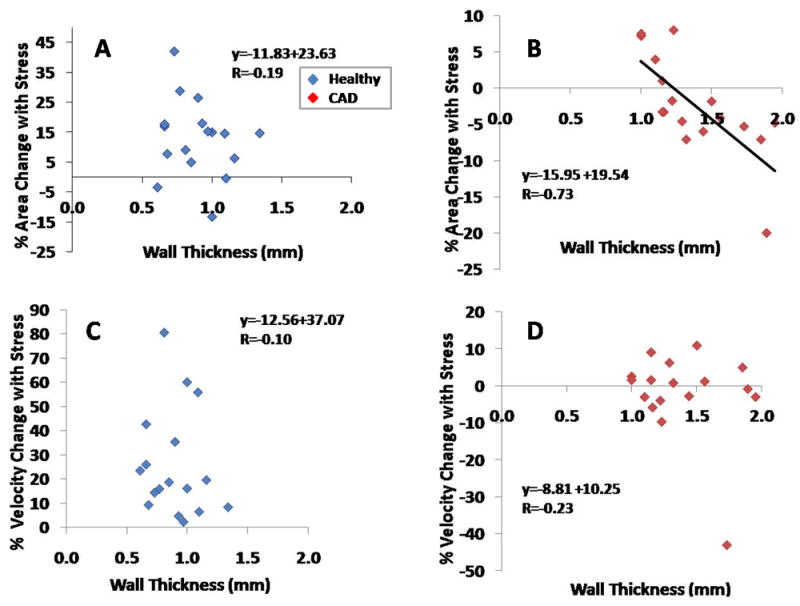
Individual data points shown for healthy subjects (blue diamonds) and for patients with coronary artery disease (red diamonds). CWT (mm) versus % coronary area change with stress is shown for (A) healthy subjects (standard error of the estimate, SEE=0.18), and (B) patients with CAD (SEE=0.22). Coronary wall thickness versus % coronary velocity change with stress is shown in (C) for healthy subjects (SEE=0.18) and (D) for CAD patients (SEE=0.31).
Figure 5.
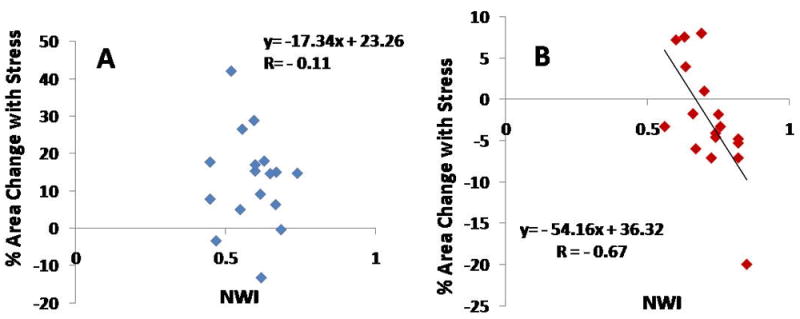
MRI measure of normalized wall index (NWI) versus % coronary cross sectional area change with isometric handgrip stress in (A) healthy subjects (standard error of the estimate, SEE=0.08) and (B) patients with coronary artery disease (SEE=0.06).
There was no significant relationship between CWT and % coronary velocity change with stress in either healthy adults (r= -0.10, p=0.66) or CAD patients (r= -0.23, p=0.37, Figure 4). Although there was a significant inverse relationship between CWT and % coronary flow change with stress in CAD patients (r= -0.58 p=0.01), this was not observed in healthy subjects (r= -0.19, p=0.46).
Because of the small sample size, multivariate analysis with respect to coronary risk factors and age could not be performed, although univariate linear regression analysis of the healthy subjects indicated a significant correlation between age and CWT (r= 0.66, p=0.009). A comparison of a subset of the seven oldest healthy subjects (mean age 54.9 ± 10.9) with CAD patients (mean age 59 ± 7.0 years, p=0.75) still showed significant differences in both CWT and % CSA change with stress between the mild CAD and healthy age-matched group (mean CWT=1.06 ± 0.2mm healthy, p=0.01 vs. mild CAD CWT; % CSA change with stress=9.0% ± 9.7, p=0.03 vs. mild CAD % CSA change with stress).
Discussion
This study demonstrated that abnormal coronary endothelial function and increased coronary wall thickness can be detected during a single, non-invasive 3T MRI exam and, importantly, that abnormal coronary endothelial-dependent vasoreactivity is present locally in mildly diseased coronaries and related to the earliest non-invasive in vivo measure of local coronary atherosclerosis, an increase in coronary artery wall thickness. We previously reported that coronary endothelial function was closely related to the degree of luminal stenosis.15 Our current pilot study in patients with non-obstructive CAD demonstrate that local functional and anatomic changes of the coronary arteries are closely related in early atherosclerosis, before the development of significant luminal stenoses, a late occurrence in the progression of atherosclerotic disease.
The present study detected significantly higher mean coronary wall thickness, wall area, and normalized wall index in patients with mild CAD compared to those of healthy subjects. This increase in wall thickness in CAD patients relative to healthy subjects and preservation of luminal area is indicative of positive arterial remodeling.4
The values for coronary wall area, wall thickness, and coronary endothelial function reported here are similar to those previously reported using MRI6-8, 15 and invasive techniques2, 10, 30-32 in separate studies, although the endothelial-dependent stressors varied across studies. The observation that mild structural and/or functional coronary disease may contribute to reduced endothelial-dependent coronary flow supports earlier PET vasomotor flow studies.33, 34 However, the ability to measure both vessel wall remodeling and endothelial-dependent coronary vasoreactivity in a single non-invasive exam, demonstrated here for the first time, enables a more complete measure of early atherosclerotic disease than previously possible. Finally, the length of the coronary protocol makes it feasible to combine the imaging sequence with an MR evaluation of left ventricular structure or function, leading to a more comprehensive cardiac exam.
In a recent report, abnormal coronary endothelial function varied among arteries in a given CAD patient and was related to the severity of luminal stenosis.15 The observation here that coronary wall thickness and local endothelial function are associated in patients with mild, non-stenotic CAD is novel and demonstrates that anatomic and functional early atherosclerosis not only co-exist in coronary arteries but are also closely related. Although structural alterations in the arterial wall may impair flow-mediated epicardial vasodilation, we previously showed that the administration of nitroglycerin to patients with significant CAD dilated the same arteries that constricted with isometric handgrip exercise.15 That demonstration that endothelial-independent mechanisms are intact shows that the likely mechanism for impaired epicardial vasodilation during isometric handgrip exercise is likely endothelial dysfunction rather than a mechanical disturbance such as may occur with heavy coronary calcification, especially in these patients with very mild atherosclerosis.
In contrast to the findings in CAD patients, there was no relationship between coronary endothelial function and CWT in healthy subjects. Though prior work demonstrated that stimuli like tobacco abuse or high circulating lipids35,36 can induce transitory abnormal endothelial function in healthy subjects, all such stimuli were avoided here by protocol design.
Although our study detected a relationship between endothelial-dependent coronary vasodilation (area change) and wall thickness in arteries with mild atherosclerosis, we found no apparent relationship between coronary velocity change with stress and early anatomic atherosclerosis in either group. These findings suggest that early local anatomic atherosclerotic changes are more closely related to measures of, and therefore, local coronary endothelial function (e.g. area change within the same epicardial coronary segment) than to endothelial function measures which incorporate non-local parameters (e.g. coronary flow velocity).
The extent of early anatomic atherosclerosis and endothelial function has been well characterized in peripheral arterial beds.37, 38 Several previous studies evaluated the relationship between brachial endothelial function (flow mediated dilation) and carotid intimal medial thickness using B-mode ultrasound and reported varying results.37-39 Although earlier studies found a positive relationship between carotid intimal medial thickness and peripheral endothelial function,37 more recent studies found no significant correlation between the two parameters.38, 39 Those findings may be explained by the fact that different vascular beds were evaluated for anatomic and functional measurements of atherosclerotic disease.
Although atherosclerosis is a systemic process, studies of different vascular territories have shown that vasoreactivity may not be uniform across vascular regions within the same individual. One study using MRI compared changes in flow and vessel radius before and during post-occlusion hyperemia in the upper (brachial) vs. lower (femoral) extremities and found that femoral but not brachial reactivity was impaired in patients with increased cardiovascular risk.40 Other data suggest that peripheral and coronary endothelial function measures may not be strongly related41, 42 possibly due to differences in vascular properties.43 Moreover, acute brachial arterial plaque rupture rarely occurs in contrast to acute coronary plaque rupture. Although peripheral and coronary endothelial vasoreactivity have not been directly compared using the same stressor and imaging modality, our previous data show significant heterogeneity of coronary endothelial function within the coronary tree.15 Thus, the non-invasive measurement of coronary endothelial function is likely more relevant for defining factors related to local coronary artery atherosclerosis and plaque progression.
In vivo human studies evaluating coronary endothelial function and its relationship to early vessel remodeling have not been performed before, likely because invasive techniques were required. The most commonly used technique to identify positive coronary arterial remodeling has been intravascular ultrasound (IVUS).44,45 Traditionally, studies of coronary endothelial function have been performed during x-ray coronary angiography with acetylcholine or cold pressor testing as the endothelial-dependent stressor.2, 9 Those techniques, however, are not suitable for screening low risk and asymptomatic populations because of their invasive nature and associated risk. Although multi-detector computed tomography (MDCT) has been used to assess positive remodeling and plaque progression in patients with CAD,46 the exposure to ionizing radiation and contrast media limit repeated studies and its use in low risk populations. It is also unable to measure coronary velocity or flow for the assessment of endothelial function. Black blood coronary wall MRI can detect coronary arterial wall changes indicative of positive arterial remodeling6, 7 with good reproducibility.28, 29 Moreover, MRI studies of the coronaries may be safely applied to low risk populations to noninvasively quantify coronary vessel wall dimensions and to measure endothelial dependent vasoreactivity. There were no noteworthy variations in image quality with respect to BMI, heart rate or other clinical parameters and the sequences performed equally well with the RCA and LAD in most cases. Lastly, in our study, the use of an MRI contrast agent (gadolinium) was not necessary, which offers the ability to safely study patients with renal insufficiency.
One limitation to the current study is that the spatial resolution of MR imaging of the coronaries is not able to distinguish separate layers of the vessel wall or plaque components. Therefore, radiofrequency intravascular ultrasonography determination of early anatomical changes in the coronary arterial wall may be more sensitive at detecting and characterizing early disease than MRI, however, we were not able to ethically justify the risk associated with invasive coronary procedures in healthy and low risk subjects studied here as an alternative validation approach. In addition, we are currently limited in the choice of the coronary segment that can be imaged. Although we primarily focused on proximal and mid coronary segments of the RCA or LAD to evaluate stress-induced area changes, the ability to also measure coronary velocity and flow permits a more global assessment of downstream endothelial function that complements the measurement of local epicardial vasoreactivity. Therefore, technical developments designed to improve spatial resolution and volumetric coverage will likely advance endothelial function studies and permit greater flexibility in the choice of the imaging plane and local characterization of plaque morphology. Another limitation to this pilot study is the relatively small sample size. However, with only 34 coronary arteries investigated, we observed significant differences in both positive arterial remodeling and vasomotor responses to a known endothelial-dependent stressor between healthy and CAD subjects. Lastly, the two groups were not age-matched. However, on further statistical analysis of a subset of healthy subjects age-matched to the CAD patients, significant differences in CWT and exercise-induced change in CSA were still observed between mild CAD patients and healthy age-matched controls. Because of the small sample size, multivariate analysis with respect to coronary risk factors and age could not be performed.
In summary, the present findings demonstrate that local coronary endothelial function is inversely related to local positive arterial remodeling in patients with non-obstructive CAD. These findings indicate that early non-stenotic atherosclerosis is associated with abnormal local endothelial function and therefore that anatomic and physiologic indicators of coronary vascular pathology are related in patients at the earliest stages of coronary atherosclerosis that can be detected non-invasively in humans. Moreover, it would be feasible to combine this imaging protocol with an MRI evaluation of left ventricular function, leading to a more comprehensive evaluation of cardiac risk. This may contribute to improved detection and monitoring of atherosclerotic disease and its response to therapy at an early, preclinical stage as well as the ability to non-invasively investigate anatomic and functional predictors of disease progression.
Clinical Summary.
This study demonstrates, for the first time 1) that abnormal coronary endothelial function and increased coronary wall thickness can be detected during a single, non-invasive 3T MRI exam and 2) that abnormal coronary endothelial-dependent vasoreactivity is present locally in mildly diseased coronaries and related to the earliest non-invasive in vivo measure of local coronary atherosclerosis, an increase in coronary artery wall thickness. Thus in a given coronary artery segment, early anatomic markers of coronary atherosclerosis are closely linked to functional markers in that segment that may occur long before the development of severe stenotic disesase. The ability to non-invasively and without contrast evaluate both structural subclinical atherosclerosis and coronary endothelial function provides an opportunity to evaluate both aspects of the coronary response to therapeutic intervention. This may also contribute to improved detection and monitoring of atherosclerotic disease and its response to therapy at an early, preclinical stage as well as the ability to non-invasively investigate anatomic and functional predictors of disease progression.
Acknowledgments
The authors thank Angela Steinberg, RN and Rob van der Geest, PhD for their assistance and the patients and healthy volunteers for their participation in this study.
Sources of Funding
This work was supported by National Institutes of Health [R01-HL084186, ARRA 3R01-Hl084186-04S1, R01-HL61912], and by the Donald W. Reynolds Foundation and the Clarence Doodeman Endowment. Dr. Kelle is supported by a scholarship from the German Cardiac Society. Dr. Hays is supported by a grant from the American Heart Association.
Footnotes
Disclosures
None.
References
- 1.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: Testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 2.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 3.Ganz P, Vita JA. Testing endothelial vasomotor function: Nitric oxide, a multipotent molecule. Circulation. 2003;108:2049–2053. doi: 10.1161/01.CIR.0000089507.19675.F9. [DOI] [PubMed] [Google Scholar]
- 4.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 5.Hodgson JM, Reddy KG, Suneja R, Nair RN, Lesnefsky EJ, Sheehan HM. Intracoronary ultrasound imaging: Correlation of plaque morphology with angiography, clinical syndrome and procedural results in patients undergoing coronary angioplasty. J Am Coll Cardiol. 1993;21:35–44. doi: 10.1016/0735-1097(93)90714-c. [DOI] [PubMed] [Google Scholar]
- 6.Fayad ZA, Fuster V, Fallon JT, Jayasundera T, Worthley SG, Helft G, Aguinaldo JG, Badimon JJ, Sharma SK. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation. 2000;102:506–510. doi: 10.1161/01.cir.102.5.506. [DOI] [PubMed] [Google Scholar]
- 7.Kim WY, Stuber M, Bornert P, Kissinger KV, Manning WJ, Botnar RM. Three-dimensional black-blood cardiac magnetic resonance coronary vessel wall imaging detects positive arterial remodeling in patients with nonsignificant coronary artery disease. Circulation. 2002;106:296–299. doi: 10.1161/01.cir.0000025629.85631.1e. [DOI] [PubMed] [Google Scholar]
- 8.Botnar RM, Stuber M, Kissinger KV, Kim WY, Spuentrup E, Manning WJ. Noninvasive coronary vessel wall and plaque imaging with magnetic resonance imaging. Circulation. 2000;102:2582–2587. doi: 10.1161/01.cir.102.21.2582. [DOI] [PubMed] [Google Scholar]
- 9.Nitenberg A, Chemla D, Antony I. Epicardial coronary artery constriction to cold pressor test is predictive of cardiovascular events in hypertensive patients with angiographically normal coronary arteries and without other major coronary risk factor. Atherosclerosis. 2004;173:115–123. doi: 10.1016/j.atherosclerosis.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 10.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 11.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 12.Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, Zhang J, Boccuzzi SJ, Cedarholm JC, Alexander RW. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481–487. doi: 10.1056/NEJM199502233320801. [DOI] [PubMed] [Google Scholar]
- 13.Schindler TH, Nitzsche EU, Munzel T, Olschewski M, Brink I, Jeserich M, Mix M, Buser PT, Pfisterer M, Solzbach U, Just H. Coronary vasoregulation in patients with various risk factors in response to cold pressor testing: Contrasting myocardial blood flow responses to short- and long-term vitamin c administration. J Am Coll Cardiol. 2003;42:814–822. doi: 10.1016/s0735-1097(03)00851-9. [DOI] [PubMed] [Google Scholar]
- 14.Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107:2805–2809. doi: 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- 15.Hays AG, Hirsch GA, Kelle S, Gerstenblith G, Weiss RG, Stuber M. Noninvasive visualization of coronary artery endothelial function in healthy subjects and in patients with coronary artery disease. J Am Coll Cardiol. 2010;56:1657–1665. doi: 10.1016/j.jacc.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 16.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 18.Stuber M, Botnar RM, Danias PG, Sodickson DK, Kissinger KV, Van Cauteren M, De Becker J, Manning WJ. Double-oblique free-breathing high resolution three-dimensional coronary magnetic resonance angiography. J Am Coll Cardiol. 1999;34:524–531. doi: 10.1016/s0735-1097(99)00223-5. [DOI] [PubMed] [Google Scholar]
- 19.Kim WY, Stuber M, Kissinger KV, Andersen NT, Manning WJ, Botnar RM. Impact of bulk cardiac motion on right coronary mr angiography and vessel wall imaging. J Magn Reson Imaging. 2001;14:383–390. doi: 10.1002/jmri.1198. [DOI] [PubMed] [Google Scholar]
- 20.Meyer CH, Hu BS, Nishimura DG, Macovski A. Fast spiral coronary artery imaging. Magn Reson Med. 1992;28:202–213. doi: 10.1002/mrm.1910280204. [DOI] [PubMed] [Google Scholar]
- 21.Keegan J, Gatehouse PD, Yang GZ, Firmin DN. Spiral phase velocity mapping of left and right coronary artery blood flow: Correction for through-plane motion using selective fat-only excitation. J Magn Reson Imaging. 2004;20:953–960. doi: 10.1002/jmri.20208. [DOI] [PubMed] [Google Scholar]
- 22.Terashima M, Meyer CH, Keeffe BG, Putz EJ, de la Pena-Almaguer E, Yang PC, Hu BS, Nishimura DG, McConnell MV. Noninvasive assessment of coronary vasodilation using magnetic resonance angiography. J Am Coll Cardiol. 2005;45:104–110. doi: 10.1016/j.jacc.2004.09.057. [DOI] [PubMed] [Google Scholar]
- 23.Weiss RG, Bottomley PA, Hardy CJ, Gerstenblith G. Regional myocardial metabolism of high-energy phosphates during isometric exercise in patients with coronary artery disease. N Engl J Med. 1990;323:1593–1600. doi: 10.1056/NEJM199012063232304. [DOI] [PubMed] [Google Scholar]
- 24.Fleckenstein JL, Archer BT, Barker BA, Vaughan JT, Parkey RW, Peshock RM. Fast short-tau inversion-recovery mr imaging. Radiology. 1991;179:499–504. doi: 10.1148/radiology.179.2.2014300. [DOI] [PubMed] [Google Scholar]
- 25.Kelle S, Hays AG, Hirsch GA, Gerstenblith G, Miller JM, Steinberg AM, Schar M, Texter JH, Wellnhofer E, Weiss RG, Stuber M. Coronary artery distensibility assessed by 3.0 tesla coronary magnetic resonance imaging in subjects with and without coronary artery disease. Am J Cardiol. 2011;108:491–497. doi: 10.1016/j.amjcard.2011.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saam T, Yuan C, Chu B, Takaya N, Underhill H, Cai J, Tran N, Polissar NL, Neradilek B, Jarvik GP, Isaac C, Garden GA, Maravilla KR, Hashimoto B, Hatsukami TS. Predictors of carotid atherosclerotic plaque progression as measured by noninvasive magnetic resonance imaging. Atherosclerosis. 2007;194:e34–42. doi: 10.1016/j.atherosclerosis.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doucette JW, Corl PD, Payne HM, Flynn AE, Goto M, Nassi M, Segal J. Validation of a doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85:1899–1911. doi: 10.1161/01.cir.85.5.1899. [DOI] [PubMed] [Google Scholar]
- 28.Desai MY, Lai S, Barmet C, Weiss RG, Stuber M. Reproducibility of 3d free-breathing magnetic resonance coronary vessel wall imaging. Eur Heart J. 2005;26:2320–2324. doi: 10.1093/eurheartj/ehi357. [DOI] [PubMed] [Google Scholar]
- 29.Hazirolan T, Gupta SN, Mohamed MA, Bluemke DA. Reproducibility of black-blood coronary vessel wall mr imaging. J Cardiovasc Magn Reson. 2005;7:409–413. doi: 10.1081/jcmr-200053464. [DOI] [PubMed] [Google Scholar]
- 30.von Birgelen C, Klinkhart W, Mintz GS, Papatheodorou A, Herrmann J, Baumgart D, Haude M, Wieneke H, Ge J, Erbel R. Plaque distribution and vascular remodeling of ruptured and nonruptured coronary plaques in the same vessel: An intravascular ultrasound study in vivo. J Am Coll Cardiol. 2001;37:1864–1870. doi: 10.1016/s0735-1097(01)01234-7. [DOI] [PubMed] [Google Scholar]
- 31.Brown BG, Lee AB, Bolson EL, Dodge HT. Reflex constriction of significant coronary stenosis as a mechanism contributing to ischemic left ventricular dysfunction during isometric exercise. Circulation. 1984;70:18–24. doi: 10.1161/01.cir.70.1.18. [DOI] [PubMed] [Google Scholar]
- 32.Nabel EG, Ganz P, Gordon JB, Alexander RW, Selwyn AP. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation. 1988;77:43–52. doi: 10.1161/01.cir.77.1.43. [DOI] [PubMed] [Google Scholar]
- 33.Gould KL, Nakagawa Y, Nakagawa K, Sdringola S, Hess MJ, Haynie M, Parker N, Mullani N, Kirkeeide R. Frequency and clinical implications of fluid dynamically significant diffuse coronary artery disease manifest as graded, longitudinal, base-to-apex myocardial perfusion abnormalities by noninvasive positron emission tomography. Circulation. 2000;101:1931–1939. doi: 10.1161/01.cir.101.16.1931. [DOI] [PubMed] [Google Scholar]
- 34.Schindler TH, Facta AD, Prior JO, Cadenas J, Zhang XL, Li Y, Sayre J, Goldin J, Schelbert HR. Structural alterations of the coronary arterial wall are associated with myocardial flow heterogeneity in type 2 diabetes mellitus. Eur J Nucl Med Mol Imaging. 2009;36:219–229. doi: 10.1007/s00259-008-0885-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams MJ, Sutherland WH, McCormick MP, de Jong SA, Walker RJ, Wilkins GT. Impaired endothelial function following a meal rich in used cooking fat. J Am Coll Cardiol. 1999;33:1050–1055. doi: 10.1016/s0735-1097(98)00681-0. [DOI] [PubMed] [Google Scholar]
- 36.Rudolph TK, Ruempler K, Schwedhelm E, Tan-Andresen J, Riederer U, Boger RH, Maas R. Acute effects of various fast-food meals on vascular function and cardiovascular disease risk markers: The hamburg burger trial. Am J Clin Nutr. 2007;86:334–340. doi: 10.1093/ajcn/86.2.334. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto M, Eto M, Akishita M, Kozaki K, Ako J, Iijima K, Kim S, Toba K, Yoshizumi M, Ouchi Y. Correlation between flow-mediated vasodilatation of the brachial artery and intima-media thickness in the carotid artery in men. Arterioscler Thromb Vasc Biol. 1999;19:2795–2800. doi: 10.1161/01.atv.19.11.2795. [DOI] [PubMed] [Google Scholar]
- 38.Yan RT, Anderson TJ, Charbonneau F, Title L, Verma S, Lonn E. Relationship between carotid artery intima-media thickness and brachial artery flow-mediated dilation in middle-aged healthy men. J Am Coll Cardiol. 2005;45:1980–1986. doi: 10.1016/j.jacc.2004.12.079. [DOI] [PubMed] [Google Scholar]
- 39.Lind L, Andersson J, Hansen T, Johansson L, Ahlstrom H. Atherosclerosis measured by whole body magnetic resonance angiography and carotid artery ultrasound is related to arterial compliance, but not to endothelium-dependent vasodilation - the prospective investigation of the vasculature in uppsala seniors (pivus) study. Clin Physiol Funct Imaging. 2009;29:321–329. doi: 10.1111/j.1475-097X.2009.00871.x. [DOI] [PubMed] [Google Scholar]
- 40.Silber HA, Lima JA, Bluemke DA, Astor BC, Gupta SN, Foo TK, Ouyang P. Arterial reactivity in lower extremities is progressively reduced as cardiovascular risk factors increase: Comparison with upper extremities using magnetic resonance imaging. J Am Coll Cardiol. 2007;49:939–945. doi: 10.1016/j.jacc.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 41.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 42.Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol. 1998;82:1535–1539. doi: 10.1016/s0002-9149(98)00702-4. [DOI] [PubMed] [Google Scholar]
- 43.Hirooka Y, Egashira K, Imaizumi T, Tagawa T, Kai H, Sugimachi M, Takeshita A. Effect of l-arginine on acetylcholine-induced endothelium-dependent vasodilation differs between the coronary and forearm vasculatures in humans. J Am Coll Cardiol. 1994;24:948–955. doi: 10.1016/0735-1097(94)90854-0. [DOI] [PubMed] [Google Scholar]
- 44.McPherson DD, Sirna SJ, Hiratzka LF, Thorpe L, Armstrong ML, Marcus ML, Kerber RE. Coronary arterial remodeling studied by high-frequency epicardial echocardiography: An early compensatory mechanism in patients with obstructive coronary atherosclerosis. J Am Coll Cardiol. 1991;17:79–86. doi: 10.1016/0735-1097(91)90707-g. [DOI] [PubMed] [Google Scholar]
- 45.Nissen SE, Gurley JC, Grines CL, Booth DC, McClure R, Berk M, Fischer C, DeMaria AN. Intravascular ultrasound assessment of lumen size and wall morphology in normal subjects and patients with coronary artery disease. Circulation. 1991;84:1087–1099. doi: 10.1161/01.cir.84.3.1087. [DOI] [PubMed] [Google Scholar]
- 46.Achenbach S, Ropers D, Hoffmann U, MacNeill B, Baum U, Pohle K, Brady TJ, Pomerantsev E, Ludwig J, Flachskampf FA, Wicky S, Jang IK, Daniel WG. Assessment of coronary remodeling in stenotic and nonstenotic coronary atherosclerotic lesions by multidetector spiral computed tomography. J Am Coll Cardiol. 2004;43:842–847. doi: 10.1016/j.jacc.2003.09.053. [DOI] [PubMed] [Google Scholar]


